Abstract
The activity and substrate specificity of the ubiquitously expressed phosphatase PP2A is determined by the type of regulatory (B) subunit that couples to the catalytic/scaffold core of the enzyme. We determined that the Bβ subunit (PPP2R2B) is expressed in resting T cells, its transcription is down-regulated during T-cell activation, and up-regulated in conditions of low IL-2. Specifically, high levels of PP2A Bβ were produced during IL-2 deprivation-induced apoptosis, whereas Fas ligation had no effect. Forced expression of the Bβ subunit in primary human T cells was sufficient to induce apoptosis, whereas silencing using siRNA protected activated T cells from IL-2 withdrawal-induced cell death. Because T-cell apoptosis is known to be altered in T cells from patients with systemic lupus erythematosus, we analyzed the regulation of PP2A Bβ in this autoimmune disease. We found that levels of PP2A Bβ did not increase upon IL-2 deprivation in 50% of the patients. Remarkably, this defect was accompanied by resistance to apoptosis. Importantly, kinetics of cell death were normal in cells of patients that up-regulated PP2A Bβ in a normal manner. We have identified a unique role for the phosphatase PP2A, particularly the holoenzyme formed by PP2A Bβ. Bβ appears to trigger apoptosis of T cells in the absence of IL-2 and probably contributes to the termination of a no-longer-needed immune response. We propose that defective production of PP2A Bβ upon IL-2 deprivation results in apoptosis resistance and longer survival of autoreactive T cells, in a subset of SLE patients.
Phosphorylation represents the major posttranslational modification that determines the fate and function of proteins (1). The most commonly phosphorylated amino acid residues are serine and threonine, which are the substrates of more than 400 serine/threonine kinases. Intriguingly, a much smaller number of serine/threonine phosphatases (∼30) oppose their action (2). In great part, this imbalance is explained by the fact that the main serine/threonine phosphatases, including protein phosphatase 2A (PP2A), have flexible compositions that alter their function, localization, and activity, by interchanging different regulatory subunits (2, 3). Thus, the term PP2A actually refers to a group of phosphatases that share a common catalytic/scaffolding (C/A) core.
PP2A is an abundant and ubiquitously expressed, highly conserved enzyme (3). It regulates a myriad of cellular processes, including cell cycle progression and cell division, cell death, cytoskeleton dynamics, and signaling pathways (4, 5). PP2A is composed of a scaffold subunit (A), a catalytic subunit (C), and a regulatory (B) subunit. The catalytic and scaffold subunits are each coded by two closely homologous genes (PP2A C α, PPP2CA and β, PPP2CB; PP2A A α, PPP2R1A and β, PPP2R1B). In contrast, the regulatory subunits are coded by a large variety of genes that have been grouped in three families (B, B′, and B′′) (3). A functional PP2A holoenzyme is formed when the relatively invariant catalytic/scaffold core heterodimer couples with one of the B subunits. The choice of the regulatory B subunit determines the substrate specificity of the enzyme, as well as its intracellular distribution (2). Therefore, consideration of the nature of the B subunits involved in a specific function of PP2A is essential to the understanding of that process.
Disturbances in the expression and/or function of PP2A have been linked to human diseases, including cancer (6) and neurodegenerative (7) and autoimmune diseases (8). Patients with systemic lupus erythematosus (SLE), a chronic autoimmune condition (9), have abnormally high levels and activity of PP2A C in T cells (8). Increased PP2A expression has been shown to contribute to altered expression of transcription factors and cytokines (8). Because PP2A is involved in several T-cell functions that could theoretically contribute to disease expression in patients with SLE, we decided to identify which B subunits are expressed in T cells and determine if they contribute to the abnormal T-cell phenotype observed in the disease.
In this communication we describe the expression of PP2A regulatory (B) subunits in human T cells and dissect the factors that regulate the expression of PP2A Bβ. We show that the subunit Bβ is involved in the regulation of programmed cell death triggered by IL-2 deficiency and identify a subset of patients with SLE in which altered regulation of PP2A Bβ is associated with resistance to IL-2 deprivation-induced apoptosis.
Results
Multiple PP2A Regulatory Subunits Are Expressed in Human T Cells.
PP2A is ubiquitously expressed. However, tissue distribution, intracellular location, and the function of each PP2A holoenzyme depend on the particular regulatory subunit associated with the A/C core heterodimer (2, 3). Thus, the repertoire of B subunits expressed by a particular cell type determines the function of PP2A in that specific cell. To identify which PP2A B subunits are present in T cells, we isolated RNA from resting and activated cells. We detected mRNA from all known B subunits except for Bγ (PPP2R2C) and B′′α (PPP2R3A; Fig. S1A). Levels of RNA from B subunits were down-regulated following cell stimulation with anti-CD3 and anti-CD28 antibodies. Notably, low levels of Bβ (PPP2R2B) were detected in resting cells, and they virtually disappeared after cell activation (Fig. S1B and Fig. 1A).
