Abstract
Complex organisms consist of a multitude of cell types arranged in precise spatial relation to each other. Arabidopsis roots generally exhibit radial tissue organization; however, within a tissue layer, cells are not identical. Specific vascular cell types are arranged in diametrically opposed longitudinal files that maximize the distance between them and create a bilaterally symmetric (diarch) root. Mutations in the LONESOME HIGHWAY (LHW) gene eliminate bilateral symmetry and reduce the number of cells in the center of the root, resulting in roots with only single and xylem and phloem poles. LHW does not appear to be required for the creation of any specific cell type, but coordinately controls the number of all vascular cell types by regulating the size of the pool of cells from which they arise. We cloned LHW and found that it encodes a protein with weak sequence similarity to basic helix-loop-helix (bHLH) domain proteins. LHW is a transcriptional activator in vitro. In plants, LHW is nuclear localized and is expressed in the root meristems where we hypothesize it acts independently of other known root patterning genes to promote the production of stele cells, but may also indirectly feed into established regulatory networks for the maintenance of the root meristem.
INTRODUCTION
Multicellular organisms must coordinate division and expansion of each tissue’s constituent cell types to ensure organized development. Plants develop through the activity of continuously dividing and self-renewing populations of cells called meristems. Two major meristematic populations, the shoot apical meristem (SAM) and root meristem (RM), are formed in the embryo and generate and pattern the bulk of the above and below ground portions of the plant, respectively. Other populations, however, also generate new cells; these include shoot auxiliary meristems, lateral root meristems and dispersed groups of cells such as stomatal meristemoids. Each of these populations maintains a constant size during normal development requiring that cell division and production of differentiated offspring are tightly controlled.
The root meristem has a stem-cell population that divides asymmetrically to create each of the tissue layers. Cells in this population (initial cells) maintain competence to divide by their proximity to the quiescent center (QC) which is specified by the coordinate activity of GRAS family transcription factors, SCARECROW (SCR) and SHORTROOT (SHR) (Sabatini et al., 2003) and the hormone auxin as mediated through the AP2 class transcription factors PLETHORA1 (PLT1) and PLT2 (Aida et al., 2004). Although the SHR protein is a transcription factor, it also serves as a positional cue by virtue of its regulated movement from the center of the root to the neighboring cell layers where it activates SCR (Nakajima et al., 2001). Downstream of these regulators that position the root stem-cells, the Arabidopsis homologue of the Retinoblastoma gene, RETINOBLASTOMA-RELATED (RBR), appears to behave similarly to its animal counterparts in repressing cell divisions within the stem cell population (Wildwater et al., 2005). Although it appears that initial cells for each of the different tissue types are regulated by this common pathway, very little is known about the initial cells for central tissues in the root.
These central tissues are collectively referred to as the stele. Clonal analysis of Arabidopsis embryos indicated that all of the tissues of the stele--the pericycle, vascular elements xylem and phloem and some ground tissue-- share a common origin (Dolan et al., 1993; Kidner et al., 2000). When viewed in cross-section, the tissues in the stele exhibit a stereotyped, species-specific arrangement. The small Arabidopsis root invariantly has two xylem poles diametrically opposed (diarch; Fig 1A) whereas other plants, such as wild-grown radish, may be diarch to heptarch (reviewed in (Turner and Sieburth, 2002)). Lateral roots are produced by postembryonic divisions in the pericycle. In roots of many species, including Arabidopsis, only the pericycle cells adjacent to the xylem poles are capable of initiating laterals. This leads to a predictable pattern of root growth somewhat analogous to the arrangement of organs in the shoot known as phyllotaxis.
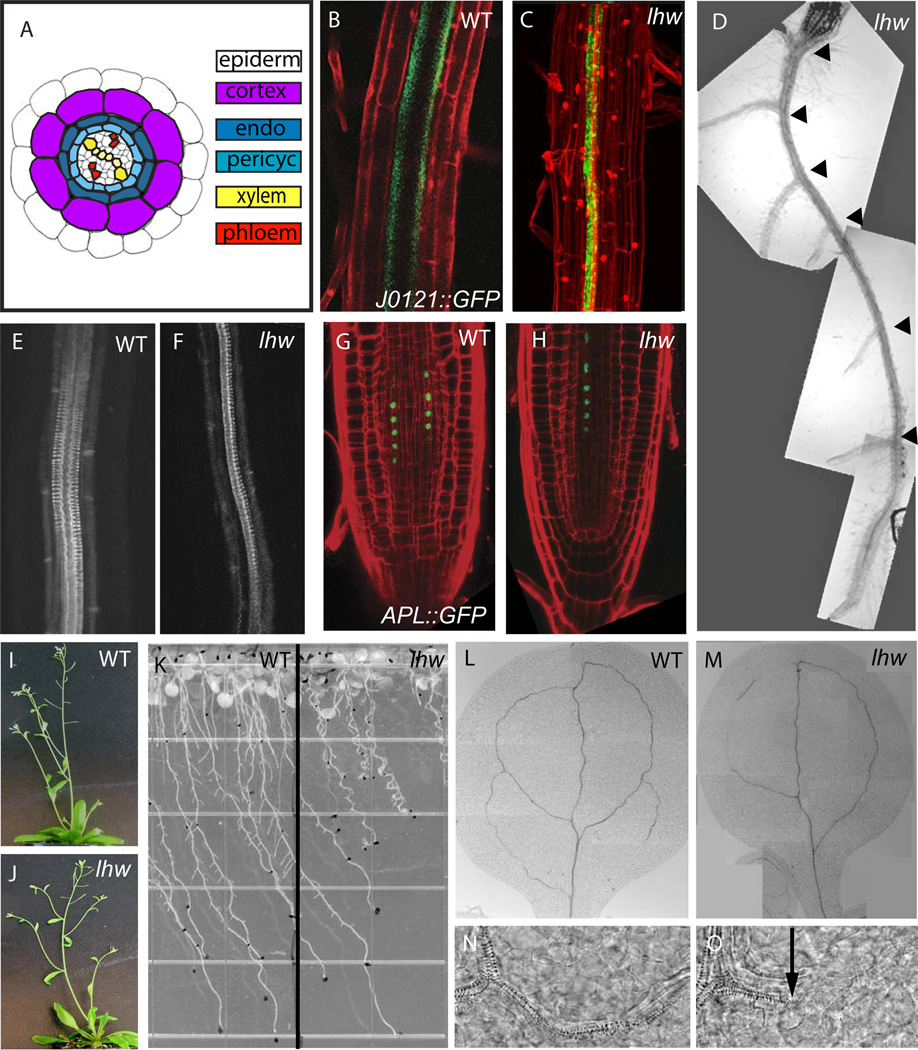
Figure 1. Phenotype of LONESOME HIGHWAY mutants.
(A) Cross section diagram of a mature Arabidopsis root; tissues are arranged radially from outside in: epidermis (white), cortex (light purple), endodermis (dark blue) and stele. The stele consists of a ring of pericycle cells (light blue), surrounding xylem (yellow) and phloem (red) arranged in bilateral symmetry. (B–C) Confocal images of wild type (B) and lhw-1 (C) expressing xylem-associated pericycle marker J0121::GFP in green. Roots are counterstained with propidum iodide (PI, red) to visualize outlines of cells. (D) Brightfield image of lhw-1 root with all lateral roots (black arrowheads) emerging from single side of primary root. (E–F) Confocal image of basic fucshin staining of xylem in wildtype (E) and lhw-1 (F). (G–H) Confocal images of wild type (G) and lhw-1 (H) expressing phloem marker APLpro::APL-GFP in green. (I–J) Whole plant phenotypes of WT Col (I) and lhw SALK_079402 (J). (K) Root growth of wild type (left) and lhw-1 (right) on agar plates at 20-dpg. Note root waving and short root phenotypes in lhw-1. (L–M) vascular pattern in mature (13 dpg) WT (L) and lhw-1 (M) cotyledons (N–O) higher magnification images of xylem from images L and M, respectively. Black arrow points to end of mature xylem elements, to the right, elongated cells typical of procambium are still seen. For each marker, the WT and lhw image pair are at same magnification.
Several genes and growth regulators have been implicated in root vascular development. ALTERED PHLOEM DEVELOPMENT (APL) encodes a MYB transcription factor required for the production of phloem. In the absence of APL, critical proliferative divisions in the vascular cylinder do not take place and the phloem is not specified (Bonke et al., 2003). WOODEN LEG (WOL) (also known as CRE1 or AHK4) is also required for proliferation of the vascular cylinder. Plants homozygous for the wol-1 mutation have fewer cells in the stele and fail to produce phloem (Mahonen et al., 2000; Scheres et al., 1995). WOL/CRE1 encodes a histidine kinase that functions in cytokinin response (Inoue et al., 2001; Mahonen et al., 2000). Further work with this kinase family as well as classic physiology experiments has implicated cytokinins in control of cell proliferation and cell fate in both shoot and root vascular development (de Leon et al., 2004; Higuchi et al., 2004; Mahonen et al., 2006; Mahonen et al., 2006; Nishimura et al., 2004).
