Abstract
BACKGROUND AND PURPOSE
Recent evidence has suggested that nicotine decreases blood pressure (BP) and heart rate (HR) in the nucleus tractus solitarii (NTS), indicating that nicotinic acetylcholine receptors (nAChRs) play an important role in BP control in the NTS. However, the signalling mechanisms involved in nAChR-mediated depressor effects in the NTS are unclear. Hence, the aim of this study was to investigate these signalling mechanisms.
EXPERIMENTAL APPROACH
Depressor responses to nicotine microinjected into the NTS of Wistar-Kyoto rats were elicited in the absence and presence of an antagonist of α7 nAChR, the calcium chelator ethylene glycol tetraacetic acid, a calmodulin-specific inhibitor, nitric oxide (NO) synthase (NOS) inhibitor, endothelial NOS (eNOS)-selective inhibitor or neuronal NOS (nNOS)-specific inhibitor.
KEY RESULTS
Microinjection of nicotine into the NTS produced a dose-dependent decrease in BP and HR, and increased nitrate levels. This depressor effect of nicotine was attenuated after pretreatment with a nAChR antagonist or blockers of the calmodulin-eNOS pathway. In contrast, N5-(1-Imino-3-butenyl)-L-ornithine (vinyl-L-NIO), nNOS-specific inhibitor, did not diminish these nicotine-mediated effects. Calmodulin was found to bind eNOS after nicotine injection into NTS. However, nicotine did not affect the eNOS phosphorylation level or eNOS upstream extracellular signal-regulated kinases (ERK)1/2 and Akt phosphorylation levels. Furthermore, pretreatment with an ERK1/2 or Akt inhibitor did not attenuate nicotine-induced depressor effects in the NTS.
CONCLUSIONS AND IMPLICATIONS
These results suggest that the nAChR-Ca2+-calmodulin-eNOS-NO signalling pathway, but not nNOS, plays a significant role in central BP regulation, and neither the ERK1/2 nor Akt signalling pathway are significantly involved in the activation of eNOS by nAChRs in the NTS.
Keywords: blood pressure, calmodulin, endothelial nitric oxide synthase, nicotine, nucleus tractus solitarii, nicotinic acetylcholine receptors
Introduction
The nucleus tractus solitarii (NTS) is located in the dorsal medulla of the brain stem and is the primary integrative centre for cardiovascular control in the central nervous system. Our previous studies showed that several neuromodulators participate in cardiovascular control in the NTS, including adenosine, (Ho et al., 2008) nicotine (Tseng et al., 1993), angiotensin II (Cheng et al., 2010), nitric oxide (NO; Cheng et al., 2010) and insulin (Huang et al., 2004). Among these neuromodulators, nicotine has been reported to play a key role in central cardiovascular control (Tseng et al., 1993; 1994;).
The receptor for nicotine, nicotinic acetylcholine receptor (nAChR), belongs to the superfamily of ligand-gated ion channels. The structure of nAChRs arises from five polypeptide subunits assembled like staves of a barrel around a central water-filled pore (Cooper et al., 1991; Paterson and Nordberg, 2000). Various subunit combinations can produce many different nAChR subtypes, which include neuronal heteropentamers (α2-α10 and β2-β4) and the neuronal homopentamers (α7) (Paterson and Nordberg, 2000; Carlisle et al., 2007). The α7 nAChR subtypes are widely expressed in the central and peripheral nervous system (Bond et al., 2009), with high calcium permeability and rapid activation and desensitization kinetics. Several studies have also indicated that α7 nAChRs are activated by intracellular Ca2+ signals, which reveal the physiological role of these receptor-channels (Gilbert et al., 2009).
Previous evidence demonstrated NO synthase (NOS) activity in various components of the central nervous system, such as the NTS, rostral ventrolateral medulla (RVLM) and dorsal motor nucleus of the vagus. Throughout the cardiac autonomic nervous system, NO participates in central cardiovascular regulation (Cheng et al., 2010). We have also found that the microinjection of NOS inhibitors L-NG-monomethyl arginine citrate and N-nitro-L-arginine methyl ester (L-NAME) attenuates the haemodynamic effects produced by activation of the baroreceptor reflex (Lo et al., 1996). These findings demonstrate that NO within the NTS may play an important role in the regulation of cardiovascular function. Furthermore, Gerzanich et al. (2001) showed that chronic nicotine alters NO signalling of calcium channels in cerebral arterioles (Gerzanich et al., 2001). In addition, it has been demonstrated that nicotine microinjection into the NTS decreases blood pressure (BP) and heart rate (HR); however, this trend was reversed in the RVLM (Papadopolou et al., 2004; Zhao et al., 2007). NAChR-mediated stimulation of NO-generation has been observed in neurones, aortic endothelial cells and the NTS. Based on these results, we hypothesize that nicotine regulates BP and HR by a mechanism involving the nAChR-Ca2+/calmodulin-NO signalling pathway.
In this study, we investigated whether the hypotensive effect induced by stimulation of nAChRs is mediated by Ca2+ signalling and activation of endothelial NOS (eNOS) and subsequent NO release in the NTS. The results demonstrate that nAChRs modulate central BP through direct activation of calcium influx; this was not associated with the induction of eNOS phosphorylation. Our results suggest the possible involvement of the nAChR-Ca2+-calmodulin-eNOS-NO signalling pathway in the regulation of depressor effects in the NTS.
Methods
Reagents and chemicals
Experimental drugs such as urethane, Triton-X100, NaCl, L-glutamate, heparin, nicotine, ethylene glycol-bis[β-aminoethyl ether]-N,N,N′,N′-tetraacetic acid (EGTA), L-NAME were obtained from Sigma-Aldrich (Sigma Chemical Co., St. Louis, MO, USA). N(5)-(-iminoethyl)-L-ornithine (L-NIO) was obtained from Calbiochem (EMD Biosciences, Darmstadt, Germany), N(5)-(1-imino-3-butenyl)-ornithine (vinyl-L-NIO) was obtained from ALEXIS (ALEXIS Corporation, Lausen, Switzerland); W-7 hydrochloride was obtained from Tocris (Tocris Cookson Ltd, Bristol, UK). Opti-MEM was obtained from Invitrogen (Carlsbad, CA, USA). The nAChR antagonist (α-bungarotoxin, α-BTX) was a kind gift from Dr Long-Sian Chang of National Sun Yat-Sen University, Kaohsiung, Taiwan.
Animals
Male Wistar-Kyoto (WKY) rats (weight 230–300 g) were obtained from the National Science Council Animal Facility (Taipei, Taiwan) and housed in the animal room of Kaohsiung Veterans General Hospital (Kaohsiung, Taiwan). The rats were given normal rat chow (Purina; St. Louis, MO, USA) and tap water ad libitum. All animal research protocols were approved by the Research Animal Facility Committee of Kaohsiung Veterans General Hospital.