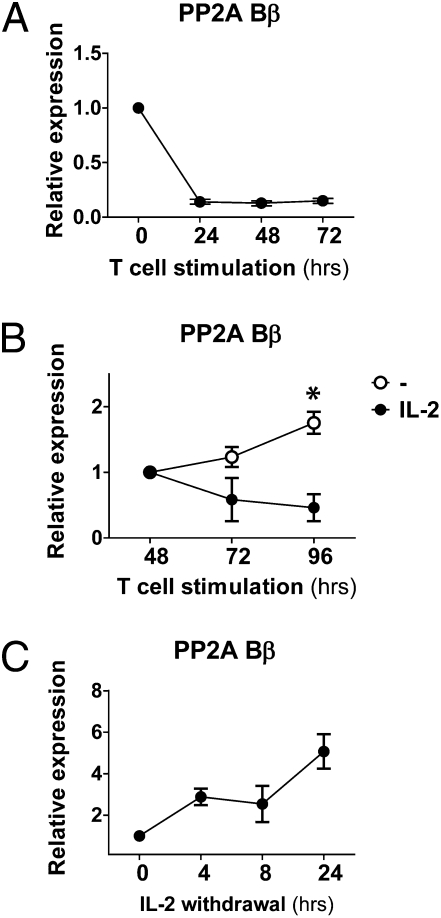
Fig. 1.
PP2A Bβ is down-regulated during T-cell activation and induced in the absence of IL-2. (A) T cells were stimulated in the presence of plate-bound anti-CD3 (1 μg/mL) and anti-CD28 (1 μg/mL). mRNA levels of PP2A Bβ were assessed by real-time PCR. The results were normalized using β-actin expression and are expressed as relative to levels of unstimulated cells (mean ± SEM). (B) T cells stimulated as in A were incubated in the absence (○) or presence (●) of IL-2 (100 U/mL) during the indicated time. PP2A Bβ was quantified as in A. Expression levels (mean ± SEM) are relative to those of cells at 48 h. *P < 0.05. (C) T cells were stimulated as in A and expanded in IL-2–containing medium for 7 d. Next, cells were washed and incubated in IL-2–free medium in the presence of a neutralizing anti-human IL-2 antibody (10 μg/mL). At the indicated time points, PP2A Bβ was quantified. Expression levels (mean ± SD) are relative to those of cells not deprived of IL-2.
PP2A Bβ Transcription Is Elicited by IL-2 Withdrawal.
The modulation of PP2A Bβ induced by T-cell activation suggested that the abundance of this subunit is regulated at the transcriptional level. Moreover, PP2A Bβ is known to be important in the regulation of neuron survival: ectopic expression causes cell death in neuronal cell lines (10, 11), and expansion of a trinucleotide repeat in the 5′ noncoding region of its gene is responsible for the neurodegenerative disease spinocerebellar ataxia type 12 (12). Thus, we hypothesized that the function of this PP2A regulatory subunit in T cells could be related to the regulation of cell cycle and death.
To define the conditions in which PP2A Bβ is transcribed, we performed time-course activation experiments with human T cells stimulated through the T-cell receptor-associated complex (CD3) and the main costimulatory molecule CD28. As shown in Fig. 1A, T-cell activation caused a rapid and profound down-regulation of PP2A Bβ. The decrease in its mRNA levels coincided with the peak of secretion of IL-2, which occurs ∼12 h after T-cell activation. Levels of PP2A Bβ started to rise at late time points (72–96 h), when IL-2 production has ceased (13). To determine if IL-2 concentrations played a role in its regulation and lack of IL-2 was indeed causing the up-regulation of PP2A Bβ, we supplemented cell cultures after 48 h of activation with IL-2. In sharp contrast to cell cultures devoid of exogenous IL-2, cells failed to increase PP2A Bβ if IL-2 had been added (Fig. 1B). The inhibitory effect of IL-2 on PP2A Bβ expression was dose dependent (Fig. S2). This suggested that IL-2 interferes with the expression of PP2A Bβ. To determine if absence of IL-2 could indeed trigger PP2A Bβ transcription, we incubated activated T cells in IL-2–free medium in the presence of an anti–IL-2 antibody to neutralize IL-2 production. As shown in Fig. 1C, levels of PP2A Bβ increased as early as 4 h after IL-2 withdrawal and neutralization. These results indicate that PP2A Bβ is down-regulated in T cells after activation and transcribed again upon IL-2 deprivation.
PP2A Bβ Is Up-Regulated During IL-2 Withdrawal-Induced Apoptosis.
