Summary
Background
Expanded access to combination antiretroviral therapy (ART) in the resource-poor setting is dependent on “task-shifting” from doctors to other health care providers. We compared “doctor-initiated-nurse-monitored” care to the current standard of care, “doctor-initiated-doctor-monitored” ART.
Methods
A randomised strategy trial to determine whether treatment outcomes of “nurse-monitored” ART were non-inferior to “doctor-monitored” ART was conducted at two South African primary-care clinics. HIV-positive individuals with a CD4 count of <350cells/mm3 or WHO stage 3 or 4 disease were eligible. The primary objective was a composite end-point of treatment limiting events, incorporating mortality, viral failure, treatment-limiting toxicities and visit schedule adherence. Intention-to-treat analyses were performed. This study is registered with ClinicalTrials.gov, NCT00255840.
Findings
The hazard ratio for composite failure was 1.09 (95% CI= 0.89-1.33) which lay within the limits for non-inferiority. The analysis was performed on 812 HIV-positive adults with either doctor-(n=408) or nurse-monitored ART (n=404). At baseline 573 (70%) patients were female, 282 (34.7%) had prior AIDS diagnoses and the median CD4 was 164 cells/mm3. After a median follow-up of 24.3 months, deaths (10 vs. 11), virological failures (44 vs. 39), CD4 gain (270 vs. 248 cells/mm3), toxicity failures (68 vs. 66) and program losses (70 vs. 63) were similar in nurse and doctor arms respectively. 371(46%) patients reached an endpoint of treatment failure; 192(47.5%) and 179(43.9%) in the nurse and doctor arms respectively.
Interpretation
Nurse-monitored ART was shown to be non-inferior to doctor-monitored therapy. This study supports task-shifting to appropriately trained nurses for monitoring ART.
Introduction
Combination drug therapy has had a remarkable impact in reducing AIDS-related morbidity and mortality [1]. In industrialised countries antiretroviral management is administered by specialist physicians who prescribe from the full range of available antiretroviral drugs, supported by frequent laboratory monitoring including resistance testing [2]. Several studies in industrialised settings have demonstrated that outpatients cared for by a physician with HIV expertise have better outcomes, including quality of care and survival, [3-7] which may reflect the complexities of HIV infection and its management [2]. In contrast to the relatively small epidemic in resource rich countries, there are 22.4 million people living with HIV in sub-Saharan Africa [8] with an estimated 3.8 million in urgent need to treatment [9]. Globally, there is a shortage of 4.3 million health workers [9] and in South Africa there are only 17.4 medical practitioners per 100,000 people, largely concentrated in urban areas [10, 11]
In contrast to the individualised approach to HIV care in developed countries, the World Health Organisation (WHO) has proposed a public-health approach to antiretroviral therapy (ART) to enable scaling-up access to treatment for large numbers of HIV-positive adults and children in developing countries [12]. An approach using standardised simplified treatment protocols and decentralised service delivery was developed to enable lower level health-care workers to deliver care [13]. Models of care have explored task shifting to clinical officers [14] and a combination of nurses and community workers [15]; however, nurse-led models of antiretroviral delivery have been one of the most widely implemented models of HIV care in poor-resourced African settings [15-18]. A control trial has demonstrated that work-site treatment of hypertension by specially trained nurses led to significantly improved blood pressure control and medication adherence [19]. To date no randomized prospective study has been published to demonstrate the effectiveness of nurse-monitored antiretroviral therapy. The HIV/AIDS strategic plan of South Africa, a medium income country with the world's largest national antiretroviral therapy programme, envisions increasing reliance on nurses for monitoring of antiretroviral therapy [20]. With increasing deployment of nurses for HIV care there is an urgent need for operational research to determine if nurse-led models of care are safe and effective.
We therefore conducted a prospective randomised controlled strategy trial comparing “doctor-initiated-nurse-monitored” to the current standard of care, “doctor-initiated-doctor-monitored” ART. Efficacy was assessed using a composite endpoint which reflected both treatment outcomes and patient management. Endpoint criteria included death, loss to follow up, viral suppression, drug interruptions due to toxicity and adherence to schedule of visits.
Methods
The study was a community-based ART strategy trial performed as part of the Comprehensive International Program for Research in AIDS in South Africa (CIPRA-SA - NCT00255840) [21]. The trial was conducted at two primary healthcare sites in South African townships: Masiphumelele in Cape Town and Soweto in Johannesburg.
Study Population
The eligible study population consisted of HIV-1 infected antiretroviral drug naïve adults (< 6 weeks) over 16 years of age with a CD4+ count <350 cells/mm3 or a prior AIDS-defining illness [22] and not in first trimester of pregnancy. Women who had received short course ART for prevention of mother to child transmission were not excluded. Screening laboratory investigations for renal function, liver enzymes and hematology were required to be less than grade 3 by the National Institutes of Health Division of AIDS toxicity grading scale [23]. An active opportunistic infection was exclusionary if the patient's treatment status was not considered stable (i.e. treatment for < 7 days) or in the case of tuberculosis (TB) if the treatment had been for less than eight weeks (amended in October 2005 to < 2 weeks of TB treatment). Other exclusion criteria included; concomitant treatment with systemic myelosuppressive, neurotoxic, pancreatotoxic, hepatotoxic, or cytotoxic treatment within 30 days of randomization; acute hepatitis, intractable diarrhea (lasting > 6 weeks), bilateral peripheral neuropathy of grade 2 or higher and drug or alcohol abuse considered by the investigator to potentially interfere with study compliance.
The study was approved at the institutional review boards of the University of Cape Town and University of the Witwatersrand, and written informed consent was obtained from all participants prior to the initiation of study procedures.