In this study we identify a new locus, LONESOME HIGHWAY (LHW) required to establish and maintain the normal vascular cell number and pattern in primary and lateral roots. Using a map-based cloning approach, we identified the LHW gene and found that it defines the first member of a clade of plant-specific genes. Further characterization of protein localization and activity suggests that LHW encodes a transcriptional activator, suggesting that LHW plays a regulatory role in establishing a “set point” for the radial extent of the root vascular population.
MATERIALS AND METHODS
Screen
An ethylmethane-sulfonate (EMS) mutagenized population of approximately 4000 M1s was created from plants homozygous for the enhancer trap J0121::GFP (ABRC stock CS9090, C24 ecotype) using standard Arabidopsis mutagenesis procedures. Approximately 48,000 roots of 5-day old M2 seedlings were scored for deviations in J0121::GFP pattern. All lines were backcrossed at least twice before further analysis. Through backcrosses and complementation crosses, five mutations that resulted in presence of a single J0121::GFP stripe were found to be recessive to WT and allelic to each other. The locus defined by mutations w305, w279, w130, w123 and w116 was designated LONESOME HIGHWAY and the mutant alleles renamed lhw-1 through lhw-5, respectively. All LHW mutants were also crossed to Landberg erecta (Ler) to establish mapping populations.
Phenotypic characterization
Markers of cell fate used were: SCR::GFP (gift of J. Long, SALK), APLproAPL::GFP ((Bonke et al., 2003)), QC25::GUS (gift of B. Scheres, U. Utrecht), J0121::GFP (ABRC stock CS9090), Q1630::GFP (ABRC stock CS9227), VH1::GUS (Clay and Nelson, 2002) DR5::GUS (Ulmasov et al., 1997), CYCB1;1::GUS (Colon-Carmona et al., 1999;Donnelly et al., 1999). Unless otherwise indicated, the wild type control for experiments with lhw-1 and lhw-2 is the unmutagenized parental line CS9090 (C24 ecotype). Seedlings were grown vertically on plants containing 0.5X MS, 1% agar for indicated times. Expression of GFP markers was analyzed on a Bio-Rad 1024 confocal microscope, with propidium iodide counterstaining to observe cell morphology. Xylem was visualized by staining with 0.01% basic fucshin. Root cross sections were prepared according to (Scheres et al., 1995). Growth curves were performed by marking root lengths on the underside of plates every 24 hours during the growth of lhw and control parental plants grown side-by-side. Auxin analogue 2,4-Dichlorophenoxyacetic Acid (2,4D)) and cytokinin (kinetin) effects on primary root growth were assayed at 5-dpg with the indicated concentration of hormone. Seedlings grown on plates containing 20 µM 1-N-naphthylphthalamic acid (NPA) were scored at 7-dpg for rescue and at 21-dpg for terminal phenotypes. Images were processed for figures using Adobe Photoshop consistent with guidelines for image manipulation specified in the instructions for authors.
Map-based cloning of LHW
All alleles were individually mapped using a standard set of PCR-based mapping primers (Lukowitz et al., 2000). Recombinants between CER459215 and CER460427 were identified from ~800 F2 individuals from a mapping outcross of lhw-1 to Ler and scored for additional SSLP markers, localizing LHW to an 80KB region on BAC F12K2. T-DNA insertion alleles for 24/34 of the genes in the region were screened for root phenotypes and SALK_079402 (At2g27230) exhibited a single xylem pole phenotype. Mutations leading to stop codons in the predicted open reading frame of At2g27230 were identified in 4 LHW alleles. Using numbering derived from AY035151, mutations were found in lhw-1 g→a at 575; lhw-2g→a at 1944; lhw-3 c→t at 1883; lhw-4 g→a at 1066. lhw-1 is predicted to truncate the protein at amino acid 23 and is likely a null. All phenotypic characterization was carried out with both lhw-1 and lhw-2, and some tests with SALK_079402, to ensure that the lhw phenotype was not ecotype dependent. All alleles behaved similarly so only results from lhw-1 are reported except as noted. Full length cDNA AY035151 was obtained from RIKEN, Japan. An error in the cDNA that introduced an additional G at position 918 was corrected by PCR. CaMV35S expression of this cDNA was capable of rescuing the xylem phenotype of lhw-1 (Fig. 5B) in the T1 generation (6/14 independent lines).
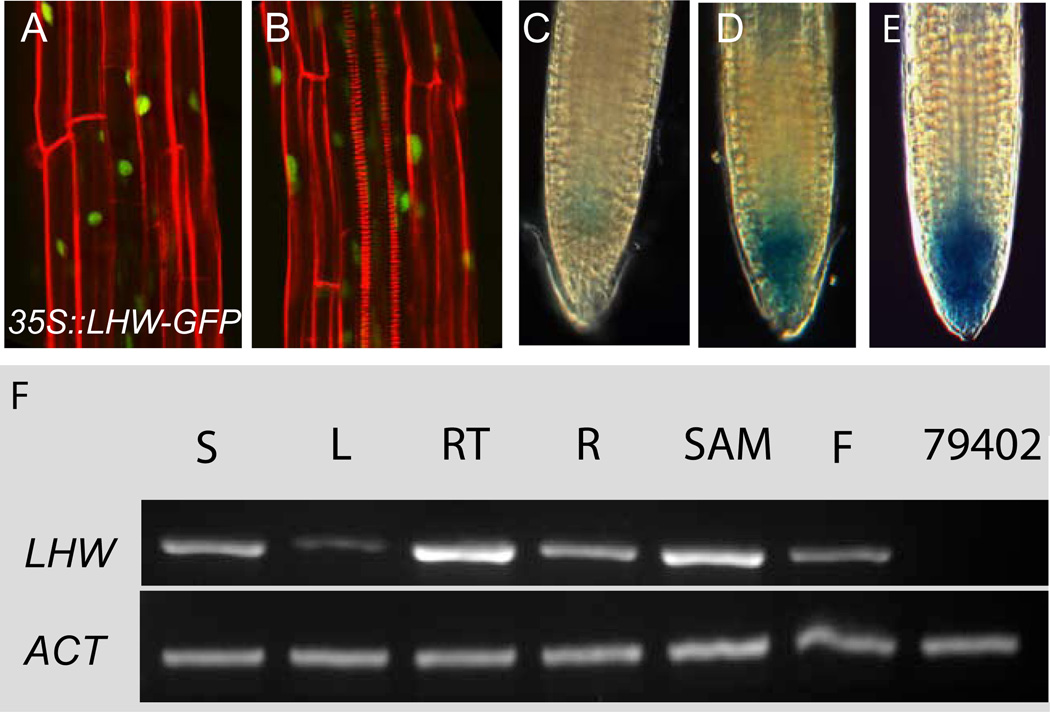
Figure 5. LHW expression.
(A–B) Confocal images of lhw-2 roots expressing 35S::LHW-GFP in nuclei (green). Expression of this transgene is sufficient to rescue the lhw-2 xylem phenotype (B). (C-E) LHWpro::GUS reporter expression in root tips, staining for 1hr, 4hr and 8hr, respectively (F). RT-PCR of LHW and actin (ACT) expression. S, 14-dpg seedling; L, expanded rosette leaf; RT, 5mm of 14-dpg root tip; R, whole 14-dpg root; SAM, transition SAM and youngest leaves; F, flower stage 8-15; 79402, 14-dpg seedling of SALK_79402.
Yeast two hybrid assay and screen
LHW and other clones were PCR amplified from cDNA clones or by RT-PCR and cloned into Clontech Matchmaker vectors pGBK (bait) and pGAD (prey). Saccharomyces cerevisiae strains AH109 or Y187 were used as hosts. Bait clones were tested for transcriptional auto-activation by co-transformation with an empty prey vector. Direct interactions between plasmids were tested by retransformation of plasmids in pairwise comparisons. A screen of ~800,000 colonies was performed using the LHW bHLH domain and C-terminus (DB-bC) as bait and a prey library in pACT constructed by Kim and Theologis (ABRC stock CD4-22). Positive clones were tested to ensure a single plasmid was responsible for the interaction, sequenced, and then retransformed into a strain containing prey for confirmation of the interaction. Quantitative analysis of β-gal expression was performed by transforming LHW variants into yeast strain Y187 and following procedures in Clontech’s yeast protocol guide.
Expression studies
Total RNA for semi-quantitative RT-PCR was isolated from plant tissues using a micro-midi RNA isolation kit (Invitrogen). 100ng of RNA was used in first strand synthesis with superscript III (Invitrogen), followed by PCR with gene specific primers lhwrtf1- gatcgtgtcaaagagctgcg and lhwrtr1- ttcgaaagcccatgttgctcc and control primers actinF-ggcgatgaagctcaatccaaacg and actinR- ggtcacgaccagcaagatcaagacg amplified for 32 and 25 cycles, respectively for 15 sec at 95° C, 30 sec at 52° C and 1 min at 68° C. A β-glucuronidase (GUS) reporter for LHW expression was created by PCR amplifying 2.8 KB of genomic sequence 5’ of the translational start site and cloning the piece into pCAMBIA 1303. Subcellular localization was determined by cloning the the LHW cDNA from translational start to one codon before the translational stop into pEZN (Cutler et al., 2000). Constructs were introduced into Arabidopsis plants via Agrobacterium-mediated transformation (Clough and Bent, 1998).