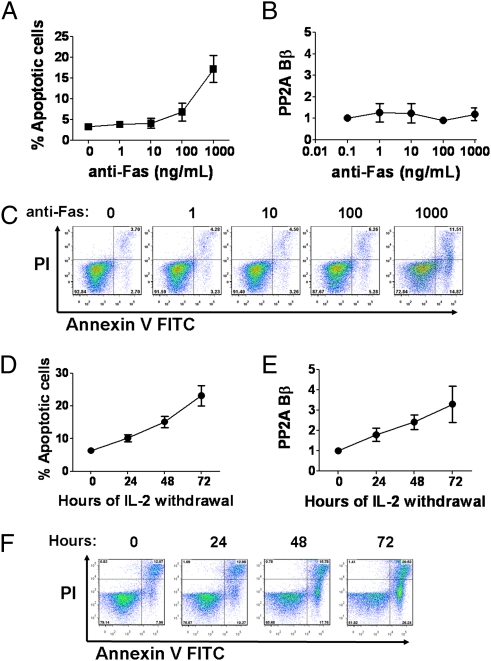
Antigen-specific T-cell clones that have expanded during the course of an immune response are deleted when the stimulus ceases. Deletion is accomplished through apoptosis induced by ligation of Fas (or other death receptors) or cytokine withdrawal (14). To determine whether PP2A Bβ is expressed during Fas-mediated or cytokine withdrawal-induced apoptosis, we stimulated primary human T cells with plate-bound anti-CD3 and anti-CD28 for 3 d followed by a period of 7 d in the presence of IL-2, before incubating them with anti-Fas, or depriving them of IL-2 (15). As shown in Fig. 2 A and C, anti-Fas antibody caused apoptosis in a dose-dependent fashion. Notably, mRNA levels of PP2A Bβ were not affected during this process (Fig. 2B). As expected, IL-2 withdrawal caused a significant degree of apoptosis (Fig. 2 D and F). In contrast with the cells incubated with anti-Fas, IL-2 deprivation was associated with a robust induction of PP2A Bβ (Fig. 2E). Taken together, these data indicate that transcription of PP2A Bβ is elicited in states of IL-2 withdrawal and is not affected by Fas engagement. Moreover, the presence of PP2A Bβ is intimately associated with T-cell apoptosis.
Fig. 2.
Expression of PP2A Bβ is elicited during IL-2 withdrawal-induced apoptosis. (A and C) T cells were stimulated with plate-bound anti-CD3 (1 μg/mL) and anti-CD28 (1 μg/mL) during 3 d and expanded in IL-2–containing medium (7 d). Then, cells were washed and incubated in the presence of increasing concentrations of plate-bound anti-Fas antibody. Early apoptotic cells [annexin V+ propidium iodide (PI)−] were quantified by flow cytometry. Cumulative data (mean ± SEM) from one experiment are shown in A; dot plots from a representative individual are shown in C. (B) RNA was extracted from a fraction of the cells, and PP2A Bβ levels were assessed by real-time PCR. Expression relative to that of cells incubated in the absence of anti-Fas is shown (fold change, mean ± SEM, n = 3). One of three independent experiments is depicted. (D and F) Cells stimulated and expanded as in A were washed and incubated in the absence of IL-2. At the indicated time points, cells were harvested and apoptosis was quantified as in C. (E) A fraction of the IL-2–deprived cells was used to isolate RNA and quantify PP2A Bβ as in B.
Forced Expression of PP2A Bβ Is Associated with T-Cell Apoptosis.
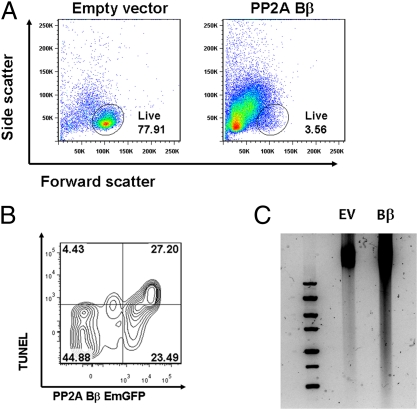
To study the effects of PP2A Bβ expression in T cells, we cloned the human PP2A Bβ into an expression vector. Transfection of primary human T cells with the PP2A Bβ-containing vector caused high degrees of cell death evident in flow cytometry analyses after 24 and 48 h by decreased cellular size (forward scatter) and increased cellular complexity (side scatter; Fig. 3A). Decreased cell viability was confirmed manually in cell aliquots stained with trypan blue. To determine if the observed cell death was attributable to apoptosis, we performed TUNEL staining. As shown in Fig. 3B, a large fraction of the efficiently transfected T cells (EmGFP+ cells) was TUNEL+. In contrast, only a small percentage of TUNEL+ cells was observed among cells that did not express the PP2A Bβ-containing vector (9.0 ± 0.07% vs. 53.7 ± 0.07%, respectively; P < 0.05). Further confirmation of apoptosis in cells transfected with PP2A Bβ was obtained by demonstrating DNA fragmentation in agarose gels (Fig. 3C). These data demonstrate that ectopic expression of PP2A Bβ in resting human T cells is sufficient to induce apoptosis.
Fig. 3.
Forced expression of PP2A Bβ causes apoptosis. (A) Human PP2A Bβ (PPP2R2B) was cloned into a bicistronic vector expressing PP2A Bβ and emerald GFP (EmGFP) under separate promoters. Resting primary human T cells were transfected by nucleoporation, and flow cytometry was performed after 24 h. Shown are representative dot plots of cells transfected with the PP2A Bβ-containing vector or an otherwise identical empty plasmid. The gate indicates the position of viable cells according to forward and side scatter parameters. (B) After fixing and permeabilizing, cells were stained using a fluorescent TUNEL assay. A representative contour plot shows that even though there are virtually no TUNEL+ cells among the cells that fail to express the plasmid (EmGFP− cells), a large fraction of EmGFP+ cells are TUNEL+, which indicates that the presence of PP2A Bβ induces apoptosis in effectively transfected cells. These experiments were repeated five times. (C) T cells transfected as in A were lysed, and DNA was extracted and analyzed in a 1.5% agarose gel. DNA from cells transfected with an empty vector (EV) was intact and did not migrate. In contrast, DNA migration was obviously increased in cells transfected with PP2A Bβ, indicating the presence of small fragments of digested DNA.
