Abstract
Immunologic memory is a critical feature of the adaptive immune system to fight recurrent infections. However, the mechanisms that shape the composition and function of the human memory T-cell pool remain incompletely understood. We here demonstrate that post-thymic human T-cell differentiation was associated with the downregulation, but not loss, of the inhibitory molecule CD5. The sensitivity of human CD8+ and CD4+ memory T cells to interleukin (IL)–15 was inversely associated with the level of CD5 expression. CD5 expression was downregulated by IL-15–mediated signaling in vitro and CD5lo memory T cells accumulated in the bone marrow. Persistent antigenic stimulation, as in the case of cytomegalovirus infection and rheumatoid arthritis (RA), was also associated with an increased number of CD5lo memory T cells. In conclusion, CD5 may be a useful marker to identify memory T-cell subsets with distinct responsiveness to the homeostatic cytokine IL-15.
Keywords: Human, Memory T cell, CD5, Interleukin-15, Bone marrow, Rheumatoid arthritis
1. Introduction
The ability to develop and maintain highly functional memory T cells after infection or immunization is a hallmark of the adaptive immune response. However, the human memory T-cell pool is heterogeneous, and comprises distinct populations that can be distinguished based on homing markers and effector functions [1]. Cytokines have been shown to be essential for the long-term maintenance of functional CD8+ and CD4+ memory T cells [2,3]. Yet, cytokine-induced signaling can modulate phenotypic and functional characteristics of memory T cells [4–7]. In addition, chronic viral infections and autoimmune diseases have been shown to induce distinct phenotypic and functional changes within the memory T-cell pool [8,9]. Thus, antigens and cytokines dynamically shape the memory T-cell pool throughout life [10].
So far, the role of the transmembrane glycoprotein CD5 in the process of memory T-cell differentiation and maintenance has not been addressed. CD5 surface expression is tightly regulated during T-cell development, with low levels expressed on immature, double negative CD4–CD8– thymocytes, followed by an approximately sixfold increase in CD5 at the double positive CD4+CD8+ stage and a further three- to fivefold increase in CD5 expression on mature, single positive CD4+ and CD8+ thymocytes [11]. All mature T lymphocytes and a subset of B cells, B-1a cells, express CD5 [11,12]. Data from CD5–/– mice indicate that CD5 acts as a negative regulator of cellular activation by being recruited into lipid rafts and dephosphorylating signaling molecules [13–16]. Recently, it has been demonstrated in mice that the level of CD5 on naive T cells does not correlate only with the strength of TCR-mediated signaling but also with the strength of responsiveness to cytokines, such as interleukin (IL)–15 [17]. Furthermore, the level of the inhibitory molecule CD5 on naive T cells could be downregulated by cytokines, such as IL-7 and IL-21, which rendered the cytokine-primed naive T cells more responsive to antigenic stimulation [18].
In this study we demonstrate that the expression of the inhibitory molecule CD5 is decreased during human memory T-cell differentiation and is associated with the strength of responsiveness to IL-15. In addition, we show which environmental cues may be involved in regulating CD5 expression on post-thymic human memory T cells.
2. Subjects and methods
2.1. Peripheral blood and bone marrow samples
Peripheral blood samples were obtained from healthy volunteers. All participants had given their informed written consent, and the study was approved by the Ethics Committee of the Medical University Innsbruck. Cytomegalovirus (CMV) seropositivity was determined by enzyme-linked immunosorbent assay (ELISA) using ENZYGNOST (Dade Behring, Vienna, Austria). Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Hypaque density gradient centrifugation (Amersham Biosciences).