Study Design
The CIPRA-SA study was a prospective, unblinded, randomised controlled trial comparing two treatment monitoring strategies. Subjects were allocated to either receive their primary care from doctors (hereafter referred to as doctor arm) or from primary health care nurses (hereafter referred to as nurse arm). The standard of care strategy (doctor) was consistent with the routine management of patients in the current South African ART program which is based on doctor initiated and monitored treatment [13]. The experimental nurse monitoring strategy utilized doctor-initiated primary health care nurse-monitored ART with participants aware of their randomised treatment assignment. To ensure that all procedures and overall study management conformed to the national [24], and NIH guidelines for research on human subjects [25], a clinical safety team was established consisting of research experienced clinicians. The clinical safety team was responsible for the recruitment of participants including consent, screening processes, initiating therapy and providing ongoing telephonic consultation support to study nurses and doctors. The on-site pharmacist assigned the patients to their treatment arms once all inclusion criteria had been met by opening a sequential sealed envelope revealing the assigned treatment arm. Participants were randomised 1:1 within sites in pre-defined blocks of six.
At each site the experimental arm (nurse) utilised two experienced primary health care nurses. Primary health care nurses are a nationally registered cadre of nurses who have undergone one further year of clinical training in primary health care. The control arm (doctor) consisted of two doctors at each site. Primary-care providers who had limited or no prior experience with antiretroviral therapy were selected for both arms of the study. Each new clinician (nurse or doctor) received similar structured didactic and clinical training in HIV and the use of ART from the clinical safety team. In order to limit contamination between randomised arms, work activity and monitoring schedules were separated with routine visits scheduled on different days of the week, although at least one clinician was available to conduct unscheduled visits in the alternate arm of the study. Whenever possible the participant saw the same clinician within the treatment arm. Both arms were supported by a clinic nurse who ensured the participant saw the correct clinician, performed routine clinic procedures, and scheduled further patient visits, as well as by a team of lay community counselors trained in treatment adherence counseling. A pharmacist oversaw ordering and dispensing of antiretroviral drugs at each site.
The primary study outcome was a composite end-point of possible treatment limiting events that may occur on first-line therapy. The composite end-point was chosen to represent both biological measures of treatment safety, efficacy and disease progression, as well as more subtle measures of patient dissatisfaction with the treatment strategy, such as loss-to-follow-up. These included the following: 1) all-cause mortality, 2) loss-to-follow-up, 3) virologic failure, 4) toxicity failure, 5) withdrawn consent, 6) defaulting clinic schedule, and 7) HIV-disease progression.
Virologic failure was defined as either a decline of < 1.5 log10 in viral load from baseline to 12 weeks of treatment (early failure), or two consecutive viral loads 4 weeks apart of >1000 copies/ml (late failure).
Toxicity failure was defined as Grade 3 and 4 adverse events or other events requiring treatment interruption for more than 42 days [23]. However, single drug substitution as a result of drug related toxicity was not considered failure if treatment was interrupted for less than 42 days.
Patients who missed three consecutive study visits and were not able to be contacted by the study team were defined as lost to follow-up. Defaulting clinic schedule was defined as missing three or more consecutive scheduled clinic appointments with a study visit window of seven days but able to be traced.
Disease progression was defined by new AIDS-defining clinical events, as defined in the revised case definition of the Centers for Disease Control and Prevention [22]. Tuberculosis (TB) is hyperendemic in South Africa and therefore pulmonary TB was not included in the composite endpoint but was analyzed separately.
Throughout the study, a data monitoring team reviewed data from all study visits to identify any default or loss-to-follow-up. An end-point review committee reviewed all events classified as death and toxicity failure to ascertain if the correct assignment to study regimen and procedure was undertaken. An independent data and safety monitoring board reviewed the safety and efficacy of the CIPRA-SA study at six monthly intervals.
Antiretroviral therapy
ART was provided and specified by the South African Department of Health. Regimens initially prescribed by the Clinical Safety Team [26] included a nucleoside backbone of stavudine and lamivudine, with a choice of efavirenz, nevirapine or lopinavir/ritonavir. The initial dose of stavudine was 40 mgs daily for individuals over 60 kgs, which was reduced to 30 mgs for all patients from mid 2007 in line with WHO recommendations [27]. Efavirenz was the preferred non-nucleoside for men and women not wishing to become pregnant and willing to maintain both barrier and hormonal contraception throughout the study. Nevirapine and lopinavir/ritonavir were prescribed to women of child bearing potential depending on whether their CD4+ lymphocyte count was above or less than 250 cells/mm3 respectively. Pregnant women, who were allowed to enroll after their first trimester, were prescribed either nelfinavir or lopinavir/ritonavir.
Data collection and monitoring
After consenting and randomization by the Clinical Safety Team, the primary-care provider of the assigned arm undertook responsibility for treatment initiation, adherence counseling and follow up visits. Patients were scheduled for study visits at baseline and then at weeks 2, 4, 8, 12, and 12 weekly thereafter. Clinical records were maintained by the primary-care providers in each arm. Study coordinators at each site extracted relevant study data into case report forms which were relayed to a central database using Datafax®.
Statistical analysis and sample size
The sample size was calculated based on an 18-month accrual and 96 weeks follow-up period with 80% power and alpha of 0.05. Non-inferiority of the nurse arm over the doctor arm for cumulative treatment failure was pre-specified as an upper 95% confidence limit for the hazard ratio that was below 1.40. An initial sample size of 850 subjects accounted for potential clustering of multiple enrolled subjects within households. As significant household clustering was not observed, enrollment was able to be discontinued after 812 subjects with no compromise of pre-established study power.
Baseline differences in randomization groups were described using simple proportions for categorical variables and means and standard deviations for continuous variables. The primary analysis was an intention-to-treat analysis of any treatment failure using Cox proportional hazards regression. Differences in specific reasons for treatment failure (e.g. lost to follow-up, toxicity, death, etc.) were compared by treatment group using hazard ratios and 95% confidence intervals. Finally, differences in time to failure used Kaplan-Meier analyses. Group comparisons using the log-rank statistic, were considered significant if p-values were < 0.05.