RESULTS
Identification of LONESOME HIGHWAY
To identify novel genes required for root cell fate specification, we screened for mutations that cause cell identity defects within the seedling stele. The screen was facilitated by the use of enhancer trap line J0121 to specifically mark xylem-adjacent pericycle cells (Laplaze et al., 2005). In wild type roots, two J0121::GFP-positive stripes of cells become visible in the elongation zone and extend to the root/hypocotyl junction (Fig 1B). Seedling roots were screened for alterations in the pattern of this marker 5–7 days post germination (dpg). Five completely recessive and allelic (see methods) mutations were found in that result in plants expressing J0121::GFP in only a single stripe (Fig. 1C and Fig. S1 in supplementary material). These five alleles define a new locus, LONESOME HIGHWAY (LHW). The absence of GFP expression correlates with a change in xylem-adjacent pericycle cell identity and/or function as seen by the production of lateral roots from only one side of the primary root (Fig. 1D).
Phenotypic analysis of lhw defects
Closer examination of lhw roots revealed that the bilaterally symmetric (diarch) organization of the stele was reduced to a monarch arrangement. Two protoxylem strands normally run the length of the root (Fig. 1E). In lhw, only one protoxylem strand was observed, and in most cases (34/40) was displaced from the center of the root. J0121::GFP expression was always adjacent to the single remaining xylem strand. In mature parts of the root, 2–5 files of metaxylem elements are normally found between the two protoxylem poles (Mahonen et al., 2000). In lhw plants, cells with the morphological characteristics of metaxylem are made, and they bear the same spatial relationship to the protoxylem pole, but there appear to be only half as many metaxylem cells (Fig. 1F and Fig. 2). In addition to two xylem poles, Arabidopsis roots normally have two phloem poles. Phloem organization can be visualized by APLpro::APL-GFP expression (Bonke et al., 2003). In wild type root tips, APLpro::APL-GFP is seen in nuclei of two cell files corresponding to maturing protophloem (Fig. 1G) In lhw, only a single APLpro::APL-GFP marked file is visible (Fig. 1H). Despite the reduced cell number in the root vasculature, LHW plants are healthy and fertile. Plants with mutations in LHW do not exhibit dramatically altered phyllotaxis, nor do they have any gross morphological abnormalities in their leaf and floral organs (Fig. 1I–J). lhw mutations in the C24 background lead to plants that are slightly agravitropic as seen in waving of lhw roots as they grow down a slanted agar surface (Fig. 1K). In cotyledons, lhw vein development is delayed relative to WT (Fig. 1L–M) and xylem gaps are still visible in the mature organs (Fig 1N–O); however, leaf venation patterns appear normal (not shown).
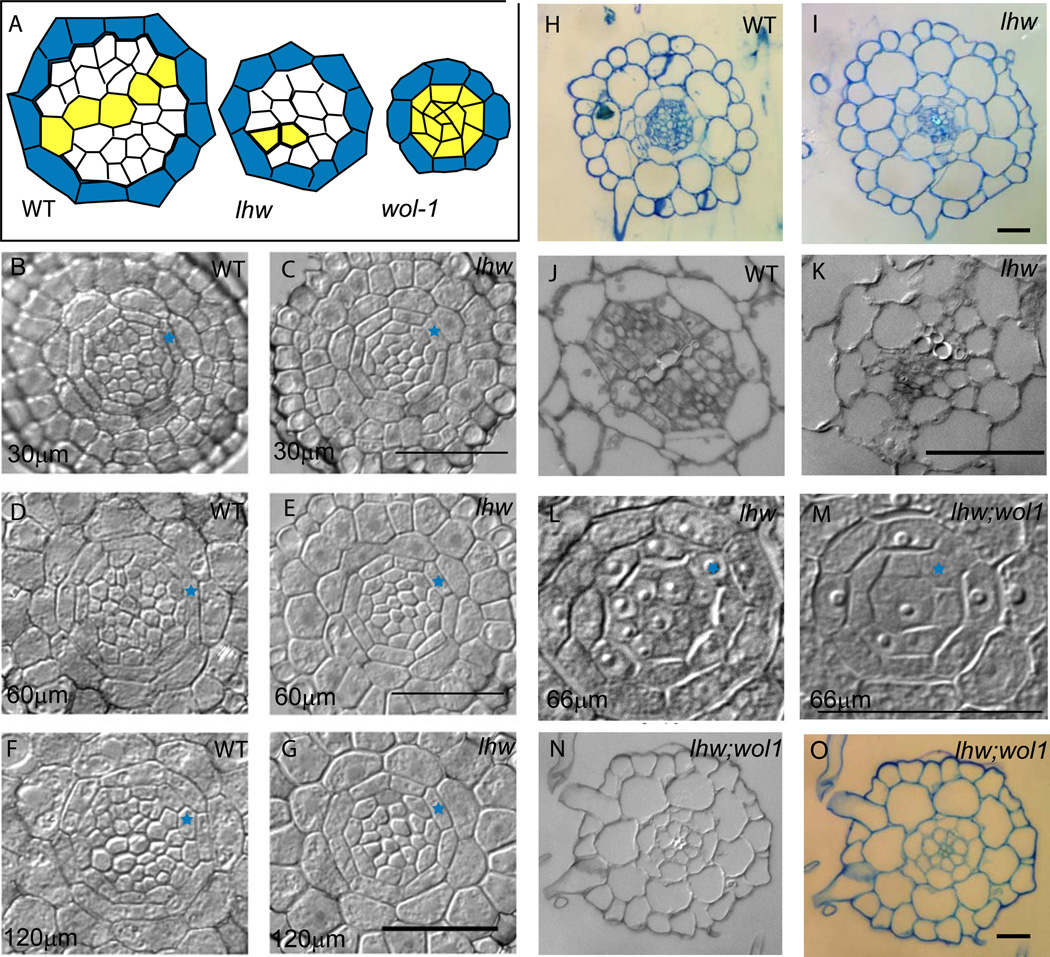
Figure 2. Cross sections of lhw roots and hypocotyls.
(A) Schematic of stele in cross sections from WT, lhw and wol-1; pericycle in blue and xylem in yellow; WT and lhw were traced from 2F and 2G, respectively. (B–K) Brightfield images of WT and lhw root sections taken at increasing distance from tip of root; one pericycle cell marked with blue star for orientation. (B) WT, 30 µm (C) lhw-2, 30 µm (D) WT, 60 µm (E) lhw-2, 60 µm (F) WT, 120 µm (G) lhw-2, 120 µm. (H–I) Toluidine blue staining of WT (H) and lhw-2 (I) in mature zone. (J–K) section through lower third of hypocotyl in WT (J) and lhw-2 (K). (L) Center of wol-1 root, 66 µm from tip (M) Center of wol-1;lhw-1 root, 66 µm from tip. (N–O) DIC and toluidine blue images of wol-1;lhw-1 mature roots showing the very reduced stele filled with xylem elements. Each image pair is at the same magnification. Scale bars = 20 µm.
The root vasculature phenotypes suggest that lhw does not have a defect in the production of any specific differentiated cell type, but that LHW is required to produce the normal arrangement and number of these cell types. In dicot roots there is a strong correlation between the size of the stele and the number of xylem poles, and the experimental manipulation of cell number in some dicot roots leads to variation in vascular pole number (Torrey, 1955). In Arabidopsis primary roots, the stele is usually comprised of 12–13 pericycle cells (Dolan et al., 1993)) and 25–28 internal cells at stages when mature xylem and phloem elements are found (Dolan et al., 1993) and Fig 2F. To determine the number of cells in the lhw stele, we made cross sections of roots from the level of the meristem (Fig. 2 B–C) through the mature zone (where root hairs are visible; Fig. 2H–I) and up into the hypocotyl (Fig. 2 J–K). In cross sections of a wild type root (30 µm above tip), the epidermis consists of ~ 25 cells, the cortex and endodermal layers each consist of 8 cells and the stele (pericycle, xylem and phloem) is approximately 25 cells (Table 1). In lhw roots, the normal number of cortex and endodermal cells are present, the epidermal number is slightly reduced, but the number of cells in the lhw stele is reduced to half as many as wild type (Fig. 2; Table 1). This affects all cell types in the stele; in addition to the reduction in cells from which the xylem and phloem arise, the lhw pericycle is reduced from the normal 13 cells to 8 cells (Fig. 2; Table 1).
Table 1.
Cell numbers in the primary root
| 30µm | 60 µm | 120µm | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| total | ||||||||||||
| stele | pericycle | inside | total stele | pericycle | inside | total stele | pericycle | inside | endodermis | cortex | epidermis | |
| WT | 33 | 11.5 | 21.5 | 41±1 | 13.33±1.5 | 27.67±1.5 | 41.67±.58 | 13±0 | 28.67±.58 | 8±0 | 8±0 | 24.3±2.1 |
| lhw-1 | 19± 1.7 | 8.5±.55 | 10.5±1.5 | 20.33±1.5 | 8.5±.55 | 11.83±1.2 | 21.83±1.6 | 8.33±1.2 | 13.17±1.5 | 8±0 | 8±0 | 20.6±3.6 |
| wol-1 | 16 | 8 | 8 | 19 | 9 | 10 | 19 | 9 | 10 | |||
| lhw-1;wol-1 | 14±3 | 7.67±1.5 | 6.33±1.5 | 14.67±3.2* | 8±1 | 6.67±2.3 | 15.33±2.9** | 8±1 | 7.33±2.1 | |||
Number of roots scored/genotype: WT = 3, lhw-1 = 8, wol-1 = 2, lhw-1;wol-1 = 3.