Paired blood and bone marrow (BM) samples were obtained from individuals who had given informed written consent and the study was approved by the Ethics Committee of the Medical University Innsbruck. Bone from the iliac crest, which otherwise would have been discarded based on necessary bone molding/recontouring before insertion into other areas of the body, in particular facial regions was harvested at the Department of Cranio-Maxillofacial and Oral Surgery at the Medical University, Innsbruck. Exclusively bone biopsies from systemically healthy individuals who did not receive immunomodulatory drugs or who had diseases known to influence the immune system, including autoimmune diseases and cancer, were used. The bone biopsy samples were washed with complete MEM (Invitrogen, Lofer, Austria), fragmented, and treated with highly purified collagenase (Worthington, Lakewood, NJ; 20 U/ml) for 1 h at 37°C. Thereafter, collagenase-treated cells were centrifuged and BM mononuclear cells (BMMC) were purified by density gradient centrifugation (Ficoll-Hypaque; Amersham Biosciences, Vienna, Austria).
Peripheral blood samples were obtained from 10 individuals who fulfilled the American College of Rheumatology (ACR) classification criteria for RA [19]. Median disease duration was 18 years (range 5–25 years), and 80% of the patients were positive for rheumatoid factor. The RA patients had moderate clinical disease activity (median disease activity score (DAS)-28 4.3; range 1.7–6.6) and were treated with methotrexate (n = 5), other conventional disease-modifying antirheumatic drugs (DMARDs) (n = 3), or tumor necrosis factor (TNF)–α blocking agents (n = 2). The study was approved by the Ethics Committee of the Medical University Graz.
2.2. Flow cytometry and cell culture experiments
Immunofluorescence surface staining was performed by adding a panel of directly conjugated mAbs to freshly prepared PBMC and BMMC. The antibodies used were CD3 (PE-Cy7 and APC-Cy7), CD4 (PerCP), CD5 (PE and APC), CD28 (APC), CD45RA (APC), CD62L (APC-Cy7), CD69 (PE), CD122 (PE), CD132 (PE), and TCRαβ (PE) (all BD Pharmingen, San Diego, CA), CD8 (PerCP) (BioLegend, San Diego, CA), and CCR7 (FITC) (R&D Systems, Minneapolis, MN). The labeled cells were measured by a FACSCanto II (BD Biosciences, San Diego, CA), and the data were analyzed using FACSDiva software (BD Biosciences, San Diego, CA).
Cell culture experiments were performed with RPMI 1640 (Invitrogen, Lofer, Austria) supplemented with 10% FCS (Sigma-Aldrich, Vienna, Austria) and 1% kanamycin sulfate (Invitrogen, Lofer, Austria). To analyze the responsiveness of memory T-cell subsets to IL-15, peripheral blood T cells were stimulated with IL-15 (50 ng/ml; Sigma-Aldrich, Vienna, Austria) for 4 days and CD69 was measured on CD8+ and CD4+ memory T-cell subsets. The gating of memory T cells was performed by excluding CCR7+CD45RA+ naive T cells. Low expression of CD5 was defined by gating on the lower third of CD5-expressing T cells and the gating was confirmed by additional CD45RA and CD28 staining. Thus, CD5lo T cells had a CD45RA+/–CD28– phenotype. To determine the regulation of CD5 expression, peripheral blood CD8+ and CD4+ T cells were labeled with the fluorescent dye carboxyfluorescein diacetate succinimidyl ester (CFSE) as described previously [20] and stimulated with IL-15, 50 ng/ml for 7 days in the presence of irradiated autologous PBMC.
2.3. Statistical analysis
Differences between groups were evaluated using two-tailed Student's t test. A value of p < 0.05 was considered statistically significant.