Role of funder
Staff of the major funder, the Division of AIDS (DAIDS) of the National Institutes of Allergy and Infectious Diseases, at the National Institutes of Health, contributed to study design, data interpretation and review of the final manuscript. The corresponding author had full access to all data generated by the study and shared final responsibility for publication of the manuscript with the first author.
Results
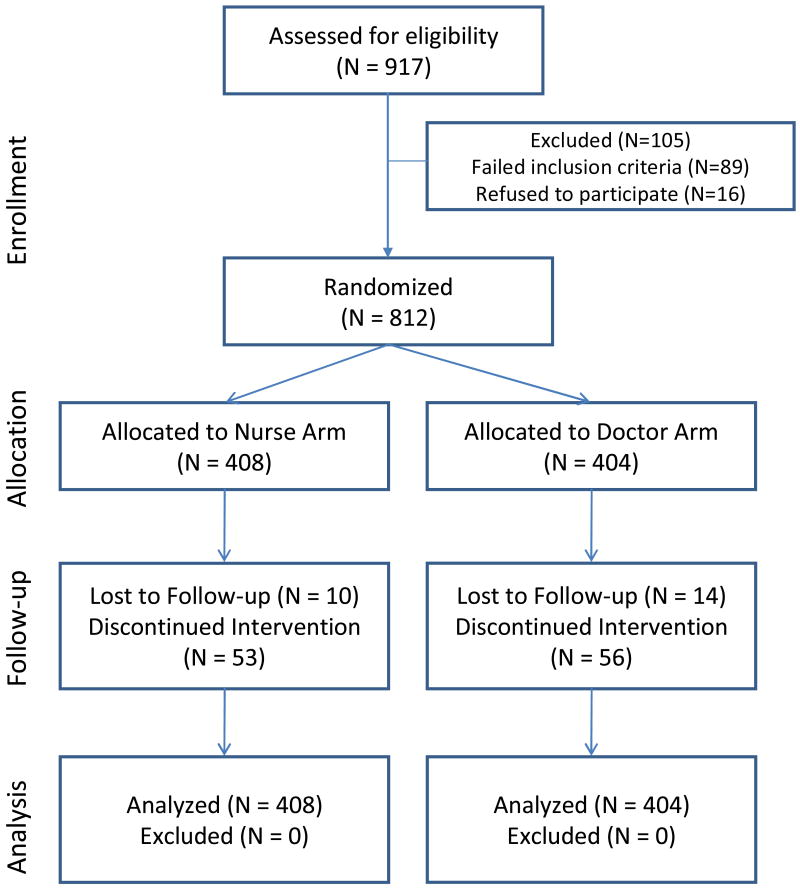
The study enrolment flow diagram is shown in figure 1. Between February 2005 and January 2007, 917 subjects were screened for study enrolment, of whom 828 met eligibility criteria and 812 consented and were randomised. Of the 89 patients excluded from the study, 32 did not meet the ART initiation criteria, 22 had acute medical conditions, 18 were considered unsuitable by investigators or failed to return, 8 had laboratory results out of eligible range and 9 were unable to take oral medication or were on excluded medications. Study subjects were 99% black African and 70% were female. Four hundred and eight individuals were randomised to the nurse arm and 404 to the doctor arm. The median follow-up was 120 weeks (IQR 60-144) with no difference between the nurse and doctor arms (median 119 vs 120 weeks respectively). The total follow-up period was 815.7 patient years and 830.9 patient years for the nurse and doctor arms, respectively.
Figure 1.
CONSORT study flow chart and participant disposition for the CIPRA-SA trial, a randomised trial of doctor vs. nurse monitored antiretroviral therapy in South Africa.
Baseline characteristics together with prior antiretroviral exposure and initial regimens are shown for each study arm in table 1. The study cohort had a median age of 32 years and had advanced HIV-disease as manifested by 35% with prior AIDS, 57% with viral loads >100,000 copies per millilitre (cpm) and a median of 164 CD4 cell/mm3. Patients in the nurse arm were slightly more likely to be female (73% vs 68%) and be in CDC stage A (40% vs 35%) than patients in the doctor arm but differences were small and non-significant. Despite the slight preponderance of females in the nurse arm, prior exposure to antiretroviral prophylaxis as part of mother-to-child prophylaxis was evenly distributed between study arms.
Table 1. Baseline demographic and clinical characteristics of 812 subjects randomised to doctor or nurse monitored antiretroviral therapy in the CIPRA-SA trial in South Africa.