Values are averages ± S.D. P values for difference between number of cells in lhw and lhw;wol stele = * P=.08;
P=.04
The total number of cells in the stele is a product of the initial pool in the embryo and divisions postembryonically. The number of stele cells visible in cross section of the lhw root at 30 µm and 120 µm is virtually unchanged (Table 1), suggesting that postembryonic divisions rarely occur. In the embryo, the stele is derived from the uppermost tier of the root meristem (Dolan et al., 1993). Early embryogenesis in lhw was indistinguishable from wild type in terms of orientation of cell divisions (Fig. S2A–B in supplementary material). The only defect seen at a significant frequency (3/15 lhw globular embryos and 11/15 lhw late heart stage embryos) was a delay relative to WT in divisions in the base of the embryo, in cells that will later become the RM (Fig. S1 C vs. D; E and G vs. F and H, in supplementary material). At the torpedo stage, lhw embryos appear to have a well formed vascular cylinder, but it is narrower in lhw than in wild type (Fig. S1 J vs. I in supplementary material).
Because lhw mutants still make some lateral roots, we could examine the organization of these post-embryonically formed organs. Arabidopsis lateral roots originate from a stereotyped series of divisions in three pericycle cell files adjacent to a xylem pole. Lateral roots normally have the same tissue organization as primary roots, although control over the number of cells in the cortex and endodermis is somewhat relaxed (Dolan et al., 1993). Despite early division patterns that are indistinguishable between WT and lhw (Fig. S1K-P in supplementary material), lhw lateral roots generate only a single protoxylem pole (100% n=40), a single APLpro::APL-GFP marked phloem pole (100% n=20) and a single J0121::GFP marked xylem-adjacent pericycle file (97% n=40), suggesting that LHW is required to establish normal cell numbers in the stele of these organs.
The relationship between LHW and auxin
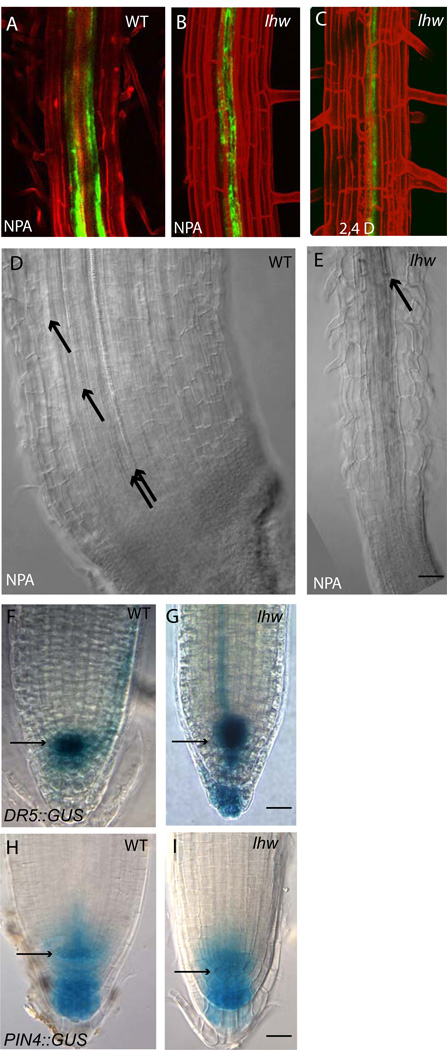
Defects in lateral root formation and xylem differentiation suggest that lhw might have defects in auxin synthesis, transport or perception. However, lhw does respond to exogenous auxins (IAA and 2,4 D) by producing root hairs and lateral roots and inhibiting primary root elongation (Fig. S3 in supplementary material and not shown) yet these auxin treatments do not rescue the xylem or pericycle defects (Fig. 3C and not shown). We visualized local auxin response near the RM by scoring the expression pattern and intensity of markers DR5::GUS and PIN4::GUS (Friml et al., 2002; Ulmasov et al., 1997). In lhw plants, expression of both markers is similar to WT in intensity and in position of the maximum (Fig. 3G vs. F and I vs. H).
Figure 3. Measures of auxin response in lhw.
(A–C) confocal images of WT (A) and lhw-1 (B–C) expressing J0121::GFP. (A–B) treatment with 20 µM NPA for 7 days (C) treatment with 30nM 2,4D for seven days. (D–E) DIC images of roots treated with 20 µM NPA for 21 days. Black arrows point to xylem elements. (F–I) Brightfield images 7-dpg seedling root tips; (F-G) DR5::GUS expression; (H–I) PIN4::GUS expression. Each image pair (and A–C) is at the same magnification. Scale bars = 20 µm.
Germination and growth on media containing the auxin transport inhibitor NPA can lead to excessive root meristem proliferation and xylem production (Mattsson et al., 1999). lhw and WT plants grown on MS agar plates containing 20 µM NPA were sampled at 7 and 21 dpg for xylem vessel formation and expression of the J0121::GFP marker in roots. At neither timepoint was expression of J0121::GFP seen in two stripes nor was the second xylem pole restored in lhw (Fig. 3B vs. 3A and not shown). Morphology of the root tip was strikingly different between wildtype and lhw at 21-dpg. In wild type, the roots became extensively fasciated and produced 8–10 xylem files (Fig. 3D). The lhw root tips were only slightly wider than untreated roots, failed to undergo excess cell proliferation and never produced more than a single differentiated xylem cell file (Fig. 3E). These data indicate that although lhw plants appear to perceive auxin and respond in terms of primary root inhibition and production of root hairs, they are unable to respond to auxin in the formation of xylem and pericycle. The simplest explanation for these phenotypes is that LHW is not a core component of auxin signaling but that lhw mutants are defective in a downstream process.
The relationship between LHW and cytokinin receptor WOL
Cytokinins, like auxin, are required for longitudinal proliferation in the root, but cytokinins also have significant roles in radial proliferation (Ferreira and Kieber, 2005; Mahonen et al., 2006). The wol-1 mutation in the WOL/CRE1 cytokinin receptor severely reduces cell proliferation in the stele (Mahonen et al., 2000). We tested whether WOL and LHW acted in the same genetic pathway by constructing double mutants between the two. wol-1 roots are short and number of cells interior to the pericycle is reduced to less than 10, all of which become xylem (Mahonen et al., 2000; Table 1). Double mutants between lhw and wol-1 exhibit the root length defect of wol however, the presence of the wol-1 mutation further reduces the number of cell in the lhw stele (Fig 2L–O; Table 1), suggesting that LHW promotes the production of stele cells in a somewhat WOL-independent manner. In addition to defects in cell proliferation, wol-1 mutations eliminate phloem production and result in a stele consisting solely of protoxylem. In terms of cell identity, the wol-1 mutation is epistatic to lhw because the interior of the lhw;wol-1 root resembles wol-1. The presence of multiple xylem poles in this double mutant indicates that there is no explicit requirement for LHW in production of this cell type.
