Abstract
Expression of the neuropeptide galanin is known to be upregulated in the brain of patients with Alzheimer’s disease (AD). We and others have shown that galanin plays a neuroprotective role in a number of excitotoxic injury paradigms, mediated by activation of the second galanin receptor subtype (GAL2). In the present study, we investigated whether galanin/GAL2 plays a similar protective role against amyloid-β(Aβ) toxicity. Here we report that galanin or the GAL2/3-specific peptide agonist Gal2-11, both equally protect primary dispersed mouse wildtype (WT) neonatal hippocampal neurons from 250 nM Aβ1–42 toxicity in a dose dependent manner. The amount of Aβ1–42 induced cell death was significantly greater in mice with loss-of-function mutations in galanin (Gal-KO) or GAL2 (GAL2-MUT) compared to strain-matched WT controls. Conversely, cell death was significantly reduced in galanin over-expressing (Gal-OE) transgenic mice compared to strain-matched WT controls. Exogenous galanin or Gal2-11 rescued the deficits in the Gal-KO but not the GAL2-MUT cultures, confirming that the protective effects of endogenous or exogenous galanin are mediated by activation of GAL2. Despite the high levels of endogenous galanin in the Gal-OE cultures, the addition of exogenous 100 nM or 50 nM galanin or 100 nM Gal2-11 further significantly reduced cell death, implying that GAL2-mediated neuroprotection is not at maximum in the Gal-OE mice. These data further support the hypothesis that galanin over-expression in AD is a neuroprotective response and imply that the development of a drug-like GAL2 agonist might reduce the progression of symptoms in patients with AD.
Keywords: Alzheimer’s disease, amyloid toxicity, galanin, GAL2, neuroprotection, transgenic models
INTRODUCTION
The pathological hallmarks of AD are the presence of neurofibrillary tangles and senile plaques. The amyloid-β (Aβ) peptide, a predominantly 40–42 amino-acid fragment of the amyloid-β protein precursor (AβPP) derived from the cleavage of AβPP by β- and γ-secretases [1], is the major component of neuritic plaques [2]. Aβ deposition triggers a long-term neuropathological cascade, which includes neuronal loss and markedly distorted axons and dendrites, which lead to dendritic regression and spine loss [3, 4]. Dystrophic neurites are typically associated with senile plaques and both are correlated with cognitive deficits [5]. Neurites contain a large number of neurotransmitter and neuromodulatory substances, which are for the most part, reduced in the AD brain [6]. By contrast, the expression of the neuropeptide galanin [7, 8] is increased in AD [9, 10]. In AD, thickened galanin-immunoreactive fibres hyper-innervate surviving cholinergic basal forebrain (CBF) neurons, the locus coeruleus, as well as cortical and hippocampal projection neurons [9-12]. Further, galanin peptide levels as well as galanin receptor binding sites are increased in the neocortex, hippocampus, amygdala, and basal forebrain in the AD brain [13-17]. Studies using mice transgenic for AβPP bearing familial AD-related gene mutations exhibit age-dependent increases in hippocampal galanin neurite formation in close opposition to amyloid containing plaques [18, 19]. These hypertrophied galaninergic fibers are thickened with bulbous endings [18], very similar to that observed in AD.
The above findings have led to a number of functional studies addressing the role played by galanin in the modulation of acetylcholine (ACh) release, learning, and memory. The injection of galanin into the rat ventral hippocampus inhibits scopolamine-stimulated ACh release and impairs spatial learning acquisition in the water maze [20, 21]. Conversely, application of the peptide into the dorsal hippocampus stimulates the release of ACh and facilitates spatial learning [22, 23]. These site-specific effects are mirrored by the findings that galanin binding sites are present in both the ventral and dorsal hippocampus, but with five-fold higher concentrations in the ventral hippocampus [24]. Transgenic mice with ectopic over-expression of galanin localized to the adrenergic neurons (using the DBH promoter) have deficits in spatial learning and acquisition in the water maze [25], while mice with more widespread ectopic galanin over-expression (using the PDGF promoter) have normal learning and memory retention in the water maze task, compared to wild-type (WT) controls [26]. These studies have led some investigators to postulate that the up-regulation of galanin in AD might be detrimental and would further impair cognition [20, 25]. In contrast, it has also been hypothesized that amyloid-induced galanin fiber over-expression in AD plays a trophic and neuroprotective role to minimize the impact of amyloid toxicity upon neuronal function and cell survival.