Intra-NTS microinjection and haemodynamic measurements
Rats were anaesthetized with urethane (1.0 g·kg−1 i.p., supplemented with 300 mg·kg−1 i.v. if necessary). A polyethylene catheter was placed in the femoral vein for drug administration. BP was measured directly through a catheter placed in the femoral artery and connected to a pressure transducer (P23 ID, Gould Electronics, Eichstetten, Germany) and polygraph (RS3800, Gould Electronics). HR was monitored continuously by a tachograph preamplifier (13-4615-65, Gould Electronics). Tracheostomy was performed to maintain airway patency during the experiment. For brain stem nuclei microinjection, the rats were placed in a stereotaxic instrument (Kopf, Tujunga, CA, USA), with the head flexed downward at a 45° angle. The dorsal surface of the medulla was exposed by limited craniotomy, and the rats were rested for at least 1 h before the experiments. Single-barrel glass catheter (0.031-inch OD, 0.006-inch ID; Richland Glass Co, Vineland, NJ, USA) that had external tip diameters of 40 µm were prepared. To verify that the needle tip of the glass electrode was exactly in the NTS, L-glutamate (0.154 nmol·60 nL−1) was microinjected. This would induce a characteristically abrupt decrease in BP (ΔBP ≥−35 mmHg) and HR (ΔHR ≥−50 beats·min−1) if the needle tip was located precisely in the medial site of the intermediate one-third of the NTS with the coordinates of anteroposterior, 0.0 mm; mediolateral, 0.5 mm; and vertical, 0.4 mm with the obex as reference (Tseng et al., 1996). To investigate whether nAChR signalling participates in the depressor effect of nicotine in the NTS, the electrode was filled with one of the following chemicals: α-BTX (66 pmol·60 nL−1), calcium chelator EGTA (60 pmol·60 nL−1), calmodulin inhibitor W7 (0.1 nmol·60 nL−1), NOS inhibitor L-NAME (33 nmol·60 nL−1), eNOS inhibitor L-NIO (6 nmol·60 nL−1), vinyl-L-NIO, a neuronal NOS (nNOS)-specific inhibitor (600 pmol·60 nL−1), dissolved in reduced-serum medium (Opti-MEM I; Invitrogen).
Measurement of NO in the NTS
NTS (10–20 mg) were deproteinized by microcon YM-30 (Millipore, Bedford, MA, USA). That total amount of NO in the samples was determined by a modification of the procedure defined using the purge system of Sievers Nitric Oxide Analyzer (NOA 280i; Sievers Instruments, Boulder, CO, USA) based on chemiluminescence. (Li et al., 2005) Sample (10 µL) was injected into a reflux column containing 0.1 mol·L−1 of VCl3 in 1 mol·L−1 of HCl at 80°C to reduce any nitrates and nitrites into NO. NO then combines with O3 produced by the analyser to form NO2. The resulting emission from the excited NO2 was detected by a photomultiplier tube and recorded digitally (mV). The values were then interpolated to a standard curve of NaNO2 concentrations concurrently determined. Measurements were made in triplicate for each sample. The NO levels measured were corrected for the NTS of studied rats.
Western blot analysis
Two groups of rats (six rats per group) were enrolled in the experiment. The NTS was dissected by use of a micropunch (1-mm inner diameter) from a 1-mm thick brainstem slice at the level of obex under a microscope. Total protein was prepared by homogenizing the NTS tissue in lysis buffer with protease inhibitor cocktail and phosphatase inhibitor cocktail. Samples were then incubated for 1 h at 4°C. Protein extracts (20 µg per sample as assessed by bicinchoninic acid protein assay, Pierce Chemical Co., Rockford, IL, USA) were fractionated in 6–8% polyacrylamide gel and transferred to a polyvinylidene difluoride membrane (GE Healthcare, Buckinghamshire, UK). The membranes were incubated with the appropriate anti-α7 nAChR (Abcam, Cambridge, UK), anti-P-ERK1/2T202/Y204 (Cell Signaling Technology, Danvers, MA, USA), anti-ERK1/2 (Cell Signaling Technology), anti-p-AKTS473 (Cell Signaling Technology), rabbit anti-Akt (Cell Signaling Technology), anti-p-eNOSS1177 (BD Biosciences, San Jose, CA, USA), anti-eNOS (BD Biosciences) and anti-actin (1:10 000; Millipore, Bedford, MA, USA) antibodies, which were diluted 1:1000 in phosphate buffer saline tween-20 with bovine serum albumin and incubated at 4°C overnight, followed by incubation with horseradish peroxidase-labelled secondary goat anti-rabbit or anti-mouse antibody at 1:10 000 dilution. The membrane was developed with the enhanced chemiluminescence-Plus detection kit (GE Healthcare).
Immunohistochemistry analysis
Immunohistochemical (IHC) staining was performed to determine whether α7 nAChRs occur in situ in the NTS of WKY rats. We incubated samples with anti-α7 nAChR antibody (1:100; Abcam) at 4°C overnight. Afterwards, sections were incubated in biotinylated secondary antibody (1:200; Vector Laboratories, Burlingame, CA, USA) for 1 h and in AB complex (1:100) for 30 min at room temperature. Sections were visualized with a DAB substrate kit (Vector Laboratories) and counterstained with haematoxylin and eosin. The sections were then photographed with a microscope equipped with a charge-coupled device camera.
Immunofluorescent staining analysis
The rats were perfused with saline, followed by a solution of 4% formaldehyde and finally a 30% sucrose solution. Sections of 20 µm of the brain stem were stained with cresyl violet, and proper placement of the pipette tip in the NTS was verified by examination of the sections under the microscope.
Brain stem sections were incubated in a mixture of mouse-anti-NeuN antibody (1:20; Chemicon, Bedford, MA, USA) and rabbit-anti-α7 nAChR (1:20; Abcam) or rabbit-anti-t-eNOS (1:20, BD Transduction Laboratories, BD Biosciences, San Jose, CA, USA). After being washed with phosphate buffer saline, sections were incubated with rhodamine-conjugated goat anti-rabbit IgG (1:50; Sigma-Aldrich) and fluorescein isothiocyanate-conjugated goat anti-mouse IgG (1:200; Sigma-Aldrich) at 25°C for 1.5 h. Sections were analysed by using fluorescence microscopy and Zeiss Image (Carl Zeiss MicroImaging, Jena, Germany).
Co-immunoprecipitation assay
The NTS was dissected by use of a micropunch (1-mm inner diameter) from a 1-mm thick brainstem slice at the level of the obex under a microscope. The NTS lysis were incubated with 5 µL mouse anti-eNOS (BD Biosciences), rabbit anti-calmodulin (Abcam) antibodies, and the Catch and Release immunoprecipitation system was applied (Upstate Biotechnology, Upstate, Waltham, MA, USA), according to the manufacturer's instructions. The proteins were subjected to immunoblotting analysis using anti-eNOS (BD Biosciences) and anti-calmodulin (Abcam) antibodies.
Statistical analysis
Student's paired t-test was used to compare BP measurements (before and after pretreatments), and a one-way analysis of variance (anova) with Scheffe post hoc, comparison ware applied to compare group differences. Differences with P < 0.05 were considered significant. All data are expressed as means ± SEM.
Results
nAChRs are involved in nicotine-induced depressor effects in NTS
Previously, we showed that microinjection of nicotine into the NTS produced depressor effects (Tseng et al., 1993; 1994;). Nicotine exerts its effects through binding to nAChRs. Here, we first determined whether nAChRs existed in NTS neurones.