| Nurse Arm | Doctor Arm | RR (95% CI) or pvalue** | |
|---|---|---|---|
| (N=404) | (N=408) | ||
| Female | 297 (73.5%) | 276 (67.7%) | 1.16 (0.99-1.36) |
| Age years median (IQR) | 32.3 (28.0-36.6) | 32.2 (28.0-37.4) | 0.8344 |
| BMI (kg/m2) (IQR) | 23.5 (21.3-27.6) | 23.5 (20.4-26.8) | 0.1044 |
| CDC Classification | |||
| Class A (%) | 160 (39.6%) | 141 (34.6%) | Reference |
| Class B (%) | 111 (27.5%) | 118 (28.9%) | 0.91 (0.77-1.08) |
| Class C (%) | 133 (32.9%) | 149 (36.5%) | 0.89 (0.75-1.04) |
| CD4+ Count (cells/ml) | |||
| <200 (%) | 260 (64.4%) | 257 (63.0%) | |
| 200-350 (%) | 119 (29.5%) | 131 (32.1%) | 0.95 (0.81-1.11) |
| 350-500 (%) | 23 (5.7%) | 18 (4.4%) | 1.12 (0.84-1.48) |
| > 500 (%) | 2 (0.5%) | 2 (0.5%) | 0.99 (0.37-2.66) |
| Median (IQR) | 165 (105-235) | 164 (110-225) | 0.7042 |
| Viral load (copies/ml) | |||
| ≤ 100,000 (%) | 181 (44.8%) | 170 (41.7%) | Reference |
| > 100,000 (%) | 223 (55.2%) | 238 (58.3%) | 0.94 (0.82-1.08) |
| Log10 mean viral load (std) | 4.99 (0.75) | 5.09 (0.73) | 0.939 |
| Baseline regimen prescribed | |||
| Stavudine, Lamivudine, Efavirenz | 293 (72.5%) | 304 (74.5%) | Reference |
| Stavudine, Lamivudine, Nevirapine | 72 (17.8%) | 81 (19.9%) | 0.96 (0.8-1.16) |
| Stavudine, Lamivudine, Lopinavir/rtv † | 35 (8.7%) | 20 (4.9%) | 1.30 (1.04-1.61) |
| Stavudine, Lamivudine, Nelfinavir‡ | 4 (1%) | 3 (0.7%) | 1.16 (0.61-2.22) |
| Prior exposure to antiretrovirals* | |||
| Single Dose Nevirapine (%) | 81 (20%) | 86 (21.1%) | Reference |
| Zidovudine (%) | 2 (0.5%) | 4 (1.0%) | 0.69 (0.22-2.15) |
| Nevirapine, Zidovudine (%) | 14 (3.5%) | 15 (3.7%) | 1.00 (0.66-1.5) |
| Triple drug therapy (%) | 1 (0.2%) | 0 (0%) | 2.06 (1.76-2.41) |
Protease inhibitor containing regimens were prescribed to pregnant women or women of childbearing potential with CD4+ count >250 who were unable or unwilling to use both a barrier contraceptive and a progesterone contraceptive. These women could not receive either a Nevirapine or Efavirenz containing regimen
Prior exposure to antiretroviral therapy for prevention of transmission, either from mother-to-child or in post-sexual exposure prophylaxis was permitted by the protocol for up to 6 weeks of treatment.
pvalue is from a Kalmogorov-Smirnoff test. RR = relative risk, CI = Confidence interval. RR is a row relative risk.
Most subjects commenced with non-nucleoside based therapy (92%) together with a nucleoside backbone of stavudine and lamivudine which reflected the prevailing South African national treatment guidelines.
Cumulative treatment failure
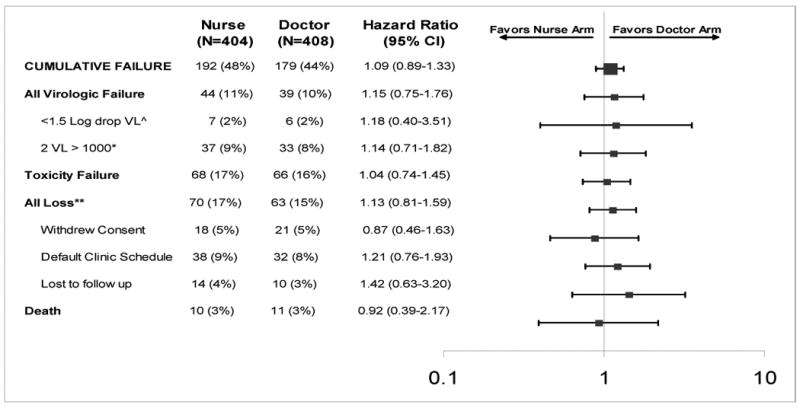
The primary study end-point of cumulative treatment failure was reached by 371 (45.7%) patients after a total of 1647 patient-years of follow-up. One hundred and ninety two (48%) of the subjects in the nurse arm reached the composite treatment failure endpoint and 179 (44%) in the doctor's arm. Using proportional hazards regression there was a nine percent increased risk of failure in the nurse arm (Hazard Ratio = 1.09 (95% confidence interval 0.89-1.33). The hazard ratio and 95% confidence limits lie below the pre-defined study criterion for inferiority (1.4). The Kaplan-Meier estimated time to composite failure was similar for each arm (Figure 2).
Figure 2.
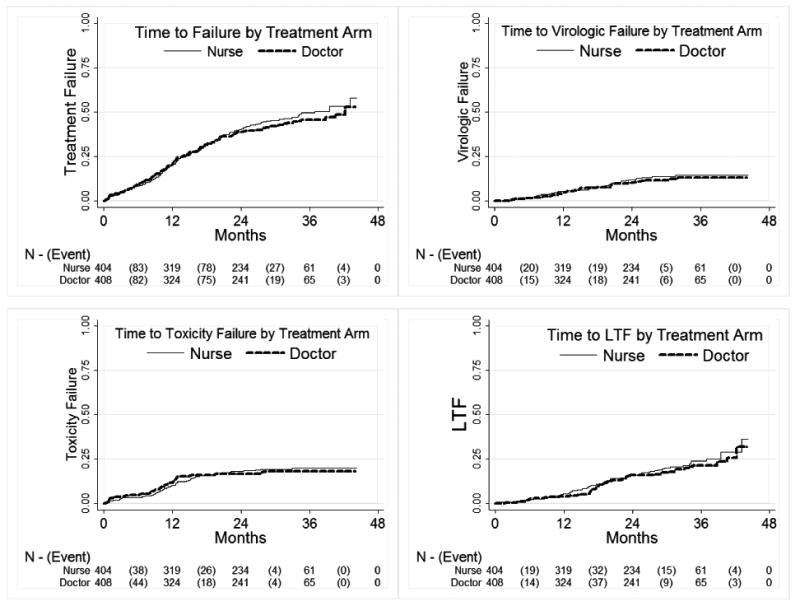
Figure 2a: Kaplan-Meier curves of time to cumulative treatment failure by study arm among 812 subjects randomised to doctor or nurse monitored antiretroviral therapy in the CIPRA-SA trial in South Africa; Figure 2b-d: Kaplan-Meier curves of time to specific reasons for treatment failure by study arm among 812 subjects randomised to doctor or nurse monitored antiretroviral therapy in the CIPRA-SA trial in South Africa*
* a) a Kaplan-Meier curve demonstrating the composite end-point of cumulative treatment failure. The primary health care nurse arm of the study is non-inferior to the doctor arm (log-rank p-value 0.4238). b) shows time to virologic failure stratified by treatment ARM (log-rank p-value = 0.5340); c) shows time to toxicity failure stratified by treatment ARM (log-rank p-value = 0.4678); d) shows time to loss to follow-up stratified by treatment ARM (log-rank p-value = 0.8358);
The hazard ratios for individual treatment failure parameters of the composite end-point are shown in Table 2. Deaths contributed 5.7% of the total events, viral failure 22.4%, toxicity failure 36% and protocol defined lost to follow up failure 36% of end-points. The subcategories of the composite endpoint hazard ratios are all closely distributed to 1.0 (0.92-1.15). There were no significant differences between study arms for Kaplan-Meier estimates of time to death, viral failure, toxicity failure and lost to follow up (Figure 2).