To further study the role of galanin in neuronal survival and growth, we have generated transgenic mice bearing loss- or gain-of-function mutations in the galanin gene [27, 28], and demonstrated that the neuropeptide acts as a survival factor to subsets of neurons in the dorsal root ganglia (DRG) and CBF [29, 30]. Further, the peptide is a trophic factor to adult sensory neurons, which are dependent upon galanin for neurite extension after injury [31], mediated by activation of the second galanin receptor subtype (GAL2) [32]. We have also demonstrated that galanin and Gal2-11 (which has 500-fold selectivity for GAL2 and GAL3 compared to GAL1 [33, 34]) both play a neuroprotective role in the central nervous system (CNS) by reducing cell death in in vivo and in vitro hippocampal models of excitotoxicity [35]. We have extended these findings using the above injury paradigms of neuronal injury by applying them to the Gal-KO, GAL2-MUT, and Gal-OE mice. Collectively, these data demonstrate that the neuroprotective role played by galanin in the hippocampus is mediated by activation of GAL2, and is abolished in GAL2-MUT mice [36]. Of note, there is no evidence to date that the developmental cell survival role played by galanin in the basal forebrain alters or modulates the neuroprotective role played by galanin/GalR2 in the adult after neuronal injury.
Most recently, several studies have demonstrated that the addition of exogenous galanin or Gal2-11 is neuroprotective against Aβ toxicity in primary rat hippocampal [37] or cholinergic [38] cultures, human fetal brain cultures [39], and in the SN56 cholinergic cell line [40], and maintains or increases the expression of cell survival genes in the AD basal forebrain [41]. Here we show by modulation of endogenous levels of galanin or GAL2 in transgenic mice, that the neuropeptide has a significant neuroprotective role against Aβ toxicity via activation of GAL2. These data further support the hypothesis that galanin over-expression in AD is a neuroprotective response and imply that the development of a drug-like GAL2 agonist might reduce the progression of symptoms in patients with this disease.
MATERIALS AND METHODS
Animals
All animals were fed standard chow and water ad libitum and animal care and procedures were performed within the United Kingdom Home Office protocols and guidelines.
Galanin over-expressing (Gal-OE) mice
Details of the strain and breeding history are as described [27, 42]. In brief, galanin over expressing mice, bred to homozygosity, were generated using a ~25 kb transgene containing the entire murine galanin coding region and 19.9 kb of upstream sequence. The transgene was excised by restriction digest and microinjected into fertilized oocytes. The transgenic line denoted OE2 was then bred and characterized (see Bacon et al. for further details [42]). The line has remained inbred on the CBA × C57BL6 (CBA/Bl6) F1 hybrid background. WT mice that were strain-, age-, and gender-matched were used as controls in all experiments.
Galanin knockout (Gal-KO) mice
Details of the strain and breeding history are as described [28]. In brief, mice homozygous for a targeted mutation in the galanin gene were generated using the E14 cell line. A PGK-Neo cassette in reverse orientation was used to replace exons 1–5, and the mutation was bred to homozygosity and has remained inbred on the 129OlaHsd strain. Strain-, age-, and gender-matched WT mice were used as controls in all experiments.
GAL2 mutant (GAL2-MUT) mice
Details of the strain and breeding history are as described [32]. In brief, mice deficient for the GALR2 gene were generated and licensed from Lexicon Genetics. The 5.17 kb gene-trap vector VICTR48 (VIral Construct for TRapping) was inserted within the single intron of the murine GAL2 gene in a 129 Sv/EvBrd ES cell line clone [43]. Omnibank clone OST105469 was used to obtain germ-line transmission of the disrupted GALR2 allele. Heterozygote pairs on the C57BL6 × 129SvEvBrd (Bl6/129 Sv) background were transferred to the University of Bristol and then bred to homozygosity and have been maintained on that background. Strain-, age-, and gender-matched WT mice were used as controls in all experiments.
Preparation of primary neuronal cultures
Hippocampi from 2- to 3-day-old mouse pups were dissected and placed into 4°C collection buffer prepared with Hanks’ balanced salt solution (calcium and magnesium free) (GIBCO/BRL), 10% 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES, ICN), 10 μg/ml Penstrep (GIBCO/BRL), and 0.5% Bovine Serum Albumin (ICN). Enzymatic digestion, isolation, and culture of hippocampal neurons were performed as previously described [35], with the exception of the substitution of 0.83 U/ml Dispase (Sigma) instead of Trypsin. To inhibit glial cell growth, 5′Fluoro 2′ Deoxyuridine (Sigma), was added (10 μg/ml) to the cultures after 24 h. Cells were counted and plated at 40,000 cells/well onto DL-polyornithine-coated (Costar) black-wall 96-well plates (Corning, Arlington, UK). Cells were placed in Minimal Essential Media (GIBCO/BRL), with 5% Fetal Bovine Serum (GIBCO/BRL), 5% Horse Serum (GIBCO/BRL), 2 mM L-Glutamine (GIBCO/BRL), 5 μg/ml Insulin (Sigma), 10 ug/ml Penstrep and 5 mM HEPES for 2 h before media was changed to Neurobasal media, as described [35]. Cultures were incubated at 37°C with ambient oxygen and 5% CO2 for 5 days before experimentation and the media was changed on the fourth days.
