Abstract
BACKGROUND AND PURPOSE
While arachidonyl ethanolamine (anandamide) produces pharmacological effects mediated by cannabinoid CB1 receptors, it is also an agonist at the transient receptor potential vanilloid type 1 (TRPV1) ion channel. This study examined the cellular actions of anandamide in the midbrain periaqueductal grey (PAG), a region implicated in the analgesic actions of cannabinoids, and which expresses both CB1 receptors and TRPV1.
EXPERIMENTAL APPROACH
In vitro whole cell patch clamp recordings of glutamatergic excitatory postsynaptic currents (EPSCs) were made from rat and mouse PAG slices.
KEY RESULTS
Capsaicin (1 µM) increased the rate, but not the amplitude of miniature EPSCs in subpopulations of neurons throughout the rat and mouse PAG. Capsaicin had no effect on miniature EPSCs in PAG neurons from TRPV1 knock-out mice. In mouse PAG neurons, anandamide (30 µM) had no effect on the rate of miniature EPSCs alone, or in the presence of either the CB1 antagonist AM251 (3 µM) or the TRPV1 antagonist iodoresiniferatoxin (300 nM). Anandamide produced a decrease in miniature EPSC rate in the presence of the fatty acid amide hydrolase (FAAH) inhibitor URB597 (1 µM). By contrast, anandamide produced an increase in miniature EPSC rate in the presence of both URB597 and AM251, which was absent in TRPV1 knock-out mice.
CONCLUSIONS AND IMPLICATIONS
These results suggest that the actions of anandamide within PAG are limited by enzymatic degradation by FAAH. FAAH blockade unmasks both presynaptic inhibition and excitation of glutamatergic synaptic transmission which are mediated via CB1 receptors and TRPV1 respectively.
Keywords: endocannabinoid, cannabinoid CB1, TRPV1, synaptic transmission, periaqueductal grey, pain
Introduction
The midbrain periaqueductal grey (PAG) plays a pivotal role in coordinating physiological responses to threat, stress and pain, and is a major site of action of analgesic and anxiolytic drugs (Keay and Bandler, 2001; Graeff, 2004; Moreira et al., 2009). For example, microinjection of synthetic cannabinoid agonists, such as HU210 and WIN55,212-2, into the PAG produces cannabinoid CB1 receptor-mediated analgesia and anxiolysis (Martin et al., 1995; Lichtman et al., 1996; Finn et al., 2003; Moreira et al., 2007; receptor nomenclature follows Alexander et al., 2008), although CB1 receptor-mediated hyperalgesia has also been reported (Maione et al., 2006).
It is becoming apparent that endogenous cannabinoids (endocannabinoids) also play an important role in pain and anxiety via their actions at cannabinoid CB1 receptors (Bradshaw and Walker, 2005; Pacher et al., 2006; Hill et al., 2009). The two main endocannabinoids, N-arachidonyl ethanolamide (anandamide) and 2-arachidonyl glycerol (2-AG), are synthesized and released on demand, and are metabolized by fatty acid amide hydrolase (FAAH) and monoacylglyceride lipase (MAGL) respectively (Piomelli, 2003; Di Marzo et al., 2005). Endocannabinoids, via cannabinoid CB1 receptors, mediate a component of stress-induced analgesia within the dorsal half of the PAG (Hohmann et al., 2005). Interestingly, anandamide and 2-AG levels within the PAG are increased by stress and painful stimuli (Walker et al., 1999; Hohmann et al., 2005). Furthermore, the FAAH inhibitor URB597 enhances anandamide levels within the PAG and potentiates endocannabinoid stress-induced analgesia (Hohmann et al., 2005; Maione et al., 2006). In addition to cannabinoid CB1 receptors, anandamide also activates the transient receptor potential vanilloid type 1 (TRPV1) ligand-gated ion channel (Melck et al., 1999; Zygmunt et al., 1999; Smart et al., 2000). Microinjection of the TRPV1 agonist capsaicin into the PAG has been reported to produce both antinociception and hyperalgesia (Palazzo et al., 2002; McGaraughty et al., 2003; Maione et al., 2006; Starowicz et al., 2007), and modulate anxiety (Terzian et al., 2009). Endocannabinoids also modulate pain from within the PAG because microinjection of URB597 into this brain structure produces both antinociception and hyperalgesia, which are abolished by cannabinoid CB1 receptor and TRPV1 channel antagonists respectively (Maione et al., 2006).
Cannabinoids are thought to produce analgesia by suppressing GABAergic inhibition of PAG output neurons which project along a descending analgesic pathway which projects via the medulla to the dorsal horn (Fields et al., 2006). The glutamatergic system also has an important role in the actions of analgesics within the PAG (see Palazzo et al., 2008). For example, synthetic cannabinoids act via presynaptic CB1 receptors to inhibit not only GABAergic, but also glutamatergic synaptic transmission throughout the PAG (Vaughan et al., 2000). In addition, it has been demonstrated that the TRPV1 agonist capsaicin presynaptically enhances glutamatergic synaptic transmission within a dorsolateral subdivision of the PAG (Xing and Li, 2007). The aim of the present study was to examine the role of cannabinoid CB1 receptors, TRPV1 ion channels and FAAH in the modulation of glutamatergic synaptic transmission by anandamide within the PAG.
Methods
Slice preparation
All animal care and experimental procedures followed the guidelines of the National Health and Medical Research Council ‘Australian code of practice for the care and use of animals for scientific purposes’ and were approved by the Royal North Shore Hospital Animal Care and Ethics Committee. We used male and female Sprague-Dawley rats (2–6 weeks old), male wild-type C57B16/J mice (4–10 weeks old) and transient receptor potential vanilloid subtype 1 receptor (TRPV1) knock-out mice (8–10 weeks old) that were generated as described previously (Caterina et al., 2000). Animals were deeply anaesthetized with isoflurane, decapitated and coronal midbrain slices (280 µm) containing the PAG were cut using a vibratome (VT1000S, Leica Microsystems, Nussloch, Germany) in ice-cold artificial cerebrospinal fluid (ACSF), of the following composition (in mM): NaCl 126, KCl 2.5, NaH2PO4 1.4, MgCl2 1.2, CaCl2 2.4, glucose 11, NaHCO3 25, as described previously (Drew et al., 2008). The slices were maintained at 34°C in a submerged chamber containing ACSF equilibrated with 95% O2 and 5% CO2. Individual slices were then transferred to a chamber and superfused continuously (1.8 mL·min−1) with ACSF at 34°C.
