Abstract
Although individuals with obesity and type 2 diabetes are insulin resistant, pancreatic beta cell failure is the core defect that distinguishes individuals who eventually develop diabetes. This process is known to occur well before the onset of hyperglycemia. Although clinical trial data support the effectiveness of intensive lifestyle modification in delaying the onset of diabetes in obese subjects, less is known about the effects of and mechanisms underlying bariatric surgery, particularly gastric bypass surgery, on diabetes. The paper under evaluation clarifies the role of both lifestyle intervention and gastric bypass surgery on pancreatic beta cell function and raises questions regarding the role of weight loss versus incretin related mechanisms on recovery of beta cell failure.
Keywords: obesity, type 2 diabetes, gastric bypass surgery, weight loss, lifestyle modification, beta cell function, insulin resistance
The current pandemic of type 2 diabetes and obesity has created an urgent need to identify effective therapeutic interventions targeting both of these chronic debilitating conditions. Obesity and diabetes are closely interrelated in that risk factors such as physical inactivity and poor diet lead to weight gain and precipitate insulin resistance in important insulin sensitive tissues, particularly skeletal muscle, liver, and adipose tissue. It is known that obese and insulin resistant diabetic patients have a positive energy balance, high fat and high carbohydrate intake, increased abdominal adipose tissue, elevated free fatty acids, increased secretory products of adipocytes mediating inflammation including tumor necrosis factor alpha (TNFα) and interleukin-6 (IL-6), and reduced secretion of adiponectin (1,2). These factors have been shown to be part of the underlying mechanisms of glucose intolerance and contribute to reduced skeletal muscle glucose disposal and increased hepatic glucose output (1). Although insulin resistance is common in obese patients, diabetes is not always present because the pancreas augments insulin production as a way to offset the severity of impaired insulin action. The transition from a mild state of insulin resistance to type 2 diabetes is heralded by progressive pancreatic beta cell dysfunction and eventual failure to secrete adequate amounts of insulin; all of this occurs well before the inception of diabetes. In obese individuals with a familial tendency for type 2 diabetes, impaired insulin secretion is documented early on in the spectrum of glucose tolerance. In certain high risk populations, up to 80% of pancreatic beta cell failure is observed to occur during the fasting state and manifests in a condition now known as impaired fasting glucose (1). According to Defronzo and colleagues, as fasting hyperglycemia develops insulin secretion decreases progressively, and in patients with fasting glucose levels of 180–200 mg/dl, there is an absolute deficiency of insulin (3). Some major non-heritable factors implicated in beta cell failure, particularly in obesity, include elevated free fatty acids and inflammatory cytokines, i.e., “lipotoxocity” (2), and incretin deficiency and/or resistance (4).
Modest weight loss of 5–10% body weight is known to improve diabetes by reducing insulin resistance in obese individuals (5). In clinical trials, caloric restriction, exercise, and weight loss have been shown to prevent and reduce diabetes in obese individuals (5,6) in part by attenuating insulin resistance and subsequent hyperinsulinemia, thereby preserving beta cell function (7,8). It has also been shown that weight loss surgery, particularly gastric bypass surgery, has a profound effect on metabolism and can induce remission of type 2 diabetes defined by normal glycemic control without the need for diabetic medications (9). The fundamental questions brought forth regarding these latter observations include: Can weight loss effectively reverse pancreatic beta cell dysfunction? If so, which specific therapeutic weight loss modality (ie. lifestyle, pharmacotherapy or surgery) produces the most effective and sustained reversal of pancreatic beta cell dysfunction in individuals with diabetes or a predisposition towards diabetes?
Summary of methods and results
Hofso et al. (10) compared the effects of either lifestyle intervention or gastric bypass surgery on pancreatic beta cell function in subjects with morbid obesity (mean BMI 45.5 kg/m2) without known diabetes. At the time of baseline testing, some subjects were found to have either impaired fasting glucose, impaired glucose tolerance, or diabetes. Subjects self-selected (thus not randomized) their treatment options, either Roux-en-Y gastric bypass (RYGB) surgery or lifestyle. The lifestyle group engaged in a program of organized physical activity and psychosocial intervention with inpatient stays totaling 7 weeks. A standard glucose tolerance test was performed before and at 1 year of follow-up. Insulin sensitivity was assessed by HOMA, and insulin secretion was measured by the insulinogenic index and the Stumvoll first phase index during a standard oral glucose tolerance test. Beta cell function was determined from the disposition index and the proinsulin/insulin ratio. Mean weight loss following gastric bypass was 30%, and 9% in the lifestyle group. Glucose intolerance was resolved in all patients who underwent RYGB and in 41% of those who followed the lifestyle intervention. However, it is noteworthy that post-challenge hypoglycemia was significantly more prevalent after RYGB. Insulin sensitivity was significantly improved after both interventions, but the magnitude of improvement was greater after surgery. Overall insulin secretion was reduced after both interventions, but there was an increase in the early-phase insulin secretion after surgery. Looking at the study population as a whole, percentage weight change was correlated with the change in the disposition index. The change in the disposition index was also correlated with the change in proinsulin/insulin ratio. The authors conclude that these physiological changes may contribute to restored glycemic control following bariatric surgery, but may also in part be responsible for the higher incidence of post-prandial hypoglycemia among the surgery group.