Preparation of fibrillar Aβ (fAβ1–42)
Aβ1–42 (American Peptide Company Inc., USA) was dissolved in dimethyl sulfoxide (DMSO, Sigma) and then diluted to a concentration of 1 mM in phosphate buffered saline (PBS, Sigma) and incubated at 22°C or 37°C for 24 or 48 h. Electron microscopy was then undertaken at the University of Bristol central imaging facility. A 5 μl drop of the Aβ1–42 suspension was placed on a formvar and carbon coated copper grid and left to dry for 5 min. Excess fluid was removed using a filter paper and the grid was placed on top of a 3% Uranyl acetate solution in water. After 1 min excess fluid was removed using filter paper and air dried. The sample was examined using a FEI Tecnai12 Biotwin equipped with a 4*4 k bottom-mounted EAGLE CCD camera. Results demonstrated that incubating the peptide at 37°C for 48 h resulted in material that displayed a predominantly fibrillar form (Supplementary Fig. 1; available online: http://www.j-alz.com/issues/25/vol25-3.html#supplementarydata01). That protocol was then used to generate fAβ1–42 for all future experiments.
Treatments
Dispersed primary hippocampal cultures were cultured with or without the addition of the following: fAβ1–42, L-glutamic acid (Sigma), galanin peptide (Bachem), or Gal2-11 (Astra-Zeneca, Montreal).
Aβ1–42 Hippocampal toxicity
Dispersed primary hippocampal cultures were exposed to concentrations varying from 1 μM to 500 μM fAβ1–42 for 48 h or glutamic acid for 3 h with or without the addition of varying concentrations of galanin or Gal2-11. Neuronal injury was measured by the presence of propidium iodide. After membrane injury, the dye enters cells, binds to nucleic acids, and accumulates, rendering the cell brightly fluorescent. The viability of neurons was measured by counting of both live and dead neurons using high content microscopy and analysis using the IN Cell Analyser 1000 microscope as previously described [44, 45]. Image acquisition and quantification of fluorescence intensity and localization was performed using IN Cell Analyzer Workstation 3.5 software (IN Cell Investigator, GE Healthcare, Amersham, UK). Red channel (propidium iodide) and blue channel using 4′,6-diamidino-2-phenylindole (DAPI) images were used to define whole-cell and nuclear regions, respectively. Images were acquired with two single fields of view (0.6 mm2) and a 10 × objective. A total of 300–500 cells per field were typically analyzed, and up to two fields per well were captured in experiments performed in triplicate, meaning that in each experiment, data were normally derived from at least 1000 individual cells per treatment.
Statistical analysis
Data are presented as the mean±SEM. Student T-test or one-way ANOVA with appropriate post-hoc comparison tests were used to analyze difference between genotypes and the different ligands. Level of significance was set at p < 0.05.
RESULTS
Exogenous galanin and Gal2-11 protect WT hippocampal cultures from fAβ1–42-induced cell death
We first studied fAβ1–42-induced cell death in dispersed neonatal hippocampal cultures from the three WT strains. In all strains 48 h exposure to fAβ1–42-induced a concentration-dependent increase in cell death with little or no consistent cell death at less than 25 nM, whereas at concentrations above 500 nM virtually all cells died and detached from the culture plate and therefore, could not be accurately counted (data not shown). 250 nM fAβ1–42 reproducibly induced cell death in all three WT strains (varying between 31.6% and 36.5%, Figs. 1 and 2 and Supplementary Fig. 2; available online: http://www.j-alz.com/issues/25/vol25-3.html#supplementarydata01) and this concentration was used for all future experiments. As a positive control 5–6 mM glutamate was used to induce a similar amount of apoptosis (varying between 28.9% and 34.9%) to that observed with 250 nM fAβ1–42 (Figs. 1 and 2).
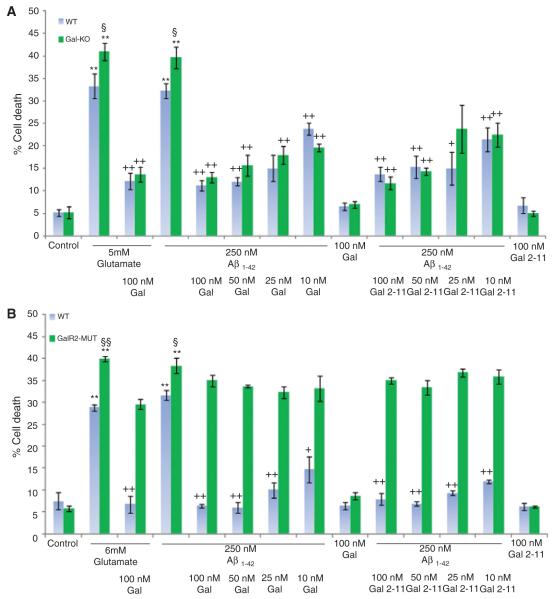
Fig. 1.