LHW encodes a member of a novel, plant specific, family of proteins
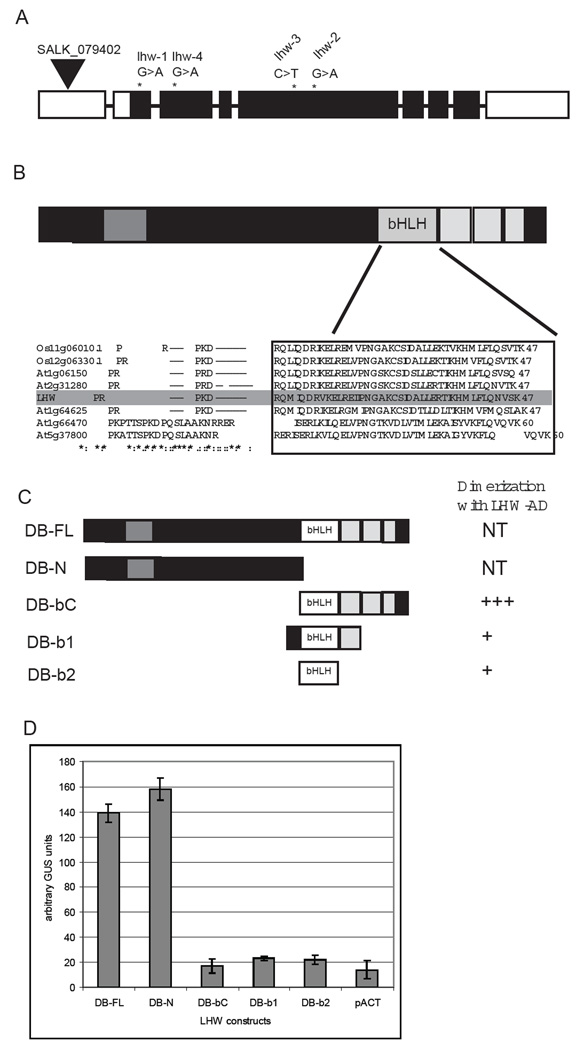
LHW appears to play a central role in defining the number of stele cells. In the root, a variety of biochemical functions have been defined by mutational analysis to be required for patterning the RM and balancing cell proliferation and differentiation. These include: core cell cycle regulators, components of cell signaling and hormone perception, and transcriptional regulators (for example: Aida et al., 2004; Blilou et al., 2002; Blilou et al., 2005; Friml et al., 2002; Mahonen et al., 2006; Sabatini et al., 2003; Wildwater et al., 2005). To understand how LHW might control root development, we used map-based cloning approach and found that LHW corresponds to At2g27230, a locus that encodes a protein of 650 amino acids (see methods). Initial searches of databases with LHW revealed that it was a plant specific protein of unknown function. LHW is closely related to three other uncharacterized proteins in Arabidopsis (encoded by At1g06150, At1g64625 and At2g31280) and two proteins in rice (encoded by Os12g06330 and Os11g06010). The highest similarities among these proteins are in an N-terminal and a C-terminal region (Fig. 4B and Fig. S4 in supplementary material). Although the N-terminal region does not resemble any domains of known biochemical function, part of the C-terminal domain is weakly similar to basic helix-loop-helix (bHLH) transcription factors (Fig. 4B). Alignments of LHW with typical bHLHs (At1g66470 and At5g37800) revealed that this similarity is most convincing in the predicted dimerization domain (boxed in Fig. 4B), however, the canonical DNA-contacting residues are not conserved in LHW, and LHW was not considered a bHLH by two independent groups doing a comprehensive analysis of the family (Heim et al., 2003; Toledo-Ortiz et al., 2003).
Figure 4. LHW gene and protein structure and behavior in two hybrid screen.
(A) LHW gene structure with exons represented as boxes and introns as lines. LHW coding region is in black. The location and nature of lhw mutant alleles is indicated above the exons. (B) LHW protein structure. Two domains conserved with other plant proteins are indicated as grey boxes. Part of the C-terminal conserved region resembles the bHLH domain of transcriptional regulators. A sequence alignment of the putative bHLH domain (boxed) is diagrammed for LHW and related proteins from Arabidopsis and rice. (C) Graphical representation of LHW protein fragments used in yeast two hybrid assay and their ability to dimerize with full length LHW. (D) GAL4 transcriptional activation activity of LHW variants; pACT is included as a negative control. GUS measurements based on 4 replicates/sample. Error bars ± SEM.
Transcriptional activation and HLH dimerization activity of LHW
Proteins in the bHLH class generally interact with DNA and regulate transcription as dimers. They can partner with a variety of protein classes including a class of proteins (the inhibitor of differentiation (Id) proteins) that have HLH dimerization domains but lack a DNA binding domain and antagonize bHLH function (Chen et al., 1996). We examined whether LHW had any properties consistent with it acting as a transcriptional regulator and/or interacting with canonical bHLH proteins.
LHW can activate transcription when fused to a DNA binding domain in a yeast two hybrid assay (DB-FL; Fig. 4C–D). A series of deletion constructs established that the N-terminus (DB-N) was responsible for this activity (Fig. 4D). Neither the bHLH and C-terminal domain (DB-bC), nor the bHLH domain alone (DB-b1 and DB-b2) could activate transcription. To test whether LHW could homodimerize, a non autoactivating portion of the protein (DB-bC) was co-transformed with variants of LHW fused to the GAL4 activation domain (AD). LHW (DB-bC) interacts strongly with full length LHW (AD-FL) and LHW missing the N-terminus (AD-bC) and weakly with versions of LHW containing only the bHLH domain (AD-b1 and AD-b2) (Fig. 4C).
We then performed a two hybrid screen using a library made from seedling cDNA (see methods) to identify other potential partners of LHW. In a screen of 800, 000 colonies, 12 prey constructs interacted with LHW (DB-bC) under stringent conditions. 9 of the clones corresponded to 4 bHLH genes: At5g08130 (5 clones); At1g68810 (2 clones); At1g29950 (1 clone) and At3g25710 (1 clone). This suggests that LHW readily binds to typical bHLH proteins. The bHLH proteins that were identified in the 2-hybrid screen have not been extensively characterized. However, it is interesting that in silico, transcripts for each of these bHLHs are enriched in root tips or found in xylem cell populations (Birnbaum et al., 2005); http://bbc.botany.utoronto.ca/efp
Expression pattern of LHW
The defects seen in lhw mutants suggested that LHW would be required in the RM, particularly in the vascular initials. By semi-quantitative RT-PCR, LHW expression is highest in the meristematic regions of both the root and shoot, and is lowest in mature tissues (Fig. 5F). A transcriptional reporter containing 2.8 KB of sequence 5’ of the start codon fused to GUS was expressed in the root tip including, but not exclusively within, the meristem (Fig. 5C–E). This expression pattern is consistent with root transcriptional profiling data that finds At2g27230 to be enriched in stage 1 (closest to the meristem) roots (Birnbaum et al., 2003) and enriched in the QC cell population relative to other cell types (Nawy et al., 2005).
To determine the subcellular localization of LHW, roots of Arabidopsis stably transformed with 35Spro::LHW-GFP were examined. GFP expression was visible in nuclei (Fig. 5A–B). Expression of these constructs in lhw-1 mutant plants was sufficient to rescue the xylem pole defect in T1s (Fig. 5B), but expression in wild type did not result in any obvious phenotypes in root length, vasculature or overall plant morphology in T1 plants (not shown). Silencing of the LHW transgene was often observed in T2 lines as a reduction in GFP expression and the appearance of a single xylem pole in plants with a wild type genomic copy of LHW (not shown).
Analysis of the requirement for LHW in root meristem maintenance
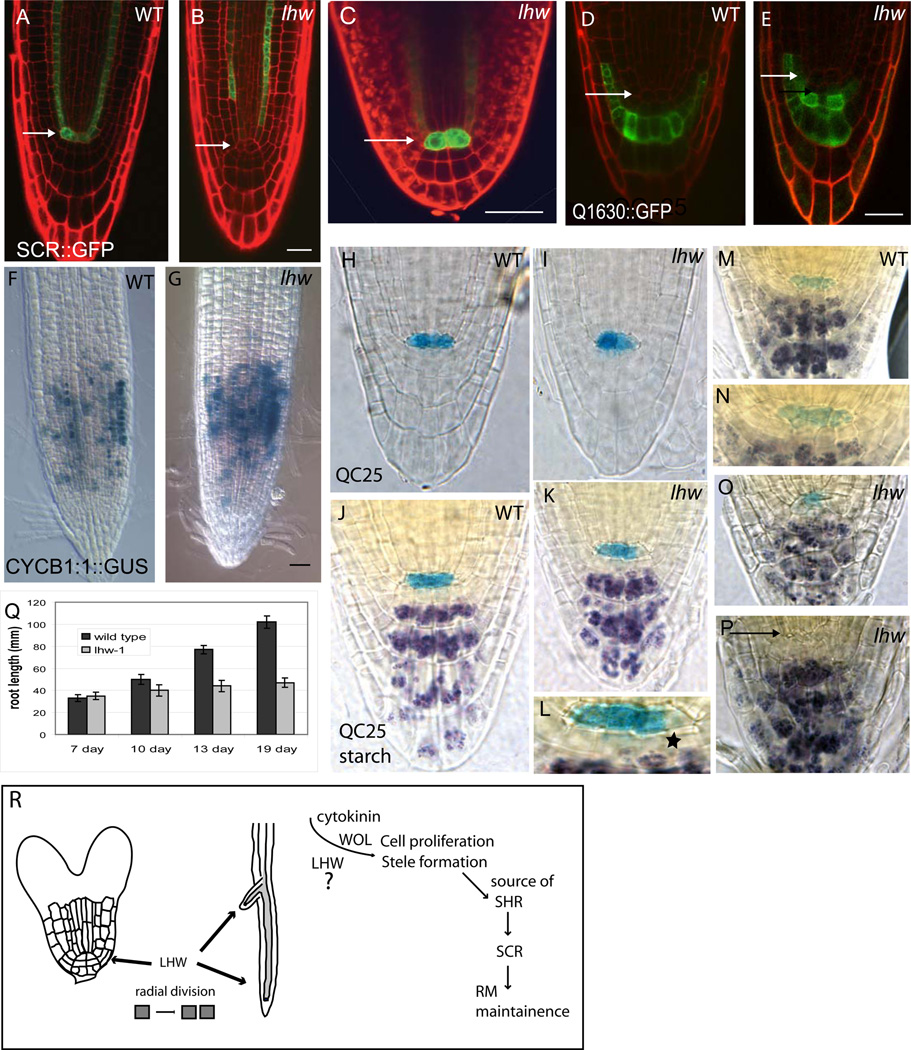
Given its identity, activities and expression pattern, we hypothesized that LHW was required in the meristem to promote cell divisions that establish the normal size of the stele. Similar roles are played by transcriptional regulators such as SCR and SHR that are important for regulating both radial and longitudinal growth. SCR is normally expressed in the QC, endodermal/cortex initials and in the maturing endodermis of the root (Di Laurenzio et al., 1996). Mosaic analysis revealed that SCR has a cell autonomous role in maintaining the QC (Heidstra et al., 2004; Sabatini et al., 2003). When we examined the expression of SCRpro::GFP in 7-dpg lhw, we found, unexpectedly, that it was present in the endodermis, but not in the QC (0/40 lhw plants compared to 38/40 wild type plants; Fig. 6B vs. 6A). In torpedo stage lhw embryos, however, SCRpro::GFP is present in QC cells (Fig. 6C), suggesting that SCR expression is lost over time.
