Summary
A minority of individuals experiencing traumatic events develop anxiety disorders. The reason for the lack of correspondence between the prevalence of exposure to psychological trauma and the development of anxiety is unknown. Extracellular proteolysis contributes to fear-associated responses by facilitating neuronal plasticity at the neuron-matrix interface1-4. Here we show that the serine protease neuropsin is critical for stress-related plasticity in the amygdala by regulating the dynamics of EphB2/NMDA receptor interaction, the expression of Fkbp5 and anxiety-like behaviour. Stress results in neuropsin-dependent cleavage of EphB2 in the amygdala causing dissociation of EphB2 from the NR1-subunit of NMDA receptor and promoting membrane turnover of EphB2 receptors. Dynamic EphB2/NR1 interaction enhances NMDA receptor current, induces the Fkbp5 gene expression and enhances behavioural signatures of anxiety. Upon stress, neuropsin-deficient mice do not show EphB2 cleavage and its dissociation from NR1 resulting in a static EphB2/NR1 interaction, attenuated induction of the Fkbp5 gene and low anxiety. The behavioural response to stress can be restored by intra-amygdala injection of neuropsin into neuropsin-deficient mice and disrupted by the injection of either anti-EphB2 antibodies or silencing the Fkbp5 gene in the amygdala of wild-type animals. Our findings establish a novel neuronal pathway linking stress-induced proteolysis of EphB2 in the amygdala to anxiety.
Fear helps organisms recognize, memorize and predict danger, thereby promoting their survival. However, severe stress can trigger maladaptive forms of neuronal remodelling leading to generalization of fear and high anxiety5.
Traumatic events are memorized due to the capacity of synaptic connections and the surrounding matrix to undergo experience-dependent functional or morphological changes1, 6. Extracellular proteases are strategically poised to remodel the neuron-extracellular matrix interface and facilitate fear and anxiety2-4. Eph-receptor tyrosine kinases constitute an important group of molecules subject to modulation by extracellular proteases7. While Ephs promote neuronal plasticity8, 9 their involvement in behavioural responses to environmental stimuli is not clear.
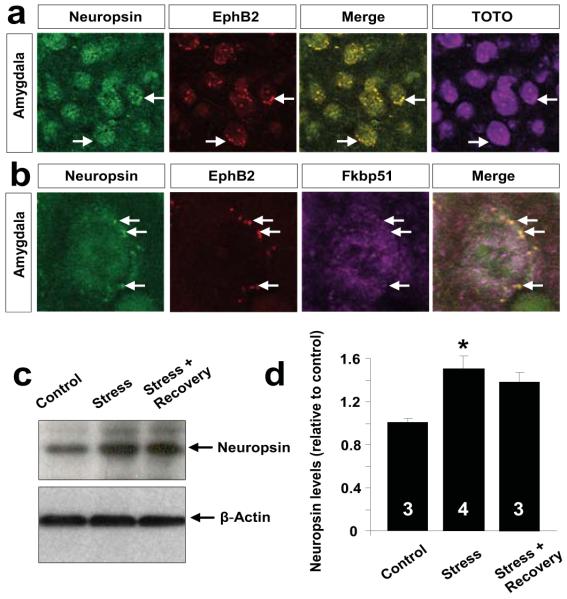
Neuropsin is a serine protease uniquely positioned to facilitate stress-induced plasticity due to its high expression in the amygdala and hippocampus10. To investigate if neuropsin and Ephs co-localize we performed immunohistochemistry. Consistent with previous reports10, 11 we found robust expression of both neuropsin and EphB2 in the amygdala (Figure 1 and Suppl. Fig. 1) and the hippocampus (not shown). Double immunohistochemistry revealed high levels of neuropsin co-localizing with EphB2-rich clusters on amygdala neurons (Figure 1a).
Figure 1. Neuropsin and EphB2 colocalize in neurons of the basolateral complex of the amygdala.
(a) Double immunohistochemistry showed neuropsin (green) and EphB2 (red) co-localize in lateral amygdala neurons (arrows show EphB2-rich clusters at neuropsin detection sites). Cells were highlighted with TOTO-3 stain. (b) Triple immunohistochemistry confirmed the presence of neuropsin/EphB2-rich clusters on the neuronal surface and low degree of co-localization with cytoplasmic Fkbp51. (c, d) Western blotting revealed amydala neuropsin upregulation following 6 hour restraint stress (F(2, 7) =8.81; p<0.05). Digits inside columns indicate n number. *p<0.05. Results are shown as mean±SEM.
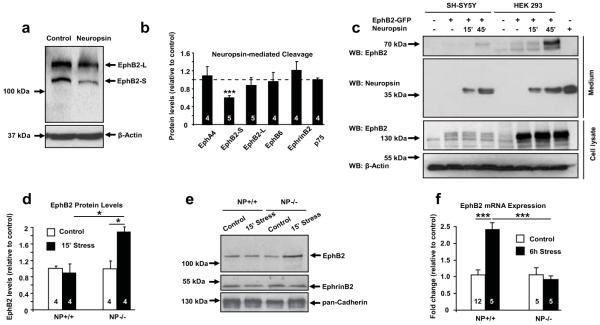
To assess whether Ephs are modulated by neuropsin we treated SH-SY5Y cells with neuropsin and measured the levels of Eph receptors by Western blotting. We found that neuropsin (but not other proteases; Suppl. Fig. 2) cleaved EphB2 (decrease by 41%, p<0.001), while the levels of other Ephs or their ligand ephrinB2 remained unchanged (Figure 2a, b and Suppl. Fig. 3a). When we expressed either GFP-tagged EphB2, GFP-tagged EphA4 or unlinked GFP in SH-SY5Y cells (Suppl. Fig. 4 and 5) and treated them with neuropsin we saw a similar decrease in the EphB2-associated signal (Suppl. Fig. 5; p<0.05).
Figure 2. Neuropsin cleaves EphB2 and regulates its expression both in vitro and in the amygdala after stress.