Inhibition of PP2A Bβ Protects T cells from Apoptosis Induced by IL-2 Withdrawal.
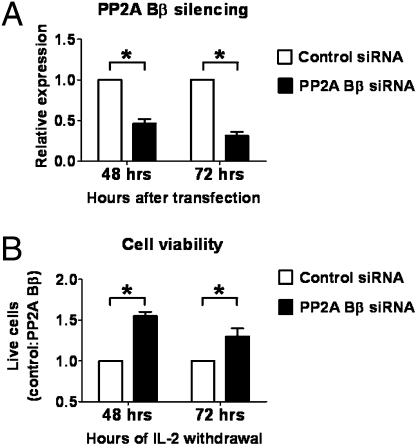
Together, our data suggest that during conditions of low IL-2 levels, PP2A Bβ is up-regulated and apoptosis ensues. To investigate whether PP2A Bβ is necessary for apoptosis triggered by IL-2 deprivation, we stimulated T cells with anti-CD3 and anti-CD28 for 3 d and then expanded them in the presence of IL-2 for 7 d. At the end of this stimulation period, cells were washed, counted, and transfected with different doses (0.25–2.0 μM) of a PP2A Bβ-specific siRNA or a scrambled control siRNA. Effective knockdown was achieved during ≥72 h using 1 μM siRNA (Fig. 4A). To determine if PP2A Bβ silencing could indeed block cell death, we analyzed the effect of siRNA transfection after 48 and 72 h of IL-2 deprivation. Fig. 4B shows the relative numbers of live cells in wells transfected with control siRNA and PP2A Bβ siRNA. PP2A Bβ knockdown increased significantly the number of live cells at 48 (55 ± 5%, P < 0.01) and 72 h (30 ± 10%, P < 0.05). Inhibition of apoptosis was confirmed using a different PP2A Bβ-specific siRNA (s10970; Fig. S3). These results indicate that PP2A Bβ is essential in the process of apoptosis induced by IL-2 withdrawal, because its inhibition protects cells from death induced by IL-2 deprivation.
Fig. 4.
PP2A Bβ silencing decreases cell death in conditions of low IL-2. (A) Human T cells were stimulated and expanded for 10 d and then washed and counted before transfection with either a siRNA-specific for PP2A Bβ (s10971) or a control siRNA (1μM). Levels of PP2A Bβ were assessed at the indicated time points using real-time PCR and are expressed as relative to the levels of cells transfected with control siRNA. Shown is the cumulative data from four independent experiments. *P < 0.005. (B) Cells expanded and transfected as in A were incubated in IL-2–free medium. Live cells were quantified at the indicated time points. Results are expressed as the ratio of live cells in wells transfected with control siRNA and PP2A Bβ siRNA. Shown is the cumulative data from three independent experiments. *P < 0.05.
Up-Regulation of PP2A Bβ and Apoptosis Induced by IL-2 Withdrawal Are Deficient in a Subset of Patients with SLE.
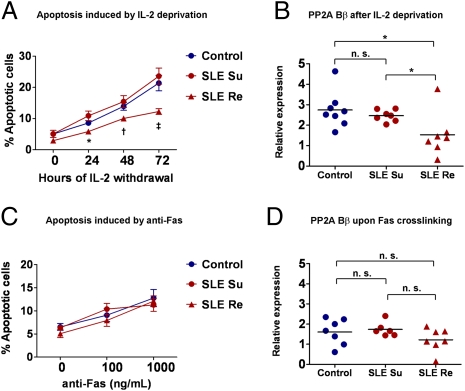
SLE is a chronic autoimmune disease associated with defective T and B lymphocyte activation (16). T cells from SLE patients exhibit a complex array of biochemical abnormalities that include abnormal apoptosis. Spontaneous apoptosis is increased (17), whereas activation-induced apoptosis is defective (18, 19). Defective apoptosis following T-cell activation is particularly important in SLE, because defects in this process could potentially contribute to the accumulation of autoreactive pathogenic T cells. To evaluate if apoptosis induced by IL-2 deprivation occurs normally in SLE, we stimulated T cells from patients with SLE following the 10-d stimulation procedure described above, and subjected the cells to Fas cross-linking or IL-2 deprivation. We noted that cells from half of the patients were resistant to apoptosis upon IL-2 withdrawal. In contrast, cells from the other half of the patients underwent apoptosis with kinetics indistinguishable from those of healthy subjects. Accordingly, we divided the patients in two groups based on their susceptibility or resistance to apoptosis in conditions of low IL-2. As shown in Fig. 5A, a significantly lower percentage of T cells from apoptosis-resistant patients (SLE Re) were apoptotic at 24, 48, and 72 h following IL-2 deprivation (P < 0.05) than cells from healthy individuals or apoptosis-susceptible patients (SLE Su). Interestingly, apoptosis induced by Fas cross-linking was not altered in cells of any of the SLE patients, irrespective of their behavior during IL-2 deprivation (Fig. 5C). To determine if altered regulation of PP2A Bβ was involved in this phenotype, we assessed the levels of PP2A Bβ after IL-2 withdrawal. As shown in Fig. 5B, levels of PP2A Bβ increased (∼threefold) in cells from healthy individuals upon 48 h of IL-2 deprivation. A normal elevation of PP2A Bβ levels was likewise observed in cells from SLE patients with normal apoptosis kinetics (SLE Su). In sharp contrast, PP2A Bβ levels exhibited only a mild, nonsignificant increase from basal levels after IL-2 withdrawal in cells from SLE Re patients. As expected, levels of PP2A Bβ did not increase significantly after Fas cross-linking in any of the groups (Fig. 5D). To determine if differences in basal T-cell activation related to disease activity affected susceptibility to apoptosis, we analyzed the SLE disease activity index (SLEDAI) of the studied patients. As shown in Fig. S4, there was no difference in disease activity between resistant and susceptible patients. These findings indicate that T cells from a large fraction of patients with SLE are resistant to apoptosis induced by low levels of IL-2, and suggest that defective regulation of PP2A Bβ is responsible for this phenotype.