Table 2. Cumulative treatment failure (primary end-point) and accompanying reasons by study arm for 812 subjects randomised to doctor or nurse monitored antiretroviral therapy in the CIPRA-SA trial in South Africa†.
|
The two arms of the study were compared using a composite end-point or cumulative treatment failure. The composite consisted of each of the reasons listed below.
Early virologic failure was defined when a participant failed to demonstrate a serologic viral load decline of more than 1.5 logarithm within 12 weeks of initiating treatment.
Late virologic failure was defined as rebound in viral load from undetectable to more than 1000 copies/ml confirmed within one month.
Any loss was defined as: 1) withdrawn consent was if the patient withdrew from participating in the study for whatever reason and represents in most cases a transfer away from the site to another geographic location and clinic; 2) defaulting clinic schedule was a protocol defined measure of adherence to the clinic schedule, any participant who missed three consecutive visits was considered to be defaulting; (3) Loss to follow-up was consider if a participant did not return to the clinic for three consecutive clinic visits and could not be traced.
Deaths and disease progression
A total of 21 deaths, 10 in nurse arm and 11 in the doctor arms, were included in the analysis. One further death was not included in the analysis as the participant had met a protocol defined end-point of toxicity failure before the death occurred. All the deaths were reviewed by a blinded end-point review committee to assign the cause of death as study related, treatment related, disease related or non-study related. Two deaths were assessed as due to lactic acidosis and 2 deaths were considered to be non-study and non-HIV related. None of the participants met the protocol defined criteria for disease progression.
Immunology
Immune response was not a component of the primary endpoint; however, CD4 cell count increases from base line were 155 cells (IQR 119-193) and 158 cells (IQR 125-169) at 1 year and 239 cells (IQR 217-290) and 220 cells (IQR 174-274) at 2 years for the nurse and doctor arms, respectively. Cumulative treatment failure was not impacted significantly by either low baseline CD4+ count <200 cells/mm3 nor high viral load > 100,000 cpm. However, there were a non-significant trends for i/ncreased failure in the nurse arm for individuals with CD4 cell count <200cells/mm3 (p=0.14) and with viral loads >100,000 copies/ml (p=0.09).
Safety monitoring and toxicity
Grade 3 and 4 toxicities which occurred during the study are shown in table 3. The most frequent laboratory abnormalities were anemia and neutropenia, raised lactate and abnormal hepatic enzymes occurring at 10.2, 10.1 and 7.0 per 100 patient years respectively. The high frequency of hyperlactatemia resulted in a data safety monitoring board recommendation in 2007, for additional training and management of raised lactate. Grade 3 and 4 and dose limiting toxicities were more commonly reported in the doctor arm compared to the nurse arm (incidence rate of 55.5 vs 45.5 respectively). However, a retrospective review by the CIPRA clinical safety team of all laboratory investigations performed throughout the study was consistent with the equal distribution of laboratory defined adverse events between arms. For clinical HIV related (IRR = 0.32 (95% CI=0.16-0.65) and non-HIV related neurologic events (IRR= 0.39 (95% CI= 0.14-1.10) doctors were more likely than nurses to make a grade 3 or 4 neurologic diagnosis.
Table 3. Rate of laboratory and clinical dose limiting or Aids Clinical Trial Group grade three and four defined toxicities 30 by study arm among 812 subjects randomised to doctor or nurse monitored antiretroviral therapy in the CIPRA-SA trial in South Africa*†.
| TOXICITY | Nurse arm | Doctor arm | IRR (95% CI)** | ||
|---|---|---|---|---|---|
| Event in | Event in | ||||
| 815.74 py | Rate/100 py | 830.88 py | Rate/100 py | ||
| Laboratory: | |||||
| Haematology | 62 | 7.6 | 106 | 13 | 0.60 (0.44 - 0.82) |
| Biochemistry | 1 | 0.1 | 2 | 0.2 | 0.51 (0.05 - 5.62) |
| Liver | 44 | 5.4 | 72 | 8.7 | 0.62 (0.43 - 0.91) |
| Hyperlactataemia | 80 | 9.8 | 87 | 10 | 0.94 (0.69 - 1.27) |
| Pancreatic | 4 | 0.5 | 4 | 0.5 | 1.02 (0.25 - 4.07) |
| Renal | 3 | 0.4 | 1 | 0.1 | 3.06 (0.32 - 29.4) |
| Drug related rash | 4 | 0.5 | 5 | 0.6 | 0.81 (0.22 - 3.03) |
| Clinical HIV events: | |||||
| Tuberculosis | 28 | 3.4 | 31 | 3.7 | 0.92 (0.55 - 1.53) |
| Cervical dysplasia | 1 | 0.1 | 4 | 0.5 | 0.25 (0.03 - 2.28) |
| Neurologic | 10 | 1.2 | 32 | 3.9 | 0.32 (0.16 - 0.65) |
| Intestinal | 8 | 1.0 | 7 | 0.8 | 1.16 (0.42 - 3.21) |
| Skin | 1 | 0.1 | 2 | 0.2 | 0.51 (0.05 - 5.62) |
| Lipodystrophy, lipoatrophy | 45 | 5.5 | 51 | 6.1 | 0.90 (0.60 - 1.34) |
| Miscellaneous | 23 | 2.8 | 23 | 2.8 | 1.02 (0.57 - 1.82) |
| Clinical Non-HIV events: | |||||
| CNS | 5 | 0.6 | 13 | 1.6 | 0.39 (0.14 - 1.10) |
| Obstetric/gynaecology | 10 | 1.2 | 7 | 0.8 | 1.46 (0.55 - 3.82) |
| Miscellaneous | 34 | 4.2 | 37 | 4.5 | 0.94 (0.59 - 1.49) |
PHCN arm had 815.7 total person-years while the MO arm had 830.9
Active reporting of adverse events was undertaken by the primary care giving nurse or doctor, and a retrospective review by the study team of all laboratory adverse events greater than or equal to grade three was undertaken.