Percentage cell death in (A) Gal-KO and (B) GAL2-MUT both compared to strain matched WT hippocampal cultures, treated with 250 nM fA_1-42 in the presence of varying concentrations of galanin or Gal2-11. In both loss-of-function mutations there is a significant increase in fA_1–42 and glutamate-induced cell death compared to strain-matched WT cultures. Addition of galanin or Gal2-11 significantly rescues the deficits in the Gal-KO but not the GAL2-MUT cultures. **p < 0.01 treatment versus control. §p < 0.05 Gal-KO or GAL2-MUT versus WT. §§p < 0.01 GAL2-MUT versus WT. +p < 0.05 treatment different to fA_1–42 or glutamate alone. ++p < 0.01 treatment different to fA_1–42 or glutamate alone.
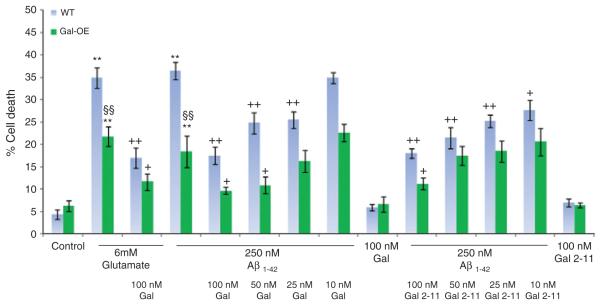
Fig. 2.
Percentage cell death in Gal-OE and strain matched WT hippocampal cultures treated with 250 nM fA_1–42 in the presence of varying concentrations of galanin or Gal2-11. In the Gal-OE mice there is a significant decrease in fA_1–42 and glutamate-induced cell death compared to strain-matched WT cultures. Addition of 100 nM and 50 nM galanin or 100 nM Gal2-11 further and significantly rescues the cell death. **p < 0.01 treatment versus control. §§p < 0.01 Gal-OE versus WT. +p < 0.05 treatment different to fA_1–42 or glutamate alone. ++p < 0.01 treatment different to fA_1–42 or glutamate alone.
Galanin and the GAL2/3 peptide agonist Gal2-11 both demonstrated similar degrees of significant dose-dependent neuroprotection in dispersed hippocampal neurons from all three wildtype strains when exposed to 250 nM fAβ1–42 (Figs. 1 and 2 and Supplementary Fig. 2). Maximum neuroprotection was obtained with 100 nM galanin varying between 52% and 81% and 51% and 84% for 100 nM Gal2-11. Similarly, 100 nM galanin was neuroprotective after exposure to glutamate, and significantly reduced cell death between 51% and 76%, similar to our previously findings [35, 36]. Treatment with galanin or Gal2-11 alone had no effect on cell death in any of the WT strains (Figs. 1 and 2). Of note, galanin and Gal2-11 both demonstrated greater neuroprotection in the Bl6/129 Sv mice, especially at lower concentrations, than in the two other strains studied (Figs. 1 and 2).
Transgenic manipulation of endogenous galanin and GAL2 levels modulate fAβ1–42-induced hippocampal cell death and responses to exogenous galanin and Gal2-11
Cell death in Gal-KO and GAL2-MUT mice after exposure to fAβ1–42 was significantly increased by 22% and 23% respectively, and by 23% and 38% respectively with glutamate, when compared to strainmatched WT controls (Fig. 1). Conversely, cell death was significantly reduced in Gal-OE mice by 51% and 38% after exposure to fAβ1–42 or glutamate, respectively when compared to strain-matched WT controls (Fig. 2).
Exogenous galanin and Gal2-11 both fully and equally rescued the above deficits in the Gal-KO mice after exposure to fAβ1–42 or glutamate (Fig. 1A). In contrast, neither peptide had any effect in the GAL2-MUT cultures (Fig. 1B). Despite the high levels of endogenous galanin in the Gal-OE cultures and the significant reduction in cell death, the addition of either exogenous 100 nM or 50 nM galanin or 100 nM Gal2-11 further significantly reduced cell death in these mice (Fig. 2).
DISCUSSION
We and others have shown that transgenic manipulation of the endogenous levels of galanin or GAL2, or the addition of exogenous galanin or Gal2-11 modulate the survival of cultured hippocampal neurons under excitotoxic conditions where apoptotic cell death occurs [35, 36, 46]. More recently these studies have been extended to Aβ-induced neuronal apoptosis. Treatment of cultured human embryonic cortical neurons with galanin significantly inhibited Aβ-induced cell death [39]. Similarly, galanin and Gal2-11 both significantly reduced Aβ toxicity in primary rat hippocampal [37] or cholinergic [38] neuronal cultures, and in cholinergic septal neuron 56 cells (a hybrid of mouse septal neurons and N18TG2 neuroblastoma cells) [40].