Results from Western blot and IHC staining analyses revealed that the α7 nAChR subtype is present in the NTS of WKY rats (Figure 1B,C). By using double-immunofluorescence staining for α7 nAChRs and neuronal nuclei (NeuN), we further found that α7 nAChR protein is expressed in cells with NeuN protein (Figure 1D). These results suggest that the α7 nAChR subtype exists in the neuronal cells of the NTS in WKY rats.
Figure 1.
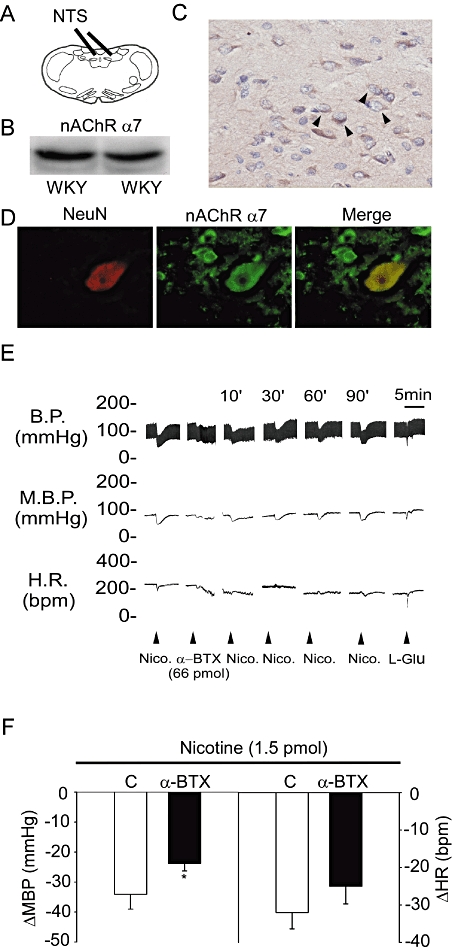
The expression of nAChRs and their effects on blood pressure in the NTS of WKY rats. (A) A diagrammatic representation of the rat NTS. (B) The immunoblot depicts the levels of α7 nAChR expression in the NTS of WKY. (C) In situ qualitative analysis by immunohistochemical staining of α7 nAChR-positive cells. The arrowhead indicates the α7 nAChR positive cells. (D) In situ qualitative analysis by immunostaining for NeuN and eNOS. (E) Representative tracings demonstrate the depressor effects of nicotine (1.5 pmol) administered into the unilateral NTS before and 10 min after pretreatment with α-BTX (66 pmol). (F) Histogram reveals the effects of a pretreatment with α-BTX on the modulation of MBP and HR induced by a microinjection of nicotine into the unilateral NTS. Nicotine was injected in the absence (C) or presence of α-BTX. The original magnification for C and D was ×400. Values are shown as mean difference ± SEM, n = 6. *P < 0.05 versus control group. α-BTX, α-bungarotoxin; BP, blood pressure; HR, heart rate; MBP, mean blood pressure; nAChRs, nicotinic acetylcholine receptors; NeuN, neuronal nuclei; NTS, nucleus tractus solitarii; WKY, Wistar-Kyoto rats.
The depressor effects of nicotine in the NTS of urethane-anaesthetized male WKY rats were further confirmed in this study. The results showed that the BP response to nicotine was attenuated by prior microinjection of α-BTX in the NTS of WKY rats (−34.1 ± 4.9 vs. −23.8 ± 2.5 mmHg, P < 0.05; and −32.0 ± 4.4 vs. −25.0 ± 4.7 beats·min−1, paired t-test; n = 6; Figure 1E,F). These results suggest that nicotine may modulate central BP via nAChRs in the NTS of WKY rats.
Ca2+ signalling participated in nAChR-modulated depressor effects in the NTS
To determine whether Ca2+/calmodulin is involved in the BP modulatory effects of nAChRs in the NTS, we investigated the effects of a Ca2+ chelator, EGTA, and a calmodulin inhibitor, N-(6-aminohexyl)-5-chloro-1-naphthalenesulphonamide (W7; 0.1 nmol·60 nL−1), on the depressor effects induced by nAChRs in the NTS. The results show that the BP response to nicotine was attenuated 10 min after treatment with EGTA (Figure 2A,B). Similarly, the BP response to nicotine was attenuated by prior microinjection of W7 in the NTS of WKY rats (−32.9 ± 3 vs. −17.1 ± 3 mmHg and −25.6 ± 5 vs. −12.8 ± 4 beats·min−1, respectively; P < 0.05, paired t-test; n = 6; Figure 2C,D). These results indicate that Ca2+ signalling is involved downstream of nAChR stimulation in mediating nicotine-induced depressor effects.
Figure 2.
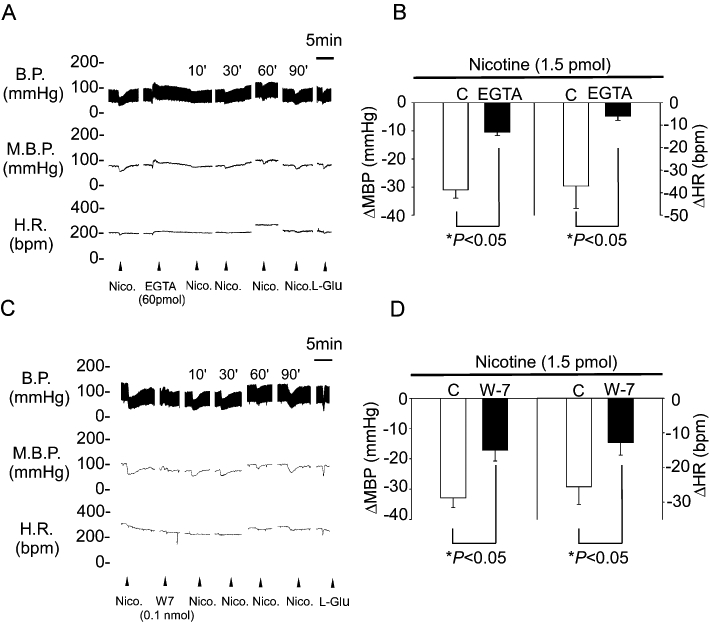
The Ca2+ signalling pathway participated in the nAChR-mediated blood pressure response. (A) Representative traces demonstrating the depressor effect of nicotine (1.5 pmol) injected into the unilateral NTS before and 10 min after pretreatment with EGTA (60 pmol). (B) Reveals the effects of pretreatment with EGTA (60 pmol) on MBP and HR responses induced by injection of nicotine into unilateral NTS. (C) Representative traces demonstrating the depressor effects of nicotine (1.5 pmol) administered into the unilateral NTS before and 10 min after pretreatment with W7 (0.1 nmol). (D) Reveals the effects of pretreatment with W7 on changes in MBP and HR induced by microinjection of nicotine into the unilateral NTS. Nicotine was injected in the absence (C) or presence of W7. Values are shown as mean change ± SEM, n = 6. *P < 0.05 versus control group. BP, blood pressure; EGTA, ethylene glycol tetraacetic acid; HR, heart rate; MBP, mean blood pressure; nAChRs, nicotinic acetylcholine receptors; NTS, nucleus tractus solitarii; W7, N-(6-aminohexyl)-5-chloro-1-naphthalenesulphonamide.