IRR = incidence rate ratio. CI = confidence interval
Discussion
This study reports the findings of the first prospective, randomised, controlled study comparing of nurse versus doctor managed ART. Mortality, viral suppression, CD4 cell count response and a composite end-point reflecting multiple aspects of ART delivery, demonstrated that nurse monitored therapy was not inferior to doctor monitored therapy. These findings support observational data from other treatment programmes reporting successful use of task shifting in HIV care in both resource-limited (South Africa, Mozambique, Rwanda and Lesotho) and resource-rich countries (Netherlands, United Kingdom) [28-33] as well as for other disease management [34].
Expansion of ART services is urgently required in resource-poor countries in order to achieve universal access targets by 2010 [35] and further expansion will be needed with initiation of universal testing and treating strategies [36]. There was no difference in mortality, viral failure or immune recovery between the study arms although there was a non-significant trend toward increased failure in the nurse arm for patients with advanced HIV disease. This study therefore supports the strategy of “task shifting” and indicates that HIV management by nurses can be safe and effective, probably even for those patients commencing therapy with advanced HIV infection, although further studies over longer duration may be required in this sub-group.
Although both study strategies successfully managed drug related toxicities, the study does highlight a high frequency of lipomorphologic changes and lactate elevation associated with use of regimens including stavudine. Recent WHO and South African guidelines have moved away from reliance on stavudine [37, 38] however, it remains widely used in resource-poor HIV therapy programs [12]. In our study the overall drug toxicity frequency appeared to be lower than earlier reports of stavudine based toxicities which resulted in drug substitutions in excess of 20% after three years [39]. The dose reduction of stavudine to 30 mgs after the first year of the study which was in line with WHO recommendations [27] may have reduced drug limiting toxicities somewhat. However, two of the study deaths were due to hyperlactatemia, a recognised complication of stavudine use.
Randomised controlled studies are frequently considered the “gold standard” on which treatment policies should be based. However, there may be some caveats in applying trial findings to non-study settings and other populations. The study did not necessarily replicate the typical conditions under which therapy is presently delivered in resource-poor settings. For instance, in addition to structured training in the use of ART, all the clinical staff in the study received protocol-specific training in the conduct of ethical research including Good Clinical Practice, didactic clinical management and had access to ongoing telephonic clinical support if required. However, widespread “task shifting” will require increased training, a redefinition of scope of practice for nurses and a clinical support structure. The study results also cannot be generalized to settings where multiple first-line antiretroviral therapy options may be used to individualize patient treatment, which in-turn may reduce dose-limiting treatment toxicities.
A strength of our study was that it was performed at busy primary care clinics located in South African communities with a high-burden of HIV where large scale task shifting will be required. The cadré of nurse used in our study, the primary care nurse, are the staff whose role as clinician is being increasingly utilised in HIV and other fields such as tuberculosis in the South African health care system. In order to limit the “contamination” between the arms of the study, once the participants were randomised scheduled visits were booked for different days of the week. A weakness of the study is the limited time over which participants were on-study. At only two years of follow-up the chance of divergence of the arms may still have been limited.
The study demonstrated a high overall composite endpoint rate in both nurse and doctor treated arms (48% vs. 44%). A stringent definition of treatment strategy failure included the traditional virologic failure (10-11%), dose-limiting toxicity (16-17% using d4T regimens), death (3%) and all clinic losses (15-17%) that translate to failure of the treatment strategy to maintain patients on antiretroviral therapy. These rates are not dissimilar to other studies despite our use of a more stringent definition of study loss, and use of a Stavudine-based antiretroviral therapy regimen with stringent criteria for hyperlactataemia and clinical toxicity [40]. There was also a high rate of loss to follow-up on this study, but again not dissimilar to other studies in resource constrained settings [40]. The future of large-scale antiretroviral programmes make it important to understand how this loss evolves over time. There were some small differences in diagnosing some grade three or four laboratory adverse events as well as some clinical diagnoses, which could have an impact on wider roll-out of nurse based ART care. These could be addressed by more focused training on monitoring of laboratory data as well as implementation of simple algorithms of when further neurologic workup by a doctor is necessary.
It should be noted that the study design did not address “nurse initiated” antiretroviral therapy because the prescription of licensed medication in South Africa is restricted to doctors. Implementation of nurse initiated therapy would therefore require additional changes to the existing legislation. However, the new national HIV strategic plan does envisage initiation of therapy by doctors together with wide scale task shifting to nurses for ongoing patient management [20].
In conclusion, primary health care nurses were shown to be non-inferior to doctors in monitoring first-line antiretroviral therapy in a public health ART program in South Africa. The results of this study strongly support the expanded access to treatment using models of task shifting in primary health care.
Acknowledgments
We would like to acknowledge the support of the Gauteng and Western Cape Provincial Health Authorities, the work of the CIPRA-SA Study Team, the Endpoint Review Committee (Gary Maartens, David Spencer), and especially the contribution of our patients.
Funding sources: Funding for the CIPRA-SA trial was by the Division of AIDS (DAIDS) of the National Institutes of Allergy and Infectious Diseases, the National Institutes of Health, through grant No 1U19AI53217-01. Funding was also provided by the United States Agency for International Development (USAID) under the terms of agreement 674-A-00-08-00007-00 with Right to Care (RTC). The project was also supported by Awards K01AI083097 and P30-AI50410 from the National Institute of Allergy And Infectious Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Division of AIDS, the National Institute of Allergy and Infectious Diseases, the National Institutes of Health, USAID, or other parties.