Discussion/Significance of Results
The authors are to be commended for conducting this relatively large clinical trial examining both intensive lifestyle and surgical approaches in morbidly obese subjects, since these strategies represent two important treatment modalities for weight loss, and share common physiological outcomes of improved insulin sensitivity and insulin secretion. As acknowledged by the authors the main strengths of their study includes the controlled design and the intensiveness of the lifestyle approach. Previous studies of bariatric surgery for diabetes have been primarily observational with the lack of appropriate control groups that encompass non-surgical/medical weight loss. However, a major weakness when comparing two groups that are not randomized is that differences in baseline characteristics may account for different outcomes. For example, the surgical group was substantially younger and heavier than the lifestyle group. However, despite the difference in baseline characteristics, the prevalence of glucose intolerance and baseline levels of insulin sensitivity and secretion were similar between the two groups. A second weakness pertains to the failure to examine the incretin hormone hypothesis, which is considered an important mechanism underlying improvement in beta cell function, particularly after obesity surgery. Other weaknesses include the use of oral glucose tolerance test derived indices for insulin sensitivity and beta cell function since these have not been validated for bariatric surgery, the lack of stratification and separate analysis of normal glucose tolerant, impaired glucose tolerant, and type 2 diabetic patients, and the inclusion of primarily Caucasian subjects. As expected, surgery resulted in a three-fold greater weight loss and 1.5-fold greater improvement in insulin sensitivity as compared to the lifestyle intervention. Other studies have observed similar improvements in insulin sensitivity, particularly in skeletal muscle, after gastric bypass surgery (11–13). The data reported by Hofso and colleagues is consistent with these studies, and is supportive of previously published work from our group in which we examined the effects of gastric bypass and gastric restrictive surgery in patients with type 2 diabetes (14). In that study we noted a markedly greater increase in insulin sensitivity (determined during hyperglycemic clamp studies) with gastric bypass versus other gastric restrictive procedures at 1 month after surgery when both groups had lost an equivalent amount of body weight (~10%). Whether these effects are related to reduced intra- or extra-myocellular lipid, lipid mobilization, or changes in lipid species such as ceramides, all of which have previously been linked with impaired insulin action in obesity, is currently not clear. More studies like those of Hofso are needed to identify the specific mechanistic defects that are being targeted by surgery and/or lifestyle-induced weight loss.
Expert Commentary
The two fold plus increase in the disposition index in normal glucose tolerant and abnormal glucose tolerant individuals noted by Hofso et al (10) following gastric bypass surgery is of tremendous scientific interest helps to explain the restoration of glucose homeostasis in diabetes. The timing of improved glycemic control following bariatric surgery is important. In most cases, a dramatic decrease in fasting glucose levels is seen within days to weeks following surgery, before major weight loss, but in the setting of enforced caloric restriction (9,15). Persistent cases of diabetes after this initial period gradually improve in parallel with weight loss (16). Patients who undergo malabsorptive procedures improve sooner and maintain glucose control for longer periods than do patients treated by purely restrictive procedures. However, compared with patients following a very low calorie diet who achieve similar weight loss, patients who undergo RYGB experience increased glucose-induced secretion of insulin, c-peptide, glucagon like peptide (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) consistent with an incretin effect (15). When we studied glucose regulation in morbidly obese patients with type 2 diabetes following similar amounts of weight loss related to enforced caloric restriction at one and four weeks following gastric restrictive (sleeve gastrectomy and gastric banding) versus RYGB procedures, we also noted greater improvement in fasting and postprandial glucose levels following RYGB (14). We attributed this to enhanced insulin secretion and beta cell responsiveness. Importantly, we observed this effect in response to a mixed meal, but the effect was not evident during intravenous glucose administration, highlighting a potent and important incretin effect. Greater improvement in insulin sensitivity during glucose infusion was also noted acutely after RYGB but not after gastric restrictive procedures. The incretin effect is characterized by robust increases in GLP-1 and either no change or increases in GIP levels (14,17,18). In subjects with profound hypoglycemia and neuroglycopenia following RYGB, Goldfine et al. (19) observed markedly higher postprandial insulin and GLP-1 levels as compared to those without hypoglycemia following RYGB. Collectively, these data provide insights into the metabolic effects of RYGB to increase insulin sensitivity and preserve beta cell function, and to enhance insulin secretion through an incretin-mediated mechanism. Whether these effects, particularly hypoglycemia following RYGB, are specific to increased GLP-1 or GIP levels are currently under investigation.