3. Results and discussion
3.1. CD5 is downregulated during post-thymic CD8+ and CD4+ memory T-cell differentiation in human beings
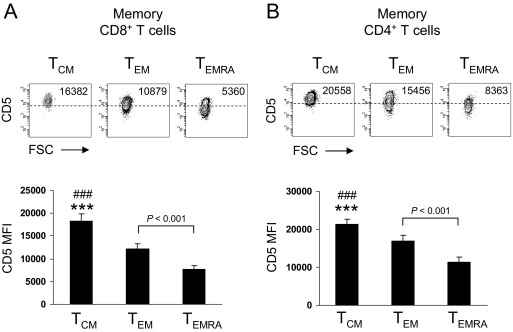
By using flow-cytometric analysis, we could demonstrate that CD5 was highly expressed on central-memory CD8+ T cells, whereas its expression was downregulated in CD45RA–CCR7– and CD45RA+CCR7– effector-memory CD8+ T cells (Fig. 1A). Similarly, when using CD45RA and CD28 to characterize memory T-cell subsets, CD45RA+CD28– effector-memory T cells displayed the lowest level of CD5 expression (Figs. S1A and S1B). However, the expression of CD3 and TCRαβ were not downregulated during CD8+ memory T-cell differentiation (Fig. S1A). In CD4+ T cells, the expression of CD5 was also downregulated during post-thymic memory T-cell differentiation (Fig. 1B and Figs. S1C and S1D), whereas the expression of CD3 and TCRαβ was not (Fig. S1C). These results indicate that the expression of CD5 is not only regulated during thymic development [11], but highlight a role for CD5 during post-thymic CD8+ and CD4+ memory T-cell differentiation in human beings.
Fig. 1.
CD5 is downregulated during peripheral human CD8+ and CD4+ memory T-cell differentiation. (A) Density plots show expression of CD5 on human CD8+ memory T-cell subsets. Memory subsets were classified as central-memory (TCM; CD45RA–CCR7+), effector-memory (TEM; CD45RA–CCR7–), and effector-memory CD45RA+ (TEMRA; CD45RA+CCR7–). Numbers indicate CD5 mean fluorescence intensity (MFI). Data are representative of 12 experiments. Bar graph shows CD5 MFI on human CD8+ memory T-cell subsets (n = 12). Statistical analysis was performed using Student's t test. ***p < 0.001, TCM vs TEM and ###p < 0.001 TCM vs TEMRA. (B) Density plots show the expression of CD5 on human CD4+ memory T-cell subsets. Memory subsets were classified as described above. Numbers indicate CD5 MFI. Data are representative of eight experiments. Bar graph shows CD5 MFI on human CD4+ memory T-cell subsets (n = 8). Statistical analysis was performed using Student's t test. ***p < 0.001, TCM vs TEM and ###p < 0.001 TCM vs TEMRA.
3.2. Expression level of CD5 on human CD8+ and CD4+ memory T cells is inversely associated with the strength of responsiveness to IL-15
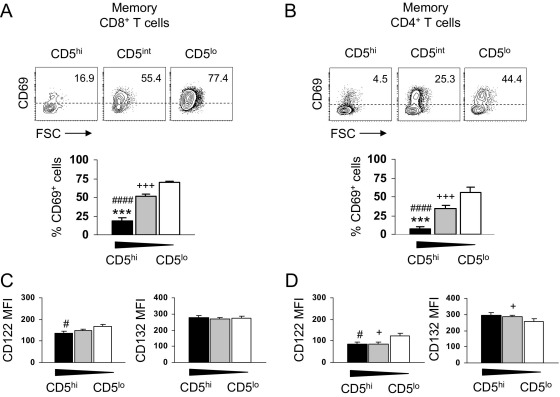
CD5 has been shown to act as a negative regulator of cellular activation by dephosphorylating signaling molecules [13,15,16] and to modulate cytokine responsiveness of naive CD8+ T cells [17]. We thus analyzed whether memory T cells with distinct CD5 levels differ in their responsiveness to IL-15, which is known to be an important cytokine for memory T-cell survival and proliferative renewal [2,21]. Although memory T-cell subsets did not express CD69 in the absence of IL-15 (data not shown), IL-15 led to the upregulation of the activation molecule CD69 on CD8+ and CD4+ memory T cells and was inversely associated with the level of CD5 (Figs. 2A, 2B). One explanation for the hyper-responsiveness of CD5lo CD8+ and CD4+ memory T cells to IL-15 could be that these cells have a higher number of cytokine receptors. However, this is unlikely, because there were no marked differences in the expression of the signaling chains CD122 (IL-2Rβ) and CD132 (γc) between memory T cells with different levels of CD5 (Figs. 2C, 2D). Interestingly, a high expression of monosialotetrahexosylganglioside (GM) 1–containing lipid rafts was associated with a high responsiveness to IL-2 and IL-15 in naive CD8+ T cells and was directly correlated with the expression of CD5 [17]. Accordingly, a recent study in human subjects demonstrated that central-memory CD4 T cells expressed a high membrane lipid order (GM1lo), whereas effector-memory CD4 T cells expressed an intermediate membrane lipid order (GM1int) [22]. This indicates that the GM1 content directly correlates with the responsiveness to IL-15 in memory T-cell subsets but inversely correlates with the level of CD5.