Footnotes
The CIPRA-SA study team included the authors as well as, listed alphabetically, Sharlaa Badal-Faesen, Mildred Botile, Nastassja Choonilal, Jennipher Gelant, Janet Grab,Veronica Graham, Najma Hafejee, Lynda Hamber, Sindesh Harduth, Johean Hendricks, Colleen Herman, Mellissa Hero, Richard Kaplan, Nicola Killa, Daniella Klemp, Faisel Laher, Thandi Mabiletsa, Zanele Madlala, Ntombekaya Mafukuzela, Bontle Mahlatsi, Helgard Marias, Nomakhaya Mfundisi, Buang Motloba, Cindy Moyo, Mcebisi Mtshizana, Lundi Ncana, Kevin Newell, Sean Palmer, Deborah Pearce, Mary-Ann Potts, Daphne Radebe, Anne Reyneke, Anna Segeneco, Jennifer Sekgale, Jan Steyn, Pinky Thebe, Handre Truter, Diederik van Niekerk, Frieda Verheye-Dua, Karlien Voges and Helen Woolgar.
Conflict of interest: The authors have each completed the ICMJE disclosure forms for potential conflict of interest. No conflicts of interest were identified.
Author contributions: Each of the authors participated in the study design and protocol development. Patient recruitment was either conducted or supervised by the clinical investigators Ian Sanne, Robin Wood, Catherine Orrell, Prudence Ive, Francesca Conradie, Jennifer Zeinecker and Mohammed Rassool. Laboratory and data management was performed by Ravindre Panchia, Handré Truter, Charlotte Ingram, Wendy Stevens and Christie Heiberg, and statistical design and analysis was conducted by Rene Gonin and Mathew Fox. The interpretation of the data and review of the publication was performed by all the authors, with the overall scientific oversight conducted by Ian Sanne, Robin Wood and James McIntyre.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Egger M, May M, Chêne G, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. The Lancet. 2002;360(9340):1178. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 2.Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. [accessed 25th November 2009];2008 November 3; Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
- 3.Kitahata MM, Van Rompaey SE, Shields AW. Physician experience in the care of HIV-infected persons is associated with earlier adoption of new antiretroviral therapy. J Acquir Immune Defic Syndr. 2000;24(2):106–14. doi: 10.1097/00126334-200006010-00004. [DOI] [PubMed] [Google Scholar]
- 4.Landon BE, Wilson IB, McInnes K, et al. Physician specialization and the quality of care for human immunodeficiency virus infection. Arch Intern Med. 2005;165(10):1133–9. doi: 10.1001/archinte.165.10.1133. [DOI] [PubMed] [Google Scholar]
- 5.Kitahata MM, Koepsell TD, Deyo RA, et al. Physicians' experience with the acquired immunodeficiency syndrome as a factor in patients' survival. N Engl J Med. 1996;334(11):701–6. doi: 10.1056/NEJM199603143341106. [DOI] [PubMed] [Google Scholar]
- 6.Laine C, Markson LE, McKee LJ, et al. The relationship of clinic experience with advanced HIV and survival of women with AIDS. AIDS. 1998;12(4):417–24. doi: 10.1097/00002030-199804000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Romanelli F, Matheny SC. HIV infection: the role of primary care. Am Fam Physician. 2009;80:946–952. [PubMed] [Google Scholar]
- 8.AIDS Epidemic Update: November 2009. [accessed December 30, 2009]; http://data.unaids.org/pub/Report/2009/2009_epidemic_update_en.pdf. 14.; World Health Organization, United Nations Children's Fund, UNAIDS. Towards universal access: scaling up priority HIV/AIDS interventions in the health sector. Geneva: World Health Organization; 2009. [Google Scholar]
- 9.World Health Organization. The World Health Report 2006: working together for health. Geneva: WHO; 2006. [accessed December 30, 2009]. http://www.who.int/whr/2006/en/ [Google Scholar]
- 10.A National Human Resources Plan for Health to provide skilled human resources for healthcare adequate to take care of all South Africans. [accessed Dec 30, 2009]; http://www.doh.gov.za/docs/discuss/2006/hrh_plan/chapt2.pdf.
- 11.Lehmann U. Strengthening Human Resources for Primary Health Care. In: Barron P, Roma-Reardon J, editors. South African Health Review 2008. Health Systems Trust; 2008. [accessed Dec 30, 2009]. pp. 163–177. http://www.hst.org.za/publications/841. [Google Scholar]
- 12.Gilks CF, Crowley S, Ekpini R, et al. The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet. 2006 Aug 5;368(9534):505–10. doi: 10.1016/S0140-6736(06)69158-7. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization; Geneva: 2002. Scaling up antiretroviral therapy in resource-limited settings. Guidelines for a public health approach. http://www.who.int/hiv/pub/prev_care/pub18/en. [PubMed] [Google Scholar]
- 14.Muula AS, Chipeta J, Siziya S, et al. Human resources requirements for highly active antiretroviral therapy scale up in Malawi. BMC Health Serv Res. 2007 Dec 19;7:208. doi: 10.1186/1472-6963-7-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bedelu M, Ford N, Hilderbrand K, Reuter H. Implementing antiretroviral therapy in rural communities: The Lusikisiki model of decentralized HIV/AIDS care. JID. 2007;196:S464–8. doi: 10.1086/521114. [DOI] [PubMed] [Google Scholar]
- 16.Miles K, Clutterbuck DJ, Seitio O, Sebegod M, Riley A. Antiretroviral treatment roll-out in a resource-constrained setting: capitalizing on nursing resources in Botswana. Bulletin of the World Health Organization. 2007;85:555–560. doi: 10.2471/BLT.06.033076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Griensven J, De Naeyer L, Uwera J, et al. Success with antiretroviral treatment for children in Kigali, Rwanda: experience with health center/nurse-based care. BMC Pediatr. 2008 Oct 2;8:39. doi: 10.1186/1471-2431-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Assefa Y, Kloos H. The public health approach to antiretroviral (ART) service scale up in Ethiopia: the first two years of free ART. Ethiop Med J. 2008 Oct;46(4):401–6. [PubMed] [Google Scholar]
- 19.Logan AG, Milne BJ, Achber C, Campbell WP, Haynes RB. Work-site treatment of hypertension by specially trained nurses. A controlled trial. Lancet. 1979 Dec 1;2(8153):1175–8. doi: 10.1016/s0140-6736(79)92397-3. [DOI] [PubMed] [Google Scholar]
- 20.South African National Department of Health; HIV and AIIDS and STI strategic plan for South Africa, 2007-2011. http://www.doh.gov.za/docs/misc/stratplan-f.html. [Google Scholar]
- 21.Comprehensive International Program for Research on AIDS in South Africa. http://www.cipra-sa.com/index.aspx.