Electrophysiology
Periaqueductal grey neurons were visualized using infrared Dodt-tube contrast gradient optics on an upright microscope (BX50; Olympus, Tokyo, Japan). Whole-cell voltage-clamp recordings at −65 mV were made using an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA, USA) with an internal solution containing (in mM): CsCl 140, HEPES 10, EGTA 0.2, MgCl2 1, MgATP 2, NaGTP 0.3 and QX-314 3; pH 7.3 and osmolality 280–285 mosmol·L−1. Series resistance (<25 MΩ) was compensated by 80% and continuously monitored during experiments. Liquid junction potentials of −4 mV were corrected. Spontaneous non-NMDA mediated miniature EPSCs were obtained in the presence of tetrodotoxin (TTX) (300 nM), picrotoxin (100 µM) and the glycine receptor antagonist strychnine (5 µM). Spontaneous GABAA receptor-mediated miniature IPSCs were recorded in the presence of TTX (300 nM), the non-NMDA receptor antagonist, 6-cyano-7-nitroquinoxaline-2,3-dione disodium (CNQX; 5 µM) and strychnine (5 µM).
Slices were pre-incubated in URB597, JZL184, AM251 and iodoresiniferatoxin for at least 40–60 min before application of anandamide or capsaicin. Only one cell was studied per slice and the entire system was washed with 20% ethanol between slices.
Inhibitory postsynaptic currents and EPSCs were filtered (2 and 5 kHz low-pass filter) and sampled (5 and 10 kHz) for online and later off-line analysis (Axograph X, Sydney, Australia). Miniature IPSCs and EPSCs were sampled in 4 and 5 s epochs every 6 s for analysis. IPSCs and EPSCs above a preset threshold (4.0–5.0 standard deviations above baseline noise) were automatically detected by a sliding template algorithm and then manually checked offline. Capsaicin was applied to slices for 4 min, and EPSC/IPSC values were compared over a 2 min interval before and from 1–3 min during capsaicin. Anandamide was applied to slices for 15 min, and EPSC/IPSC values were compared over a 5 min interval before and from 10–15 min during anandamide. Neurons were considered to be capsaicin responders if there was an increase in miniature EPSC/IPSC rate of at least 20% which recovered following washout.
Data analysis
All numerical data are expressed as mean ± SEM, averaged across all neurons tested. Normalized cumulative distribution plots of IPSC and EPSC inter-event interval and amplitude were constructed and compared using the Kolmogorov–Smirnov test. Statistical comparisons of mean drug effects were made using paired Student's t-test, and comparisons between multiple treatment groups with a one-way anova (using Newman-Keuls correction for post hoc comparisons). Differences were considered significant if P < 0.05.
Materials
6-Cyano-7-nitroquinoxaline-2,3-dione disodium, strychnine hydrochloride and picrotoxin were from Sigma (Sydney, Australia). (5Z,8Z,11Z,14Z)- N-(2-hydroxyethyl)icosa- 5,8,11,14- tetraenamide (anandamide), 1-(2,4-dichlorophenyl) -5-(4-iodophenyl) -4-methyl-N-1 –piperidinyl -1H-pyrazole -3-carboxamide (AM251), [3-(3-carbamoylphenyl)phenyl] N-cyclohexylcarbamate (URB597), 4-nitrophenyl-4-[bis(1,3-benzodioxol-5-yl)(hydroxy)methyl]piperidine-1-carboxylate (JZL184) were from Cayman Chemicals (Ann Arbor, MI, USA). QX-314 bromide, 2-(3-carboxypropyl)-3-amino-6-(4 methoxyphenyl)pyridazinium bromide (SR95531) and TTX were from Ascent Scientific (Bristol, UK). Capsaicin and 6,7-deepoxy-6,7- didehydro-5- deoxy-21-dephenyl- 21-(phenylmethyl)- daphnetoxin,20- (4-hydroxy-5-iodo-3-methoxybenzeneacetate) (iodoresiniferatoxin) were from Tocris Cookson (Bristol, UK). Stock solutions of all drugs were made in distilled water, except AM251, URB597, iodoresiniferatoxin and JZL184 which were made in dimethyl sulphoxide, and capsaicin in ethanol. All cannabinoid and TRP ligands, plus other agents were diluted to working concentrations in ACSF immediately before use and applied by superfusion (dimethyl sulphoxide and ethanol ≤0.03% v·v–1 in final ACSF solution).
Results
Capsaicin acts via TRPV1 channels to enhance glutamatergic synaptic transmission throughout the PAG
It has previously been reported that the TRPV1 agonist capsaicin increases miniature EPSC rate in neurons within the rat dorsolateral PAG (Xing and Li, 2007). We examined the effect of a maximal concentration of capsaicin (1 µM) (Marinelli et al., 2002) on miniature EPSCs in neurons within the different longitudinal columns of both rat and mouse PAG. In PAG neurons from rats, miniature EPSCs were readily observed which had an average rate and amplitude of 1.2 ± 0.2 s−1 and 25 ± 1 pA, and were abolished by CNQX (10 µM, n = 4). Superfusion of capsaicin (1 µM) produced an increase in the rate of miniature EPSCs in 57% (n = 17/30) of rat PAG neurons tested. When averaged across all rat PAG neurons, capsaicin produced a significant increase in the rate of miniature EPSCs, but did not significantly affect their amplitude (Figure 1G, P = 0.0001 and 0.2, n = 30). Capsaicin produced an increase in miniature EPSC rate in similar proportions of neurons from the ventrolateral, lateral and dorsolateral PAG (Figure 1F, P = 0.9, χ2 = 0.3, n = 6/10, 6/10, 5/10 respectively).
Figure 1.