Fig. 2.
Responsiveness of human CD8+ and CD4+ memory T-cell subsets with distinct CD5 levels to IL-15. (A) Representative density plots that show the expression of the activation molecule CD69 upon IL-15–mediated stimulation of CD8+ memory T cells with distinct CD5 level. Bar graph shows percentage of CD69-expressing CD8+ memory T cells with high (filled bars), intermediate (gray bars), and low (open bars) expression of CD5 after stimulation with IL-15, 50 ng/ml for 4 days. Statistical analysis was performed using paired Student's t test (n = 11). (B) Representative density plots that show the expression of CD69 upon IL-15–mediated stimulation of CD4+ memory T cells with distinct CD5 level. Bar graph shows percentage of CD69-expressing CD4+ memory T cells with high (CCR7+CD45RA–; filled bars), intermediate (CCR7–CD45RA–; gray bars), and low (CCR7–CD45RA+; open bars) expression of CD5 after stimulation with IL-15, 50 ng/ml for 4 days. Statistical analysis was performed using paired Student's t test (n = 11). ***p < 0.001, CD5hi vs CD5int; ###p < 0.001, CD5hi vs CD5lo and +++p < 0.001, CD5int vs CD5lo. (C) Flow-cytometric analysis of CD122 MFI and CD132 MFI in CD8+ memory T cells with high (filled bars), intermediate (gray bars), and low (open bars) expression of CD5 (n = 8). (D) Flow-cytometric analysis of CD122 MFI and CD132 MFI in CD4+ memory T cells with high (filled bars), intermediate (gray bars) and low (open bars) expression of CD5 (n = 8). Statistical analysis was performed using Student's t test. *p < 0.05, CD5hi vs CD5int; #p < 0.05, CD5hi vs CD5lo and +p < 0.05, CD5int vs CD5lo.
3.3. CD5 is downregulated by IL-15–mediated signaling and CD5lo memory T cells accumulate in the human BM
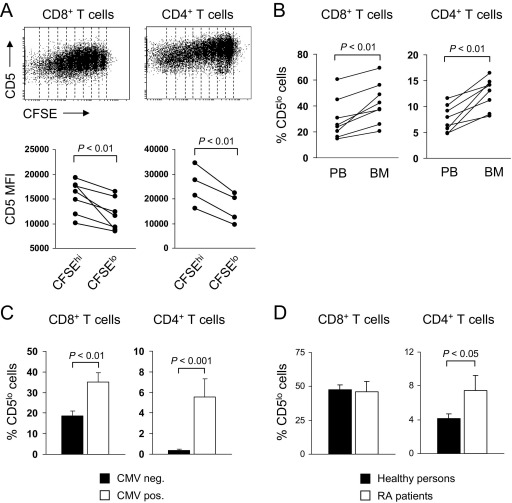
We next analyzed which factors may contribute to the downregulation of CD5 on human T cells. We therefore stimulated purified, CFSE-labeled CD8+ and CD4+ T cells with IL-15 for 7 days. Although CD8+ and CD4+ T cells did not proliferate in the absence of IL-15 (data not shown), CD5 was progressively downregulated in proliferating CD8+ and CD4+ T cells stimulated with IL-15 (Fig. 3A). Comparison of CD5 levels between CD8+ T cells that have not proliferated (CFSEhi) and cells that have undergone four or more cell divisions (CFSElo) demonstrated that IL-15–mediated signaling decreased the CD5 mean fluorescence intensity (MFI) by 21% (Fig. 3A). Similarly, CD5 was downregulated in proliferating CD4+ T cells stimulated with IL-15 and IL-15–mediated signaling decreased the CD5 MFI by 36% (Fig. 3A). The BM has been shown to be a preferred site for IL-15–mediated proliferation of memory T cells [23] and a higher expression of IL-15 was identified in BMMC compared with PBMC [24]. (Moreover, CD4+ and CD8+ T cells were in close contact with IL-15–producing cells in the human BM [24]. We therefore determined the expression of CD5 on memory T cells in the human BM. Our results demonstrate that a higher number of CD5lo CD8+ and CD4+ memory T cells accumulated in the human BM compared with peripheral blood (Fig. 3B). Thus, our results highlight a so far unappreciated role for IL-15–mediated signaling in modulating the expression of the inhibitory molecule CD5, which is associated with the strength of responsiveness to IL-15. Intriguingly, as memory T-cell homeostasis is critically dependent on IL-15–dependent signaling [2], increased sensitivity of CD5lo memory T cells to IL-15 may explain the selective survival and/or expansion of CD5lo memory T cells in the human BM.