Modest improvements in beta cell function following lifestyle intervention as documented by Hofso et al. (10) are consistent with a number of other studies (20–23) as well as our own (24–27). Exercise training has been shown to improve insulin sensitivity independent of weight loss (23). These effects are primarily due to improved insulin action in skeletal muscle leading to increased glucose and lipid oxidation, as well as effects independent of insulin. Dela et al. (21) and others (20,22,23) have provided strong evidence of exercise training-induced increases in beta cell function in response to intravenous glucose in type 2 diabetic and obese subjects. Additionally, some studies have found elevated insulin responses to an oral glucose load after caloric restriction and exercise-induced weight loss, and more recently we demonstrated a possible incretin effect related to GIP that may contribute to increased insulin secretion following a hypocaloric diet and exercise training intervention (27). Following a moderate to high intensity 12-week aerobic exercise program, older obese normal glucose tolerant subjects demonstrated marked improvements in insulin sensitivity and a reduction in fasting and postprandial hyperinsulinemia, which was associated with a decrease in GIP secretion. In contrast, type 2 diabetic individuals showed increases in both insulin secretion and the disposition index. These changes directly corresponded to increased GIP secretion. It is noteworthy that these subjects only had a modest weight-loss of ~5%, similar to levels observed in the study by Hofso.
Alterations in dietary composition also have profound effects on beta cell function. We studied older obese pre-diabetic individuals participating in a moderate to high intensity 12-week aerobic exercise program in combination with isocaloric diets consisting of either high- or low-glycemic index carbohydrates(25). Both groups lost ~9% body weight and had equal improvements in insulin sensitivity and basal insulin secretion rates (ISR), but only the low glycemic diet group reduced glucose-stimulated ISR, and was associated with with a decrease in glucose-stimulated GIP secretion. After correction for improved insulin sensitivity, oral glucose-induced ISR was not different from pre-intervention in the low glycemic diet group, while it was increased in the high glycemic diet group, indicating further beta cell dysfunction. Thus, diet alone may have profound effects on beta cell function and incretin secretion. Considering the findings of these studies, it is evident that incretin hormones play a pivotal role in lifestyle-induced changes in glucose metabolism, and that these changes differ based upon where an individual is on the beta cell dysfunction spectrum. Besides the effect on insulin secretion, incretin hormones induce satiety. This may account for weight loss or maintenance of weight loss following lifestyle interventions in individuals with type 2 diabetes, and may further decrease lipotoxicity, leading to improvements in insulin sensitivity. Alternatively, in nondiabetic or prediabetic populations, lifestyle-induced reductions in incretin hormones may decrease beta cell secretion independent of weight loss.
Five-Year View
Future research in this area will require randomized controlled trials examining the effects of intensive lifestyle modification versus various bariatric procedures on clinical outcomes in patients with type 2 diabetes in the setting of morbid and modest obesity. Feasibility and pilot studies are well underway at several centers. Given the potential role of the intestine in treating type 2 diabetes, future studies will target manipulations (surgical and/or biochemical via pharmacotherapy and lifestyle modifications) of intestinal biology to reverse pancreatic beta cell dysfunction, insulin resistance and cardiovascular risk associated with diabetes. Investigations into less invasive and safer methods for weight loss that achieve similar efficacy and durability following bariatric surgery are underway. Finally, consideration must be given to developing new drugs that mimic the effects of bariatric surgery and produce durable weight loss and remission of type 2 diabetes.