(a, b) EphB2-S band density in SH-SY5Y cells decreased upon 15 min neuropsin treatment (F(3,18)=11.24; p<0.001). Neuropsin did not cleave other molecules of the same class (b and Suppl. Fig. 3a). (c) Exposure of EphB2-GFP-transfected SH-SY5Y or HEK293 cells to neuropsin (15 or 45 min) resulted in the appearance of a ~70kDa N-terminal EphB2 fragment in the medium (Suppl. Fig. 6). (d, e) A 2-fold increase in the membrane-associated EphB2 in neuropsin−/− (F(3, 12)=6.4; p<0.05 vs non-stressed) but not wild-type mice was observed after stress (p<0.05 vs stressed neuropsin−/− mice). (f) qRT-PCR revealed a 2-fold upregulation of the EphB2 gene expression following 6 hour stress (F(3, 23)=13.48; p<0.001), not observed in neuropsin-deficient animals (p<0.001 vs. stressed wild-type mice). EphB2-S and EphB2-L describe short and long splice variants, respectively. NP – neuropsin. Digits inside columns indicate n number. *p<0.05; **p<0.01; ***p<0.001. Results shown as mean±SEM.
When we used the above protocol to examine the composition of the SH-SY5Y or HEK293 cell culture medium following the application of neuropsin, we found a new ~70kDa extracellular fragment of EphB2 released into the media (Figure 2c) whose size was consistent with neuropsin cleaving EphB2 close to the cell membrane (Suppl. Fig. 6a, b). Next, we subjected wild-type and neuropsin−/− mice to restraint stress to activate the basolateral complex of the amygdala12. Neuropsin levels increased by 50% after stress and gradually normalized during recovery in this brain region (Figure 1c, d; p<0.05). Western blotting revealed a 2-fold increase in membrane-associated amygdalar EphB2 levels after 15 minutes of restraint stress in neuropsin−/− mice (Figure 2d, e and Suppl. Fig. 7; p<0.05) indicative of new EphB2 receptors being incorporated into the membrane. This increase was not observed in wild-type animals consistent with neuropsin-mediated EphB2 cleavage during stress. Cleavage of EphB2 in the amygdala of wild-type mice was followed by a 2-fold raise in the expression of the EphB2 gene (Figure 2f; p<0.001). The cleavage was substrate-specific because stress did not alter the levels of either ephrinB2 (Suppl. Fig. 9a) or a presynaptic neuropsin substrate, NCAM-L113 (Suppl. Fig. 3b,c).
To determine the structural basis of neuropsin-specific EphB2 cleavage we analysed the fibronectin type III domain of EphB2 (Suppl. Fig. 8) looking for similarities with the previously published neuropsin cleavage sequences14. We found a critical amino acid pair Gly-Arg at position 517 of EphB2 but not EphB1, EphB6 or EphA4. Consistent with our experimental findings cleavage of EphB2 at this site would result in the release of a ~70kDa extracellular fragment.
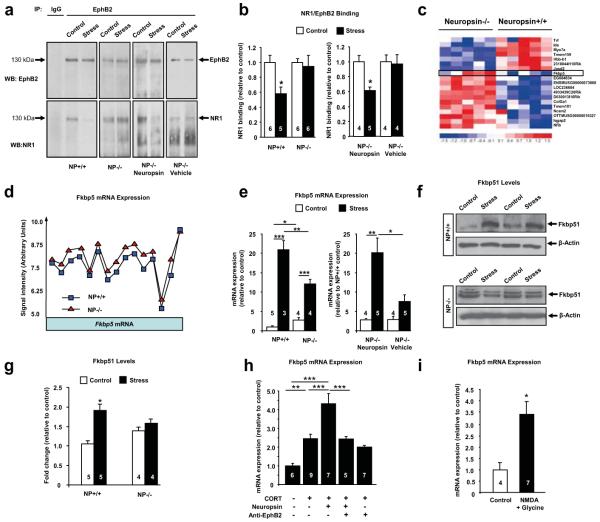
The EphB2 receptors cluster and associate with NMDA receptors at excitatory synapses15-17. Indeed, co-immunoprecipitation revealed NR1 bound to EphB2 in the amygdala (Figure 3a). Restraint stress reduced the amount of EphB2 associated with NR1 at 15 minutes by 42% (Figure 3a, b; p<0.05) while NR1 levels were not altered (Suppl. Fig. 9b-e). Stress-induced decrease in the EphB2/NR1 association was not observed in neuropsin−/− mice but restored by intraamygdalar administration of neuropsin into these animals (Figure 3a, b), consistent with neuropsin cleaving the extracellular portion of EphB2 during stress and triggering its dissociation from NR1. These results, together with stress-induced EphB2 membrane trafficking (Figure 2d, e), suggest that neuropsin increases the dynamics of EphB2/NR1 interaction following stress.
Figure 3. Neuropsin regulates the dynamics of EphB2/NR1 interaction and controls the expression of Fkbp5.
(a, b) EphB2 immunoprecipitation (before and after 15 minutes of restraint stress) from amygdalae revealed dissociation of EphB2/NR1 complexes in wild-type (F(3, 19)=4.2; p<0.05), but not neuropsin−/− mice. EphB2/NR1 dissociation was restored in neuropsin−/− mice by intraamygdalar neuropsin injections (F(3, 13)=4.7; p<0.05). Microarray analysis of wild-type and neuropsin−/− amygdalae revealed differential expression of Fkbp5 (heatmap in c, Suppl. Fig. 10). Exon-specific Fkbp5 probes showed an upregulation of the whole transcript (d) confirmed by qRT-PCR (e; F(3, 12)=72.15; p<0.001). qRT-PCR revealed attenuated stress-induced upregulation of Fkbp5 in neuropsin−/− mice (e; p<0.01 for wild-type after stress vs. neuropsin−/− after stress) rescued by intraamygdalar neuropsin injections (F(3, 14)=9.2; p<0.01). (f, g) Fkbp51 protein levels were upregulated in wild-type mice (F(3, 14)=8.95; p<0.001) but not in neuropsin−/− mice by stress. (h) Neuropsin-mediated upregulation of Fkbp5 in amygdala neuronal cultures (F(4, 29)=19.04; p<0.0001) was blocked by anti-EphB2 antibody and mimicked by stimulation of NMDA receptors (i; p<0.05). *p<0.05; **p<0.01; ***p<0.001. Results are shown as mean±SEM. NP- neuropsin. Digits inside columns indicate n number.