Fig. 3.
Impact of environmental cues, persistent CMV infection, and RA on the frequency of CD5lo T cells. (A) Regulation of CD5 surface expression by IL-15–mediated proliferation in CD8+ and CD4+ T cells. Histograms show expression of CD5 in CFSE-labeled CD8+ and CD4+ T cells stimulated with IL-15, 50 ng/ml for 7 days in the presence of irradiated PBMC. One representative experiment is shown. Graphs show CD5 MFI in CFSEhi (not divided) and CFSElo CD8+ and CD4+ T cells (four or more cell divisions) stimulated with IL-15 for 7 days. Statistical analysis was performed using paired Student's t test. (B) Graphs show percentage of CD5lo cells within the CD8+ and CD4+ T-cell pool in PB and bone marrow (BM) of healthy individuals (definition and phenotype of CD5lo T cells is explained in Subjects and methods section). (C) Left bar graph shows percentage of CD5lo cells within the CD8+ T-cell pool in CMV seronegative persons (n = 26; mean age ± SEM: 41 ± 4 years) and seropositive persons (n = 15; 41 ± 5 years). Right bar graph shows percentage of CD5lo cells within the CD4+ T-cell pool in CMV seronegative persons (n = 23; mean age ± SEM: 38 ± 4 years) and seropositive persons (n = 13; 41 ± 5 years). (D) Left bar graph shows percentage of CD5lo cells within the CD8+ T-cell pool in healthy persons (n = 38; mean age ± SEM: 72 ± 1 years) and RA patients (n = 10; 71 ± 3 years). Right bar graph shows percentage of CD5lo cells within the CD4+ T-cell pool in healthy persons (n = 28; mean age ± SEM: 71 ± 1 years) and RA patients (n = 10; 71 ± 3 years). Healthy persons and RA patients were all CMV seropositive. Statistical analysis was performed using Student's t test.
3.4. Persistent CMV infection and RA drive the accumulation of CD5lo memory T cells
We next examined whether persistent infection with CMV, which is known to drive the accumulation of highly differentiated T cells [25,26], may alter the frequency of CD5lo memory T cells in human beings. Indeed, the number of CD5lo CD8+ and CD4+ memory T cells was increased in CMV seropositive compared with CMV seronegative persons (Fig. 3C). In addition, a higher percentage of CD5lo CD4+ memory T cells was identified in the peripheral blood of patients with RA compared with age- and CMV-matched healthy individuals (Fig. 3D). Thus, our results indicate that persistent infection with CMV and pro-inflammatory autoimmune diseases, such as RA, characteristically shape the functional composition of the human memory T-cell pool by driving the accumulation of CD5lo memory T cells. The lower expression level of CD5 on these highly differentiated memory T cells is also associated with an increased responsiveness to the homeostatic cytokine IL-15, which may represent a survival advantage and may drive their accumulation in the BM. In the case of RA, IL-15 is increased in the synovium and BM [27,28] and may decrease the activation threshold of highly differentiated, IL-15–sensitive CD5lo memory T cells, thereby contributing to increased T-cell activation and inflammation [28].