Fig. 5.
Defective up-regulation of PP2A Bβ upon IL-2 withdrawal is associated with apoptosis resistance in patients with SLE. (A) T cells isolated from healthy donors (n = 11) or patients with SLE (n = 14) were stimulated and expanded for 10 d and then deprived of IL-2. Apoptosis was quantified by flow cytometry (annexin V and PI staining). Patients with SLE were divided in two groups (susceptible, SLE Su; resistant, SLE Re) based on the observed apoptosis kinetics. Each group was comprised of seven patients. *P = 0.01 vs. SLE Su; †P = 0.02 vs. SLE Su; ‡P = 0.001 vs. SLE Su, P = 0.04 vs. control. (B) RNA was isolated after 48 h of IL-2 deprivation, and PP2A Bβ levels were assessed by real-time PCR. Shown is cumulative data from six experiments. Results are expressed as relative to PP2A Bβ levels of cells not deprived of IL-2. *P < 0.05. (C) T cells stimulated and expanded as in A were incubated in the presence of immobilized anti-Fas. Apoptosis was quantified as in A. No differences were observed between the three groups. (D) PP2A Bβ mRNA of anti-Fas–treated cells was quantified as in B. Expression levels are relative to cells not treated with anti-Fas. n.s., not significant.
Discussion
We have provided evidence that the Bβ regulatory subunit of protein phosphatase 2A is expressed in human T cells when IL-2 levels decrease. Moreover, expression of PP2A Bβ is associated with the induction of apoptosis, and its forced expression in T cells triggers programmed cell death. Silencing of PP2A Bβ in activated T cells deprived of IL-2 decreases cell death, indicating that PP2A Bβ plays an essential role in this process. Finally, we have found that T cells from half of patients with SLE are resistant to IL-2 withdrawal-induced apoptosis, and that such resistance is associated with failure to up-regulate PP2A Bβ in low IL-2 conditions.
Apoptosis is an essential phenomenon that limits the duration of immune responses and maintains the diversity of the lymphoid repertoire (20). The importance of this process is well known, and deficiency of central molecules involved in lymphocyte apoptosis causes lymphoproliferative and autoimmune diseases in mice and humans (15, 21–24). Apoptosis induced by IL-2 deprivation is triggered by intrinsic cellular signals (14). The balance between anti- and proapoptotic Bcl-2 family proteins determines the maintenance of the mitochondrial membrane potential. In the presence of IL-2, Bad is phosphorylated and sequestered in the cytoplasm by 14-3-3 proteins (25–28). Bim, another proapoptotic molecule, is absent, and levels of antiapoptotic Bcl-2 and Bcl-x are high. During IL-2 deprivation, Bad becomes dephosphorylated, dissociates from 14-3-3, and translocates to the mitochondrial membrane where it binds to Bcl-2 and Bcl-x and neutralizes their antiapoptotic capacity (26, 29). This process results in the loss of the mitochondrial membrane potential and leads to apoptosis. Two major serine/threonine phosphatases, PP1 (30) and PP2A, have been shown to dephosphorylate Bad (31, 32). In fact, IL-2 deprivation-induced Bad dephosphorylation can be blocked by okadaic acid and calyculin A, powerful PP2A and PP1 inhibitors (33). PP2A has also been shown to dephosphorylate 14-3-3 (34).
Cell cycle regulation and apoptosis induction are linked processes controlled in T cells by IL-2. IL-2 promotes Akt phosphorylation by phosphoinositide 3-kinase. This induces Bcl-2 and c-myc, which inhibit apoptosis and stimulate cell cycle progression (35). PP2A has been shown to inactivate Akt by dephosphorylation, which induces p27kip1, causing cell cycle arrest and apoptosis in cancer cells (36).
Apoptosis induction in response to IL-2 deprivation and subsequent mitochondrial depolarization is associated with a distinct gene transcription profile (37–39). In fact, it requires gene transcription and can be blocked by cycloheximide and actinomycin D. Our results indicate that PP2A Bβ is one of the genes induced during this process. The fact that cell death induced by IL-2 deprivation can be decreased by silencing PP2A Bβ indicates that its role is important and probably upstream of effector molecules.
Thus, PP2A is involved in the regulation of multiple players that determine the fate of the T cell in response to IL-2 levels. The identity of the particular B regulatory subunit(s) associated with each of these effects is unknown. The death-inducing effect of PP2A Bβ ectopic expression could depend on Bad or Akt dephosphorylation, or on a yet-unknown function of PP2A. Further work will determine if Bβ is the regulatory subunit that confers PP2A the capacity to act upon Bad or 14-3-3. The expression kinetics of PP2A Bβ suggests that it may act as a negative regulator of cell cycle progression—present in resting T cells (mostly in G0), disappearing after T-cell activation, and transcribed again in response to growth factor depletion.