The activation of nAChRs induced NOS activity and NO production
Previously, nicotine was reported to induce NO production (Papadopolou et al., 2004; Zhao et al., 2007). We investigated the effects of an NOS inhibitor, L-NAME, on the depressor effects of nicotine in the NTS. Pretreatment with L-NAME (33 nmol·60 nL−1) elicited slight depressor effects (Figure 3A). Interestingly, the depressor and bradycardic responses to the same dose of nicotine in the NTS were attenuated after L-NAME treatment (−37 ± 9 vs. −23 ± 10 mmHg and −32 ± 3 vs. −13 ± 3 beats·min−1, respectively; P < 0.05, paired t-test; n = 6; Figure 3A,B). The depressor effect of nicotine in the NTS recovered gradually 30 min after L-NAME treatment (Figure 3A). We also directly measured the metabolites of NO after treatment with nicotine in the NTS. As shown in Figure 3C, microinjection of nicotine into the NTS significantly increased nitrate levels (NO3; 1.24 ± 0.24 vs. 2.22 ± 0.52, P < 0.05, n = 6). These results indicate that nAChRs may induce the production of nitrate in the NTS.
Figure 3.
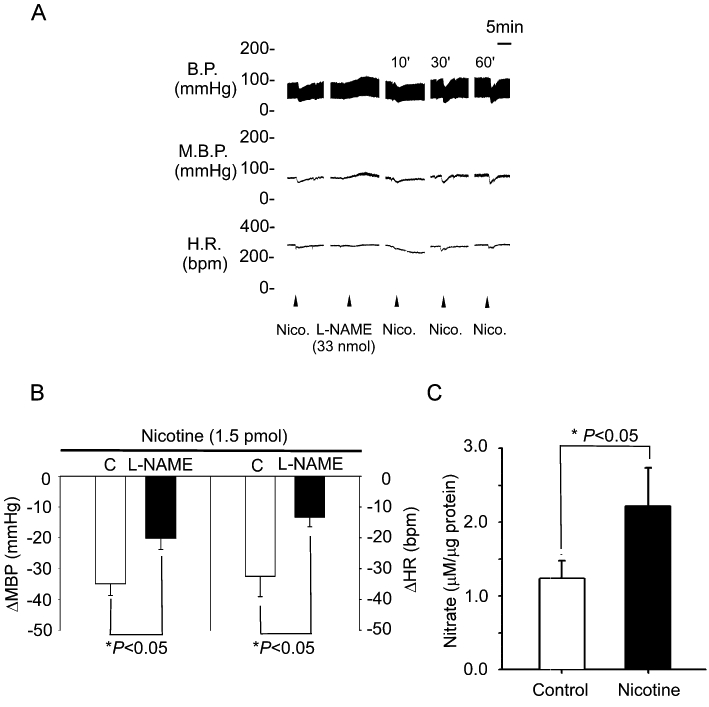
The NO system participates in the modulation of blood pressure mediated by nAChRs. (A) Representative tracings demonstrate the depressor effects of nicotine (1.5 pmol) injected into the unilateral NTS before and 10 min after pretreatment with L-NAME (33 nmol) in the anaesthetized WKY rats. (B) Reveals the effects of pretreatment with L-NAME on the changes in MBP and HR induced by microinjection of nicotine into unilateral NTS. (C) Reveals the effects of nicotine on NO3− levels in the NTS of WKY rats. Values are shown as mean change ± SEM, n = 6. *P < 0.05 versus control group. BP, blood pressure; EGTA, ethylene glycol tetraacetic acid; HR, heart rate; L-NAME, N-nitro-L-arginine methyl ester; MBP, mean blood pressure; NTS, nucleus tractus solitarii.
Effects of eNOS but not nNOS on depressor response mediated by nAChRs in the NTS
Our previous studies suggested that depressor responses to nAChRs are achieved through activation of NOS in the NTS. To identify which constitutive NOS contributes to these depressor effects of nAChRs in the NTS, we investigated the effects of a selective eNOS inhibitor, L-NIO, on the depressor response mediated by nAChRs in the NTS. Ten minutes after pretreatment with L-NIO (6 nmol·60 nL−1), the depressor and bradycardic responses to the same dose of nicotine were attenuated significantly (−35 ± 8 vs. −8 ± 3 mmHg and −41 ± 13 vs. −14 ± 8 beats·min−1, respectively; P < 0.05, paired t-test; n = 6; Figure 4A,B). In contrast, pretreatment with vinyl-L-NIO (600 pmol·60 nL−1) a nNOS-specific inhibitor, did not diminish the nicotine-mediated pressor (−34 ± 4 vs. −28 ± 6 mmHg, P > 0.05, n = 6) and bradycardic effects (−30 ± 4 vs. −29 ± 8 beats·min−1, P > 0.05, n = 6). These results indicate that eNOS but not nNOS may play a role in nAChRs-mediated depressor and bradycardic responses in the NTS.
Figure 4.
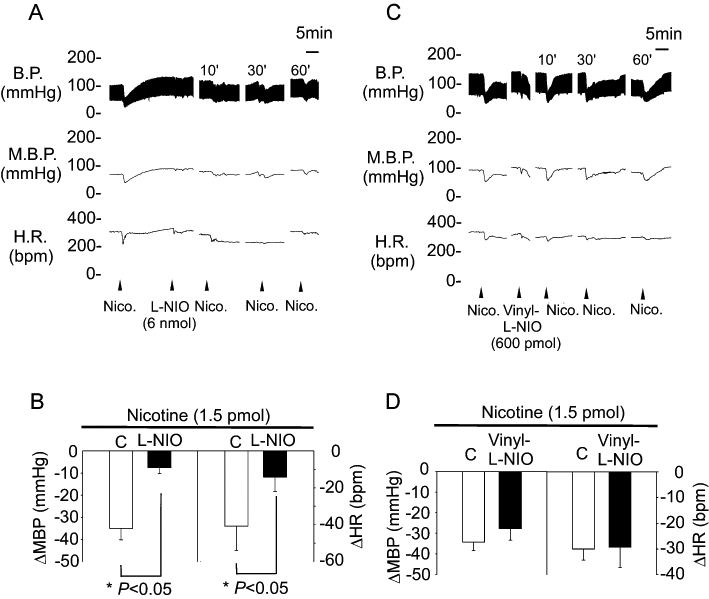
The depressor effects of nAChRs in the NTS before and after administration of selective nitric oxide synthase inhibitors. (A) Representative traces demonstrate the depressor effects of nicotine (1.5 pmol) in unilateral NTS before and 10 min after pretreatment with L-NIO (6 nmol). (B) Reveals the effects of pretreatment with L-NIO (6 nmol) on MBP and HR after microinjection of nicotine into unilateral NTS. (C) Representative traces demonstrate the depressor effects of nicotine (1.5 pmol) in unilateral NTS before and 10 min after pretreatment with vinyl-L-NIO (600 pmol). (D) Reveals the effects of pretreatment with vinyl-L-NIO (600 pmol) on MBP and HR after microinjection of nicotine into unilateral NTS. Values are shown as mean change ± SEM, n = 6. *P < 0.05 versus control group. BP, blood pressure; EGTA, ethylene glycol tetraacetic acid; HR, heart rate; L-NIO, N(5)-(-iminoethyl)-L-ornithine; MBP, mean blood pressure; nAChRs, nicotinic acetylcholine receptors; NTS, nucleus tractus solitarii; vinyl-L-NIO, N5-(1-Imino-3-butenyl)-L-ornithine.