Regulating EphB2/NMDA-receptor interaction results in modulation of the expression of NMDA receptor-dependent genes facilitating synaptic plasticity17. To examine if neuropsin-mediated regulation of the EphB2/NR1 assembly affect gene expression in the amygdala we used microarrays in neuropsin−/− and wild-type mice. We found 19 differentially expressed transcripts with a marked upregulation of the Fkbp5 gene (Figure 3c, d; Suppl. Fig. 10, 11; p<0.0005). This gene encodes Fkbp51 protein regulating glucocorticoid receptor sensitivity. Fkbp5 has been implicated in the development of anxiety, depression and post-traumatic stress disorder (PTSD)18-20. Quantitative RT-PCR confirmed an increase in the Fkbp5 gene expression in the amygdalae of stress-naïve neuropsin−/− animals (Figure 3e; p<0.05).
The extent of upregulation of Fkbp5 mRNA shortly after trauma correlates with the development of PTSD21. If neuropsin regulates Fkbp5 gene expression then the magnitude of its stress-related regulation should be altered in neuropsin-deficient mice. When we analysed stress-induced Fkbp5 gene expression we found a 21-fold upregulation in wild-type amygdalae (Figure 3e; p<0.001) but an attenuated upregulation in neuropsin−/− mice. The increase in the Fkbp5 gene expression was accompanied by a 2-fold upregulation of Fkbp51 protein levels in wild-type mice but not neuropsin−/− animals (Figure 3f, G; p<0.05). These results indicate that neuropsin is a key regulator of the Fkbp5 gene and protein expression.
Neuropsin is an extracellular protease and thus unlikely to alter the expression of the Fkbp5 gene directly. Although the Fkbp5 gene can be regulated by glucocorticoids (Suppl. Fig. 12), the above differences in Fkbp5 expression after stress cannot be attributed to corticosterone levels (Suppl. Fig. 13). Interference with EphB2 signalling has recently been linked to the regulation of the Fkbp5 gene22. Indeed, when we mimicked stress in vitro by adding corticosterone into neuronal amygdala cultures, neuropsin-mediated upregulation of Fkbp5 was hindered by anti-EphB2 antibody (Figure 3h; p<0.001) and imitated by NMDA receptor stimulation (Figure 3i; p<0.05).
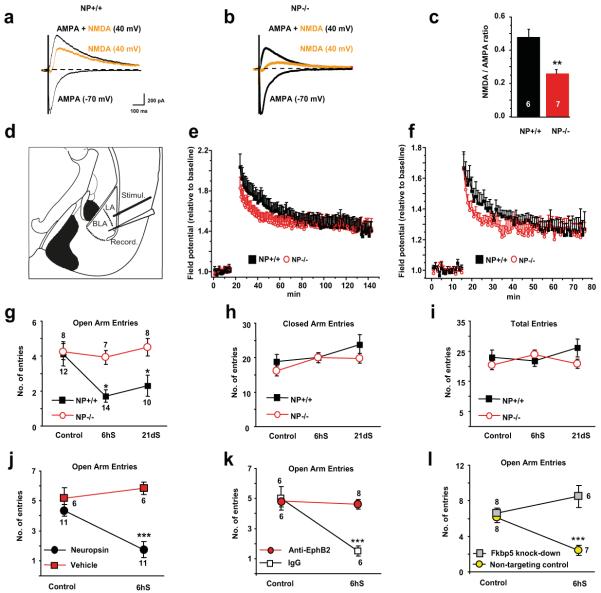
To address the effect of neuropsin on NMDA receptors directly we measured evoked NMDA/AMPA current ratio in principal neurons of the basal amygdala in wild-type and neuropsin −/− mice. We found that, unlike in the hippocampus13, the NMDA current was markedly reduced by the deletion of the neuropsin gene resulting in a ~50% drop in the NMDA/AMPA ratio (Figure 4a-c; p<0.01).
Figure 4. Neuropsin controls NMDA receptor current, E-LTP and stress-induced anxiety.
(a-c) Whole-cell recordings from basal nucleus neurons of neuropsin −/− animals demonstrated lower NMDA currents compared to wild-type mice. Induction of LTP in the lateral-basal pathway (d) using a strong (e) or weak (f) protocol revealed an impairment of E-LTP in neuropsin−/− mice. The elevated plus maze test following acute or chronic restraint stress demonstrated lack of anxiety in neuropsin −/− mice as indicated by the number of entries into open arms (g). General locomotor activity was similar in both genotypes (h, i). The behavioural phenotype was reversed by bilaterally injecting neuropsin back into the amygdala of neuropsin−/− mice (j). Stress-induced anxiety in wild-type animals was disrupted by blocking EphB2 (k) or silencing the Fkbp5 gene (l) in the amygdala. NP – neuropsin; LA-lateral amygdala; BLA – basal amygdala; CA – central amygdala; MeA – medial amygdala. Digits inside columns or near symbols indicate n number. 6hS – six hour stress, 21dS – 21 days of daily restraint. *p<0.05, **p<0.01, ***p<0.001. Results shown as mean±SEM.
We next asked if the neuropsin pathway affects neuronal plasticity in the amygdala. We induced early (E-LTP) or sustained (L-LTP) long term potentiation in the amygdala lateral-basal pathway of wild-type and neuropsin−/− mice. While basal synaptic responses were not altered (Suppl. Fig. 14a), E-LTP was impaired in neuropsin−/− mice (Figure 4d-f and Suppl. Fig. 14b, c; p<0.001 vs. wild-type at 20 min post-tetanus). These changes temporally correlated with neuropsin-mediated cleavage of EphB2, its dynamic interaction with NR1 and with the involvement of NMDA receptors in E-LTP in the lateral-basal pathway (Suppl. Fig. 15).