Acknowledgments
The authors thank Michael Keller for excellent technical assistance. This work was supported by the Austrian Science Fund (S9308-B05) as well as by the EU funded Network of Excellence Lifespan (FP6 036894). D.H.-B. was supported by a European Future Leaders of Ageing Research in Europe (FLARE) fellowship funded by the Austrian Federal Ministry of Science and Research.
Available online 15 April 2011
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.humimm.2011.03.028.
Supplementary data
References
- 1.Sallusto F., Geginat J., Lanzavecchia A. Central memory and effector memory T-cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 2.Surh C.D., Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Woodland D.L., Kohlmeier J.E. Migration, maintenance and recall of memory T cells in peripheral tissues. Nat Rev Immunol. 2009;9:153–161. doi: 10.1038/nri2496. [DOI] [PubMed] [Google Scholar]
- 4.Kim H.R., Hwang K.A., Kang I. Dual roles of IL-15 in maintaining IL-7RalphalowCCR7-memory CD8+ T cells in humans via recovering the phosphatidylinositol 3-kinase/AKT pathway. J Immunol. 2007;179:6734–6740. doi: 10.4049/jimmunol.179.10.6734. [DOI] [PubMed] [Google Scholar]
- 5.Geginat J., Lanzavecchia A., Sallusto F. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood. 2003;101:4260–4266. doi: 10.1182/blood-2002-11-3577. [DOI] [PubMed] [Google Scholar]
- 6.Geginat J., Sallusto F., Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4(+) T cells. J Exp Med. 2001;194:1711–1719. doi: 10.1084/jem.194.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiu W.K., Fann M., Weng N.P. Generation and growth of CD28nullCD8+ memory T cells mediated by IL-15 and its induced cytokines. J Immunol. 2006;177:7802–7810. doi: 10.4049/jimmunol.177.11.7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Appay V., Dunbar P.R., Callan M., Klenerman P., Gillespie G.M., Papagno L. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379–385. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 9.Weyand C.M., Fujii H., Shao L., Goronzy J.J. Rejuvenating the immune system in rheumatoid arthritis. Nat Rev Rheumatol. 2009;5:583–588. doi: 10.1038/nrrheum.2009.180. [DOI] [PubMed] [Google Scholar]
- 10.Harty J.T., Badovinac V.P. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8:107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- 11.Azzam H.S., Grinberg A., Lui K., Shen H., Shores E.W., Love P.E. CD5 expression is developmentally regulated by T-cell receptor (TCR) signals and TCR avidity. J Exp Med. 1998;188:2301–2311. doi: 10.1084/jem.188.12.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antin J.H., Emerson S.G., Martin P., Gadol N., Ault K.A. Leu-1+ (CD5+) B cells: A major lymphoid subpopulation in human fetal spleen: phenotypic and functional studies. J Immunol. 1986;136:505–510. [PubMed] [Google Scholar]
- 13.Tarakhovsky A., Kanner S.B., Hombach J., Ledbetter J.A., Muller W., Killeen N. A role for CD5 in TCR-mediated signal transduction and thymocyte selection. Science. 1995;269:535–537. doi: 10.1126/science.7542801. [DOI] [PubMed] [Google Scholar]
- 14.Brossard C., Semichon M., Trautmann A., Bismuth G. CD5 inhibits signaling at the immunological synapse without impairing its formation. J Immunol. 2003;170:4623–4629. doi: 10.4049/jimmunol.170.9.4623. [DOI] [PubMed] [Google Scholar]
