Abstract
Objective
This study analyzed relationship between prenatal mood states and serological evidence of immune response to Toxoplasma gondii (T.gondii). A secondary aim was to determine if thyroid peroxidase (TPO) autoantibody status was related to T.gondii status.
Study Design
Pregnant women (N=414) were measured between 16 to 25 weeks gestation with demographic and mood questionnaires and a blood draw. All plasma samples were analyzed for TPO and T. gondii IgG, tryptophan, kynurenine and neopterin. Cytokines were available on a subset (N=142).
Results
Women with serological evidence of exposure to T. gondii (N=44) showed positive correlations between IgG levels, and the Profile of Mood Scales (POMS) depression and anxiety subscales. Plasma TNF-α was higher in T. gondii positive women.
Conclusions
Higher T. gondii IgG titers in infected women were related to anxiety and depression during pregnancy. Reactivation of T.gondii or immune responses to T.gondii may alter mood in pregnant women.
Keywords: Toxoplasma gondii, depression, pregnancy
Introduction
The pathophysiology of prenatal depression, which occurs in up to 20% of pregnant women, is incompletely understood. While genetics, environment, and endocrine influences play roles in the etiology, another potential pathway is through pregnancy related biological phenomena. Thyroid disease, chronic latent infection, and the immune changes of pregnancy are also possible factors that contribute to prenatal depression. Thyroid peroxidase autoantibodies (TPO) are present in about 10% of pregnant women, and half of these women develop postpartum thyroiditis.1 Depression is known to be higher in TPO positive women during the postpartum period.2. One of the authors (Yolken) had recently contributed to a study relating TPO antibody status with Toxoplasmosis gondii infection in a large sample of pregnant women, suggesting that T.gondii activation might prodiuce a bystander effect upregualting autoimmunity.3 We had previously collected clinical and psychiatric data and blood samples from pregnant women in a study of TPO positive and negative pregnant women. These samples and data form the basis of the current study.
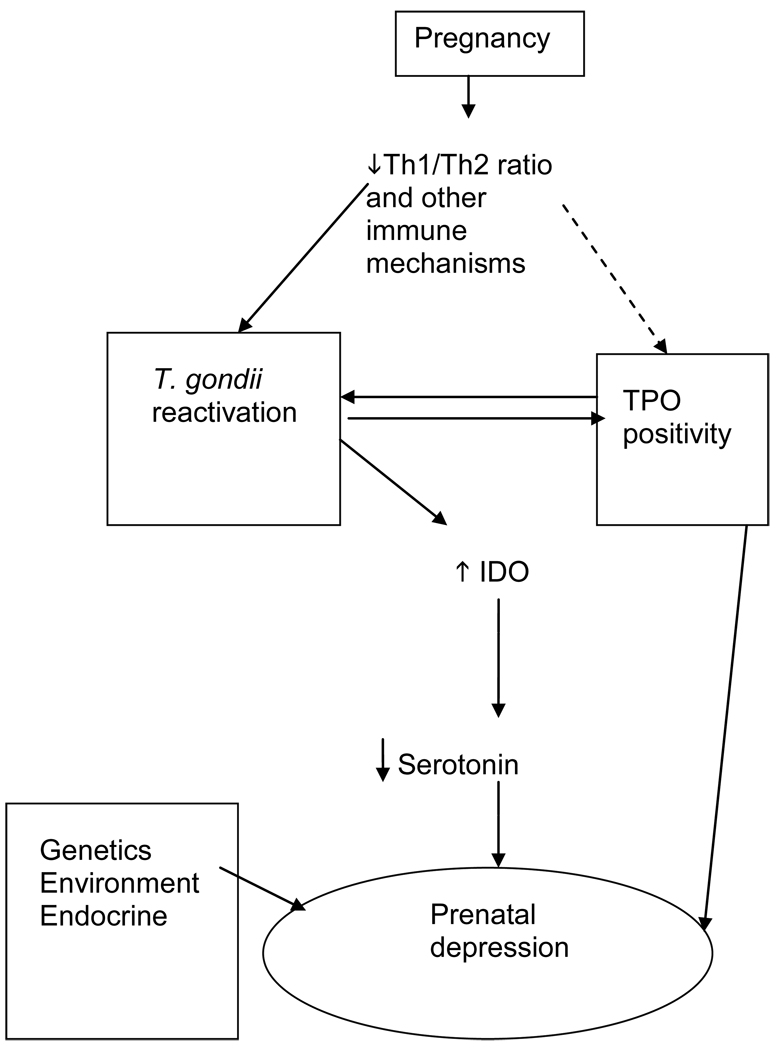
Toxoplasma gondii (T gondii) is a protozoan of the family apicomplexa with worldwide distribution. T. gondii often persists after acute infection in a chronic, intracellular form that is usually contained by T helper 1 (Th1) and Natural Killer (NK) mediated immune processes. It has been estimated from the measurement of antibodies to T.gondii in the blood that in the United States 22.5% of the population 12 years and older have been infected with Toxoplasma [CDC (2008).4 Toxoplasma cysts are found in brain, muscle, lymph nodes and other tissues. T.gondii has previously been associated with mood disturbances in non-pregnant populations, possibly through changes in the levels of brain dopamine and serotonin. Serotonin depletion has been postulated to be related to IFN-γ activation in T. gondii infection resulting in activation of indoleamine 2,3-dioxygenase which metabolizes tryptophan to kynurenine and depletes serotonin. 5, 6 Acknowledging that the immune system in pregnancy undergoes dramatic and remarkable changes from the non-pregnant state, we postulated that it would be of interest to analyze T.gondii titers in relationship to prenatal depression in this population. Moreover, significant decline of tryptophan and increase of kynurenine concentrations has been documented in normal pregnancy. 