The regulation of nAChRs-induced eNOS activity in the NTS is not achieved through phosphorylation by ERK or Akt
The above results demonstrate that eNOS is involved in nAChR-mediated depressor and bradycardic responses in the NTS. To confirm the localization of eNOS expression, we analysed the expression of eNOS in the NTS of WKY rats by double-immunostaining analysis. Figure 5A shows that expression of eNOS (red fluorescence) was found in the same cells with NeuN expression (green fluorescence). These results indicate that nAChR-induced eNOS activity occurs in the neurones of the NTS.
Figure 5.
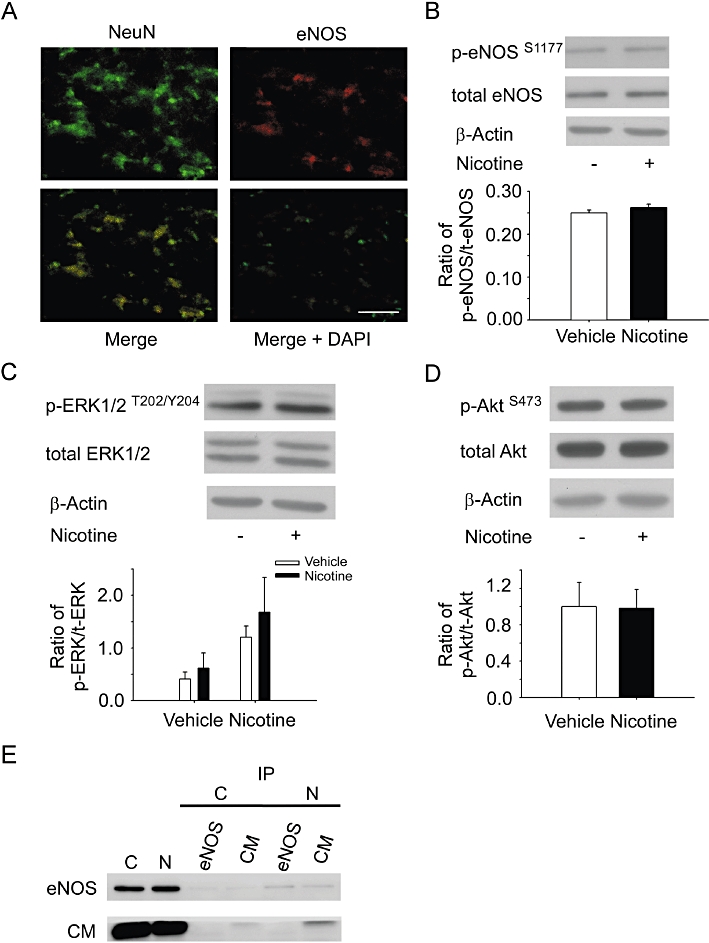
The regulation of eNOS activity by nicotine microinjected into the NTS. (A) The expression of NeuN (a) and eNOS (b) in the NTS of WKY rats as determined by immunofluorescent staining. (c) The merged image of NeuN-FITC (green, panel a) and eNOS-Rhod (red, panel c). (d) The merged image of NeuN-FITC, eNOS-Rhod and DAPI. The original magnification was ×400. Scale bar = 100 µm. (B) The immunoblot depicts the phosphorylation of eNOSS1177 proteins in the NTS without (lane 1) or with (lane 2) nicotine treatment. Phosphorylated eNOSS1177 was not increased in the nicotine-microinjected group as compared with the control group. (C) The immunoblot depicts the phosphorylation of ERK1/2T202/Y204 proteins in the NTS without (lane 1) or with (lane 2) nicotine treatment. Phosphorylated ERK1/2T202/Y204 was not increased in the nicotine-microinjected group compared with the control group. (D) The immunoblot depicts the phosphorylation of AktS473 proteins in the NTS without (lane 1) or with (lane 2) nicotine treatment. (E) Lysates from NTS were assayed and used for co-immunoprecipitation of calmodulin and eNOS. Total lysates were also analysed by immunoblot with antibodies to eNOS and calmodulin. eNOS interacts with calmodulin. Values are shown as mean ± SEM, n = 6. P > 0.05 versus control group. BP, blood pressure; DAPI, 2-(4-Amidinophenyl)-6-indolecarbamidine dihydrochloride; eNOS, endothelial nitric oxide synthase; ERK, extracellular signal-regulated kinases; FITC, fluorescein isothiocyanate; HR, heart rate; NeuN, neuronal nuclei; nNOS, neuronal nitric oxide synthase; NTS, nucleus tractus solitarii.
Previous studies have indicated that eNOS may be regulated by the ERK1/2 or Akt signalling pathway in the NTS (Ho et al., 2008; Hsiao et al., 2008). To investigate whether these pathways are activated by stimulation of nAChRs in the NTS of rats, we determined the phosphorylation levels of ERK1/2T202/Y204, AktS473, eNOSS1177 after nicotine injection into the NTS. Our results show that nicotine did not significantly increase the levels of phosphorylated eNOSS1177 (Figure 5B), ERK1/2T202/Y204 (Figure 5C) or AktS473 (Figure 5D) in the NTS (1.00 ± 0.02 vs. 1.05 ± 0.03 for eNOS; 1.0 ± 0.1 vs. 1.5 ± 0.3 for ERK1; 1.0 ± 0.3 vs. 1.0 ± 0.2 for Akt, P > 0.05; n = 6). Furthermore, prior microinjection of PD98059 [meiosis-specific serine/threonine protein kinase (MEK1) inhibitor] or LY294002 [phosphoinositide 3-kinase (P13K) inhibitor] did not attenuate nicotine-induced depressor and bradycardic effects in the NTS of WKY rats (Supporting Information Figure S1). These results indicate that neither the ERK1/2 nor the Akt signalling pathway plays a significant role in the activation of eNOS by nAChRs in the NTS.
eNOS activation is mediated by binding to calmodulin
We further investigated whether calmodulin binds to eNOS to induce its activation in the NTS after nicotine injection. Co-immunoprecipitation (IP) assay was performed to analyse calmodulin-NOS binding capacity. NTS lysate was immunoprecipitated with anti-eNOS or anti-calmodulin antibody and then probed with t-eNOS and calmodulin respectively. Our results in Figure 5E show that stimulation of nAChRs induces eNOS co-IP with calmodulin. This suggests that, indeed, calmodulin binds to eNOS and this is involved in nAChR-mediated depressor effects in the NTS.