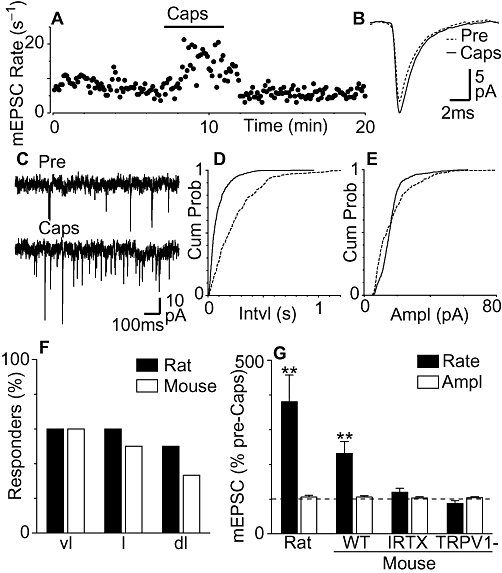
Capsaicin produces transient receptor potential vanilloid type 1 (TRPV1) channel-mediated enhancement of miniature excitatory postsynaptic currents (EPSCs) in subpopulations of rat and mouse periaqueductal grey (PAG) neurons. (A) Time course of miniature EPSC (mEPSC) rate during superfusion of capsaicin (Caps, 1 µM) in a mouse PAG neuron. (B) Average traces of miniature EPSCs before (Pre) and during capsaicin (Caps). (C) Raw current traces of miniature EPSCs before and during capsaicin. Cumulative probability distribution plots of miniature EPSC (D) inter-event interval and (E) amplitude before and during capsaicin. (F) Bar chart of the percentage of cells responding to capsaicin in the ventrolateral (vl), lateral (l) and dorsolateral (dl) PAG columns (n = 6/10, 6/10, 5/10). (G) Bar chart of the mean rate and amplitude of miniature EPSCs in the presence of capsaicin in PAG neurons from rats (n = 30), wild-type mice (WT, n = 24), wild-type mice in the presence of iodoresiniferatoxin (IRTX, n = 7) and TRPV1 knock-out mice (TRPV1-, n = 7), expressed as a percentage of the pre-capsaicin level (averaged across all neurons tested). In (G), **P < 0.01, significantly different from values before capsaicin. Traces in (A)–(E) are from the same neuron.
We next examined the effect of capsaicin in mouse PAG neurons and whether this was mediated by TRPV1 channels. In PAG neurons from wild-type mice, miniature EPSCs were readily observed which had an average rate and amplitude of 3.8 ± 0.4 s−1 and 21 ± 3 pA, and were abolished by CNQX (10 µM, n = 6). Capsaicin (1 µM) produced an increase in the rate of miniature EPSCs in 50% (n = 12/24) of PAG neurons (Figure 1A and C). In some neurons the increase in miniature EPSC rate rapidly desensitized, while in others the increase in rate was maintained during capsaicin application and decreased towards baseline levels following washout. The increase in miniature EPSC rate was reflected as a leftward shift in the miniature EPSC inter-event interval cumulative probability distribution (Figure 1D). By contrast, capsaicin had no effect on the amplitude and kinetics of miniature EPSCs, nor did it have an effect on the cumulative probability distributions of miniature EPSC amplitudes (Figure 1B and E). When averaged across all neurons, capsaicin produced a significant increase in miniature EPSC rate (P = 0.009), but did not significantly affect miniature EPSC amplitude (P = 0.2) (Figure 1G). The increase in miniature EPSC rate produced by capsaicin was similar for neurons within the ventrolateral, lateral and dorsolateral PAG columns (Figure 1F, P = 0.6, χ2 = 1.1, n = 6/10, 4/8, 2/6 respectively). The capsaicin-induced increase in miniature EPSC rate was concentration-dependent (average rate = 104 ± 9%, 117 ± 6%, 209 ± 29% and 214 ± 74% of pre-capsaicin levels at 0.1, 0.3, 1 and 3 µM, respectively, n = 5, 6, 24, 10). Capsaicin had no significant effect on the rate and amplitude of miniature EPSCs in mouse PAG neurons from slice pre-incubated in iodoresiniferatoxin (300 nM) (Figure 1G, P = 0.5 and 0.3, n = 5). In addition, capsaicin had no significant effect on the rate and amplitude of miniature EPSCs in PAG neurons from TRPV1 knock-out mice (Figure 1G, P = 0.2 and 0.2, n = 7).
We also examined the effect of capsaicin on GABAA receptor-mediated miniature IPSCs. In PAG neurons from wild-type mice, miniature IPSCs were readily observed that had an average rate and amplitude of 1.6 ± 0.2 s−1 and 41 ± 3 pA (n = 15), and were abolished by the GABAA receptor antagonist SR95531 (10 µM, n = 5). Capsaicin (1 µM) had no effect on the rate of miniature IPSCs, or on the cumulative probability distributions of miniature IPSC inter-event intervals in most neurons (Figure 2A, B and D, n = 14/15). In addition, capsaicin had no effect on the amplitude and kinetics of miniature IPSCs, nor did it have an effect on the cumulative probability distributions of miniature IPSC amplitudes in these neurons (Figure 2C and E). On average, the rate and amplitude of miniature IPSCs in the presence of capsaicin (1 µM) was 102 ± 7% and 103 ± 3% of the pre-capsaicin values respectively (Figure 2F, P = 0.8, 0.4, n = 15). It might be noted, however, that capsaicin produced a significant increase in miniature IPSC rate in one ventrolateral PAG neuron (rate = 168% of pre-capsaicin) which reversed upon washout.
Figure 2.
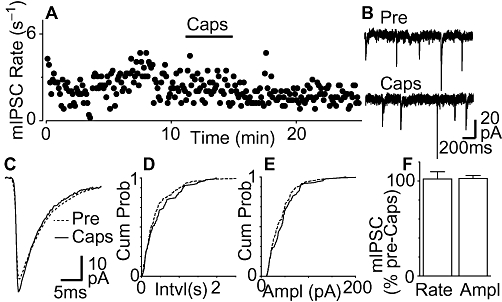
Capsaicin has no effect on miniature inhibitory postsynaptic currents (IPSCs). (A) Time course of miniature IPSC rate during superfusion of capsaicin (1 µM) in a mouse periaqueductal grey (PAG) neuron. (B) Raw current traces of miniature IPSCs before (Pre) and during capsaicin (Caps). (C) Average traces of miniature IPSCs before and during capsaicin. Cumulative probability distribution plots of miniature IPSC (D) inter-event interval and (E) amplitude before and during capsaicin. (F) Bar chart of the mean rate and amplitude (Ampl) of miniature IPSCs during application of capsaicin, expressed as a percentage of the pre-capsaicin level (averaged across all neurons tested, n = 15). (A–E) are taken from the same neuron.
Anandamide alone does not affect glutamatergic synaptic transmission
We next examined the effect of a maximal concentration of anandamide (Vaughan et al., 2000) on miniature EPSCs in neurons within the lateral/ventrolateral PAG of wild-type mice at a concentration that was likely to act on both cannabinoid CB1 receptors and TRPV1 ion channels. In wild-type mice, superfusion of anandamide (30 µM) had no effect on the rate, or amplitude of miniature EPSCs (Figure 3A, B and E, P = 0.8, 0.9, n = 8). This was reflected as a lack of effect on the cumulative probability distributions of miniature EPSC inter-event intervals and amplitudes (Figure 3C and D). In addition, anandamide had no effect on the kinetics of miniature EPSCs (Figure 3B).
Figure 3.