7 We therefore explored the links between prenatal dysphoric moods, IgGs against T. gondii, tryptophan metabolism, cytokines and TPO autoantibody status. The first hypothesis that was tested was that T. gondii positive women would show higher scores on a measure of depressive and anxiety symptoms. A second hypothesis was that TPO antibody status would be related to T. gondii antibody titer. A third very exploratory hypothesis was that T gondii antibodies would be associated with plasma levels of IFN-γ and TNFα, tryptophan, kynurenine and neopterin. The directions of these hypothesized relationships are depicted in Figure 1.
Figure 1. Framework for the Study.
Prenatal depression is due to multiple and interacting factors. Proposed is that immune escape of T. gondii bradyzoites from cysts in brain and other tissues may occur due to immune changes of pregnancy. This could then cause depression through activation of indoleamine 2,3-dehydroxylase (IDO) resulting in serotonin decrease. TPO positivity may interact with T.gondii reactivation
Materials and Methods
Participants
Healthy prenatal participants (N=414) in a study of postpartum thyroiditis were measured for TPO autoantibody titers and were characterized thereafter as TPO positive or TPO negative. The study was approved by the university Institutional Review Board and all participants gave informed consent. Recruitment took place in 2 large university obstetrical practices by trained recruiters. They received information about the study at a prenatal visit and were then enrolled in a subsequent visit after having the opportunity to review all materials and ask questions. Participants were between 16 and 25 gestational weeks of pregnancy. Exclusion criteria for the study included immune diseases, immune altering medications, in vitro fertilization, HIV disease, illegal drug abuse, extreme thinness, and age under 18 years or over 45 years. Participants completed a demographic questionnaire and the Profile of Mood States (POMS).
Measures
The POMS was used in this study. It is a 65-item dysphoric moods instrument, with 7 subscales: depression-dejection, tension-anxiety, confusion-bewilderment, fatigue-inertia, anger-hostility, vigor-activity, and a total mood disturbance score. Respondents indicated their responses over the past week to items using 4-point rating scales with verbal anchors. The POMS-depression/dejection score (POMS-D) has been shown to be highly correlated with the (Beck’s Depression Index BDI-II (r=0.81). 9 The POMS-D is also reported to correlate with the modified Hopkins Symptom Distress Scales (ranging from 0.43 to 0.86), Taylor Manifest Anxiety Scale (ranging from 0.51 to 0.80), and the MMPI-2 Depression scale (0.65),(POMS Manual). The POMS-Tension/Anxiety (POMS-T) score correlates with the Spielberger State Trait Anxiety scale score at 0.71.10
TPO antibody status was measured on all participants at time of data collection. Aliquots of frozen plasma were analyzed for antibodies to Toxoplasma gondii using previously published methods. A set of aliquots was shipped to Professor Dietmar Fuchs at the University of Innsbruck, Austria for analysis of tryptophan, kynurenine, and neopterin. Cytokines were measured in batches in frozen plasma samples.