Discussion and conclusions
Nicotine has been demonstrated to play an important role in hypotensive modulation in the NTS (Tseng et al., 1993; 1994;). However, the underlying molecular mechanism of nAChRs activity is still unclear. Previous studies show that nAChR subtypes can be found in several areas of the brain. These receptors are characterized by rapid desensitization and high permeability to calcium, as well as local and global calcium signals associated with the opening of neuronal α7-nAChR (Gilbert et al., 2009). Smith and Uteshev demonstrated that nAChR are expressed in neurones of the caudal NTS, which were found to be randomly distributed between presynaptic and somatic/dendritic sites (Smith and Uteshev, 2008). In our present study, we have also demonstrated that α7 nAChR subtypes are immunochemically positive in the NTS (Figure 1C). Double immunostaining revealed that the α7 nAChR subtype co-localized with the neuronal marker NeuN in NTS neurones (Figure 1D). These results indicate that the nAChR-mediated BP response might occur in the neurones of the NTS. The results of the present study confirm and extend our previous observations that nAChRs participate in the effect of nicotine on BP in the NTS of WKY rats (Figure 1E,F). Recently, Wu et al. (2009) reported that the nAChRs play a predominant role in nicotine-induced cell signalling. Several studies have indicated that α7 nAChRs have high calcium permeability and are rapidly activated by intracellular Ca2+ signals. However, Ca2+ is well known to be an important signal-transducing molecule in the neuronal cell. Constitutive isoforms of NOS include Ca2+/calmodulin-dependent enzymes and contain a conserved amino acid sequence for the calmodulin-binding site (Montgomery et al., 2003). The binding of calmodulin to constitutive NOS is essential for the activation of constitutive NOS activity induced by calcium (Kone et al., 2003). In endothelial cells, the main signal-transduction pathway for agonist-stimulated eNOS activation depends on Ca2+/calmodulin/caveolin (Dudzinski and Michel, 2007). Ca2+/calmodulin/caveolin are reported to regulate NO production by phosphorylation of eNOS, which facilitates the association of the enzyme with calmodulin, thus reducing its inhibitory interaction with caveolin (Michel et al., 1997).
Several neuromodulators modulate central BP effects via different signalling pathways in the NTS. For example, our previous study showed that the adenosine-ERK-eNOS signalling pathway exists and plays an important role in the modulation of cardiovascular responses to adenosine in the NTS (Ho et al., 2008). In addition, we also demonstrated that Ang II may modulate central BP effects via ROS to down-regulate ERK1/2, ribosomal protein S6 kinase and nNOS (Cheng et al., 2010). Several studies have investigated the possibility that IGF-1-stimulated NO production is mediated via PI3K-dependent Akt activation on Ser473, involving phosphorylation of eNOS at Ser1177 (Isenovic et al., 2001; 2003;). Recent studies have also shown that selective inhibition of nNOS in the brain increases BP in spontaneously hypertensive rats (SHR) but not in WKY rats (Qadri et al., 1999). These results indicate that different neuromodulators may modulate central BP effects via different signalling pathways in the NTS. In our opinion, the neuromodulator NO plays a major role in hypotensive effects induced in the NTS (Tseng et al., 1996). In addition, it has been reported that nAChRs mediate the increased generation of NO in neurones of the NTS (Zayas et al., 2002; Papadopolou et al., 2004; Zhao et al., 2007). In the present study, nicotine-induced depressor effects in the NTS were reduced by prior administration of the NOS inhibitor L-NAME (Figure 3A,B). However, nAChR-mediated BP responses in the NTS were attenuated by L-NIO but not by vinyl-L-NIO (Figure 4B,D). This suggests that eNOS might be one of the downstream targets of nAChRs that is involved in NO production, which then modulates BP in the NTS of WKY rats. We have also demonstrated that intra-NTS administration of eNOS genes induces a depressor response in the SHR (Tai et al., 2004). Waki et al. (2006) showed that eNOS-induced production of NO in the NTS plays a role in the control of baroreflex gain and arterial pressure. In the present study, we not only demonstrated the pharmacological effects of eNOS but also nNOS inhibitors on cardiovascular parameters (Figure 4). Our results suggested that nAChR-mediated depressor effects are achieved by the activity of eNOS rather than nNOS in the NTS.
Interestingly, several studies have indicated that the eNOS is a constitutive and strictly Ca2+/CaM-dependent enzyme. When the intracellular calcium concentration increases, the caveolin is displaced by calcium-calmodulin, which results in stimulation of phosphorylation at serine 1177 of the eNOS (Fleming et al., 2001). This, in turn, enhances enzyme activity in vitro. The activity of eNOS was also modulated by MEK kinase (Ho et al., 2008). eNOS is inactivated by calmodulin dissociation, which allows the re-binding of caveolin, accompanied by re-nitrosylation of the enzyme and de-phosphorylation of eNOS at its stimulating phosphorylation sites. Recently, a low dose of nicotine was found to improve the angiogenesis of human embryonic stem cells and prevent apoptosis mediated by mitogen-activated protein kinase and Akt signalling pathways during hypoxia (Yu et al., 2009). Serine/threonine kinase Akt also appears to be activated by nicotine in non-immortalized human airway epithelial cells in vitro (West et al., 2003). However, in our study, we showed by immunoblot analysis that microinjection of nicotine in the NTS does not induce Akt or ERK1/2 phosphorylation (Figure 5C,D). Furthermore, prior microinjection of PD98059 (MEK1 inhibitor) or LY294002 (PI3K inhibitor) did not attenuate nicotine-induced depressor or bradycardic effects in the NTS of WKY rats (Supporting Information Figure S1). Therefore, these results suggest that the Akt and ERK pathway are not involved in nAChR-regulated downstream signalling control of BP. In our study, we verified through a co-IP assay that calmodulin interacts with eNOS in the NTS. The data show that stimulation of nAChRs increases the association of eNOS with calmodulin (Figure 5E). These results indicate that the increase of eNOS activity induced via Ca2+/calmodulin binding may be responsible for the depressor response triggered by nicotine binding to and activating nAChRs in the NTS. The findings of the present study also confirm and validate our hypothesis that the Ca2+/calmodulin-eNOS signalling pathway participates in the regulation of BP by nAChRs in the NTS (Figure 5).
In this study, we propose a novel nAChRs-calmodulin-eNOS-NO pathway in the NTS. Our results support the BP modulation of nAChRs by Ca2+ signalling and activation of eNOS with subsequent NO release in the NTS (Figure 6). Our present studies further support a possible interaction between nAChRs and Ca2+/calmodulin in the NTS. The results demonstrates that nAChRs may modulate central BP effects via eNOS-induced NO production, as mediated by Ca2+/calmodulin binding.
Figure 6.
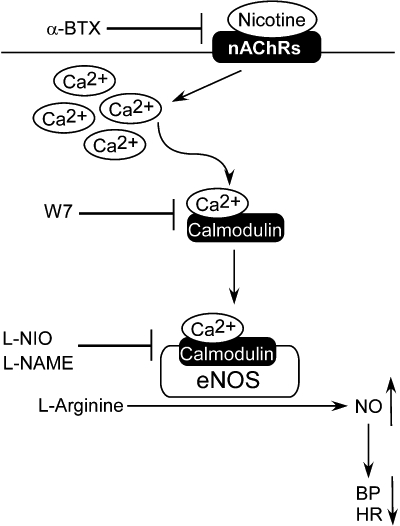
Proposed pathway by which nAChRs regulate blood pressure and heart rate in the NTS. Nicotine binds to the nAChRs, leading to Ca2+ influx; eNOS may then be activated by binding to calmodulin. This ultimately leads to increased NO concentrations in the NTS and decreased blood pressure. α-BTX, α-bungarotoxin; BP, blood pressure; eNOS, endothelial nitric oxide synthase; HR, heart rate; L-NAME, N-nitro-L-arginine methyl ester; L-NIO, N(5)-(-iminoethyl)-L-ornithine; nAChRs, nicotinic acetylcholine receptors; NeuN, neuronal nuclei; NO, nitric oxide; NTS, nucleus tractus solitarii; W7, N-(6-aminohexyl)-5-chloro-1-naphthalenesulphonamide.