The regulation of T-cell death following activation is known to be altered in patients with SLE (18, 19, 40). Here we have confirmed and expanded those findings. Our results indicate that the kinetics of apoptosis following IL-2 deprivation is affected in a fraction of patients with SLE. Importantly, induction of PP2A Bβ upon IL-2 withdrawal was suboptimal or completely absent in these patients, which confirms the importance of PP2A Bβ as a molecule induced in cytokine withdrawal apoptosis and suggests that its faulty expression may underlie the observed phenotype. Mitochondrial hyperpolarization (MHP) could also contribute to the apoptosis resistance observed in SLE patients upon IL-2 deprivation (18, 41). A deeper understanding of the mechanisms whereby PP2A Bβ induces apoptosis will determine if MHP and low PP2A Bβ are associated. The activator protein (AP)-1 transcription factors (c-fos and c-jun) are up-regulated in T cells upon IL-2 deprivation (37). Levels of these transcription factors have been reported to be low in patients with SLE (8, 42). This defect may underlie the low PP2A Bβ induction observed in patients with deficient apoptosis.
Cellular levels of the catalytic subunit of PP2A are abnormally high in patients with SLE (8). This defect affects the activity of several transcription factors (8, 43, 44) and has been proposed to contribute to the altered phenotype of T cells from SLE patients (9). The fact that levels of PP2A Bβ are abnormally low in some patients with SLE underscores the notion that each component of the PP2A holoenzyme is regulated separately, and hints at its complex regulation (2). Further work will clarify whether increased levels of PP2Ac and faulty up-regulation of PP2A Bβ are associated.
Therapeutic modulation of PP2A is not feasible because of the large number of essential roles it plays in cellular physiology (45). Thus, PP2A could only become a suitable pharmacologic target when the expression and/or function of specific B subunits involved in pathologic pathways are identified. Here we have described which B subunits are expressed in T lymphocytes and assigned a role for one of them as a key regulator of apoptosis upon IL-2 deprivation.
Materials and Methods
Patients and Control Subjects.
Blood samples were obtained from 16 healthy platelet donors from the Kraft Family Blood Donor Center (Dana Farber Cancer Institute, Boston) and from 14 patients with SLE that fulfilled the revised classification criteria for SLE from the American College of Rheumatology (46). The clinical and demographic characteristics of the patients are provided in Fig. S4A. Information of the healthy donors is omitted, because we do not have access to it per policy of the Kraft Family Blood Donor Center. T cells were isolated by negative selection (RosetteSep; Stemcell Technologies). Purity was always ≥96%. The study was approved by the Institutional Review Board of the Beth Israel Deaconess Medical Center. All study participants signed informed consent forms.
RNA Isolation and PCR.
RNA was isolated using TRIzol (Invitrogen). cDNA was produced from 500 ng of RNA (Reverse Transcription System; Promega). Real-time PCR was performed using SYBR Green (LightCycler 480 SYBR Green I Master; Roche). Primer sequences and amplification conditions are available upon request.
T-Cell Stimulation and Apoptosis Induction.
T cells were stimulated with plate-bound anti-CD3 (clone OKT-3, 1 μg/mL; BioXCell) and anti-CD28 (clone CD28.2, 1 μg/mL; Biolegend) in full RPMI (3 × 106 T cells in 2 mL of RPMI) during 3 d. Next, cells were replated in 6 mL of fresh, full RPMI containing human recombinant IL-2 (100 U/mL; R&D Systems). IL-2 was replenished every 48 h. After 7 d in RPMI supplemented with IL-2, cells were washed and replated in the presence of IL-2 (100 U/mL; control), anti-Fas (clone DX2, 1–1,000 ng/mL; BD Biosciences), or medium (IL-2 deprivation). In some experiments, an anti–IL-2 antibody (clone MQ1-17H12, 10 μg/mL; Biolegend) was used to neutralize IL-2.
Apoptosis Detection.
In most experiments, apoptosis was assessed by annexin V binding and propidium iodide exclusion. Data were acquired using a LSRII flow cytometer (BD Biosciences) and analyzed using FlowJo (Tree Star, Inc.). For some experiments, cells were stained with TUNEL (In Situ Cell Death Detection Kit; Roche) and analyzed with flow cytometry. For the detection of DNA fragmentation, DNA was precipitated from the phenol phase of the TRIzol cell lysate and run in a 1.5% agarose gel stained with SYBR Safe (Invitrogen).
PP2A Bβ Cloning and Expression.
The mRNA sequence of PPP2R2B (NM_181676.2) was obtained from the Gene database (www.ncbi.nlm.nih.gov/gene) and cloned from human total brain cDNA (US Biological). The sequence was inserted into the pcDNA6.2/EmGFP-Bsd/V5-Dest plasmid (Invitrogen). An empty plasmid was expanded as control. T cells (106) were transfected with 5 μg of plasmid by nucleoporation using the Amaxa Human T-cell Nucleofector Kit (Lonza). Cells were assayed at 24 and 48 h posttransfection.
PP2A Bβ Silencing.