Procedures
Each participant completed a demographic questionnaire, the POMS, and provided a 15 ml heparinized blood sample collected by venipuncture at their prenatal provider’s visit between 16 and 25 weeks postpartum. The blood was brought to the lab within 2 hours in a cold biohazard container, and then centrifuged at 3800 rpm for 25 minutes at 4°C. The plasmas were aliquotted into Eppendorf tubes and stored at −80°C until TPO antibodies, and cytokines were measured in batches.
TPO antibodies were measured in plasma by an ELISA (ALPCO, Salem, New Hampshire) and a value of 20 IU/mL or above was considered TPO positive. Cytokines (IFN-γ, IL-10, TNF-α) were measured in undiluted plasma samples by Luminex technology using multiplex kits from Millipore (St. Louis). Dr. Robert Yolken (Johns Hopkins University) performed the T. gondii serology, The analysis was done by solid phase immunoassay. The results are expressed in terms of ratios to arbitrary standards and are thus useful for comparison within groups. Values greater than 1 were considered positive for all titers except for HHV6, which was considered positive if greater than 6. T. gondii serotyping for type 1, type 2, and type 3 was also performed as previously described. Dr. Dietmar Fuchs (University of Innsbruck, Austria) analyzed the plasma for tryptophan (µmol/L) and kynurenine(µmol/L) by high performance liquid chromatography(HPLC) The kynurenine-to-tryptophan ratio was calculated (expressed as µmol kynurenine per mmol tryptophan). 8 Neopterin was measured by ELISA (BRAHMS, Hennigsdorf, Germany) according to the manufacturer's instructions, with a detection limit of 2 nmol/l.
Statistical Analysis
Data were examined for normality and log transformed if necessary. Pearson Product-Moment correlations were performed between T.gondii IgG titers and POMS scores. The T. gondii positive group (N=44) was compared to the negative group (N=370) by t-tests. The T.gondii positive group was evaluated separately, and correlational analyses were performed between depression and T.gondii titers in this group. The effect of race and age on this relationship was analyzed by linear regression, controlling for race and age. Correlations were also performed on the kynurenine to tryptophan ratio and neopterin in the total sample and in the Toxoplasmosis positive subgroup.
Chi Square analysis was done to examine the relationship between Toxoplasmosis (T. gondii antibody status greater than 1) and TPO status.
T-tests and ANOVAs were used to compare the T.gondii positive and negative groups and serotypes on tryptophan, kynurenine, kynurenine to tryptophan ratio , neopterin, and mood states.
Cytokine data (IL-10, TNF-α and IFN-γ) were available on a subset of the population (T.gondii positive, N=38; T.gondii negatives, N=104). The levels of these cytokines in the 2 groups were compared by t-tests.
Results
Table 1 depicts the racial distribution for T. gondii. The percentage of positive titers in the 414 participants was 10.3% overall, but in Hispanics the frequency was 25%. When comparing African Americans, Caucasians and Hispanics, the T.gondii titer was statistically significantly higher in Hispanics (F (2,383)=15.6 (p<0.001). African Americans had the highest depression scores, Hispanics the second highest and Caucasians the lowest when comparing the three major groups. This difference was significant for African Americans (F (2,338)=7.34 (p<0.001). The other POMS scores did not differ by racial group.
Table 1.