In the present study, we provide additional data demonstrating that exogenous galanin or Gal2-11 both provide significant neuroprotection against Aβ or glutamate toxicity in three different WT strains, confirming the above data sets. Moreover, we have now extended these findings using a panel of previously characterized transgenic mice with loss-of-function mutations in galanin or GAL2 and over-expression of galanin in the CNS using the previously described 20 kb galanin promoter region [35]. Aβ-induced cell death is significantly increased in Gal-KO and GAL2-MUT mice and reduced in Gal-OE transgenic mice, each compared to strain-matched WT controls. Importantly, exogenous galanin or Gal2-11 fully rescue the deficits in the Gal-KO but have no effect in the GAL2-MUT mice lacking a functional GAL2. Since activation of GAL1 and GAL3 by galanin, and GAL3 by Gal2-11 had no protective effects in the GAL2-MUT mice, these findings add further weight to the hypothesis that the galanin over-expression seen in AD is a neuroprotective response, mediated by activation of GAL2. We are currently extending these in vitro findings into an in vivo model of Aβ-induced neuronal dysfunction by crossing the Gal-OE mice to an AβPP/PS1 expressing line and then studying whether the over-expression of galanin modulates the previously described cognitive deficits in that line.
The intracellular signaling pathways that mediate the neuroprotective effects of GAL2 activation have yet to be fully defined. GAL2 couples to both Gi/o and inhibits adenylyl cyclase [47] and also signals via Gq/11 to activate phospholipase C (PLC) and protein kinase C (PKC) [47, 48]. Our previous studies using cultured hippocampal or sensory neurons have shown that the addition of galanin rapidly and potently stimulates phosphorylation of the serine/threonine kinase Akt and extracellular signal-regulated kinases (ERK) [32, 36]. These findings are also consistent with previous publications that hippocampal protection after excitotoxic damage is dependent in part upon activation of ERK [49, 50] and/or Akt [51, 52]. Recent data demonstrates that the neuroprotective effects of galanin against Aβ-induced apoptosis, are mediated by: (a) reversal of the Aβ-induced reduction in pERK and pAkt [38], (b) down-regulation of Bax levels [37, 39], and (c) attenuation in the cleavage of caspase 3 [37, 38, 40].
Irrespective of which signaling pathways mediate the neuroprotective effects of GAL2 activation, our findings that the addition of exogenous galanin or Gal2-11 in Gal-OE mice further reduces apoptosis, is of considerable interest in the context of the raised levels of galanin reported in AD [41]. This result implies that GAL2-mediated neuroprotection is not yet at maximum in situations where endogenous galanin expression is raised. This further supports the premise that the development of drug-like GAL2 agonists or positive allosteric modulators (as recently described [53]) might reduce the progression of symptoms in patients with AD.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by The Wellcome Trust and National Institute on Aging grant AG10668.
Footnotes
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=784).
REFERENCES
- [1].Haass C, Selkoe DJ. Cellular processing of beta-amyloid precursor protein and the genesis of amyloid beta-peptide. Cell. 1993;75:1039–1042. doi: 10.1016/0092-8674(93)90312-e. [DOI] [PubMed] [Google Scholar]
- [2].Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- [3].Lombardo JA, Stern EA, McLellan ME, Kajdasz ST, Hickey GA, Bacskai BJ, Hyman BT. Amyloid-beta antibody treatment leads to rapid normalization of plaque-induced neuritic alterations. J Neurosci. 2003;23:10879–10883. doi: 10.1523/JNEUROSCI.23-34-10879.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Selkoe DJ. Amyloid beta-protein and the genetics of Alzheimer’s disease. J Biol Chem. 1996;271:18295–18298. doi: 10.1074/jbc.271.31.18295. [DOI] [PubMed] [Google Scholar]
- [5].Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- [6].Bierer LM, Haroutunian V, Gabriel S, Knott PJ, Carlin LS, Purohit DP, Perl DP, Schmeidler J, Kanof P, Davis KL. Neurochemical correlates of dementia severity in Alzheimer’s disease: relative importance of the cholinergic deficits. J Neurochem. 1995;64:749–760. doi: 10.1046/j.1471-4159.1995.64020749.x. [DOI] [PubMed] [Google Scholar]