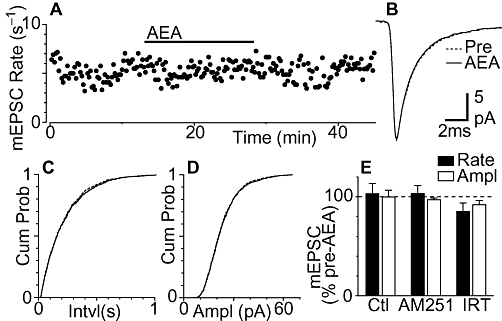
Anandamide alone has no CB1 receptor, or transient receptor potential vanilloid type 1 channel-mediated effects on miniature excitatory postsynaptic currents (EPSCs). (A) Time course of miniature EPSC rate during superfusion of anandamide (AEA, 30 µM) in a mouse PAG. (B) Average traces of miniature EPSCs before and during anandamide. Cumulative distribution plots of miniature EPSC (C) inter-event interval and (D) amplitude, before (Pre) and during anandamide (AEA; 30 µM). (E) Bar chart of the mean rate and amplitude (Ampl) of miniature EPSCs during application of anandamide alone (Control, Ctl, n = 8) and in neurons pre-incubated in AM251 (3 µM, n = 8), or iodoresiniferatoxin (IRT, 300 nM, n = 8). Data in (E) are expressed as a percentage of the pre-anandamide level (averaged across all neurons tested). (A–D) are taken from the same neuron.
The lack of effect of anandamide on miniature EPSCs may have been due to functional antagonism arising from concurrent activation of CB1 receptors and TRPV1 channels. In the presence of the TRPV1 antagonist iodoresiniferatoxin (300 nM), however, anandamide (30 µM) had no effect on the rate, or the amplitude of miniature EPSCs (Figure 3E, P = 0.1, P = 0.2, n = 8). Similarly, anandamide (30 µM) had no effect on the rate, or the amplitude of miniature EPSCs in the presence of the cannabinoid CB1 receptor antagonist AM251 (3 µM) (Figure 3E, P = 0.7, 0.2, n = 8). The basal miniature EPSC rate did not differ between neurons from control, iodoresiniferatoxin and AM251 pre-incubated slices (P = 0.5).
FAAH inhibition unmasks CB1 receptor-mediated presynaptic inhibition
The lack of effect of anandamide on miniature EPSCs may have been due to uptake and degradation, as we have previously demonstrated for IPSCs in rat PAG (Vaughan et al., 2000). We therefore examined the effect of anandamide in slices from mouse PAG pre-incubated with the FAAH inhibitor URB597 (1 µM). In the presence of URB597, anandamide produced a reduction in the rate of miniature EPSCs in all PAG neurons tested that only partially reversed upon washout (Figure 4A and E, P = 0.0008, n = 6). The anandamide-induced reduction in miniature EPSC rate was reflected as a rightward shift in the cumulative probability distributions of miniature EPSC inter-event intervals (Figure 4C). Anandamide had no effect on the amplitude (P = 0.2) and kinetics of miniature EPSCs, or on the cumulative probability distributions of miniature EPSC amplitudes in the presence of URB597 (Figure 4B, D and E). We next examined whether this anandamide-induced inhibition was enhanced by blocking TRPV1 channels. In the presence of URB597 and iodoresiniferatoxin, anandamide produced a reduction in the rate, but had no effect on the amplitude of miniature EPSCs (Figure 4E, P = 0.02, P = 0.3, n = 5). The anandamide-induced decrease in miniature EPSC rate, however, was similar in the presence of URB597 alone and URB597 plus iodoresiniferatoxin (P > 0.05). Anandamide had no effect on the rate, or amplitude of miniature EPSCs in the combined presence of URB597, iodoresiniferatoxin and AM251 (Figure 4E, P = 0.5, 0.6, n = 6). The basal miniature EPSC rate did not differ between neurons from control, URB597, URB597/iodoresiniferatoxin and URB597/iodoresiniferatoxin/AM251 pre-incubated slices (P = 0.2).
Figure 4.
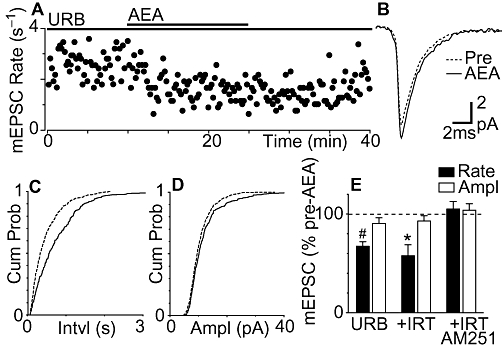
Fatty acid amide hydrolase (FAAH) inhibition unmasks CB1 receptor-mediated inhibition of miniature excitatory postsynaptic currents (EPSCs) by anandamide. (A) Time course of miniature EPSC rate during superfusion of anandamide (AEA, 30 µM) in a neuron pre-incubated with URB597 (URB, 1 µM). (B). Average traces of miniature EPSCs before (Pre) and during anandamide, in the presence of URB597. Cumulative distribution plots of miniature EPSC (C) inter-event interval and (D) amplitude, before and during anandamide, in the presence of URB597. (E) Bar chart of the mean rate and amplitude (Ampl) of miniature EPSCs during application of anandamide, in neurons pre-incubated in URB597 alone (n = 6), URB597 plus iodoresiniferatoxin (IRT, 300 nM, n = 5), or URB597 plus AM251 (3 µM, n = 6). Data in (E) are expressed as a percentage of the pre-anandamide level (averaged across all neurons tested); *P < 0.05, #P < 0.0001, significantly different from values before AEA. (A–D) are taken from the same neuron.
FAAH inhibition also unmasks TRPV1 channel-mediated presynaptic excitation
We next examined whether anandamide also produces a TRPV1 channel-mediated increase in miniature EPSC rate which is normally masked by enzymatic breakdown and cannabinoid CB1 receptor-mediated inhibition. In the combined presence of URB597 (1 µM) and AM251 (3 µM), anandamide (30 µM) produced an increase in miniature EPSC rate in 63% (n = 5/8) of PAG neurons (Figure 5A and E, P = 0.03). In the responding neurons, this increase in rate was reflected as a leftward shift in the miniature EPSC inter-event interval cumulative probability distribution (Figure 5C). Anandamide had no effect on the amplitude (P = 0.09) and kinetics of miniature EPSCs, nor did it have an effect on the cumulative probability distributions of their amplitudes in the presence of URB597 and AM251 (Figure 5B and E). The increase in miniature EPSC rate produced by anandamide was likely to be TRPV1 channel-mediated because, in TRPV1 knock-out mice, anandamide (30 µM) had no effect on the rate and amplitude of miniature EPSCs in the presence of AM251 and URB597 (Figure 5E, P = 0.2, 0.1, n = 5). It might also be noted that there was no endogenous TRPV1 channel-mediated excitation because superfusion of iodoresiniferatoxin (1 µM, for 10 min) had no effect on the rate and amplitude of miniature EPSCs in the presence of AM251 and URB597 (90 ± 5% and 98 ± 3% of pre-iodoresiniferatoxin rate and amplitude, P = 0.1, 0.4, n = 7).