T cells were stimulated and expanded during 10 d as described. Then, 107 T cells were transfected with different concentrations (0.25–2.0 μM) of either specific validated silencer siRNAs or the negative control 1 siRNA (Ambion). Silencing efficiency and cell assays were performed at 48 and 72 h.
Statistical Analyses.
Paired and nonpaired Student two-tailed t tests were used. Results are expressed as the mean ± SEM, unless noted otherwise.
Supplementary Material
Acknowledgments
We thank Dr. Vasileios Kyttaris and Michelle D. Finnell for their help with patient recruitment and sample collection. This work was supported by National Institutes of Health Grant R01 A1068787. J.C.C. is a recipient of an Arthritis Foundation Postdoctoral Fellowship Award.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1103915108/-/DCSupplemental.
References
- 1.Olsen JV, et al. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell. 2006;127:635–648. doi: 10.1016/j.cell.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 2.Shi Y. Serine/threonine phosphatases: Mechanism through structure. Cell. 2009;139:468–484. doi: 10.1016/j.cell.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Janssens V, Longin S, Goris J. PP2A holoenzyme assembly: In cauda venenum (the sting is in the tail) Trends Biochem Sci. 2008;33:113–121. doi: 10.1016/j.tibs.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Janssens V, Goris J. Protein phosphatase 2A: A highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J. 2001;353:417–439. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sontag E. Protein phosphatase 2A: The Trojan Horse of cellular signaling. Cell Signal. 2001;13:7–16. doi: 10.1016/s0898-6568(00)00123-6. [DOI] [PubMed] [Google Scholar]
- 6.Janssens V, Goris J, Van Hoof C. PP2A: The expected tumor suppressor. Curr Opin Genet Dev. 2005;15:34–41. doi: 10.1016/j.gde.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Tian Q, Wang J. Role of serine/threonine protein phosphatase in Alzheimer's disease. Neurosignals. 2002;11:262–269. doi: 10.1159/000067425. [DOI] [PubMed] [Google Scholar]
- 8.Katsiari CG, Kyttaris VC, Juang YT, Tsokos GC. Protein phosphatase 2A is a negative regulator of IL-2 production in patients with systemic lupus erythematosus. J Clin Invest. 2005;115:3193–3204. doi: 10.1172/JCI24895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crispín JC, Kyttaris VC, Juang YT, Tsokos GC. How signaling and gene transcription aberrations dictate the systemic lupus erythematosus T cell phenotype. Trends Immunol. 2008;29:110–115. doi: 10.1016/j.it.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Dagda RK, et al. The spinocerebellar ataxia 12 gene product and protein phosphatase 2A regulatory subunit Bbeta2 antagonizes neuronal survival by promoting mitochondrial fission. J Biol Chem. 2008;283:36241–36248. doi: 10.1074/jbc.M800989200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng WT, et al. Oxidative stress promotes autophagic cell death in human neuroblastoma cells with ectopic transfer of mitochondrial PPP2R2B (Bbeta2) BMC Cell Biol. 2009;10:91. doi: 10.1186/1471-2121-10-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holmes SE, et al. Expansion of a novel CAG trinucleotide repeat in the 5′ region of PPP2R2B is associated with SCA12. Nat Genet. 1999;23:391–392. doi: 10.1038/70493. [DOI] [PubMed] [Google Scholar]
- 13.Villarino AV, et al. Helper T cell IL-2 production is limited by negative feedback and STAT-dependent cytokine signals. J Exp Med. 2007;204:65–71. doi: 10.1084/jem.20061198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lenardo M, et al. Mature T lymphocyte apoptosis—immune regulation in a dynamic and unpredictable antigenic environment. Annu Rev Immunol. 1999;17:221–253. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- 15.Snow AL, et al. Restimulation-induced apoptosis of T cells is impaired in patients with X-linked lymphoproliferative disease caused by SAP deficiency. J Clin Invest. 2009;119:2976–2989. doi: 10.1172/JCI39518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crispín JC, et al. Pathogenesis of human systemic lupus erythematosus: Recent advances. Trends Mol Med. 2010;16:47–57. doi: 10.1016/j.molmed.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xue C, Lan-Lan W, Bei C, Jie C, Wei-Hua F. Abnormal Fas/FasL and caspase-3-mediated apoptotic signaling pathways of T lymphocyte subset in patients with systemic lupus erythematosus. Cell Immunol. 2006;239:121–128. doi: 10.1016/j.cellimm.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Gergely P, Jr, et al. Persistent mitochondrial hyperpolarization, increased reactive oxygen intermediate production, and cytoplasmic alkalinization characterize altered IL-10 signaling in patients with systemic lupus erythematosus. J Immunol. 2002;169:1092–1101. doi: 10.4049/jimmunol.169.2.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu L, Zhang L, Yi Y, Kang HK, Datta SK. Human lupus T cells resist inactivation and escape death by upregulating COX-2. Nat Med. 2004;10:411–415. doi: 10.1038/nm1005. [DOI] [PubMed] [Google Scholar]