- [7].Tatemoto K, Rokaeus A, Jornvall H, McDonald TJ, Mutt V. Galanin - a novel biologically active peptide from porcine intestine. FEBS Lett. 1983;164:124–128. doi: 10.1016/0014-5793(83)80033-7. [DOI] [PubMed] [Google Scholar]
- [8].Evans HF, Shine J. Human galanin: molecular cloning reveals a unique structure. Endocrinology. 1991;129:1682–1684. doi: 10.1210/endo-129-3-1682. [DOI] [PubMed] [Google Scholar]
- [9].Chan-Palay V. Galanin hyperinnervates surviving neurons of the human basal nucleus of Meynert in dementias of Alzheimer’s and Parkinson’s disease: a hypothesis for the role of galanin in accentuating cholinergic dysfunction in dementia. J Comp Neurol. 1988;273:543–557. doi: 10.1002/cne.902730409. [DOI] [PubMed] [Google Scholar]
- [10].Mufson EJ, Cochran E, Benzing W, Kordower JH. Galaninergic innervation of the cholinergic vertical limb of the diagonal band (Ch2) and bed nucleus of the stria terminalis in aging, Alzheimer’s disease and Down’s syndrome. Dementia. 1993;4:237–250. doi: 10.1159/000107329. [DOI] [PubMed] [Google Scholar]
- [11].Bowser R, Kordower JH, Mufson EJ. A confocal microscopic analysis of galaninergic hyperinnervation of cholinergic basal forebrain neurons in Alzheimer’s disease. Brain Pathol. 1997;72:723–730. doi: 10.1111/j.1750-3639.1997.tb01058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chan-Palay V. Alterations in the locus coeruleus in dementias of Alzheimer’s and Parkinson’s disease. Prog Brain Res. 1991;88:625–630. doi: 10.1016/s0079-6123(08)63839-x. [DOI] [PubMed] [Google Scholar]
- [13].Gabriel SM, Knott PJ, Haroutunian V. Alterations in cerebral cortical galanin concentrations following neurotransmitter-specific subcortical lesions in the rat. J Neurosci. 1995;15:5526–5534. doi: 10.1523/JNEUROSCI.15-08-05526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mufson EJ, Deecher DC, Basile M, Izenwasse S, Mash DC. Galanin receptor plasticity within the nucleus basalis in early and late Alzheimer’s disease: an in vitro autoradiographic analysis. Neuropharmacology. 2000;39:1404–1412. doi: 10.1016/s0028-3908(00)00011-3. [DOI] [PubMed] [Google Scholar]
- [15].Rodriguez-Puertas R, Nilsson S, Pascual J, Pazos A, Hokfelt T. 125I-galanin binding sites in Alzheimer’s disease: increases in hippocampal subfields and a decrease in the caudate nucleus. J Neurochem. 1997;68:1106–1113. doi: 10.1046/j.1471-4159.1997.68031106.x. [DOI] [PubMed] [Google Scholar]
- [16].Perez S, Basile M, Mash DC, Mufson EJ. Galanin receptor over-expression within the amygdala in early Alzheimer’s disease: an in vitro autoradiographic analysis. J Chem Neuroanat. 2002;24:109–116. doi: 10.1016/s0891-0618(02)00034-0. [DOI] [PubMed] [Google Scholar]
- [17].McMillan PJ, Peskind E, Raskind MA, Leverenz JB. Increased galanin receptor occupancy in Alzheimer’s disease. Neurobiol Aging. 2004;25:1309–1314. doi: 10.1016/j.neurobiolaging.2004.01.004. [DOI] [PubMed] [Google Scholar]
- [18].Mufson EJ, Counts SE, Perez SE, Binder L. Galanin plasticity in the cholinergic basal forebrain in Alzheimer’s disease and transgenic mice. Neuropeptides. 2005;39:233–237. doi: 10.1016/j.npep.2004.12.005. [DOI] [PubMed] [Google Scholar]
- [19].Diez M, Danner S, Frey P, Sommer B, Staufenbiel M, Wiederhold KH, Hokfelt T. Neuropeptide alterations in the hippocampal formation and cortex of transgenic mice over-expressing beta-amyloid precursor protein (APP) with the Swedish double mutation (APP23) Neurobiol Dis. 2003;14:579–594. doi: 10.1016/j.nbd.2003.08.003. [DOI] [PubMed] [Google Scholar]
- [20].Wrenn CC, Crawley JN. Pharmacological evidence supporting a role for galanin in cognition and affect. Prog Neuro-Psychoph. 2001;25:283–299. doi: 10.1016/s0278-5846(00)00156-1. [DOI] [PubMed] [Google Scholar]
- [21].Sundstrom E, Archer T, Melander T, Hokfelt T. Galanin impairs acquisition but not retrieval of spatial memory in rats studied in the Morris swim maze. Neurosci Lett. 1988;88:331–335. doi: 10.1016/0304-3940(88)90233-9. [DOI] [PubMed] [Google Scholar]
- [22].Schott PA, Hokfelt T, Ogren SO. Galanin and spatial learning in the rat. Evidence for a differential role for galanin in subregions of the hippocampal formation. Neuropharm. 2000;39:1386–1403. doi: 10.1016/s0028-3908(00)00053-8. [DOI] [PubMed] [Google Scholar]
- [23].Ogren SO, Schott PA, Kehr J, Misane I, Razani H. Galanin and learning. Brain Res. 1999;848:174–182. doi: 10.1016/s0006-8993(99)01973-3. [DOI] [PubMed] [Google Scholar]