To examine if the neuropsin pathway alters behavioural signatures of stress we subjected wild-type and neuropsin−/− mice to acute or chronic stress and measured anxiety in the elevated-plus maze (Figure 4g-i). We found that stress caused a decrease in the number of entries of wild-type mice into open arms, indicative of high anxiety levels2, 3. In contrast, after stress, neuropsin−/− mice did not develop anxiety (Figure 4g; p<0.05). Closed arm entries, the total number of entries (Figure 4h-i and Suppl. Fig. 16a, b) as well as general locomotor activity measures (Supp. Fig. 17d) were similar between the genotypes as previously reported23. Furthermore, neuropsin−/− mice demonstrated an anxiolytic phenotype in the open field test confirming a general role of neuropsin in regulating anxiety (Suppl. Fig. 17a-c). While this effect is consistent with functional deficits in the NMDA receptor function (Figure 4a-c) and E-LTP (Figure 4d-f) observed in neuropsin−/− mice it cannot be excluded that additional mechanisms, such as abnormal dendritic plasticity, may contribute to the lack of anxiety observed in neuropsin−/− mice, particularly after long-lasting stress24.
To examine whether the effect of neuropsin was acute and not associated with the lack of the protease during development we bilaterally injected neuropsin into the amygdalae of neuropsin −/− mice (Suppl. Fig. 18). The neuropsin injection restored stress-induced anxiety in these animals (Figure 4j; p<0.001). The development of anxiety was hindered by blocking EphB2 in the amygdala of wild-type mice (Figure 4k; p<0.001) consistent with neuropsin interacting with EphB2 to facilitate stress-induced behavioural changes. Similarly, stress-induced anxiety was blocked by silencing the Fkbp5 gene expression in this brain region (Figure 4l and Suppl. Fig. 19) consistent with the downstream role of Fkbp5 in the neuropsin pathway.
Our studies favour a model where, after stress, both corticosterone-induced and neuropsin-mediated components converge to modulate the Fkbp5 gene expression and trigger anxiety (Suppl. Fig. 20). Neuropsin cleaves the extracellular portion of EphB2 and facilitates the dynamic interaction of EphB2 with the NR1 subunit of the NMDA receptor. The resulting enhancement of the NMDA current causes an upregulation of Fkbp5 and promotes the development of anxiety. This novel pathway, highlighting the ability of Eph and NMDA receptors to respond to activity dependent signals from the extracellular milieu, opens new possibilities for treatment of stress-associated disorders, including various forms of anxiety disorders.
Methods Summary
Restraint stress was performed by placing the mice in wire mesh restrainers while control animals were left undisturbed. Anxiety was measured using the elevated-plus maze by counting the number of entries to closed or open arms during 5 min. Intraamygdala injections were performed through bilaterally implanted cannulae and were followed by restraint stress in plexiglass tubes. Fkbp5 gene was silenced by intraamygdala injection of lentiviral shRNA construct followed by behavioural assessment two weeks later. LTP was recorded from the lateral-basal pathway and whole-cell recordings made from basal amygdala neurons. Data were analyzed by Student t-test or ANOVA followed by Tukey’s post-test. P values of less than 0.05 were considered significant.
Supplementary Material
Acknowledgements
This work was supported by Medical Research Council Project Grant (G0500231/73852), and a Marie Curie Excellence Grant (MEXT-CT-2006-042265 from European Commission) to Robert Pawlak, Medisearch Fellowship to Benjamin Attwood, the Ministry of Education, Culture, Sports, Science and Technology of Japan (Grants-in-Aid, # 20300128) and Japan Science and Technology Agency (CREST program) to Sadao Shiosaka, GENADDICT (LSHM-CT-2004-005166 from the European Commission) and NN405 274137 from KBN to Ryszard Przewlocki. We are obliged to Drs. A. Kania, H. Castro and R. Fern for reagents. We are grateful to Drs. B. McEwen and A. Tobin for their comments on the manuscript and Drs. Todor Gerdjikov, Giovanna Mallucci and her lab members for advice.
Appendix
Extended Methods
Animals
Experiments were performed on three-month old wild-type (C57/BL6) or neuropsin−/− mice backcrossed to C57/BL6 for 12 generations. To generate neuropsin−/− mice exons 1-3 of the neuropsin gene, including the protease active site, were replaced by a neomycin resistance cassette25. A lack of full-length neuropsin transcript and proteolytic activity in the brain of these animals was confirmed by RT-PCR and amidolytic assay25, respectively. Neuropsin−/− mice were genotyped as described13. Animals were housed three to five per cage in a colony room with a 12 hour light/dark cycle (lights on at 7AM) with ad libitum access to commercial chow and tap water. The experiments were approved by the UK Home Office and the UoL Ethical Committee.
Restraint stress
C57/BL6 J and neuropsin−/− mice were kept undisturbed for at least one week in their home cages and restraint stress was performed during the light period of the circadian cycle as described3. Control animals were left undisturbed, and stressed animals were subjected to a single five minute, fifteen minute or six hour restraint stress in a separate room. The mice were placed in their home cages in wire mesh restrainers secured at the head and tail ends with clips.
qRT-PCR
Primers
The primers for EphB2 (forward 5′-CTTCCTCATCGCTGTGGTC and reverse 5′-ATGTGTCCGCTGGTGTAGTG) and Fkbp5 (forward 5′-ATTTGATTGCCGAGATGTG and reverse 5′-TCTTCACCAGGGCTTTGTC) were bought from Invitrogen. To quantify gene expression the target genes were compared against the actin gene as previously described26.
RNA extraction
Control and stressed mice were anaesthetised (intraperitoneal sodium pentobarbital 50 mg/kg) and perfused transcardially (ice cold PBS). Amygdalae were dissected from a coronal slice −0.58 to −2.3 mm relative to Bregma and stored in “RNA later” (QIAgen) at 4 °C. Alternatively RNA was extracted from primary amygdala neuronal cultures using QIAzol lysis reagent (QIAgen) and Mini Spin Columns according to the manufacturers’ instructions (RNeasy Lipid tissue mini kit, QIAgen). 2μg of RNA was converted to cDNA using Superscript III (Invitrogen) and oligo (dT) primers according to manufacturer’s instructions.
qRT-PCR reaction
Triplicate wells contained 20μl of SYBR Green Master Mix (Applied Biosystems and BioRad), forward primer (250nM), reverse primer (250nM), cDNA (1μl) and nuclease free water to a total of 40μl. PCR was performed using Chromo4/PTC-200 thermal cycler (MJ Research) under the following conditions:
95 °C for 15 minutes
94 °C for 15 seconds,
55 °C for 30 seconds
72 °C for 30 seconds
Steps 2-4 repeated 40 times
Control reactions were performed without DNA template and/or with unconverted RNA as the template.