| Race | T.gondii IgG titer (IUs) |
|---|---|
| African-American (n=93) | 3.48 |
| Caucasian (n=191) | 3.39 |
| Asian (n=12) | 3.19 |
| Hispanic (n=102) | 8.69 |
| Native American (N=2) | 1.32 |
| Others (n=14) | 3.51 |
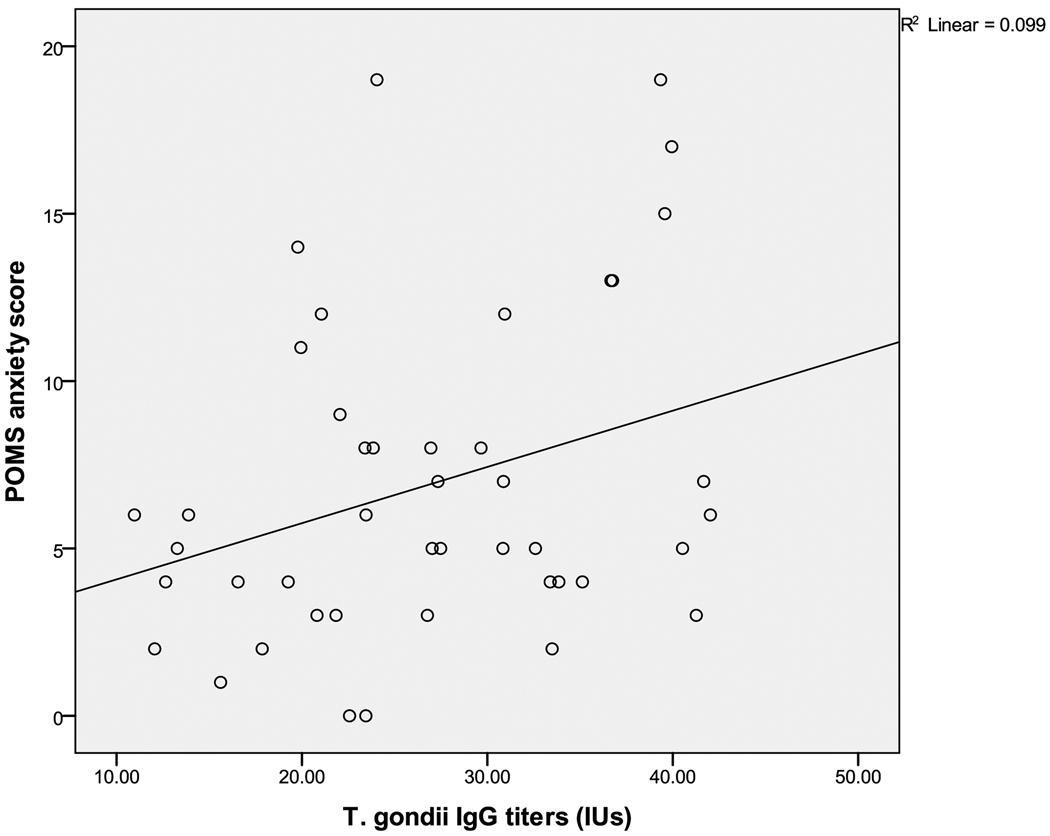
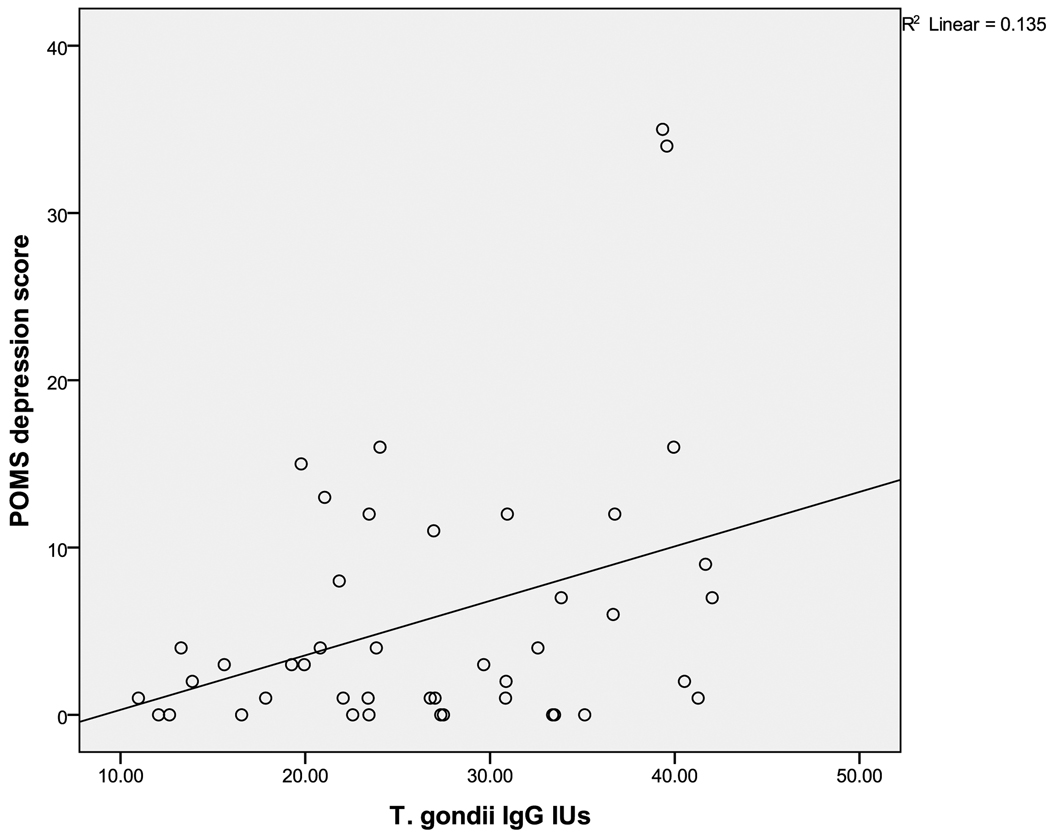
The first hypothesis was that T. gondii positive women would show higher scores on a measure of depressive symptoms. Moods did not differ significantly between positive and negative T.gondii titer groups in the entire sample. However, the T. gondii positive group (N=44) showed a positive correlation of T. gondii IgG titer with the POMS depression (r=0.37, p<0.01) and POMS anxiety (r=0.31, p<0.04) subscale scores. These relationships remained statistically significant when race and age were controlled. T.gondii serotypes were also available and analyzed. Serotypes were Type I (27%), Type II (31%), and Unclassified (42%) (showing intermediate levels of reactivity). The depression and anxiety scores were highest in Type I but this was not significant. A 3-group ANOVA showed that the POMS-Vigor score was lowest in Type II compared to the Type I or Unclassified groups (F(2,41)=3.35 (p<.05).
The second hypothesis was that TPO status would be related to T.gondii antibody titer. We did not find a relationship between TPO status and T.gondii antibody titer when we examined the TPO positives (antibody titer greater than 20 U/mL) compared to the TPO negatives. However TPO positive women had statistically significant higher LOG10 transformed anxiety scores (0.87 +/− 0.37 in TPO positives; (0.70+/−0.41 in TPO negatives) (t=2.28,df=412, p<0.02) and LOG10 transformed depression (0.74+/−0.46 in TPO positives; 0.58 +/− .49 in TPO negatives) (t=2.6,df=412, p<0.04).
The third hypothesis was that T.gondii antibody titer would be associated with plasma IFN-γ and TNF-α, tryptophan, kynurenine and neopterin. Regarding the plasma cytokines. Transformed LOG 10 TNF-α concentrations were higher in the T.gondii positive (.743=/−.45 pg/mL) compared to the negative group (.52+/−.44 pg/mL) (t=2.8, df=140, p<0.01). LOG10 