- [24].Valkna A, Jureus A, Karelson E, Zilmer M, Bartfai T, Langel U. Differential regulation of adenylate cyclase activity in rat ventral and dorsal hippocampus by rat galanin. Neurosci Lett. 1995;187:75–78. doi: 10.1016/0304-3940(95)11340-1. [DOI] [PubMed] [Google Scholar]
- [25].Steiner RA, Hohmann JG, Holmes A, Wrenn CC, Cadd G, Jureus A, Clifton DK, Luo M, Gutshall M, Ma SY, Mufson EJ, Crawley JN. Galanin transgenic mice display cognitive and neurochemical deficits characteristic of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2001;98:4184–4189. doi: 10.1073/pnas.061445598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kuteeva E, Hokfelt T, Ove OS. Behavioural characterisation of transgenic mice overexpressing galanin under the PDGF-B promoter. Neuropeptides. 2005;39:297–302. doi: 10.1016/j.npep.2005.01.004. [DOI] [PubMed] [Google Scholar]
- [27].Holmes FE, Bacon A, Pope RJ, Vanderplank PA, Kerr NC, Sukumaran M, Pachnis V, Wynick D. Transgenic overexpression of galanin in the dorsal root ganglia modulates pain-related behavior. Proc Natl Acad Sci U S A. 2003;100:6180–6185. doi: 10.1073/pnas.0937087100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wynick D, Small CJ, Bacon A, Holmes FE, Norman M, Ormandy CJ, Kilic E, Kerr NCH, Ghatei M, Talamantes F, Bloom SR, Pachnis V. Galanin regulates prolactin release and lactotroph proliferation. Proc Natl Acad Sci U S A. 1998;95:12671–12676. doi: 10.1073/pnas.95.21.12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Holmes FE, Mahoney S, King VR, Bacon A, Kerr NCH, Pachnis V, Curtis R, Priestley JV, Wynick D. Targeted disruption of the galanin gene reduces the number of sensory neurons and their regenerative capacity. Proc Natl Acad Sci U S A. 2000;97:11563–11568. doi: 10.1073/pnas.210221897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].O’Meara G, Coumis U, Ma SY, Kehr J, Mahoney S, Bacon A, Allen SJ, Holmes F, Kahl U, Wang FH, Kearns IR, Ove-Ogren S, Dawbarn D, Mufson EJ, Davies C, Dawson G, Wynick D. Galanin regulates the postnatal survival of a subset of basal forebrain cholinergic neurons. Proc Natl Acad Sci U S A. 2000;97:11569–11574. doi: 10.1073/pnas.210254597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mahoney SA, Hosking R, Farrant S, Holmes FE, Jacoby AS, Shine J, Iismaa TP, Scott MK, Schmidt R, Wynick D. The second galanin receptor GalR2 plays a key role in neurite outgrowth from adult sensory neurons. J Neurosci. 2003;23:416–421. doi: 10.1523/JNEUROSCI.23-02-00416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hobson SA, Holmes FE, Kerr NCH, Pope RJ, Wynick D. Mice deficient for galanin receptor 2 have decreased neurite outgrowth from adult sensory neurons and impaired pain-like behaviour. J Neurochem. 2006;99:1000–1010. doi: 10.1111/j.1471-4159.2006.04143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Liu HX, Brumovsky P, Schmidt R, Brown W, Payza K, Hodzic L, Pou C, Godbout C, Hokfelt T. Receptor subtype-specific pronociceptive and analgesic actions of galanin in the spinal cord: selective actions via GalR1 and GalR2 receptors. Proc Natl Acad Sci U S A. 2001;98:9960–9964. doi: 10.1073/pnas.161293598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lu X, Lundstrom L, Bartfai T. Galanin (2–11) binds to GalR3 in transfected cell lines: limitations for pharmacological definition of receptor subtypes. Neuropeptides. 2005;39:165–167. doi: 10.1016/j.npep.2004.12.013. [DOI] [PubMed] [Google Scholar]
- [35].Elliott-Hunt CR, Marsh B, Bacon A, Pope R, Vanderplank P, Wynick D. Galanin acts as a neuroprotective factor to the hippocampus. Proc Natl Acad Sci U S A. 2004;101:5105–5110. doi: 10.1073/pnas.0304823101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Elliott-Hunt CR, Pope RJ, Vanderplank P, Wynick D. Activation of the galanin receptor 2 (GalR2) protects the hippocampus from neuronal damage. J Neurochem. 2007;100:780–789. doi: 10.1111/j.1471-4159.2006.04239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cheng Y, Yu LC. Galanin protects amyloid-beta-induced neurotoxicity on primary cultured hippocampal neurons of rats. J Alzheimers Dis. 2010;20:1143–1157. doi: 10.3233/JAD-2010-091234. [DOI] [PubMed] [Google Scholar]
- [38].Ding X, MacTavish D, Kar S, Jhamandas JH. Galanin attenuates beta-amyloid (Abeta) toxicity in rat cholinergic basal forebrain neurons. Neurobiol Dis. 2006;21:413–420. doi: 10.1016/j.nbd.2005.08.016. [DOI] [PubMed] [Google Scholar]