- 22.1993 Revised Classification System for HIV Infection and Expanded Surveillance Case Definition for AIDS Among Adolescents and Adults. MMWR Recomm Rep. 1992;41(RR-17):1–19. [PubMed] [Google Scholar]
- 23.Division of Aids Table for Grading the Severity of Adult and Pediatric Adverse Events. [Accessed 16th April 2009];2004 December; http://www.ucdmc.ucdavis.edu/clinicaltrials/documents/DAIDS_AE_GradingTable_FinalDec2004.pdf.
- 24.Department of Health. Department of Health; Pretoria, South Africa: 2006. [Accessed 25th November 2009]. Guidelines for Good Practice in the Conduct of Clinical Trials with Human Participants in South Africa. Available at: URL: http://www.doh.gov.za/nhrec/norms/gcp.pdf. [Google Scholar]
- 25.National Institutes of Health. Required Education in the Protection of Human Research Participants Policy. [Accessed 25th November 2009]; Available at: URL: http://grants.nih.gov/grants/guide/notice-files/NOT-OD-00-039.html.
- 26.National Department of Health; South Africa: 2004. [Accessed 25 November 2009]. National antiretroviral treatment guidelines. Available at http://hst.org.za/uploads/files/sa_ART_Guidelines1.pdf. [Google Scholar]
- 27.World Health Organization; Geneva: 2007. [Accessed 25 November 2009]. Addendum to 2006 who guidelines on antiretroviral therapy for HIV infection in adults and adolescents New dosage recommendations for stavudine (d4t) Available at: http://www.who.int/hiv/art/ARTadultsaddendum.pdf. [Google Scholar]
- 28.Shumbushe F, van Griesven J, Lowrance D, et al. Task shifting for scale-up of HIV Care: Evaluation of nurse-centered antiretroviral treatment at rural health centers in Rwanda. Plos Medicine. 2009 Oct;6(10):e1000163. doi: 10.1371/journal.pmed.1000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen R, Lynch S, Bygrave H, et al. Antiretroviral treatment outcomes from a nurse-driven, community-supported HIV/AIDS treatment programme in Lesotho: observational cohort assessment at two years. J IAS. 2009;12:23. doi: 10.1186/1758-2652-12-23. http://www.jiasociety.org/content/12/1/23. [DOI] [PMC free article] [PubMed]
- 30.Charalambous S, Grant AD, Day JH, Pemba L, et al. Churchyard GJ. Establishing a work place antiretroviral therapy programme in South Africa. AIDS Care. 2007;19:34–41. doi: 10.1080/09500340600677872. [DOI] [PubMed] [Google Scholar]
- 31.Keitz SA, Box TL, Homan RK, Bartlett JA, Oddone EZ. Primary care for patients infected with human immunodeficiencyvirus: a randomised controlled trial. J Gen Intern Med. 2001;16:573–82. doi: 10.1046/j.1525-1497.2001.016009573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith S, Robinson J, Hollyer J, et al. Combining specialist and primary health care teams for HIV positive patients: retrospective and prospective studies. BMJ. 1996;312(7028):416–420. doi: 10.1136/bmj.312.7028.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fairall L, Zwarenstein M, Bateman ED, et al. Educational outreach to nurses improves tuberculosis case detection and primary care of respiratory illness: a pragmatic cluster randomised controlled trial. BMJ. 2005;331:750–754. doi: 10.1136/bmj.331.7519.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laurant M, Reeves D, Hermens R, et al. Substitution of doctors by nurses in primary care. CochraneDatabase of Systematic Reviews. 2004 doi: 10.1002/14651858.CD001271.pub2. [DOI] [PubMed] [Google Scholar]
- 35.Towards universal access by 2010 How WHO is working with countries to scale-up HIV prevention, treatment, care and support. World Health Organization; Geneva: 2006. [Google Scholar]
- 36.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009 Jan 3;373(9657):48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 37.World Health Organization; Geneva: 2006. [Accessed 25 November 2009]. Antiretroviral therapy for HIV infection in adults and adolescents Recommendations for a public health approach (2006 revision) Available at http://www.who.int/hiv/pub/arv/adult/en/index.html. [PubMed] [Google Scholar]
- 38.National Department of Health SA. Clinical Guidelines For The Management Of HIV & AIDS In Adults And Adolescents. 2010. [Google Scholar]
- 39.Boulle A, Orrell C, Kaplan R, et al. Substitutions due to antiretroviral toxicity or contraindication in the first 3 years of antiretroviral therapy in a large South African cohort. Antivir Ther. 2007;12(5):753–60. doi: 10.1177/135965350701200508. [DOI] [PubMed] [Google Scholar]
- 40.Fox Matthew P, Rosen Sydney. Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007–2009: systematic review. [Apr 29 2010];Trop Med Int Health. doi: 10.1111/j.1365-3156.2010.02508.x. Published Online: 5:38 AM. [DOI] [PMC free article] [PubMed] [Google Scholar]