transformed IFN-γ was also higher in T.gondii positives (+/− 0.12+/−0.4 pg/mL compared to 0.04+/−.41 pg/mL) but this was not significant. LOG10 IL-10 was lower in the T.gondii positive group (0.45+/−0.56 pg/mL compared to 0.34 +/−0.43 pg/mL) but again this difference was not significant. TNF-α was positively correlated with both tryptophan (r=0.37, p<0.02) and kynurenine (r=0.35, p<0.04) in the T.gondii positive women.
Neopterin, tryptophan and kynurenine, and their ratio, showed no significant difference in T.gondii positive women.
In the entire sample, tryptophan was positively correlated with the POMS-vigor score (r=0.15, p<0.002). There were 28 women of the 414 who had POMS-D scores higher than 20, a score that in our study prompted a referral to a health provider. Tryptophan was significantly lower in this group considered at risk for severe depression (56.3+/− 12.1 µmol/L in depressed; 63.03 +/−15.2 µmol/L in non-depressed) (t=2.3, df=404, p<0.02).
Neopterin was correlated with the kynurenine to tryptophan ratio in the entire sample (r=0.33, p<0.001) and in the depressed subgroup (r=0.43, p<0.02). This suggests that it did serve as a marker of immune activation. The correlation did not reach significance in the T.gondii positive group (r=0.21. p<.18).
Comment
Prenatal depression, which occurs in up to 20% of pregnant women, is related to many factors. A recent review identified three major influences: life stress, lack of social support, and domestic violence. 11 A significant number of prenatally depressed women continue to be depressed in the postpartum and the development of postpartum blues has been found to be related to low tryptophan concentrations. 12 We found a similar relationship in that women showing the highest POMS depression scale scores had lower tryptophan. The data presented here suggests that T.gondii infected pregnant women are at risk for the dysphoric mood states of depression and anxiety. It is most likely that these women have chronic, latent infection rather than reactivation, although the highest IgG titers may represent reactivation.