- [39].Cui J, Chen Q, Yue X, Jiang X, Gao GF, Yu LC, Zhang Y. Galanin protects against intracellular amyloid toxicity in human primary neurons. J Alzheimers Dis. 2010;19:529–544. doi: 10.3233/JAD-2010-1246. [DOI] [PubMed] [Google Scholar]
- [40].Pirondi S, Giuliani A, Del VG, Giardino L, Hokfelt T, Calza L. The galanin receptor 2/3 agonist Gal2-11 protects the SN56 cells against beta-amyloid 25–35 toxicity. J Neurosci Res. 2010;88:1064–1073. doi: 10.1002/jnr.22278. [DOI] [PubMed] [Google Scholar]
- [41].Counts SE, He B, Che S, Ginsberg SD, Mufson EJ. Galanin fiber hyperinnervation preserves neuroprotective gene expression in cholinergic basal forebrain neurons in Alzheimer’s disease. J Alzheimers Dis. 2009;18:885–896. doi: 10.3233/JAD-2009-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bacon A, Holmes FE, Small CJ, Ghatei M, Mahoney S, Bloom SR, Wynick D. Transgenic over-expression of galanin in injured primary sensory neurons. Neuroreport. 2002;13:2129–2132. doi: 10.1097/00001756-200211150-00028. [DOI] [PubMed] [Google Scholar]
- [43].Zambrowicz BP, Abuin A, Ramirez-Solis R, Richter LJ, Piggott J, BeltrandelRio H, Buxton EC, Edwards J, Finch RA, Friddle CJ, Gupta A, Hansen G, Hu Y, Huang W, Jaing C, Key BW, Jr, Kipp P, Kohlhauff B, Ma ZQ, Markesich D, Payne R, Potter DG, Qian N, Shaw J, Schrick J, Shi ZZ, Sparks MJ, Van SI, Vogel P, Walke W, Xu N, Zhu Q, Person C, Sands AT. Wnk1 kinase deficiency lowers blood pressure in mice: a gene-trap screen to identify potential targets for therapeutic intervention. Proc Natl Acad Sci U S A. 2003;100:14109–14114. doi: 10.1073/pnas.2336103100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Armstrong SP, Caunt CJ, Finch AR, Mcardle CA. Using automated imaging to interrogate gonadotrophin-releasing hormone receptor trafficking and function. Mol Cell Endocrinol. 2011;331:194–204. doi: 10.1016/j.mce.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Caunt CJ, Armstrong SP, Mcardle CA. Using high-content microscopy to study gonadotrophin-releasing hormone regulation of ERK. Methods Mol Biol. 2010;661:507–524. doi: 10.1007/978-1-60761-795-2_32. [DOI] [PubMed] [Google Scholar]
- [46].Pirondi S, Fernandez M, Schmidt R, Hokfelt T, Giardino L, Calza L. The galanin-R2 agonist AR-M1896 reduces glutamate toxicity in primary neural hippocampal cells. J Neurochem. 2005;95:821–833. doi: 10.1111/j.1471-4159.2005.03437.x. [DOI] [PubMed] [Google Scholar]
- [47].Wang S, Hashemi T, Fried S, Clemmons AL, Hawes BE. Differential intracellular signaling of the GalR1 and GalR2 galanin receptor subtypes. Biochemistry (Mosc) 1998;37:6711–6717. doi: 10.1021/bi9728405. [DOI] [PubMed] [Google Scholar]
- [48].Wittau N, Grosse R, Kalkbrenner F, Gohla A, Schultz G, Gudermann T. The galanin receptor type 2 initiates multiple signaling pathways in small cell lung cancer cells by coupling to G(q), G(i) and G(12) proteins. Oncogene. 2000;19:4199–4209. doi: 10.1038/sj.onc.1203777. [DOI] [PubMed] [Google Scholar]
- [49].Maher P. How protein kinase C activation protects nerve cells from oxidative stress-induced cell death. J Neurosci. 2001;21:2929–2938. doi: 10.1523/JNEUROSCI.21-09-02929.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ozawa H, Shioda S, Dohi K, Matsumoto H, Mizushima H, Zhou CJ, Funahashi H, Nakai Y, Nakajo S, Matsumoto K. Delayed neuronal cell death in the rat hippocampus is mediated by the mitogen-activated protein kinase signal transduction pathway. Neurosci Lett. 1999;262:57–60. doi: 10.1016/s0304-3940(99)00034-8. [DOI] [PubMed] [Google Scholar]
- [51].Culmsee C, Gerling N, Lehmann M, Nikolova-Karakashian M, Prehn JH, Mattson MP, Krieglstein J. Nerve growth factor survival signaling in cultured hippocampal neurons is mediated through TrkA and requires the common neurotrophin receptor P75. Neuroscience. 2002;115:1089–1108. doi: 10.1016/s0306-4522(02)00539-0. [DOI] [PubMed] [Google Scholar]
- [52].Gary DS, Milhavet O, Camandola S, Mattson MP. Essential role for integrin linked kinase in Akt-mediated integrin survival signaling in hippocampal neurons. J Neurochem. 2003;84:878–890. doi: 10.1046/j.1471-4159.2003.01579.x. [DOI] [PubMed] [Google Scholar]
- [53].Lu X, Roberts E, Xia F, Sanchez-Alavez M, Liu T, Baldwin R, Wu S, Chang J, Wasterlain CG, Bartfai T. GalR2-positive allosteric modulator exhibits anticonvulsant effects in animal models. Proc Natl Acad Sci U S A. 2010;107:15229–15234. doi: 10.1073/pnas.1008986107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.