Figure 6. LHW effects on RM meristem establishment and maintenance.
(A–I) Meristem markers in WT and lhw-1 7-dpg seedlings (A–B) SCR::GFP expression (C) SCR::GFP in torpedo stage lhw-1 embryo (D–E) Columella differentiation marker Q1630::GFP. (F–G) CYCB1;2::GUS expression (H–P) QC25::GUS (blue) and starch granules (purple) mark degeneration of lhw meristem over time (H–I; 7dpg, J–L; 13 dpg; M–P; 18 dpg). L is higher magnification image of K; start indicates starch granule containing cells adjacent to QC25 marked cells. N is higher magnification of M. (Q) Graph of WT (dark grey) and lhw-1 (light grey) root growth over time. For each marker, the WT and lhw image pair are the same magnification. Arrows point to QC cells. (R) Model for LHW action in generating vascular pattern. LHW is required to establish the radial extent of the root vascular tissues in the embryo and promotes postembryonic divisions in these tissues. LHW therefore acts as a meristem size-control protein for the center of the root. LHW and WOL are both required for these cell divisions, but appear to act at least somewhat independently. We propose that the eventual slowing down of longitudinal growth in lhw mutant roots is not due to direct requirement for LHW in meristem maintenance, but because LHW is required to create the tissue that normally produce SHR. Without adequate levels of SHR, SCR is not maintained in the QC and meristems eventually terminate. Each image pair (A-K and M, O, P) at the same magnification. Scale bars = 30 µm. Error bars in Q ± SEM
Loss of SCR in the QC is reminiscent of the defects in HOBBIT (Blilou et al., 2002) and SHR (Helariutta et al., 2000) both required for meristem maintenance. The arrangement of SCRpro::GFP expressing cells in lhw was also similar to that in roots provided with only endodermal expression of SCR (Sabatini et al., 2003). Because roots lacking SCR in the QC often have a compromised RM (Sabatini et al., 2003), we examined several other markers of “meristem health” in lhw including expression of QC identity markers, the longitudinal extent of the zone of proliferation and whether lhw roots exhibit determinate growth.
In WT plants, QC25::GUS is expressed specifically in the QC (Sabatini et al., 2003). At 7-dpg, lhw mutants express QC25::GUS, but interestingly, the intensity of GUS expression in the QC cells is often asymmetric (14/20 lhw plants vs. 0/10 WT; (Fig. 6H–I). The intensity of staining does not appear to be correlated with the side on which protoxylem forms (not shown). Despite this asymmetry, at 7-dpg, the lhw RM appears to have normal proliferative capacity as assayed by root growth (Fig. 6Q) and presence of columella initials. Columella initials are identified as a layer of cells between the QC and cell expressing columella marker Q1630::GFP and starch granules (Fig. 6 D–E and not shown). Several groups have used the expression of a mitotic cyclin (CYCB1;1 GUS) to measure the longitudinal extent of the proliferative zone (for example, Aida et al., 2004; Hutchison et al., 2006; Ioio et al., 2007). At 7-dpg, the region of CYCB1;1pro::GUS expressing cells is similar in WT and lhw (Fig. 6F–G; 119 µm ± 1.6 SD in WT n = 11; 125 µm ± 1.73 in lhw-1 n = 10). Together, these data suggest that LHW is not required for the establishment of a functioning RM.
Despite the normal early development, maintenance of the lhw RM fails over time. At 13 days, QC25::GUS is still expressed (Fig.6 J vs. K), but columella initials begin to differentiate and contain starch grains (star in Fig 6L). At 17 dpg, the meristem of lhw roots is visibly disorganized. In contrast to WT roots (Fig 6 M–N), lhw roots exhibit a variety of defects including a failure to express QC25::GUS (5/10; Fig. 6P), loss of columella initials (6/10; Fig 6O) and grossly abnormal QC morphology (4/10; Fig 6O). The RM abnormalities correlate with decreased growth; beginning approximately 10-dpg, lhw root growth slows relative to wild type and by 19 days, lhw primary roots cease growing (Fig. 6Q).
DISCUSSION
We have identified, characterized and cloned a new regulator of development in Arabidopsis. LHW positively regulates the size of the stele cell population and is required to establish the normal diarch pattern of root vascular tissues. One of the most striking aspects of the lhw mutant phenotype is that the vascular cylinder is not just reduced, but that lhw roots seem to have a new “set point” for the number of cells in the stele that, in the mature zone, is consistently half of the wild type number. All lhw primary and lateral roots produce single files of protoxylem, metaxylem, phloem, and lateral-root producing pericycle cells. Size and symmetry can be mechanistically connected when pattern is generated through inhibitory signals from differentiating tissues or cells. The organization of vascular tissues in plants has been hypothesized to result from both feed-forward mechanisms that promote the formation of continuous vascular strands (canalization) and lateral inhibition that creates spaces between the strands (reviewed in Turner and Sieburth, 2002). If this hypothesis is correct, then it suggests that LHW is required only for cell production, as the lhw mutants leave the lateral inhibition system intact.
LHW exhibits several characteristics consistent with it being a transcription factor; it is likely, therefore, to play a regulatory role upstream in a division/differentiation pathway. It is somewhat mysterious how mutations in such a factor consistently reduce the size of the stele cell population to half that of WT. Several possible ways to account for this are: (1) the described alleles of LHW only partially reduce function; (2) LHW paralogues partially compensate for function and/or; (3) additional inputs from unrelated transcription factors or signaling systems contribute to stele size. We think it unlikely that the lhw mutations are partial loss of function because at least six LHW alleles exhibit identical phenotypes and two of these mutations (lhw-1 and SALK_079402) are expected to produce no functional protein. In silico expression patterns of LHW paralogues At1g06150, At1g64625 and At2g31280 are consistent with these genes playing a role in root development, however, no single mutant phenotypes have been observed for T-DNA insertion alleles of these genes (DCB, unpublished). It is still possible that multiple mutant combinations might reveal the role of these genes in relation to LHW and root development.
If stele cell number is controlled by LHW in parallel with other factors, then cytokinin is a likely candidate. Cytokinin signaling is required for repression of xylem differentiation and promotion of stele cell proliferation (Mahonen et al., 2006; Mahonen et al., 2006). Like cytokinin, LHW is required to promote cell proliferation in the stele, however, LHW is also required to promote protoxylem formation--a combination of phenotypes inconsistent with a simple loss or gain of cytokinin production or response. In addition, lhw-1;wol-1 roots have significantly fewer stele cells in the mature zone than lhw mutants. The interpretation of this genetic result is complicated because WOL is one of three cytokinin receptors required for root vascular development (Higuchi et al., 2004; Mahonen et al., 2006). The wol1-1 mutation has been reported to mimic the loss of all three receptors in root vascular development (Mahonen et al., 2006; Mahonen et al., 2006); if wol-1 eliminates cytokinin perception, then LHW and cytokinin are likely to be two of several inputs that promote proliferation of the stele independently.
We interpret the appearance of a smaller provascular region in the lhw embryo and young lateral roots as meaning that the primary role of LHW is to produce the wild type number of stele initial cells in the radial direction. However, we also demonstrated that LHW is required to maintain growth in the longitudinal direction. LHW could have a direct or indirect role in maintaining the RM. In contrast to other root patterning mutants that exhibit a clear “short root” phenotype lhw mutant roots are not noticeably shorter than wild type until 10-dpg. Abnormalities in the QC cells, however, precede this growth defect, and previous studies have shown that the self-renewing properties of the RM initials are maintained through interactions with the QC cells (Aida et al., 2004; Sabatini et al., 2003; van den Berg et al., 1997; Wildwater et al., 2005). By 5-dpg, lhw roots fail to express SCR in the QC; by 7-dpg, the majority of lhw roots express QC25::GUS asymmetrically and a small fraction exhibit and morphological abnormalities in the QC cells. That SCR is missing from the QC earlier than other markers could indicate a specific requirement for LHW to promote expression of this gene. Alternatively, LHW might be indirectly required for the RM via its effects on SHR production. Disappearance of SCR from the QC is seen in reduction of function mutations of SHR (Sabatini et al., 2003). Because LHW acts early to establish the number of cells in radial direction of the stele and SHR RNA is produced exclusively in the stele, the loss of SCR and gradual slowing of root growth in lhw may be due to the reduction of the SHR source (Fig. 6R).