One limitation of this study is due to the use of anaesthetics during the experiments. Urethane was used to produce anaesthesia in the laboratory animals as it is known to provide long periods of anaesthesia with minimal physiological changes. However, due to the potential health risks of urethane, it should be used with care. Several studies have demonstrated that urethane produces far fewer respiratory effects than either gas or barbiturate anaesthesia. A number of pulmonary pharmacologists have suggested that, in the rat, for pulmonary assessments, this is the only really useful anaesthetic (Buelke-Sam et al., 1978; Field and Lang, 1988; Field et al., 1993). However, other studies have indicated that BP and HR are elevated with urethane because it stimulates endogenous catecholamine release. In our future work, we will try to use new techniques such as siRNA or lentivirus, which may help us overcome the limitations imposed in anaesthetized animals.
In conclusion, our study suggests that nAChR-modulated depressor effects in the NTS are enacted through eNOS-induced NO production, and the regulation of eNOS activity is achieved through binding with calmodulin. The nAChR-calmodulin-eNOS-NO pathway may be involved in the regulation of BP in the NTS. This is the first study to demonstrate the mechanism by which nAChRs regulate depressor effects in the NTS. Our findings suggest new insights into central nervous system regulation of BP and may be of help for further development of therapy against this disease.
Acknowledgments
The authors gratefully acknowledge the technical assistance and the invaluable inputs and supports from Miss Yi-Shan Wu, and Mr Bo-Zone Chen. The kind gift of α-bungarotoxin (α-BTX) and suggestions from Dr Long-Sian Chang of the Institute of Biomedical Sciences, National Sun Yat-Sen University, Kaohsiung, Taiwan are greatly appreciated.
This work was supported by funding from the National Science Council NSC97-2321-B-075B-002 and Kaohsiung Veterans General Hospital VGHKS99-096. (to C.J.T.)
Glossary
Abbreviations
- α-BTX
α-bungarotoxin
- BP
blood pressure
- EGTA
ethylene glycol tetraacetic acid
- eNOS
endothelial nitric oxide synthase
- ERK
extracellular signal-regulated kinases
- HR
heart rate
- L-NIO
N(5)-(-iminoethyl)-L-ornithine
- L-NMMA
L-NG-monomethyl arginine citrate
- nAChRs
nicotinic acetylcholine receptors
- nNOS
neuronal nitric oxide synthase
- NO
nitric oxide
- NTS
nucleus tractus solitarii
- P
phosphorylated
- vinyl-L-NIO
N5-(1-Imino-3-butenyl)-L-ornithine
- W7
N-(6-aminohexyl)-5-chloro-1-naphthalenesulphonamide
- WKY
Wistar-Kyoto rats
Conflict of interest
None.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 The hypotensive effects of nAChRs in the NTS before and after administration of the P13K (LY294002) and MEK (PD98059) inhibitor. (A) Representative tracings demonstrate the depressor effects of nicotine (1.5 pmol) in unilateral NTS before and 10 min after pretreatment with LY294002 (6 pmol). (B) Graph reveals the effects of pretreated LY294002 on MBP and HR after microinjection of nicotine into unilateral NTS. (C) Representative tracings demonstrate the depressor effects of nicotine (1.5 pmol) in unilateral NTS before and 10 min after pretreatment with PD98059 (6 pmol). (D) Graph reveals the effects of pretreated PD98059 on MBP and HR after microinjection of nicotine into unilateral NTS. Values are shown as mean difference ± SEM, n = 4. *P < 0.05 vs. control group.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Bond CE, Zimmermann M, Greenfield SA. Upregulation of alpha7 nicotinic receptors by acetylcholinesterase C-terminal peptides. PLoS ONE. 2009;4:e4846. doi: 10.1371/journal.pone.0004846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buelke-Sam J, Holson JF, Bazare JJ, Young JF. Comparative stability of physiological parameters during sustained anesthesia in rats. Lab Anim Sci. 1978;28:157–162. [PubMed] [Google Scholar]
- Carlisle DL, Liu X, Hopkins TM, Swick MC, Dhir R, Siegfried JM. Nicotine activates cell-signaling pathways through muscle-type and neuronal nicotinic acetylcholine receptors in non-small cell lung cancer cells. Pulm Pharmacol Ther. 2007;20:629–641. doi: 10.1016/j.pupt.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Cheng WH, Lu PJ, Ho WY, Tung CS, Cheng PW, Hsiao M, et al. Angiotensin II inhibits neuronal nitric oxide synthase activation through the ERK1/2-RSK signaling pathway to modulate central control of blood pressure. Circ Res. 2010;106:788–795. doi: 10.1161/CIRCRESAHA.109.208439. [DOI] [PubMed] [Google Scholar]
- Cooper E, Couturier S, Ballivet M. Pentameric structure and subunit stoichiometry of a neuronal acetylcholine receptor. Nature. 1991;350:235–238. doi: 10.1038/350235a0. [DOI] [PubMed] [Google Scholar]
- Dudzinski D, Michel T. Life history of eNOS: partners and pathways. Cardiovasc Res. 2007;75:247–260. doi: 10.1016/j.cardiores.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field KJ, Lang CM. Hazards of urethane (ethyl carbamate): a review of the literature. Lab Anim. 1988;22:255–262. doi: 10.1258/002367788780746331. [DOI] [PubMed] [Google Scholar]
- Field KJ, White WJ, Lang CM. Anaesthetic effects of chloral hydrate, pentobarbitone and urethane in adult male rats. Lab Anim. 1993;27:258–269. doi: 10.1258/002367793780745471. [DOI] [PubMed] [Google Scholar]
- Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, Busse R. Phosphorylation of Thr(495) regulates Ca(2+)/calmodulin-dependent endothelial nitric oxide synthase activity. Circ Res. 2001;88:E68–E75. doi: 10.1161/hh1101.092677. [DOI] [PubMed] [Google Scholar]
- Gerzanich V, Zhang F, West GA, Simard JM. Chronic nicotine alters NO signaling of Ca(2+) channels in cerebral arterioles. Circ Res. 2001;88:359–365. doi: 10.1161/01.res.88.3.359. [DOI] [PubMed] [Google Scholar]
- Gilbert D, Lecchi M, Arnaudeau S, Bertrand D, Demaurex N. Local and global calcium signals associated with the opening of neuronal alpha7 nicotinic acetylcholine receptors. Cell Calcium. 2009;45:198–207. doi: 10.1016/j.ceca.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Ho WY, Lu PJ, Hsiao M, Hwang HR, Tseng YC, Yen MH, et al. Adenosine modulates cardiovascular functions through activation of extracellular signal-regulated kinases 1 and 2 and endothelial nitric oxide synthase in the nucleus tractus solitarii of rats. Circulation. 2008;117:773–780. doi: 10.1161/CIRCULATIONAHA.107.746032. [DOI] [PubMed] [Google Scholar]