Western blotting, cell fractionation and immunoprecipitation
Mice were anaesthetised (intraperitoneal sodium pentobarbital 50 mg/kg) and perfused transcardially (ice cold PBS). The brains were removed and amygdalae dissected from a slice −0.58 to −2.3 mm relative to Bregma. Samples were homogenized in 0.1M Tris, 0.1% Triton X-100, pH 7.4, containing phosphatase inhibitors (10mM NaF, 1mM Na orthovanadate) and protease inhibitors (Complete, Roche). Protein concentration was adjusted (Bradford method; Pierce). For Fkbp51 levels samples were homogenized in RIPA buffer (150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 50mM Tris, 10mM NaF, 1mM Na orthovanadate, complete Roche protease inhibitors, pH 8.0). Reduced (DTT) and denatured (100°C for 5 minutes) samples (40μg per lane) were subjected to SDS-PAGE electrophoresis and transferred onto nitrocellulose membrane, blocked (5% skim milk for 1h at RT) and washed with TBS-T (3 × 5 mins). The membranes were probed with the following primary antibodies overnight at 4°C: goat anti- NCAM L1 (SantaCruz Biotechnology, 1:300), goat anti-EphB2, anti-EphB6 and anti-ephrinB2 (R&D, 1:500, 1:500 and 1:300 respectively), mouse anti-EphA4 (Zymed, 1:1000), rabbit anti pan-cadherin (Abcam, 1:2000), rabbit anti-p75NGF (Chemicon, 1:1000), rabbit anti-NR1 (Upstate, 1:250), rabbit anti-neuropsin (Dr. Helena C. Castro, Niterói, Brazil), rabbit anti-FKBP51 (Abcam, 1:250). The membranes were then washed in TBS-T (3 × 10 min) and incubated with a relevant HRP-conjugated secondary antibody as appropriate (Vector Labs, 1:1000, 1hr, RT). The signal was developed, after washing with TBS-T (6 × 10 min), using a Western Blot Luminol Reagent (Santa Cruz). To normalize the results the membranes were stripped, blocked, washed as above and re-blotted using mouse anti-β-actin antibody (Sigma, 1:2500, 1hr, RT). The memebranes were then prepared and developed as above. To quantify the results the band intensities were measured using Scion Image software and normalized to the actin bands.
When indicated cellular fractions of the amygdala samples were separated using a cellular protein fractionation kit (PerkinElmer) as per the manufacturers protocol, and analysed by Western blotting.
For immunoprecipitation amygdala samples were homogenised as previously described27, pre-cleared using goat IgG (Sigma, 1μg) before incubation with goat anti-EphB2 antibody (R&D, 2μg) for 1 hour (4°C). The samples were then incubated with protein G-sepharose beads overnight before being washed with PBS and analysed by Western blotting.
Cell Culture
SH-SY5Y cells at 80-90% confluence were washed with PBS three times before incubation with PBS, PBS + neuropsin (50nM; R&D) or PBS + tPA (Alteplase, Genentech; 1μg/ml) with or without human plasminogen for fifteen minutes after which the dishes were placed on ice and protease inhibitors (Complete, Roche) were added. The cells were collected and homogenised in Tris 50mM pH7.5, NaCl 150mM, EDTA 5mM, EGTA 5mM, Triton-100 1%, NP40 0.5%. The resulting protein sample was analysed by Western blotting as described above.
SHSY-5Y and HEK293 cells were transfected with mouse EphB2-GFP and incubated with PBS or PBS + neuropsin (300nM) for 15 or 45 mins. The samples were then treated as above for analysis.
For imaging SH-SY5Y cells were transfected with GFP, mouse EphB2-GFP or EphA4-GFP (gift from Dr. A. Kania) and loaded with the cell tracker (Invitrogen). Images were taken with Zeiss LSM5 Exciter before and after 15 minutes incubation with neuropsin (50nM, R&D), converted to grayscale and the intensity of the fluorescent signal was analysed using Scion Image.
Primary neuronal cultures were prepared from P1 C57/BL6J wild-type mice. The amygdalae were dissected and placed into a Petri dish (9.1 mM glucose, 25 mM HEPES, 5 mM KCl and 120 mM NaCl). Tissue was chopped and incubated in 10 ml of buffer containing 5 mg of protease (Type XIV; Sigma) and 5 mg of thermolysin (Type X; Sigma) at room temperature for 30-45 minutes. The digestion solution was replaced with 3 ml of HBSS (Gibco) plus 40 μg/ml DNAse. The mixture was titurated, centrifuged and resuspended in the plating medium (Neurobasal A medium, 10% fetal bovine serum, 2 mM Glutamax and 2% B-27 supplement, 100 μg/ml streptomycin, 100 U/ml penicillin (Invitrogen)), centrifuged and resuspended again. 20-30 μl droplets containing cells were added to the centre of poly-D-lysine (Sigma) coated coverslips and the plating medium was added one hour later. 5 μM cytosine B-D-arabinofuranoside (Ara-C; Sigma) was added to prevent proliferation of glial cells. Neurons were maintained in serum-free Neurobasal A medium at 37°C in a humidified atmosphere of 5% CO2/95%air. Half of the medium was replaced every 3-4 days. Cells were maintained for 11-16 DIV and then treated with either vehicle, corticosterone (10nM), neuropsin (50nM) or NMDA (100μM, Sigma) + glycine (10 μM, Sigma). To block EphB2, neurons were treated with anti-EphB2 antibody (2μg/ml; R&D) 10 minutes before the experiment.