Figure 5.
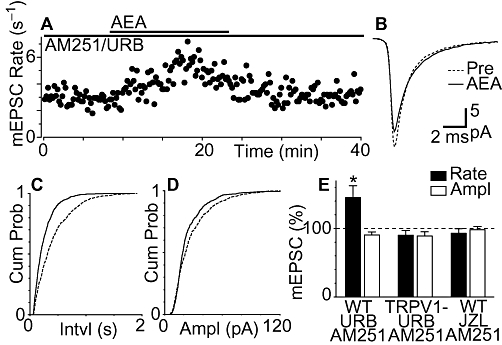
Fatty acid amide hydrolase inhibition and cannabinoid CB1 receptor blockade unmask transient receptor potential vanilloid type 1 (TRPV1) channel-mediated excitation of miniature excitatory postsynaptic currents (EPSCs) by anandamide. (A) Time course of miniature EPSC rate during superfusion of anandamide (AEA, 30 µM) in a neuron pre-incubated with URB597 (URB, 1 µM) and AM251 (3 µM). (B) Average traces of miniature EPSCs before (Pre) and during anandamide, in the presence of URB597 and AM251. Cumulative distribution plots of miniature EPSC (C) inter-event interval and (D) amplitude, before and during anandamide, in the presence of URB597 plus AM251. (E) Bar chart of the mean rate and amplitude of miniature EPSCs during application of anandamide, in neurons pre-incubated in URB597 plus AM251 from wild-type mice (WT, n = 8) and TRPV1 knock-out mice (TRPV1-, n = 5), and from neurons pre-incubated in JZL184 (JZL, 1 µM) plus AM251 from wild-type mice (n = 7). Data in (E) are expressed as a percentage of the pre-anandamide level (averaged across all neurons tested); *P < 0.05, significantly different from values before AEA. (A–D) are taken from the same neuron.
We finally examined whether the MAGL inhibitor JZL184 (Long et al., 2009) also unmasked a TRPV1 channel-mediated increase in miniature EPSC rate. In the combined presence of JZL184 (1 µM) and AM251 (3 µM) anandamide (30 µM) did not significantly affect the rate and amplitude of miniature EPSCs (Figure 5E, P = 0.3, 0.7, n = 7). The basal miniature EPSC rate did not differ between neurons from control, URB597/AM251 (including wild-type and TRPV1 knock-out mice) and JZL184/AM251 pre-incubated slices (P = 0.3).
Discussion
In the present study, we have shown that FAAH inhibition unmasks anandamide-induced presynaptic inhibition and excitation of glutamatergic synaptic transmission in the midbrain PAG, effects which are mediated by cannabinoid CB1 receptors and TRPV1 ion channels respectively. These findings indicate that, in addition to cannabinoid receptors, TRPV1 ion channels have the potential to modulate the diverse physiological functions mediated by the PAG.
The TRPV1 agonist capsaicin presynaptically enhanced glutamatergic synaptic transmission within the rat and mouse PAG, as observed previously in the rat (Xing and Li, 2007). The effect of capsaicin was likely to be presynaptic because it produced an increase in the rate of miniature EPSCs, but had no effect on EPSC amplitude, or kinetics as observed in other brain regions (Yang et al., 1998; Marinelli et al., 2002; 2003; Li et al., 2004). This capsaicin-induced presynaptic excitation was likely to be mediated by TRPV1 ion channels because it was absent in TRPV1 knock-out mice, as observed in the striatum (Musella et al., 2009), and was abolished by a TRPV1 antagonist, as observed in the PAG and other brain regions (Yang et al., 1998; Marinelli et al., 2002; 2003; Li et al., 2004; Derbenev et al., 2006; Xing and Li, 2007). Unlike its effects on glutamatergic miniature EPSCs, capsaicin had little effect on GABAergic miniature IPSCs in neurons throughout the mouse PAG, as observed previously in the rat dorsolateral PAG (Xing and Li, 2007). This is similar to other brain regions where electrophysiological evidence suggests that functional TRPV1 ion channels are located exclusively on glutamatergic nerve terminals (Marinelli et al., 2002; 2003; Li et al., 2004; Musella et al., 2009). The present and previous (Xing and Li, 2007) electrophysiological findings are at least partly consistent with anatomical evidence that TRPV1 channels are expressed in cell bodies and nerve fibres, particularly glutamatergic terminals, within the PAG and adjacent colliculus (McGaraughty et al., 2003; Cristino et al., 2006; Maione et al., 2006; 2009a,b; Starowicz et al., 2007).
In the present study anandamide had no significant effect on miniature EPSCs under basal conditions. In addition to cannabinoid CB1 receptors, the endocannabinoid anandamide is an agonist at TRPV1 ion channel (Melck et al., 1999; Zygmunt et al., 1999; Smart et al., 2000). The lack of effect of anandamide, however, was unlikely to be due to functional antagonism between presynaptic CB1 receptors and TRPV1 ion channels because anandamide had no effect on miniature EPSCs in the presence of either CB1 receptor or TRPV1 channel antagonists. Instead, the lack of effect of anandamide on miniature EPSCs under basal conditions was likely to be due to breakdown of anandamide via the endocannabinoid degrading enzyme FAAH because anandamide produced a reduction in miniature EPSCs rate in the presence of the FAAH inhibitor URB597. This enhancement of anandamide inhibition of glutamatergic synaptic transmission produced by FAAH inhibition is similar to that previously reported in hippocampal and cerebellar slices (Bajo et al., 2009; Pan et al., 2009). The inhibition produced by anandamide in the presence of URB597 was less than that produced by synthetic cannabinoid agonists (Vaughan et al., 2000), as observed in other brain regions (e.g. Ameri et al., 1999; Bajo et al., 2009; Haj-Dahmane and Shen, 2009; Pan et al., 2009), and may have been due to the lower efficacy of anandamide for cannabinoid CB1 receptors (Pacher et al., 2006).