There is a general consensus that there is a shift in the Th1/Th2 ratio in pregnancy in order to protect against cytotoxic T cell and NK cell attack of fetal cells. 13, 14, 15 While beneficial to fetal survival, the decrease in Th1 cytokines such as IL-2 and IFN-γ - with upregulation of Th2 cytokines such as IL-10, IL-4, and IL-5 may put the pregnant woman at risk for certain Th2 autoimmune diseases 16 and more severe viral infections such as influenza and varicella. 17 Pregnancy associated suppressed Th1 immunity and down-regulated IFN-γ and TNF-α levels might decrease the local T cell, natural killer (NK) cell and macrophage mediated control of T.gondii cysts, allowing immune escape of the organism. The organism survives in a chronic latent state as bradyzoites contained within tissue cysts but is reactivated in immunosuppressive states such as HIV disease and in transplant patients receiving immunosuppressive drugs. There is emerging evidence that T.gondii can be reactivated during pregnancy, and the risk for passing T. gondii infection to the fetus even in immunocompetent mothers has been well recognized as one of the main risk factors of congenital toxoplasmosis. In one study, eighteen Toxoplasma gondii positive women were followed during 35 pregnancies and seven developed ocular recurrence during pregnancy. 18 Clearly, there is a considerable risk to fetuses for congenital toxoplasmosis infection in such situations.
The increase in TNF-α in T.gondii positive women that we observed suggests that there may be reactivation of T. gondii tissue cysts during pregnancy with potential escape of infective organisms, eliciting an immune response through TNF-α. Macrophages release TNF-α upon binding of T.gondii proteins to toll-like receptors (TLRs).19 IFN-γ was increased in the same direction, but not significantly, and IL-10 was reduced in T.gondii positive women, again not significantly. TNF-α, released also by NK cells and neutrophils, plays an important function in promoting the release of IFN-γ. IFN-γ is necessary to contain the bradyzoite cysts from release and activation. 20 In pregnancy, both Th1 and NK mediated immune responses are suppressed. While this may play a role in activation of the organism, the finding of increased levels of TNF-α in association with IgG to the organism suggests that pregnant women are capable of launching an innate immune response to prevent release and spread of T.gondii. The finding that both tryptophan and its metabolite kynurenine were correlated with TNF-α in the T.gondii positive group bears examination and further study. It was anticipated that TNF-α would be associated with lower tryptophan and higher kynurenine levels if IDO was stimulated.
When the whole group of females was considered, we observed that tryptophan concentrations were significantly lower in individuals with POMS-D scores greater than 20, a group considered at risk for severe depression. However, the relationship of infection to depression in T. gondii positive women did not appear to be through indoleamine 2,3 dioxygenase (IDO), as tryptophan and kynurenine concentrations and their ratio were not different in the T.gondii positive compared to negative group. The percentage of T.gondii positive women was only a little higher than 10% in our sample and the statistical power may not be adequate to detect differences.
In determining the role of IDO and tryptrophan depletion in T.gondii positive women, one has to keep in mind that only plasma measurements were performed and serotonin concentrations were not available. However, other mechanisms than IDO activity may be important and should be explored. If local immune control of brain tissue cysts is decreased in pregnancy, metabolites from cysts may be released which could alter neurotransmitters and cause inflammation. 21 Cysts are heavily localized in areas of the brain associated with anxiety and depression such as the amygdala. Two of this report’s authors (Postolache and Yolken) have recently shown a relationship between attempted suicide in patients with recurrent mood disorder and T. gondii antibody titers. 22 Another possibility is that T. gondii infection affects behavior through endocrine mechanisms. While we found no relationship to TPO antibody status, other endocrine pathways may be involved. T. gondii infected individuals appear to have higher levels of testosterone. 23 Testosterone levels may be higher in pregnancy in infected women, and testosterone may suppress immune responses. 24 In addition, testosterone may contribute to depression in women. 25 Hispanic women are most at risk for these effects because of their higher incidence of T.gondii IgGs and depression incidence.
We did not find a relationship between T.gondii and TPO status in this study. In the Wasserman study, a relationship was found between TPO and T.gondii antibodies measured in a large sample of banked blood from pregnant women tested in the latter half of pregnancy. We evaluated TPO status earlier in pregnancy (16–25 weeks), and we had far fewer participants, so the discrepancy may be related to stage of pregnancy or an effect size that was too small to detect a relationship in our smaller sample.