Electrophysiology
For field recordings coronal slices of the amygdala (400 μm) were obtained from 8-12 weeks-old neuropsin−/− and wild-type mice. The animals were anaesthetized with ketamine/xylazine (2:1 ratio; 2.4 μl/g i.p.). Slices were prepared using a vibrating microtome (Campden Instruments; MA752) in ice-cold, low sodium ACSF (in mM: sucrose (249); KCl (2.5); NaH2PO4 (1.25); D-glucose (10); NaHCO3 (26); CaCl2 (0.1); MgSO4 (2.9); ascorbic acid (0.5), bubbled with 95% O2/5% CO2 mixture, pH 7.3). Slices were placed in a holding chamber for 30 min at 35°C and then for at least 2.5 hours (30 min for whole-cell recordings) at room temperature (25 C°) in ACSF (in mM: NaCl (124); KCl (5); NaH2PO4 (1.25); D-glucose (10); NaHCO3 (26); CaCl2 (2.4); MgSO4 (1.3)). All the experiments were performed at room temperature.
Extracellular recording were made with a bipolar tungsten electrode (WPI). For recordings glass microelectrodes (1-2 MΩ) filled with ACSF were used. To record field potentials in the lateral-basal amygdala pathway the stimulating electrode was positioned in the lateral amygdaloid nucleus close to the external capsule and the recording electrode in the basal nucleus28. The stimulus intensity was adjusted to evoke a field potential (FP) of 60-70% (0.2 ms pulse duration) of the maximal amplitude. The amygdala was stimulated every 30 sec in order to record a stable baseline for at least 15 min. Several responses were averaged and a template was created. Only the responses matching the template were analysed. Early phase of long term potentiation (E-LTP) was evoked by a single tetanic stimulation (100 Hz,1 sec). Late-long term potentiation (L-LTP) were elicited by two tetanic stimulations (100 Hz, 1 sec, 10 sec interval) repeated 4 times in 3 min intervals with the same intensity and pulse duration as the test stimuli as described29. The recordings were amplified (Multiclamp 700b, Axon Instruments), filtered (10 kHz) and digitized at 50 kHz (Digidata 1440A, Axon Instruments). pClamp 10 (Axon Instruments) and Origin 7 (Microcal Inc.) software were routinely used during data acquisition and analysis.
For whole-cell recordings coronal slices (300 μm) were obtained from 3-4 weeks old mice. The animals were anaesthetized with hypnorm/midazolam (1:1; 8 μl/g b.w., i.p.). Recordings were made from somata of principal neurons of the basal nucleus of the amygdala. Principal neurons and interneurons were distinguished by their morphological and electrophysiological properties30. After whole-cell configuration the series resistance was regularly monitored and a maximum of 10-15 MΩ tolerated. AMPA and NMDA currents were recorded by clamping the membrane potential of the cell at −70 mV and +40mV respectively (average of 5 traces each). The slice was subsequently perfused with AP-5 in order to isolate the NMDA component at +40 mV (subtraction of traces before and after perfusion with AP-5). The NMDA/AMPA ratio was obtained by measuring the peak of the AMPA current at −70 mV in the presence of AP-5 and the peak of NMDA current at +40 mV. At the end of the experiments the currents were blocked by CNQX (30 μM in DMSO) and AP-5 (50 μM in DMSO). All drugs were bath applied (perfusion rate 1.5 ml/min). The recording electrodes were borosilicate glass pipettes (2-4 MΩ). The pipettes were filled with the following solution (in mM): Cs-methyl sulfonate (130), KCl (8), EGTA (0.5), HEPES (10), glucose (5), QX314 (5). ACSF composition (in mM): NaCl (124); KCl (5); NaH2PO4 (1.25); D-glucose (10); NaHCO3 (26); CaCl2 (2); MgSO4 (1). All the experiments were performed at 25°C. Data were recorded with a Multiclamp 700B amplifier, filtered at 10 kHz and digitized at 50 kHz (Digidata 1440A, Axon instruments). pClamp 10 (Axon Instruments) and Origin 7 (Microcal Inc.) software was routinely used during data acquisition and analysis.
Analysis of the Fkbp5 promoter
The identification of over-represented transcription factor binding sites (TFBSs) in promoter region of Fkbp5 was performed using the cREMaG database (http://cremag.org). Promoter region of Fkbp5 was defined as evolutionary conserved (based on the alignment of mouse and human genes) sequence between 10000 bp upstream and 5000 bp downstream of transcription start site (TSS). The parameters of 65% conservation threshold and maximum number of top 10000 conserved TFBSs in coding and non-coding regions were used. The obtained results were compared to conserved promoter background. Over-representation was measured as the number of identified TFBSs compared to putative number obtained by chance (p<0.01).
Microarray study
Amygdalae were isolated from wild-type (n=15) and neuropsin−/− (n=15) mice using a dissecting microscope in ice-cold ACSF (in mM: glucose (25), NaCl (115), NaH2PO4·H2O (1.2), KCl (3.3), CaCl2, (2), MgSO4, (1), NaHCO3, (25.5), pH 7.4 and stored at −20°C in RNAlater solution (Qiagen). RNA was extracted using RNeasy Lipid Tissue Mini Kit (Qiagen), the ribosomal fraction of RNA reduced with RiboMinus Kit (Invitrogen) and the RNA integrity verified by electrophoresis using Agilent Bioanalyser 2100 (Agilent Technologies). RNA pulled from 3 animals has been reverse transcribed and hybridized with GeneChip Mouse Exon 1.0 ST Array (Affymetrix; 5 arrays per genotype).
Microarray data were initially processed using GeneChip Operating Software. DTT data were transferred by Transfer Tool software (Affymetrix). Chip quality and raw microarray data pre-processing were performed according to the Affymetrix guidelines using Expression Console software (Affymetrix). After background subtraction, the data were processed using RMA method and quantile normalization. The obtained results were taken as the measure of mRNA abundance derived from the level of gene expression. Significance levels (P-values) of differences in mRNA abundance between the wild-type and neuropsin−/− animals were calculated for each probe set using t-test. The P-values for all exons of each particular gene were multiplied to establish gene P value. The threshold of P < 0.05 for each gene was computed using permutation test followed by Bonferroni correction for multiple testing. All the statistical analyses were done in R software version 2.8.1 (www.r-project.org). Sources of variation were analysed by a three-way ANOVA using Partek Genomic Suite.