In the future, several lines of inquiry may illuminate whether LHW play an indirect or direct role in RM maintenance. For example, when RETINOBLASTOMA-RELATED function is inactivated specifically in the meristem (rBRr), a larger RM is created (Wildwater et al., 2005). If rRBR can rescue lhw stele size, pattern and premature termination, then it is likely that LHW acts through this cell cycle controller to reach the balance of cells in the stele, and lhw effects on longitudinal growth are largely indirect. If pattern and size are rescued, but the meristem terminates, then LHW may have a direct and independent role in creating and maintaining a functional stem cell pool in the root.
LHW is the first characterized member of a clade of proteins that represent potential transcriptional regulators in Arabidopsis and in other plants including rice, a monocot, and poplar, a woody species. Root architecture is significantly different between monocots and dicots; therefore it would be particularly interesting to see whether LHW orthologues retain a similar role in promoting vascular proliferation, and how this role manifests itself in a structurally diverse root system. As LHW represents a xylem promoting factor, its potential for promoting growth in a woody plant system might prove valuable in wood and biofuel production.
Supplementary Material
Supplemental Figure 1: Montages of sections through lhw and WT roots
Original confocal sections used in the stacked images provided in the text. In each pair, propidium iodide is on left and J0121::GFP is on right. (A–B) WT, from Fig. 1B; (C–D) lhw, from Fig. 1C; (E–F) lhw treated with 2,4 D, from Fig. 3C.
Supplemental Figure 2: Embryo and lateral root development in lhw and wild type
(A–H) DIC images of cleared embryos. (A–B) early globular embryo of WT (A) and lhw-1 (B); (C–D) late globular WT (C) and lhw-1 (D); (E–F) late heart of WT (E) and lhw-1 (F). (G–H) Higher magnification image of basal pole of embryo showing divisions in WT (white arrows in G) but no cell divisions in lhw-1 (H). (I–J) Confocal images of torpedo-stage WT (I) and lhw-1 (J) embryos. The width of vascular cylinder is indicated by white (WT) and yellow (lhw-1) bars. Both bars supplied for comparison in J. (K–P) WT and lhw-1 lateral root primordial expressing CYCB1;1::GUS (blue). (K–N) Comparison of longitudinal extent of lateral root initiation (stages as in (Malamy and Benfey, 1997), stage 1 (K–L) and stage 2 (M–N). (O–P) Head on view of stage 1–2 lateral root showing three contiguous files (black asterisks) undergoing divisions in both WT (O) and lhw-1 (P). Each staged WT and lhw image pair is at same magnification.
Figure supplemental 3: Comparison of WT and lhw root growth inhibition
Graph of root length at 7-dpg in response to germination and growth on increasing 2,4D concentrations. WT (open diamonds) and lhw-1 (filled squares).
Supplemental figure 4: ClustalW alignment of entire LHW protein with relatives from Arabidopsis and Rice.
ACKNOWLEDGMENTS
The authors wish to thank J. Long (SALK, CA), B. Scheres (U. Utrecht), Y. Helariutta (U. Helsinki) and the ABRC stock center for providing materials. We thank members of the lab and colleagues Wolfgang Lukowitz, Kathy Barton and Matt Evans for helpful discussions and/or comments on the manuscript, Cora MacAlister for statistics consulting, and Chris Somerville for support in the initial stages of the project. This work was supported by funds from US-DOE-FG02-03ER20133 to Chris Somerville and Ruth L. Kirschstein NRSA fellowship (5F32GM064273-03) and Stanford University funds to DCB.
References
- Aida M, Beis D, Heidstra R, Willemsen V, Blilou I, Galinha C, Nussaume L, Noh YS, Amasino R, Scheres B. The PLETHORA Genes Mediate Patterning of the Arabidopsis Root Stem Cell Niche. Cell. 2004;119:109–120. doi: 10.1016/j.cell.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Birnbaum K, Jung JW, Wang JY, Lambert GM, Hirst JA, Galbraith DW, Benfey PN. Cell Type-Specific Expression Profiling in Plants Via Cell Sorting of Protoplasts from Fluorescent Reporter Lines. Nat. Methods. 2005;2:615–619. doi: 10.1038/nmeth0805-615. [DOI] [PubMed] [Google Scholar]
- Birnbaum K, Shasha DE, Wang JY, Jung JW, Lambert GM, Galbraith DW, Benfey PN. A Gene Expression Map of the Arabidopsis Root. Science. 2003;302:1956–1960. doi: 10.1126/science.1090022. [DOI] [PubMed] [Google Scholar]
- Blilou I, Frugier F, Folmer S, Serralbo O, Willemsen V, Wolkenfelt H, Eloy NB, Ferreira PC, Weisbeek P, Scheres B. The Arabidopsis HOBBIT Gene Encodes a CDC27 Homolog that Links the Plant Cell Cycle to Progression of Cell Differentiation. Genes Dev. 2002;16:2566–2575. doi: 10.1101/gad.237302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heidstra R, Aida M, Palme K, Scheres B. The PIN Auxin Efflux Facilitator Network Controls Growth and Patterning in Arabidopsis Roots. Nature. 2005;433:39–44. doi: 10.1038/nature03184. [DOI] [PubMed] [Google Scholar]
- Bonke M, Thitamadee S, Mahonen AP, Hauser MT, Helariutta Y. APL Regulates Vascular Tissue Identity in Arabidopsis. Nature. 2003;426:181–186. doi: 10.1038/nature02100. [DOI] [PubMed] [Google Scholar]
- Chen CM, Kraut N, Groudine M, Weintraub H. I-Mf, a Novel Myogenic Repressor, Interacts with Members of the MyoD Family. Cell. 1996;86:731–741. doi: 10.1016/s0092-8674(00)80148-8. [DOI] [PubMed] [Google Scholar]
- Clay NK, Nelson T. VH1, a Provascular Cell-Specific Receptor Kinase that Influences Leaf Cell Patterns in Arabidopsis. Plant Cell. 2002;14:2707–2722. doi: 10.1105/tpc.005884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral Dip: A Simplified Method for Agrobacterium-Mediated Transformation of Arabidopsis Thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Colon-Carmona A, You R, Haimovitch-Gal T, Doerner P. Technical Advance: Spatio-Temporal Analysis of Mitotic Activity with a Labile Cyclin-GUS Fusion Protein. Plant J. 1999;20:503–508. doi: 10.1046/j.1365-313x.1999.00620.x. [DOI] [PubMed] [Google Scholar]
- Cutler SR, Ehrhardt DW, Griffitts JS, Somerville CR. Random GFP::CDNA Fusions Enable Visualization of Subcellular Structures in Cells of Arabidopsis at a High Frequency. Proc. Natl. Acad. Sci. U. S. A. 2000;97:3718–3723. doi: 10.1073/pnas.97.7.3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon BG, Zorrilla JM, Rubio V, Dahiya P, Paz-Ares J, Leyva A. Interallelic Complementation at the Arabidopsis CRE1 Locus Uncovers Independent Pathways for the Proliferation of Vascular Initials and Canonical Cytokinin Signalling. Plant J. 2004;38:70–79. doi: 10.1111/j.1365-313X.2004.02023.x. [DOI] [PubMed] [Google Scholar]
- Di Laurenzio L, Wysocka-Diller J, Malamy JE, Pysh L, Helariutta Y, Freshour G, Hahn MG, Feldmann KA, Benfey PN. The SCARECROW Gene Regulates an Asymmetric Cell Division that is Essential for Generating the Radial Organization of the Arabidopsis Root. Cell. 1996;86:423–433. doi: 10.1016/s0092-8674(00)80115-4. [DOI] [PubMed] [Google Scholar]
- Dolan L, Janmaat K, Willemsen V, Linstead P, Poethig S, Roberts K, Scheres B. Cellular Organisation of the Arabidopsis Thaliana Root. Development. 1993;119:71–84. doi: 10.1242/dev.119.1.71. [DOI] [PubMed] [Google Scholar]
- Donnelly PM, Bonetta D, Tsukaya H, Dengler RE, Dengler NG. Cell Cycling and Cell Enlargement in Developing Leaves of Arabidopsis. Dev. Biol. 1999;215:407–419. doi: 10.1006/dbio.1999.9443. [DOI] [PubMed] [Google Scholar]
- Ferreira FJ, Kieber JJ. Cytokinin Signaling. Current Opinion in Plant Biology. 2005/10;8:518–525. doi: 10.1016/j.pbi.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Friml J, Benkova E, Blilou I, Wisniewska J, Hamann T, Ljung K, Woody S, Sandberg G, Scheres B, Jurgens G, et al. AtPIN4 Mediates Sink-Driven Auxin Gradients and Root Patterning in Arabidopsis. Cell. 2002;108:661–673. doi: 10.1016/s0092-8674(02)00656-6. [DOI] [PubMed] [Google Scholar]