The association of T.gondii IgGs with depression and anxiety in pregnancy is important for a number of reasons. Over one third of the world population is positive for T.gondii antibodies. 26 Prenatal depression and anxiety are also common and are associated with negative pregnancy and fetal outcomes. 27 It is also a predictor for postpartum depression. This preliminary work suggests that there are relationships between depression, immunity, T.gondii IgG titers and cytokines in pregnancy that may translate into significant health risks for both mother and fetus.
A limitation to this study was the use of the POMS to determine anxiety and depression. Even though the POMS subscales scores have been shown to be highly correlated with well-validated depression and anxiety scores, the POMS measures symptoms over the previous week. Mood states could change dramatically depending upon personal circumstances.
Figure 2.
Scatter plot of T.gondii IgG titer and POMS-anxiety score
Figure 3.
Scatter plot of T.gondii IgG titer and POMS-depression score
Table 2.
Distribution of serotypes by Racial/Ethnic groups
| Racial/Ethnic group |
|||||
|---|---|---|---|---|---|
| Caucasian | African American | Asian | Hispanic | ||
| Serotype 1 | 0 | 1 | 0 | 11 | 12 |
| 2 | 4 | 3 | 0 | 7 | 14 |
| 3 | 7 | 2 | 1 | 8 | 18 |
| Total | 11 | 6 | 1 | 26 | 44 |
Acknowledgments
This research was supported in part by NIH NR05000 Technical assistance of Ms. Shaunte Williams, Ms. Brittany Hasty and Mr. Blake Rankin is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
The authors have no biomedical financial interests or potential conflicts of interest to disclose.
References
- 1.Lazarus J, Parkes A, Premawardhana L. Postpartum thyroiditis. Autoimmunity. 2003;35:169–173. doi: 10.1080/08916930290031667. [DOI] [PubMed] [Google Scholar]
- 2.Lazarus J, Hall R, Othman S, Parkesa A, Richards C, McCullogh B, Harris B. The clinical spectrum of postpartum thyroid disease. Quart J Med. 1996;89:429–435. doi: 10.1093/qjmed/89.6.429. [DOI] [PubMed] [Google Scholar]
- 3.Wasserman EE, Nelson K, Rose NR, Rhode C, Pillion JP, Seaberg E, Talor MV, Burek L, Eaton W, Duggan A, Yolken RH. Infection and thyroid autoimmunity: A seroepidemiologic study of TPOaAb. Autoimmunity. 2009;42(5):439–446. doi: 10.1080/08916930902787716. [DOI] [PubMed] [Google Scholar]
- 4.CDC. Toxoplasmosis: epidemiology & risk factors. 2008 http://www.cdc.gov/toxoplasmosis/epi.html.
- 5.Pfefferkorn ER. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc. Natl. Acad. Sci. U. S. A. 1984;81:908–912. doi: 10.1073/pnas.81.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schroeksnadel K, Wirleitner B, Winkler C, Fuchs D. Monitoring trytptophan metabolism in chronic immune activation. Clin Chim Acta. 2006;364:82–90. doi: 10.1016/j.cca.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 7.Schroeksnadel H, Baier-Bitterlich G, Dapunt O, Wachter H, Fuchs D. Decreased plasma tryptophan in pregnancy. Obstet Gynecol. 1996;88:47–50. doi: 10.1016/0029-7844(96)00084-1. [DOI] [PubMed] [Google Scholar]
- 8.Widner B, Werner ER, Schennach H, Wachter H, Fuchs D. Simultaneous measurement of serum tryptophan and kynurenine by HPLC. Clin Chem. 1997;43:2424–2426. [PubMed] [Google Scholar]
- 9.Malouff JM, Schutte NS, Ramerth W. Evaluation of a short form of the POMS-Depression scale. J Clin Psychol. 1985;41(3):389–391. doi: 10.1002/1097-4679(198505)41:3<389::aid-jclp2270410314>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 10.Nyenhuis DL, Yamamoto C, Luchetta T, Terrien A, Parmentier A. Adult and geriatric normative data and validation of the profile of mood states. J Clin Psychol. 1999;55(1):79–86. doi: 10.1002/(sici)1097-4679(199901)55:1<79::aid-jclp8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 11.Lancaster CA, Gold KJ, Flynn HA, Yoo H, Marcus SM, Davis MM. Risk factors for depressive symptoms during pregnancy: a systematic review. Am J Obstet Gynecol. 