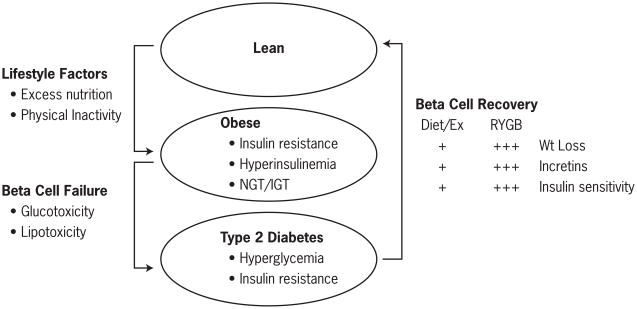
Figure 1.
illustrates the pathophysiology of type 2 diabetes in the setting of obesity. Chronic caloric surplus and physical inactivity leads to obesity and insulin resistance. Beta cell failure is required in this setting to drive the transition from obesity/insulin resistance to type 2 diabetes. Both Roux-en-Y gastric bypass (RYGB) surgery and diet/exercise contribute to beta cell recovery thru weight loss, restoration of incretin function and improvement of insulin sensitivity. Evidently, the magnitude of these effects is far greater following bariatric surgery than diet/exercise interventions represented by the + symbol.
Acknowledgments
SRK and JPK receive grant support from the National Institutes of Health, RO1 DK089547-01 and RO1 AG12834-10, and the American Diabetes Association. ESL was supported by NIH training grant T32DK007319.
Abbreviations
- RYGB
gastric bypass surgery
- GLP-1
glucagon like peptide
- GIP
glucose-dependent insulinotropic polypeptide
Footnotes
Financial and competing interests disclosure
SRK receives research funding from ethicon endo-surgery. JPK receives research funding from Nestle
Reference List
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- **1.Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773–795. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **2.Unger RH. Minireview: weapons of lean body mass destruction: the role of ectopic lipids in the metabolic syndrome. Endocrinology. 2003;144:5159–5165. doi: 10.1210/en.2003-0870. [DOI] [PubMed] [Google Scholar]
- 3.Defronzo RA. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am. 2004;88:787–835. ix. doi: 10.1016/j.mcna.2004.04.013. [DOI] [PubMed] [Google Scholar]
- *4.Vollmer K, Holst JJ, Baller B, Ellrichmann M, Nauck MA, Schmidt WE, Meier JJ. Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes. 2008;57:678–687. doi: 10.2337/db07-1124. [DOI] [PubMed] [Google Scholar]
- *5.Pi-Sunyer X, Blackburn G, Brancati FL, Bray GA, Bright R, Clark JM, Curtis JM, Espeland MA, Foreyt JP, Graves K, Haffner SM, Harrison B, Hill JO, Horton ES, Jakicic J, Jeffery RW, Johnson KC, Kahn S, Kelley DE, Kitabchi AE, Knowler WC, Lewis CE, Maschak-Carey BJ, Montgomery B, Nathan DM, Patricio J, Peters A, Redmon JB, Reeves RS, Ryan DH, Safford M, Van DB, Wadden TA, Wagenknecht L, Wesche-Thobaben J, Wing RR, Yanovski SZ. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30:1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **6.Klein S, Sheard NF, Pi-Sunyer X, Daly A, Wylie-Rosett J, Kulkarni K, Clark NG. Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies: a statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition. Diabetes Care. 2004;27:2067–2073. doi: 10.2337/diacare.27.8.2067. [DOI] [PubMed] [Google Scholar]
- 7.Camastra S, Manco M, Mari A, Baldi S, Gastaldelli A, Greco AV, Mingrone G, Ferrannini E. beta-cell function in morbidly obese subjects during free living: long-term effects of weight loss. Diabetes. 2005;54:2382–2389. doi: 10.2337/diabetes.54.8.2382. [DOI] [PubMed] [Google Scholar]
- *8.Polonsky KS, Gumbiner B, Ostrega D, Griver K, Tager H, Henry RR. Alterations in immunoreactive proinsulin and insulin clearance induced by weight loss in NIDDM. Diabetes. 1994;43:871–877. doi: 10.2337/diab.43.7.871. [DOI] [PubMed] [Google Scholar]
- **9.Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, Barakat HA, deRamon RA, Israel G, Dolezal JM. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222:339–350. doi: 10.1097/00000658-199509000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hofso D, Jenssen T, Bollerslev J, Ueland T, Godang K, Stumvoll M, Sandbu R, Roislien J, Hjelmesaeth J. Beta cell function after weight loss: a clinical trial comparing gastric bypass surgery and intensive lifestyle intervention. Eur J Endocrinol. 2011;164:231–238. doi: 10.1530/EJE-10-0804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V, Shurey S, Ghatei MA, Patel AG, Bloom SR. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243:108–114. doi: 10.1097/01.sla.0000183349.16877.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mingrone G, DeGaetano A, Greco AV, Capristo E, Benedetti G, Castagneto M, Gasbarrini G. Reversibility of insulin resistance in obese diabetic patients: role of plasma lipids. Diabetologia. 1997;40:599–605. doi: 10.1007/s001250050721. [DOI] [PubMed] [Google Scholar]