- Heidstra R, Welch D, Scheres B. Mosaic Analyses using Marked Activation and Deletion Clones Dissect Arabidopsis SCARECROW Action in Asymmetric Cell Division. Genes Dev. 2004;18:1964–1969. doi: 10.1101/gad.305504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim MA, Jakoby M, Werber M, Martin C, Weisshaar B, Bailey PC. The Basic Helix-Loop-Helix Transcription Factor Family in Plants: A Genome-Wide Study of Protein Structure and Functional Diversity. Mol. Biol. Evol. 2003;20:735–747. doi: 10.1093/molbev/msg088. [DOI] [PubMed] [Google Scholar]
- Helariutta Y, Fukaki H, Wysocka-Diller J, Nakajima K, Jung J, Sena G, Hauser MT, Benfey PN. The SHORT-ROOT Gene Controls Radial Patterning of the Arabidopsis Root through Radial Signaling. Cell. 2000;101:555–567. doi: 10.1016/s0092-8674(00)80865-x. [DOI] [PubMed] [Google Scholar]
- Higuchi M, Pischke MS, Mahonen AP, Miyawaki K, Hashimoto Y, Seki M, Kobayashi M, Shinozaki K, Kato T, Tabata S, et al. In Planta Functions of the Arabidopsis Cytokinin Receptor Family. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8821–8826. doi: 10.1073/pnas.0402887101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison CE, Li J, Argueso C, Gonzalez M, Lee E, Lewis MW, Maxwell BB, Perdue TD, Schaller GE, Alonso JM, et al. The Arabidopsis Histidine Phosphotransfer Proteins are Redundant Positive Regulators of Cytokinin Signaling. Plant Cell. 2006;18:3073–3087. doi: 10.1105/tpc.106.045674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Higuchi M, Hashimoto Y, Seki M, Kobayashi M, Kato T, Tabata S, Shinozaki K, Kakimoto T. Identification of CRE1 as a Cytokinin Receptor from Arabidopsis. Nature. 2001;409:1060–1063. doi: 10.1038/35059117. [DOI] [PubMed] [Google Scholar]
- Ioio RD, Linhares FS, Scacchi E, Casamitjana-Martinez E, Heidstra R, Costantino P, Sabatini S. Cytokinins Determine Arabidopsis Root-Meristem Size by Controlling Cell Differentiation. Curr. Biol. 2007;17:678–682. doi: 10.1016/j.cub.2007.02.047. [DOI] [PubMed] [Google Scholar]
- Kidner C, Sundaresan V, Roberts K, Dolan L. Clonal Analysis of the Arabidopsis Root Confirms that Position, Not Lineage, Determines Cell Fate. Planta. 2000;211:191–199. doi: 10.1007/s004250000284. [DOI] [PubMed] [Google Scholar]
- Laplaze L, Parizot B, Baker A, Ricaud L, Martiniere A, Auguy F, Franche C, Nussaume L, Bogusz D, Haseloff J. GAL4-GFP Enhancer Trap Lines for Genetic Manipulation of Lateral Root Development in Arabidopsis Thaliana. J. Exp. Bot. 2005;56:2433–2442. doi: 10.1093/jxb/eri236. [DOI] [PubMed] [Google Scholar]
- Lukowitz W, Gillmor CS, Scheible WR. Positional Cloning in Arabidopsis. Why it Feels Good to have a Genome Initiative Working for You. Plant Physiol. 2000;123:795–805. doi: 10.1104/pp.123.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahonen AP, Bishopp A, Higuchi M, Nieminen KM, Kinoshita K, Tormakangas K, Ikeda Y, Oka A, Kakimoto T, Helariutta Y. Cytokinin Signaling and its Inhibitor AHP6 Regulate Cell Fate during Vascular Development. Science. 2006;311:94–98. doi: 10.1126/science.1118875. [DOI] [PubMed] [Google Scholar]
- Mahonen AP, Bonke M, Kauppinen L, Riikonen M, Benfey PN, Helariutta Y. A Novel Two-Component Hybrid Molecule Regulates Vascular Morphogenesis of the Arabidopsis Root. Genes Dev. 2000;14:2938–2943. doi: 10.1101/gad.189200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahonen AP, Higuchi M, Tormakangas K, Miyawaki K, Pischke MS, Sussman MR, Helariutta Y, Kakimoto T. Cytokinins Regulate a Bidirectional Phosphorelay Network in Arabidopsis. Curr. Biol. 2006;16:1116–1122. doi: 10.1016/j.cub.2006.04.030. [DOI] [PubMed] [Google Scholar]
- Malamy JE, Benfey PN. Organization and Cell Differentiation in Lateral Roots of Arabidopsis Thaliana. Development. 1997;124:33–44. doi: 10.1242/dev.124.1.33. [DOI] [PubMed] [Google Scholar]
- Mattsson J, Sung ZR, Berleth T. Responses of Plant Vascular Systems to Auxin Transport Inhibition. Development. 1999;126:2979–2991. doi: 10.1242/dev.126.13.2979. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Sena G, Nawy T, Benfey PN. Intercellular Movement of the Putative Transcription Factor SHR in Root Patterning. Nature. 2001;413:307–311. doi: 10.1038/35095061. [DOI] [PubMed] [Google Scholar]
- Nawy T, Lee JY, Colinas J, Wang JY, Thongrod SC, Malamy JE, Birnbaum K, Benfey PN. Transcriptional Profile of the Arabidopsis Root Quiescent Center. Plant Cell. 2005;17:1908–1925. doi: 10.1105/tpc.105.031724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura C, Ohashi Y, Sato S, Kato T, Tabata S, Ueguchi C. Histidine Kinase Homologs that Act as Cytokinin Receptors Possess Overlapping Functions in the Regulation of Shoot and Root Growth in Arabidopsis. Plant Cell. 2004;16:1365–1377. doi: 10.1105/tpc.021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini S, Heidstra R, Wildwater M, Scheres B. SCARECROW is Involved in Positioning the Stem Cell Niche in the Arabidopsis Root Meristem. Genes Dev. 2003;17:354–358. doi: 10.1101/gad.252503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres B, Di Laurenzio L, Willemsen V, Hauser MT, Janmaat K, Weisbeek P, Benfey PN. Mutations Affecting the Radial Organisation of the Arabidopsis Root Display Specific Defects Throughout the Embryonic Axis. Development. 1995;121:53–62. [Google Scholar]
- Toledo-Ortiz G, Huq E, Quail PH. The Arabidopsis basic/helix-Loop-Helix Transcription Factor Family. Plant Cell. 2003;15:1749–1770. doi: 10.1105/tpc.013839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrey JG. On the Determination of Vascular Patterns during Tissue Differentiation in Excised Pea Roots. Am. J. Bot. 1955;42:183–198. [Google Scholar]
- Turner S, Sieburth LE. Vascular Patterning. The Arabidopsis Book. 2002 doi: 10.1199/tab.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ. Aux/IAA Proteins Repress Expression of Reporter Genes Containing Natural and Highly Active Synthetic Auxin Response Elements. Plant Cell. 1997;9:1963–1971. doi: 10.1105/tpc.9.11.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg C, Willemsen V, Hendriks G, Weisbeek P, Scheres B. Short-Range Control of Cell Differentiation in the Arabidopsis Root Meristem. Nature. 1997;390:287–289. doi: 10.1038/36856. [DOI] [PubMed] [Google Scholar]
- Wildwater M, Campilho A, Perez-Perez JM, Heidstra R, Blilou I, Korthout H, Chatterjee J, Mariconti L, Gruissem W, Scheres B. The RETINOBLASTOMA-RELATED Gene Regulates Stem Cell Maintenance in Arabidopsis Roots. Cell. 2005;123:1337–1349. doi: 10.1016/j.cell.2005.09.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Montages of sections through lhw and WT roots
Original confocal sections used in the stacked images provided in the text. In each pair, propidium iodide is on left and J0121::GFP is on right. (A–B) WT, from Fig. 1B; (C–D) lhw, from Fig. 1C; (E–F) lhw treated with 2,4 D, from Fig. 3C.
Supplemental Figure 2: Embryo and lateral root development in lhw and wild type
(A–H) DIC images of cleared embryos. (A–B) early globular embryo of WT (A) and lhw-1 (B); (C–D) late globular WT (C) and lhw-1 (D); (E–F) late heart of WT (E) and lhw-1 (F). (G–H) Higher magnification image of basal pole of embryo showing divisions in WT (white arrows in G) but no cell divisions in lhw-1 (H). (I–J) Confocal images of torpedo-stage WT (I) and lhw-1 (J) embryos. The width of vascular cylinder is indicated by white (WT) and yellow (lhw-1) bars. Both bars supplied for comparison in J. (K–P) WT and lhw-1 lateral root primordial expressing CYCB1;1::GUS (blue). (K–N) Comparison of longitudinal extent of lateral root initiation (stages as in (Malamy and Benfey, 1997), stage 1 (K–L) and stage 2 (M–N). (O–P) Head on view of stage 1–2 lateral root showing three contiguous files (black asterisks) undergoing divisions in both WT (O) and lhw-1 (P). Each staged WT and lhw image pair is at same magnification.
Figure supplemental 3: Comparison of WT and lhw root growth inhibition
Graph of root length at 7-dpg in response to germination and growth on increasing 2,4D concentrations. WT (open diamonds) and lhw-1 (filled squares).
Supplemental figure 4: ClustalW alignment of entire LHW protein with relatives from Arabidopsis and Rice.