Immunohistochemistry
Mice were anaesthetized (intraperitoneal sodium pentobarbital 50 mg/kg) and transcardially perfused (ice-cold PBS containing protease inhibitors; (Complete, Roche) followed by ice-cold 4% paraformaldehyde). The brains were dissected and fixed in 4% paraformaldehyde in PBS overnight at 4°C. 70μm thick coronal slices were collected on a vibrating microtome and stored at 4°C in PBS containing 0.002% sodium azide (Sigma). Slices were preincubated in PBS-T (PBS solution 0.5% bovine serum albumin, 0.02% Triton X-100 and blocking sera at 1:500) for 5 hours at room temperature, incubated with goat anti-EphB2 (1:300, R&D) or rabbit anti-neuropsin (1:200, Dr. Helena Castro; the antibody was preabsorbed on acetone powder prepared from neuropsin−/− brain for 1 hour at RT prior to use) antibodies, along with mouse anti-NeuN (1:200, Chemicon) and chicken anti-GFAP (1:1000, Dako) overnight at 4°C in PBS-T. Next, the slices were washed for 8-10 hours with PBS-T and incubated overnight with compatible FITC, Alexa Fluor 488, Alexa Fluor 546 or Alexa Fluor 647 secondary antibodies (1:500, Molecular Probes) in the same buffer. Control sections were processed with the primary antibodies omitted. For double Ephb2/neuropsin co-labelling sections were incubated in PBS-T containing anti-goat Alexa Fluor 546 as well as anti-rabbit Alexa Fluor 488 for detection of the above primary antibodies. TOTO-3 iodide (1nM, Molecular Probes) was added to the secondary antibody mixture. For the triple Ephb2/neuropsin/Fkbp5 labelling rat anti-Fkbp51 (R&D, 1:500) was additionally used along with a compatible Alexa Fluor 647 secondary antibody. Sections were then washed in PBS-T for 5 hours, mounted on glass slides using Vectamount medium (Vector Laboratories), and photographed using Zeiss LSM 5 Exciter confocal microscope.
Corticosterone levels
Mice were subjected to restraint stress of various durations with or without recovery and trunk blood was collected to measure corticosterone levels in the plasma by EIA according to the manufacturer’s instructions (Cayman Chemicals, Cat No. 500651).
Elevated Plus Maze
The elevated-plus maze test was performed as previously described3. The apparatus consisted of four non-transparent white Plexiglas arms: two enclosed arms (50 × 10 × 30 cm) that formed a cross shape with the two open arms (50 × 10 cm) opposite each other. The maze was 55 cm above the floor and dimly illuminated. Wild-type and neuropsin−/− mice were tested 12 hours after the restraint stress. Mice were placed individually on the central platform, facing an open arm, and allowed to explore the apparatus for 5 min. Behavior was recorded by an overhead camera. The number of entries of the animal from the central platform to closed or open arms was counted. The maze was cleaned with 70% alcohol after each session to avoid any odorant cues.
Open Field
Mice were placed in a 50 × 50 × 50 cm plexiglas box and were left free to move during 10 min. The box was cleaned with 70% alcohol after each session to avoid any odorant cues. An overhead camera placed above the box recorded the session. Locomotor parameters were analysed with the ANY-MAZE software (Stoelting).
Stereotaxic injections
Mice were intraperitoneally anaesthetized with ketamine/xylazine (100 and 10 mg/kg, respectively), placed in a stereotaxic apparatus and bilaterally implanted with stainless steel guide cannulae (26 gauge; Plastics One, Roanoke, VA) aimed above the basolateral complex of the amygdala (1.5 mm posterior to bregma, 3.5 lateral and 4.0 ventral). The cannulae were secured in place with dental cement. Dummy cannulae were inserted into all implanted cannulae to maintain patency. After one week dummy cannulae were replaced with the injection cannulae (projecting 0.75 mm from the tip of the guide cannulae to reach the basolateral complex of the amygdala) and the mice were injected with either anti-EphB2 antibody (R&D, 1 μl, 2 μg/ml), control IgG, recombinant neuropsin (R&D, 1 μl, 50nM) or its vehicle followed by 6-hour restraint stress in transparent plexiglass tubes. After the experiment a small volume of bromophenol blue was injected to visualize the injection site, the brains were sectioned and the cannulae placement was determined histologically.
The Fkbp5 gene silencing
To silence the Fkbp5 gene in the amygdala SMARTvector 2.0 lentiviral shRNA technology (Dharmacon, USA) employing human cytomegalovirus (hCMV) promoter and a turboGFP reporter gene was used. Three different targeting constructs were tested. First, 0.3 μl of the lentivirus was injected at point 1.7 mm posterior from bregma, 3.5 mm lateral from the midline and 4.4 mm ventral at 200 nl/min using the Nanofil syringe with a 33G needle through UMP-3.1 micropump (all from World Precision Instruments, USA) mounted on Stoelting stereotaxic frame. After 5 minutes the needle was lowered to 5 mm ventral and 0.3 μl of the virus injected. The needle remained in place for another 5 min to prevent the backflow, slowly removed and the skin closed with Vetbond (3M, USA). After two-weeks recovery the amygdalae were dissected to determine the knockdown efficiencies in vivo as compared to the non-targeting construct (TGGTTTACATGTTGTGTGA; 2.66 × 108 TU/ml) or uninjected mouse amygdalae by qRT-PCR and Western blotting as described above. The most efficient construct (~60% mRNA and protein knockdown efficiency; targeting sequence ATGCTGAGCTTATGTACGA; 3.02 × 108 TU/ml) was used to silence the Fkbp5 gene in all subsequent behavioural experiments. The restraint stress and the elevated-plus maze were performed two weeks after the intra-amygdala lentivirus injection as described above. The region specificity of lentiviral injections was verified histologically by direct observation of the turboGFP fluorescence on consecutive coronal sections spanning the amygdala, using Zeiss LSM5 Exciter confocal microscope.
Statistics
Student t-test (when two group were compared) or analysis of variance (ANOVA) followed by Tukey’s post-test were used as appropriate. P values of less than 0.05 were considered significant. The ANOVA p values are reported in the text, the results of the post-test are indicated by asterisks on graphs.