- Hsiao M, Lu PJ, Huang HN, Lo WC, Ho WY, Lai TC, et al. Defective phosphatidylinositol 3-kinase signaling in central control of cardiovascular effects in the nucleus tractus solitarii of spontaneously hypertensive rats. Hypertens Res. 2008;31:1209–1218. doi: 10.1291/hypres.31.1209. [DOI] [PubMed] [Google Scholar]
- Huang HN, Lu PJ, Lo WC, Lin CH, Hsiao M, Tseng CJ. In situ Akt phosphorylation in the nucleus tractus solitarii is involved in central control of blood pressure and heart rate. Circulation. 2004;110:2476–2483. doi: 10.1161/01.CIR.0000145116.75657.2D. [DOI] [PubMed] [Google Scholar]
- Isenovic E, Muniyappa R, Milivojevic N, Rao Y, Sowers JR. Role of P13-kinase in isoproterenol and IGF-1 induced ecNOS activity. Biochem Biophys Res Commun. 2001;285:954–958. doi: 10.1006/bbrc.2001.5246. [DOI] [PubMed] [Google Scholar]
- Isenovic ER, Divald A, Milivojevic N, Grgurevic T, Fisher SE, Sowers JR. Interactive effects of insulin-like growth factor-1 and beta-estradiol on endothelial nitric oxide synthase activity in rat aortic endothelial cells. Metabolism. 2003;52:482–487. doi: 10.1053/meta.2003.50079. [DOI] [PubMed] [Google Scholar]
- Kone BC, Kuncewicz T, Zhang W, Yu Z-Y. Protein interactions with nitric oxide synthases: controling the right time, the right place, and the right amount of nitric oxide. Am J Physiol Renal Physiol. 2003;285:F178–F190. doi: 10.1152/ajprenal.00048.2003. [DOI] [PubMed] [Google Scholar]
- Li YL, Li YF, Liu D, Cornish KG, Patel KP, Zucker IH, et al. Gene transfer of neuronal nitric oxide synthase to carotid body reverses enhanced chemoreceptor function in heart failure rabbits. Circ Res. 2005;97:260–267. doi: 10.1161/01.RES.0000175722.21555.55. [DOI] [PubMed] [Google Scholar]
- Lo WJ, Liu HW, Lin HC, Ger LP, Tung CS, Tseng CJ. Modulatory effects of nitric oxide on baroreflex activation in the brainstem nuclei of rats. Chin J Physiol. 1996;39:57–62. [PubMed] [Google Scholar]
- Michel J, Feron O, Sacks D, Michel T. Reciprocal regulation of endothelial nitric-oxide synthase by Ca2+-calmodulin and caveolin. J Biol Chem. 1997;272:15583–15586. doi: 10.1074/jbc.272.25.15583. [DOI] [PubMed] [Google Scholar]
- Montgomery HJ, Bartlett R, Perdicakis B, Jervis E, Squier TC, Guillemette JG. Activation of constitutive nitric oxide synthases by oxidized calmodulin mutants. Biochemistry. 2003;42:7759–7768. doi: 10.1021/bi027097h. [DOI] [PubMed] [Google Scholar]
- Papadopolou S, Hartmann P, Lips K, Kummer W, Haberberger R. Nicotinic receptor mediated stimulation of NO-generation in neurons of rat thoracic dorsal root ganglia. Neurosci Lett. 2004;361:32–35. doi: 10.1016/j.neulet.2003.08.064. [DOI] [PubMed] [Google Scholar]
- Paterson D, Nordberg A. Neuronal nicotinic receptors in the human brain. Prog Neurobiol. 2000;61:75–111. doi: 10.1016/s0301-0082(99)00045-3. [DOI] [PubMed] [Google Scholar]
- Qadri F, Carretero OA, Scicli AG. Centrally produced neuronal nitric oxide in the control of baroreceptor reflex sensitivity and blood pressure in normotensive and spontaneously hypertensive rats. Jpn J Pharmacol. 1999;81:279–285. doi: 10.1254/jjp.81.279. [DOI] [PubMed] [Google Scholar]
- Smith DV, Uteshev VV. Heterogeneity of nicotinic acetylcholine receptor expression in the caudal nucleus of the solitary tract. Neuropharmacology. 2008;54:445–453. doi: 10.1016/j.neuropharm.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai MH, Hsiao M, Chan JY, Lo WC, Wang FS, Liu GS, et al. Gene delivery of endothelial nitric oxide synthase into nucleus tractus solitarii induces biphasic response in cardiovascular functions of hypertensive rats. Am J Hypertens. 2004;17:63–70. doi: 10.1016/j.amjhyper.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Tseng CJ, Appalsamy M, Robertson D, Mosqueda-Garcia R. Effects of nicotine on brain stem mechanisms of cardiovascular control. J Pharmacol Exp Ther. 1993;265:1511–1518. [PubMed] [Google Scholar]
- Tseng CJ, Ger LP, Lin HC, Tung CS. The pressor effect of nicotine in the rostral ventrolateral medulla of rats. Chin J Physiol. 1994;37:83–87. [PubMed] [Google Scholar]
- Tseng CJ, Liu HY, Lin HC, Ger LP, Tung CS, Yen MH. Cardiovascular effects of nitric oxide in the brain stem nuclei of rats. Hypertension. 1996;27:36–42. doi: 10.1161/01.hyp.27.1.36. [DOI] [PubMed] [Google Scholar]
- Waki H, Murphy D, Yao ST, Kasparov S, Paton JF. Endothelial NO synthase activity in nucleus tractus solitarii contributes to hypertension in spontaneously hypertensive rats. Hypertension. 2006;48:644–650. doi: 10.1161/01.HYP.0000238200.46085.c6. [DOI] [PubMed] [Google Scholar]
- West KA, Brognard J, Clark AS, Linnoila IR, Yang X, Swain SM, et al. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J Clin Invest. 2003;111:81–90. doi: 10.1172/JCI16147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JC, Chruscinski A, De Jesus Perez VA, Singh H, Pitsiouni M, Rabinovitch M, et al. Cholinergic modulation of angiogenesis: role of the 7 nicotinic acetylcholine receptor. J Cell Biochem. 2009;108:433–446. doi: 10.1002/jcb.22270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Huang NF, Wilson KD, Velotta JB, Huang M, Li Z, et al. nAChRs mediate human embryonic stem cell-derived endothelial cells: proliferation, apoptosis, and angiogenesis. PLoS ONE. 2009;4:e7040. doi: 10.1371/journal.pone.0007040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayas R, Qazi S, Morton D, Trimmer B. Nicotinic-acetylcholine receptors are functionally coupled to the nitric oxide/cGMP-pathway in insect neurons. J Neurochem. 2002;83:421–431. doi: 10.1046/j.1471-4159.2002.01147.x. [DOI] [PubMed] [Google Scholar]
- Zhao R, Chen H, Sharp B. Nicotine-induced norepinephrine release in hypothalamic paraventricular nucleus and amygdala is mediated by N-methyl-D-aspartate receptors and nitric oxide in the nucleus tractus solitarius. J Pharmacol Exp Ther. 2007;320:837–844. doi: 10.1124/jpet.106.112474. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