2010;202(1):5–14. doi: 10.1016/j.ajog.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kohl C, Walch T, Huber R, Kemmler G, Neurater G, Fuchs D, Soelder E, Schroecksnadel H, Sperner-Unterweger B. Measurement of tryptophan, kynurenine and neopterin in women with and without postpartum blues. J Affect Dis. 2005;86:135–142. doi: 10.1016/j.jad.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Buyon JP, Nelson JL, Lockshin MD. The effects of pregnancy on autoimmune diseases. Clin. Immunol. Immunopathol. 1996;78:99–104. doi: 10.1006/clin.1996.0018. [DOI] [PubMed] [Google Scholar]
- 14.Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T. Rate of pregnancy related relapse in multiple sclerosis. Pregnancy in multiple sclerosis group. N Engl J Med. 1998;339:285–291. doi: 10.1056/NEJM199807303390501. [DOI] [PubMed] [Google Scholar]
- 15.Saito S, Nakashima A, Shima T, Ito M. Th1/Th2/Th17 regulatory T-cell paradigm in pregnancy. Am J Reprod Immunol. 2010;63:601–610. doi: 10.1111/j.1600-0897.2010.00852.x. [DOI] [PubMed] [Google Scholar]
- 16.Buyon JP. The effects of pregnancy on autoimmune diseases. J. Leukoc Biol. 1998;63:281–287. doi: 10.1002/jlb.63.3.281. [DOI] [PubMed] [Google Scholar]
- 17.Jamieson DJ, Theiler RN, Rasmussen SA. Emerging infections and pregnancy. Emerg Infect Dis. 2006;12(11):1638–1643. doi: 10.3201/eid1211.060152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garweg JG, Scherrer J, Wallon M, Kodjikian L, Peyron F. Reactivation of ocular toxoplasmosis during pregnancy. BJOG. 2005;112(2):241–242. doi: 10.1111/j.1471-0528.2004.00302.x. [DOI] [PubMed] [Google Scholar]
- 19.Stafford JL, Neumann F, Belosevic M. Macrophage-mediated innate host defense against protozoan parasites. Crit Rev Microbiol. 2002;28 doi: 10.1080/1040-840291046731. 187 248.20. [DOI] [PubMed] [Google Scholar]
- 20.Jones TC, Bienz KA, Erb P. In vitro cultivation of Toxoplasmoa gondii cysts in astrocytes in the presence of gamma interferon. Infect immun. 1986;51:147–156. doi: 10.1128/iai.51.1.147-156.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costa da Silva R, Langoni H. Toxoplasma gondii:host-parasite interactions and behavior manipulation. Parasitol Res. 2009;105:893–898. doi: 10.1007/s00436-009-1526-6. [DOI] [PubMed] [Google Scholar]
- 22.Arling TA, Yolken RH, Lapidus M, Langenberg P, Dickerson FB, Zimmerman SA, Balis T, Cabassa JA, Scrandis DA, Tonelli LH, Postolache TT. Toxoplasma gondii antibody titers and history of suicide attempts in patients with recurrent mood disorders. J Nerv Ment Dis. 2009;197(12):905–908. doi: 10.1097/NMD.0b013e3181c29a23. [DOI] [PubMed] [Google Scholar]
- 23.Flegr J. Effects of toxoplasma on human behavior. Schizophr Bull. 2007;33(3):757–760. doi: 10.1093/schbul/sbl074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flegr J, Hrdá S, Kodym P. Influence of latent 'asymptomatic' toxoplasmosis on body weight of pregnant women. Folia Parasitol (Praha) 2005;52(3):199–204. doi: 10.14411/fp.2005.026. [DOI] [PubMed] [Google Scholar]
- 25.Bromberger JT, Schott LL, Kravitz HM, Sowers M, Avis NE, Gold EB, Randolph JF, Jr, Matthews KA. Longitudinal change in reproductive hormones and depressive symptoms across the menopausal transition: results from the Study of Women's Health Across the Nation (SWAN) Arch Gen Psychiatry. 2010;67(6):598–607. doi: 10.1001/archgenpsychiatry.2010.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363(9425):1965–1976. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 27.Bansil P, Kuklina EV, Meikle SF, Posner SF, Kourtis AP, Ellington SR, et al. Maternal and fetal outcomes among women with depression. J Women's Hlth. 2010;19:329–334. doi: 10.1089/jwh.2009.1387. [DOI] [PubMed] [Google Scholar]