- 15.Perez-Villar J.J., Whitney G.S., Bowen D.H., Hewgill A.A.Aruffo, Kanner S.B. CD5 negatively regulates the T-cell antigen receptor signal transduction pathway: Involvement of SH2-containing phosphotyrosine phosphatase SHP-1. Mol Cell Biol. 1999;19:2903–2912. doi: 10.1128/mcb.19.4.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Migone T.S., Cacalano N.A., Taylor N., Yi T., Waldmann T.A., Johnston J.A. Recruitment of SH2-containing protein tyrosine phosphatase SHP-1 to the interleukin 2 receptor; loss of SHP-1 expression in human T-lymphotropic virus type I-transformed T cells. Proc Natl Acad Sci U S A. 1998;95:3845–3850. doi: 10.1073/pnas.95.7.3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho J.H., Kim H.O., Surh C.D., Sprent J. T-cell receptor-dependent regulation of lipid rafts controls naive CD8+ T-cell homeostasis. Immunity. 2010;32:214–226. doi: 10.1016/j.immuni.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gagnon J., Chen X.L., Forand-Boulerice M., Leblanc C., Raman C., Ramanathan S. Increased antigen responsiveness of naive CD8 T cells exposed to IL-7 and IL-21 is associated with decreased CD5 expression. Immunol Cell Biol. 2010;88:451–460. doi: 10.1038/icb.2009.109. [DOI] [PubMed] [Google Scholar]
- 19.Arnett FC, Edworthy DA, Bloch DJ, McShane JF, Fries NS, Cooper LA, et al. 1988; The American Rheumatism Association. 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum;31:315–24. [DOI] [PubMed]
- 20.Herndler-Brandstetter D., Schwaiger S., Veel E., Fehrer C., Cioca G., Almanzar M. CD25-expressing CD8+ T cells are potent memory cells in old age. J Immunol. 2005;175:1566–1574. doi: 10.4049/jimmunol.175.3.1566. [DOI] [PubMed] [Google Scholar]
- 21.Becker T.C., Wherry E.J., Boone D., Murali-Krishna K., Antia R. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J Exp Med. 2002;195:1541–1548. doi: 10.1084/jem.20020369. AMa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miguel L., Owen C., Lim C., Liebig J., Evans A.I., Magee Primary human CD4+ T cells have diverse levels of membrane lipid order that correlate with their function. J Immunol. 2011;186:3505–3516. doi: 10.4049/jimmunol.1002980. [DOI] [PubMed] [Google Scholar]
- 23.Becker T.C., Coley E.J.Wherry, Ahmed R. Bone marrow is a preferred site for homeostatic proliferation of memory CD8 T cells. J Immunol. 2005;174:1269–1273. doi: 10.4049/jimmunol.174.3.1269. [DOI] [PubMed] [Google Scholar]
- 24.Herndler-Brandstetter D., Landgraf K., Jenewein B., Tzankov A., Brunauer R., Brunner S. Human bone marrow hosts polyfunctional memory CD4+ and CD8+ T cells with close contact to IL-15-producing cells. J Immunol. 2011 doi: 10.4049/jimmunol.1100243. in press. [DOI] [PubMed] [Google Scholar]
- 25.Almanzar G., Schwaiger S., Jenewein B., Keller M., Herndler-Brandstetter D., Wurzner R. Long-term cytomegalovirus infection leads to significant changes in the composition of the CD8+ T-cell repertoire, which may be the basis for an imbalance in the cytokine production profile in elderly persons. J Virol. 2005;79:3675–3683. doi: 10.1128/JVI.79.6.3675-3683.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sylwester A.W., Mitchell B.L., Edgar J.B., Taormina C., Pelte C., Ruchti F. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med. 2005;202:673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thurkow E.W., van der Heijden I.M., Breedveld F.C., Smeets T.J., Daha P.M., Kluin A.E. Increased expression of IL-15 in the synovium of patients with rheumatoid arthritis compared with patients with Yersinia-induced arthritis and osteoarthritis. J Pathol. 1997;181:444–450. doi: 10.1002/(SICI)1096-9896(199704)181:4<444::AID-PATH778>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 28.Kuca-Warnawin E., Burakowski T., Kurowska W., Prochorec-Sobieszek M., Radzikowska A., Chorazy-Massalska M. Elevated number of recently activated T cells in bone marrow of patients with rheumatoid arthritis: a role for interleukin 15? Ann Rheum Dis. 2010;70:227. doi: 10.1136/ard.2009.124966. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.