Extended Methods’ References
- 25.Hirata A, et al. Abnormalities of synapses and neurons in the hippocampus of neuropsin-deficient mice. Mol Cell Neurosci. 2001;17:600–10. doi: 10.1006/mcne.2000.0945. [DOI] [PubMed] [Google Scholar]
- 26.Salter MG, Fern R. NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature. 2005;438:1167–71. doi: 10.1038/nature04301. [DOI] [PubMed] [Google Scholar]
- 27.Calo L, et al. Interactions between ephrin-B and metabotropic glutamate 1 receptors in brain tissue and cultured neurons. J Neurosci. 2005;25:2245–54. doi: 10.1523/JNEUROSCI.4956-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brambilla R, et al. A role for the Ras signalling pathway in synaptic transmission and long-term memory. Nature. 1997;390:281–6. doi: 10.1038/36849. [DOI] [PubMed] [Google Scholar]
- 29.Huang YY, Kandel ER. 5-Hydroxytryptamine induces a protein kinase A/mitogen-activated protein kinase-mediated and macromolecular synthesis-dependent late phase of long-term potentiation in the amygdala. J Neurosci. 2007;27:3111–9. doi: 10.1523/JNEUROSCI.3908-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Washburn MS, Moises HC. Electrophysiological and morphological properties of rat basolateral amygdaloid neurons in vitro. J Neurosci. 1992;12:4066–79. doi: 10.1523/JNEUROSCI.12-10-04066.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
References
- 1.Gogolla N, Caroni P, Luthi A, Herry C. Perineuronal nets protect fear memories from erasure. Science. 2009;325:1258–61. doi: 10.1126/science.1174146. [DOI] [PubMed] [Google Scholar]
- 2.Matys T, et al. Tissue plasminogen activator promotes the effects of corticotropin-releasing factor on the amygdala and anxiety-like behavior. Proc Natl Acad Sci U S A. 2004;101:16345–50. doi: 10.1073/pnas.0407355101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pawlak R, Magarinos AM, Melchor J, McEwen B, Strickland S. Tissue plasminogen activator in the amygdala is critical for stress-induced anxiety-like behavior. Nat Neurosci. 2003;6:168–74. doi: 10.1038/nn998. [DOI] [PubMed] [Google Scholar]
- 4.Pawlak R, et al. Tissue plasminogen activator and plasminogen mediate stress-induced decline of neuronal and cognitive functions in the mouse hippocampus. Proc Natl Acad Sci U S A. 2005;102:18201–6. doi: 10.1073/pnas.0509232102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–45. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 6.Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423–33. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- 7.Lin KT, Sloniowski S, Ethell DW, Ethell IM. Ephrin-B2-induced cleavage of EphB2 receptor is mediated by matrix metalloproteinases to trigger cell repulsion. J Biol Chem. 2008;283:28969–79. doi: 10.1074/jbc.M804401200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klein R. Bidirectional modulation of synaptic functions by Eph/ephrin signaling. Nat Neurosci. 2009;12:15–20. doi: 10.1038/nn.2231. [DOI] [PubMed] [Google Scholar]
- 9.Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 10.Chen ZL, et al. Expression and activity-dependent changes of a novel limbic-serine protease gene in the hippocampus. J Neurosci. 1995;15:5088–97. doi: 10.1523/JNEUROSCI.15-07-05088.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouvier D, et al. Pre-synaptic and post-synaptic localization of EphA4 and EphB2 in adult mouse forebrain. J Neurochem. 2008;106:682–95. doi: 10.1111/j.1471-4159.2008.05416.x. [DOI] [PubMed] [Google Scholar]
- 12.Perrotti LI, et al. Induction of deltaFosB in reward-related brain structures after chronic stress. J Neurosci. 2004;24:10594–602. doi: 10.1523/JNEUROSCI.2542-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsumoto-Miyai K, et al. NMDA-dependent proteolysis of presynaptic adhesion molecule L1 in the hippocampus by neuropsin. J Neurosci. 2003;23:7727–36. doi: 10.1523/JNEUROSCI.23-21-07727.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimizu C, et al. Characterization of recombinant and brain neuropsin, a plasticity-related serine protease. J Biol Chem. 1998;273:11189–96. doi: 10.1074/jbc.273.18.11189. [DOI] [PubMed] [Google Scholar]
- 15.Dalva MB, et al. EphB receptors interact with NMDA receptors and regulate excitatory synapse formation. Cell. 2000;103:945–56. doi: 10.1016/s0092-8674(00)00197-5. [DOI] [PubMed] [Google Scholar]
- 16.Grunwald IC, et al. Kinase-independent requirement of EphB2 receptors in hippocampal synaptic plasticity. Neuron. 2001;32:1027–40. doi: 10.1016/s0896-6273(01)00550-5. [DOI] [PubMed] [Google Scholar]
- 17.Takasu MA, Dalva MB, Zigmond RE, Greenberg ME. Modulation of NMDA receptor-dependent calcium influx and gene expression through EphB receptors. Science. 2002;295:491–5. doi: 10.1126/science.1065983. [DOI] [PubMed] [Google Scholar]
- 18.Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009 doi: 10.1016/j.psyneuen.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 19.Binder EB, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. Jama. 2008;299:1291–305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Binder EB, et al. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat Genet. 2004;36:1319–25. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- 21.Segman RH, et al. Peripheral blood mononuclear cell gene expression profiles identify emergent post-traumatic stress disorder among trauma survivors. Mol Psychiatry. 2005;10:500–13. 425. doi: 10.1038/sj.mp.4001636. [DOI] [PubMed] [Google Scholar]
- 22.Genander M, et al. Dissociation of EphB2 signaling pathways mediating progenitor cell proliferation and tumor suppression. Cell. 2009;139:679–92. doi: 10.1016/j.cell.2009.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horii Y, Yamasaki N, Miyakawa T, Shiosaka S. Increased anxiety-like behavior in neuropsin (kallikrein-related peptidase 8) gene-deficient mice. Behav Neurosci. 2008;122:498–504. doi: 10.1037/0735-7044.122.3.498. [DOI] [PubMed] [Google Scholar]
- 24.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–8. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.