Interestingly, we found that after FAAH blockade, anandamide produced an increase in miniature EPSC rate in the presence of AM251 which was abolished by iodoresiniferatoxin and was absent in TRPV1 knock-out mice. This is similar to the TRPV1 channel-mediated enhancement of primary afferent glutamatergic synaptic transmission by anandamide in the spinal and trigeminal dorsal horn (Morisset et al., 2001; Jennings et al., 2003), but differs from cultured hippocampal neurons where the anandamide-induced increase in miniature EPSC rate was unaffected by TRPV1 antagonists (Sang et al., 2010). The finding that the TRPV1 channel-mediated facilitation of glutamatergic synaptic transmission by anandamide was only observed after cannabinoid CB1 receptor antagonism possibly reflects the lower efficacy and affinity of anandamide for TRPV1 channels (Zygmunt et al., 1999; Toth et al., 2005; De Petrocellis and Di Marzo, 2009). We could not perform a concentration–response analysis in our slice preparation, however, because the stochastic nature of miniature EPSCs obscured responses to low micromolar concentrations of anandamide which had a slower time course and smaller magnitude (data not shown). In contrast to the effects of FAAH inhibition, anandamide had no effect on miniature EPSCs in the combined presence of the MAGL inhibitor JZL184 and AM251. The different effects of URB597 and JZL184 are consistent with FAAH and MAGL being the main enzymes responsible for the degradation of anandamide and 2-AG, respectively (Cravatt et al., 2001; Blankman et al., 2007), although it has been reported that URB597 increases both anandamide and 2-AG levels within the PAG (Maione et al., 2006).
Studies on the mechanisms underlying the analgesic effects of opioids and cannabinoids have largely focussed on the GABAergic system within the PAG. Like opioids, cannabinoids are thought to produce analgesia by suppressing GABAergic inhibition of PAG output neurons which project along a descending analgesic pathway (Fields et al., 2006). Indeed, we have previously demonstrated that exogenously applied anandamide and synthetic cannabinoid agonists, plus endogenously released endocannabinoids act via presynaptic CB1 receptors to inhibit GABAergic synaptic transmission in all neurons throughout the PAG (Vaughan et al., 2000; Drew et al., 2008; 2009; Lau and Vaughan, 2008). Cannabinoid agonists and endocannabinoids, however, also presynaptically inhibit glutamatergic synaptic transmission in all PAG neurons (Vaughan et al., 2000; Lau and Vaughan, 2008). The cannabinoid inhibition of synaptic transmission is likely to be mediated by an action at CB1 receptors in presynaptic nerve terminals (Tsou et al., 1998). The present findings indicate that presynaptic TRPV1 channel activation enhances glutamatergic, but not GABAergic, synaptic transmission onto neurons throughout the ventrolateral, lateral and dorsolateral columns PAG of both rats and mice, extending previous findings in the rat dorsolateral PAG (Xing and Li, 2007). There was, however, an incomplete overlap between presynaptic modulation of glutamatergic synaptic transmission in the midbrain PAG by TRPV1 channels and CB1 receptors because TRPV1-mediated enhancement of glutamatergic synaptic transmission was only observed in subpopulations of PAG neurons. Future studies would therefore need to determine the phenotype of the TRPV1-sensitive and insensitive neurons, including their neurochemical composition and whether they are interneurons or projection neurons.
The present findings support the notion that modulation of the glutamatergic system within the PAG by TRPV1 channel and CB1 receptors has an important role in the control of descending analgesia (Palazzo et al., 2008). Functionally, TRPV1 channel-mediated enhancement of glutamate release and CB1 receptor-mediated inhibition of GABA release would be expected to activate PAG output neurons which project along the descending analgesic pathway, while CB1 receptor-mediated inhibition of glutamate release would be expected to inhibit these neurons. This complex modulation of neuronal excitability within PAG by endocannabinoids is reflected in the disparate pain modulatory actions of cannabinoids and vanilloids within the PAG. Microinjection of synthetic cannabinoid agonists, such as HU210 and WIN55,212-2, into the PAG has been reported to produce cannabinoid CB1 receptor-mediated analgesia (Martin et al., 1995; Lichtman et al., 1996; Hohmann et al., 2005) and hyperalgesia (Maione et al., 2006). Likewise, some studies have reported that capsaicin microinjection into the ventrolateral and dorsolateral PAG produces analgesia (Palazzo et al., 2002; Maione et al., 2006; Starowicz et al., 2007), although another study has reported that capsaicin microinjection into the dorsolateral PAG produces hyperalgesia and has no effect in the ventrolateral PAG (McGaraughty et al., 2003). Finally, it has recently been shown that enhancing endocannabinoid levels within the ventrolateral PAG with microinjections of URB597 produces both analgesia and hyperalgesia, which are blocked by TRPV1 channel and CB1 receptor antagonists respectively (Maione et al., 2006; de Novellis et al., 2008). The disparities between these functional studies might also be due to differences in doses, pain assays, or the circuitry mediating the TRPV1 channel and CB1 receptor-mediated actions within specific PAG columns. In this regard, the ventrolateral, lateral and dorsolateral PAG columns mediate distinct analgesic, cardiovascular and behavioural responses to different forms of threat, stress and pain (Keay and Bandler, 2001). Thus, cannabinoids and vanilloids have the potential to influence a range of behavioural responses mediated by this complex brain structure.
In conclusion, this study has shown that both cannabinoid CB1 receptors and TRPV1 ion channels play an important role in modulating glutamatergic synaptic transmission within the PAG. This might be contrasted to the GABAergic system which is exclusively modulated by cannabinoid CB1 receptors and is not directly influenced by TRPV1 ion channels. Our findings suggest that anandamide has the potential to engage both cannabinoid CB1 receptors and TRPV1 channels, although this is normally limited by degradation via FAAH. This complex modulation of neuronal excitability might explain the mixed analgesic and hyperalgesic actions reported for agents who target cannabinoid CB1 receptors and TRPV1 ion channels. Further investigation of the interaction between cannabinoid and vanilloid receptors in descending modulatory systems may lead to novel and effective pharmacotherapies for the treatment of pain.
Acknowledgments
Supported by the National Health & Medical Research Council of Australia grant 457563 to C. W. Vaughan.
Glossary
Abbreviations
- ACSF
artificial cerebrospinal fluid
- CNQX
6-cyano-2,3-dihdroxy-7-nitro-quinoxaline
- EPSC
excitatory postsynaptic current
- FAAH
fatty acid amide hydrolase
- IPSC
inhibitory postsynaptic current
- MAGL
monoacylglyceride lipase
- NMDA
N-methyl-D-aspartate
- TRPV1
transient receptor potential vanilloid-1
- TTX
tetrodotoxin
Conflict of interest
The authors have no conflicting financial interests.