- 13.Morinigo R, Vidal J, Lacy AM, Delgado S, Casamitjana R, Gomis R. Circulating peptide YY, weight loss, and glucose homeostasis after gastric bypass surgery in morbidly obese subjects. Ann Surg. 2008;247:270–275. doi: 10.1097/SLA.0b013e31815f6e77. [DOI] [PubMed] [Google Scholar]
- *14.Kashyap SR, Daud S, Kelly KR, Gastaldelli A, Win H, Brethauer S, Kirwan JP, Schauer PR. Acute effects of gastric bypass versus gastric restrictive surgery on beta-cell function and insulinotropic hormones in severely obese patients with type 2 diabetes. Int J Obes (Lond) 2009 doi: 10.1038/ijo.2009.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *15.Laferrere B, Teixeira J, McGinty J, Tran H, Egger JR, Colarusso A, Kovack B, Bawa B, Koshy N, Lee H, Yapp K, Olivan B. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93:2479–2485. doi: 10.1210/jc.2007-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *16.Dixon JB, O’Brien PE, Playfair J, Chapman L, Schachter LM, Skinner S, Proietto J, Bailey M, Anderson M. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008;299:316–323. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]
- 17.Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis. 2007;3:597–601. doi: 10.1016/j.soard.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laferrere B, Heshka S, Wang K, Khan Y, McGinty J, Teixeira J, Hart AB, Olivan B. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care. 2007;30:1709–1716. doi: 10.2337/dc06-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **19.Goldfine AB, Mun EC, Devine E, Bernier R, Baz-Hecht M, Jones DB, Schneider BE, Holst JJ, Patti ME. Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. J Clin Endocrinol Metab. 2007;92:4678–4685. doi: 10.1210/jc.2007-0918. [DOI] [PubMed] [Google Scholar]
- 20.Bogardus C, Ravussin E, Robbins DC, Wolfe RR, Horton ES, Sims EA. Effects of physical training and diet therapy on carbohydrate metabolism in patients with glucose intolerance and non-insulin-dependent diabetes mellitus. Diabetes. 1984;33:311–318. doi: 10.2337/diab.33.4.311. [DOI] [PubMed] [Google Scholar]
- 21.Dela F, von Linstow ME, Mikines KJ, Galbo H. Physical training may enhance beta-cell function in type 2 diabetes. Am J Physiol Endocrinol Metab. 2004;287:E1024–E1031. doi: 10.1152/ajpendo.00056.2004. [DOI] [PubMed] [Google Scholar]
- *22.Henry RR, Gumbiner B. Benefits and limitations of very-low-calorie diet therapy in obese NIDDM. Diabetes Care. 1991;14:802–823. doi: 10.2337/diacare.14.9.802. [DOI] [PubMed] [Google Scholar]
- *23.Kelley DE, Wing R, Buonocore C, Sturis J, Polonsky K, Fitzsimmons M. Relative effects of calorie restriction and weight loss in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1993;77:1287–1293. doi: 10.1210/jcem.77.5.8077323. [DOI] [PubMed] [Google Scholar]
- 24.Kelly KR, Brooks LM, Solomon TP, Kashyap S, O’Leary VB, Kirwan JP. The glucose-dependent insulinotropic polypeptide (GIP) and glucose-stimulated insulin response to exercise training and diet in obesity. Am J Physiol Endocrinol Metab. 2009 doi: 10.1152/ajpendo.00112.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solomon TP, Haus JM, Kelly KR, Cook MD, Riccardi M, Rocco M, Kashyap SR, Barkoukis H, Kirwan JP. Randomized trial on the effects of a 7-d low-glycemic diet and exercise intervention on insulin resistance in older obese humans. Am J Clin Nutr. 2009;90:1222–1229. doi: 10.3945/ajcn.2009.28293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *26.Solomon TP, Haus JM, Kelly KR, Cook MD, Filion J, Rocco M, Kashyap SR, Watanabe RM, Barkoukis H, Kirwan JP. A low-glycemic index diet combined with exercise reduces insulin resistance, postprandial hyperinsulinemia, and glucose-dependent insulinotropic polypeptide responses in obese, prediabetic humans. Am J Clin Nutr. 2010;92:1359–1368. doi: 10.3945/ajcn.2010.29771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *27.Solomon TP, Haus JM, Kelly KR, Rocco M, Kashyap SR, Kirwan JP. Improved pancreatic beta-cell function in type 2 diabetic patients after lifestyle-induced weight loss is related to glucose-dependent insulinotropic polypeptide. Diabetes Care. 2010;33:1561–1566. doi: 10.2337/dc09-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]