Supporting Information
Teaching Materials; Figs 1–5 as PowerPoint slide.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edition. Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameri A, Wilhelm A, Simmet T. Effects of the endogeneous cannabinoid, anandamide, on neuronal activity in rat hippocampal slices. Br J Pharmacol. 1999;126:1831–1839. doi: 10.1038/sj.bjp.0702478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo M, Roberto M, Schweitzer P. Differential alteration of hippocampal excitatory synaptic transmission by cannabinoid ligands. J Neurosci Res. 2009;87:766–775. doi: 10.1002/jnr.21889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14:1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw HB, Walker JM. The expanding field of cannabimimetic and related lipid mediators. Br J Pharmacol. 2005;144:459–465. doi: 10.1038/sj.bjp.0706093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, et al. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci U S A. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristino L, de Petrocellis L, Pryce G, Baker D, Guglielmotti V, Di Marzo V. Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience. 2006;139:1405–1415. doi: 10.1016/j.neuroscience.2006.02.074. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Di Marzo V. Role of endocannabinoids and endovanilloids in Ca2+ signalling. Cell Calcium. 2009;45:611–624. doi: 10.1016/j.ceca.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Derbenev AV, Monroe MJ, Glatzer NR, Smith BN. Vanilloid-mediated heterosynaptic facilitation of inhibitory synaptic input to neurons of the rat dorsal motor nucleus of the vagus. J Neurosci. 2006;26:9666–9672. doi: 10.1523/JNEUROSCI.1591-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, De Petrocellis L, Bisogno T. The biosynthesis, fate and pharmacological properties of endocannabinoids. Handb Exp Pharmacol. 2005;168:147–185. doi: 10.1007/3-540-26573-2_5. [DOI] [PubMed] [Google Scholar]
- Drew GM, Mitchell VA, Vaughan CW. Glutamate spillover modulates GABAergic synaptic transmission in the rat midbrain periaqueductal grey via metabotropic glutamate receptors and endocannabinoid signaling. J Neurosci. 2008;28:808–815. doi: 10.1523/JNEUROSCI.4876-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew GM, Lau BK, Vaughan CW. Substance P drives endocannabinoid-mediated disinhibition in a midbrain descending analgesic pathway. J Neurosci. 2009;29:7220–7229. doi: 10.1523/JNEUROSCI.4362-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields HL, Basbaum AI, Heinricher MM. Central nervous systems mechanisms of pain modulation. In: McMahon SB, Koltzenburg M, editors. Textbook of Pain. 5th edn. Philadelphia, PA: Elsevier, Churchill Livingston; 2006. pp. 125–142. [Google Scholar]
- Finn DP, Jhaveri MD, Beckett SR, Roe CH, Kendall DA, Marsden CA, et al. Effects of direct periaqueductal grey administration of a cannabinoid receptor agonist on nociceptive and aversive responses in rats. Neuropharmacology. 2003;45:594–604. doi: 10.1016/s0028-3908(03)00235-1. [DOI] [PubMed] [Google Scholar]
- Graeff FG. Serotonin, the periaqueductal gray and panic. Neurosci Biobehav Rev. 2004;28:239–259. doi: 10.1016/j.neubiorev.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Haj-Dahmane S, Shen RY. Endocannabinoids suppress excitatory synaptic transmission to dorsal raphe serotonin neurons through the activation of presynaptic CB1 receptors. J Pharmacol Exp Ther. 2009;331:186–196. doi: 10.1124/jpet.109.153858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Hillard CJ, Bambico FR, Patel S, Gorzalka BB, Gobbi G. The therapeutic potential of the endocannabinoid system for the development of a novel class of antidepressants. Trends Pharmacol Sci. 2009;30:484–493. doi: 10.1016/j.tips.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, et al. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435:1108–1112. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- Jennings EA, Vaughan CW, Roberts LA, Christie MJ. The actions of anandamide on rat superficial medullary dorsal horn neurons in vitro. J Physiol (Lond) 2003;548:121–129. doi: 10.1113/jphysiol.2002.035063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keay KA, Bandler R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci Biobehav Rev. 2001;25:669–678. doi: 10.1016/s0149-7634(01)00049-5. [DOI] [PubMed] [Google Scholar]
- Lau BK, Vaughan CW. Muscarinic modulation of synaptic transmission via endocannabinoid signalling in the rat midbrain periaqueductal gray. Mol Pharmacol. 2008;74:1392–1398. doi: 10.1124/mol.108.045872. [DOI] [PubMed] [Google Scholar]
- Li DP, Chen SR, Pan HL. VR1 receptor activation induces glutamate release and postsynaptic firing in the paraventricular nucleus. J Neurophysiol. 2004;92:1807–1816. doi: 10.1152/jn.00171.2004. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Cook SA, Martin BR. Investigation of brain sites mediating cannabinoid-induced antinociception in rats: evidence supporting periaqueductal gray involvement. J Pharmacol Exp Ther. 1996;276:585–593. [PubMed] [Google Scholar]
- Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, et al. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol. 2009;5:37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaraughty S, Chu KL, Bitner RS, Martino B, El Kouhen R, Han P, et al. Capsaicin infused into the PAG affects rat tail flick responses to noxious heat and alters neuronal firing in the RVM. J Neurophysiol. 2003;90:2702–2710. doi: 10.1152/jn.00433.2003. [DOI] [PubMed] [Google Scholar]
- Maione S, Bisogno T, de Novellis V, Palazzo E, Cristino L, Valenti M, et al. Elevation of endocannabinoid levels in the ventrolateral periaqueductal grey through inhibition of fatty acid amide hydrolase affects descending nociceptive pathways via both cannabinoid receptor type 1 and transient receptor potential vanilloid type-1 receptors. J Pharmacol Exp Ther. 2006;316:969–982. doi: 10.1124/jpet.105.093286. [DOI] [PubMed] [Google Scholar]
- Maione S, Cristino L, Migliozzi AL, Georgiou AL, Starowicz K, Salt TE, et al. TRPV1 channels control synaptic plasticity in the developing superior colliculus. J Physiol (Lond) 2009a;587:2521–2535. doi: 10.1113/jphysiol.2009.171900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maione S, Starowicz K, Cristino L, Guida F, Palazzo E, Luongo L, et al. Functional interaction between TRPV1 and µ-opioid receptors in the descending antinociceptive pathway activates glutamate transmission and induces analgesia. J Neurophysiol. 2009b;101:2411–2422. doi: 10.1152/jn.91225.2008. [DOI] [PubMed] [Google Scholar]
- Marinelli S, Vaughan CW, Christie MJ, Connor M. Capsaicin activation of glutamatergic synaptic transmission in the rat locus coeruleus in vitro. J Physiol (Lond) 2002;543:531–540. doi: 10.1113/jphysiol.2002.022863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli S, Di Marzo V, Berretta N, Matias I, Maccarrone M, Bernardi G, et al. Presynaptic facilitation of glutamatergic synapses to dopaminergic neurons of the rat substantia nigra by endogenous stimulation of vanilloid receptors. J Neurosci. 2003;23:3136–3144. doi: 10.1523/JNEUROSCI.23-08-03136.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WJ, Patrick SL, Coffin PO, Tsou K, Walker JM. An examination of the central sites of action of cannabinoid-induced antinociception in the rat. Life Sci. 1995;56:2103–2109. doi: 10.1016/0024-3205(95)00195-c. [DOI] [PubMed] [Google Scholar]
- Melck D, Bisogno T, De Petrocellis L, Chuang H, Julius D, Bifulco M, et al. Unsaturated long-chain N-acyl-vanillyl-amides (N-AVAMs): vanilloid receptor ligands that inhibit anandamide-facilitated transport and bind to CB1 cannabinoid receptors. Biochem Biophys Res Commun. 1999;262:275–284. doi: 10.1006/bbrc.1999.1105. [DOI] [PubMed] [Google Scholar]
- Moreira FA, Aguiar DC, Guimaraes FS. Anxiolytic-like effect of cannabinoids injected into the rat dorsolateral periaqueductal gray. Neuropharmacology. 2007;52:958–965. doi: 10.1016/j.neuropharm.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Moreira FA, Aguiar DC, Campos AC, Lisboa SF, Terzian AL, Resstel LB, et al. Antiaversive effects of cannabinoids: is the periaqueductal gray involved? Neural Plast. 2009;2009:625469. doi: 10.1155/2009/625469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisset V, Ahluwalia J, Nagy I, Urban L. Possible mechanisms of cannabinoid-induced antinociception in the spinal cord. Eur J Pharmacol. 2001;429:93–100. doi: 10.1016/s0014-2999(01)01309-7. [DOI] [PubMed] [Google Scholar]
- Musella A, De Chiara V, Rossi S, Prosperetti C, Bernardi G, Maccarrone M, et al. TRPV1 channels facilitate glutamate transmission in the striatum. Mol Cell Neurosci. 2009;40:89–97. doi: 10.1016/j.mcn.2008.09.001. [DOI] [PubMed] [Google Scholar]
- de Novellis V, Palazzo E, Rossi F, De Petrocellis L, Petrosino S, Guida F, et al. The analgesic effect of N-arachidonoyl-serotonin, a FAAH inhibitor and TRPV1 receptor antagonist, associated with changes in rostral ventromedial medulla and locus coeruleus cell activity in rats. Neuropharmacology. 2008;55:1105–1113. doi: 10.1016/j.neuropharm.2008.06.023. [DOI] [PubMed] [Google Scholar]
- Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzo E, de Novellis V, Marabese I, Cuomo D, Rossi F, Berrino L, et al. Interaction between vanilloid and glutamate receptors in the central modulation of nociception. Eur J Pharmacol. 2002;439:69–75. doi: 10.1016/s0014-2999(02)01367-5. [DOI] [PubMed] [Google Scholar]
- Palazzo E, Rossi F, Maione S. Role of TRPV1 receptors in descending modulation of pain. Mol Cell Endocrinol. 2008;286:S79–S83. doi: 10.1016/j.mce.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Pan B, Wang W, Long JZ, Sun D, Hillard CJ, Cravatt BF, et al. Blockade of 2-arachidonoylglycerol hydrolysis by selective monoacylglycerol lipase inhibitor 4-nitrophenyl 4-(dibenzo[d][1,3]dioxol-5-yl(hydroxy)methyl)piperidine-1-carboxylate (JZL184) Enhances retrograde endocannabinoid signaling. J Pharmacol Exp Ther. 2009;331:591–597. doi: 10.1124/jpet.109.158162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Sang N, Zhang J, Chen C. Anandamide potentiation of miniature spontaneous excitatory synaptic transmission is mediated via IP3 pathway. Neurochem Int. 2010;56:590–596. doi: 10.1016/j.neuint.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart D, Gunthorpe MJ, Jerman JC, Nasir S, Gray J, Muir AI, et al. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1) Br J Pharmacol. 2000;129:227–230. doi: 10.1038/sj.bjp.0703050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starowicz K, Maione S, Cristino L, Palazzo E, Marabese I, Rossi F, et al. Tonic endovanilloid facilitation of glutamate release in brainstem descending antinociceptive pathways. J Neurosci. 2007;27:13739–13749. doi: 10.1523/JNEUROSCI.3258-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzian AL, Aguiar DC, Guimaraes FS, Moreira FA. Modulation of anxiety-like behaviour by Transient Receptor Potential Vanilloid Type 1 (TRPV1) channels located in the dorsolateral periaqueductal gray. Eur Neuropsychopharmacol. 2009;19:188–195. doi: 10.1016/j.euroneuro.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Toth A, Wang Y, Kedei N, Tran R, Pearce LV, Kang SU, et al. Different vanilloid agonists cause different patterns of calcium response in CHO cells heterologously expressing rat TRPV1. Life Sci. 2005;76:2921–2932. doi: 10.1016/j.lfs.2004.10.056. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Vaughan CW, Connor M, Bagley EE, Christie MJ. Actions of cannabinoids on membrane properties and synaptic transmission in rat periaqueductal gray neurons in vitro. Mol Pharmacol. 2000;57:288–295. [PubMed] [Google Scholar]
- Walker JM, Huang SM, Strangman NM, Tsou K, Sanudo-Pena MC. Pain modulation by release of the endogenous cannabinoid anandamide. Proc Natl Acad Sci U S A. 1999;96:12198–12203. doi: 10.1073/pnas.96.21.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J, Li J. TRPV1 receptor mediates glutamatergic synaptic input to dorsolateral periaqueductal gray (dl-PAG) neurons. J Neurophysiol. 2007;97:503–511. doi: 10.1152/jn.01023.2006. [DOI] [PubMed] [Google Scholar]
- Yang K, Kumamoto E, Furue H, Yoshimura M. Capsaicin facilitates excitatory but not inhibitory synaptic transmission in substantia gelatinosa of the rat spinal cord. Neurosci Lett. 1998;255:135–138. doi: 10.1016/s0304-3940(98)00730-7. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