- 20.Snow AL, Pandiyan P, Zheng L, Krummey SM, Lenardo MJ. The power and the promise of restimulation-induced cell death in human immune diseases. Immunol Rev. 2010;236:68–82. doi: 10.1111/j.1600-065X.2010.00917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi T, et al. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994;76:969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- 23.Turbyville JC, Rao VK. The autoimmune lymphoproliferative syndrome: A rare disorder providing clues about normal tolerance. Autoimmun Rev. 2010;9:488–493. doi: 10.1016/j.autrev.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Cohen PL. Apoptotic cell death and lupus. Springer Semin Immunopathol. 2006;28:145–152. doi: 10.1007/s00281-006-0038-z. [DOI] [PubMed] [Google Scholar]
- 25.Datta SR, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 26.Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L) Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 27.Scheid MP, Duronio V. Dissociation of cytokine-induced phosphorylation of Bad and activation of PKB/akt: Involvement of MEK upstream of Bad phosphorylation. Proc Natl Acad Sci USA. 1998;95:7439–7444. doi: 10.1073/pnas.95.13.7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pastorino JG, Tafani M, Farber JL. Tumor necrosis factor induces phosphorylation and translocation of BAD through a phosphatidylinositide-3-OH kinase-dependent pathway. J Biol Chem. 1999;274:19411–19416. doi: 10.1074/jbc.274.27.19411. [DOI] [PubMed] [Google Scholar]
- 29.Yang E, et al. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995;80:285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- 30.Ayllón V, Martínez-A C, García A, Cayla X, Rebollo A. Protein phosphatase 1alpha is a Ras-activated Bad phosphatase that regulates interleukin-2 deprivation-induced apoptosis. EMBO J. 2000;19:2237–2246. doi: 10.1093/emboj/19.10.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiang CW, et al. Protein phosphatase 2A activates the proapoptotic function of BAD in interleukin-3-dependent lymphoid cells by a mechanism requiring 14-3-3 dissociation. Blood. 2001;97:1289–1297. doi: 10.1182/blood.v97.5.1289. [DOI] [PubMed] [Google Scholar]
- 32.Chiang CW, et al. Protein phosphatase 2A dephosphorylation of phosphoserine 112 plays the gatekeeper role for BAD-mediated apoptosis. Mol Cell Biol. 2003;23:6350–6362. doi: 10.1128/MCB.23.18.6350-6362.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weller M, Malipiero U, Groscurth P, Fontana A. T cell apoptosis induced by interleukin-2 deprivation or transforming growth factor-beta 2: Modulation by the phosphatase inhibitors okadaic acid and calyculin A. Exp Cell Res. 1995;221:395–403. doi: 10.1006/excr.1995.1390. [DOI] [PubMed] [Google Scholar]
- 34.Martin M, et al. Protein phosphatase 2A controls the activity of histone deacetylase 7 during T cell apoptosis and angiogenesis. Proc Natl Acad Sci USA. 2008;105:4727–4732. doi: 10.1073/pnas.0708455105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmed NN, Grimes HL, Bellacosa A, Chan TO, Tsichlis PN. Transduction of interleukin-2 antiapoptotic and proliferative signals via Akt protein kinase. Proc Natl Acad Sci USA. 1997;94:3627–3632. doi: 10.1073/pnas.94.8.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim SW, Kim HJ, Chun YJ, Kim MY. Ceramide produces apoptosis through induction of p27(kip1) by protein phosphatase 2A-dependent Akt dephosphorylation in PC-3 prostate cancer cells. J Toxicol Environ Health A. 2010;73:1465–1476. doi: 10.1080/15287394.2010.511553. [DOI] [PubMed] [Google Scholar]
- 37.Colotta F, Polentarutti N, Sironi M, Mantovani A. Expression and involvement of c-fos and c-jun protooncogenes in programmed cell death induced by growth factor deprivation in lymphoid cell lines. J Biol Chem. 1992;267:18278–18283. [PubMed] [Google Scholar]
- 38.Devireddy LR, Green MR. Transcriptional program of apoptosis induction following interleukin 2 deprivation: Identification of RC3, a calcium/calmodulin binding protein, as a novel proapoptotic factor. Mol Cell Biol. 2003;23:4532–4541. doi: 10.1128/MCB.23.13.4532-4541.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fleischer A, et al. Cascade of transcriptional induction and repression during IL-2 deprivation-induced apoptosis. Immunol Lett. 2007;112:9–29. doi: 10.1016/j.imlet.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 40.Kovacs B, Vassilopoulos D, Vogelgesang SA, Tsokos GC. Defective CD3-mediated cell death in activated T cells from patients with systemic lupus erythematosus: Role of decreased intracellular TNF-alpha. Clin Immunol Immunopathol. 1996;81:293–302. doi: 10.1006/clin.1996.0192. [DOI] [PubMed] [Google Scholar]
- 41.Fernandez D, Bonilla E, Mirza N, Niland B, Perl A. Rapamycin reduces disease activity and normalizes T cell activation-induced calcium fluxing in patients with systemic lupus erythematosus. Arthritis Rheum. 2006;54:2983–2988. doi: 10.1002/art.22085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kyttaris VC, Juang YT, Tenbrock K, Weinstein A, Tsokos GC. Cyclic adenosine 5′-monophosphate response element modulator is responsible for the decreased expression of c-fos and activator protein-1 binding in T cells from patients with systemic lupus erythematosus. J Immunol. 2004;173:3557–3563. doi: 10.4049/jimmunol.173.5.3557. [DOI] [PubMed] [Google Scholar]
- 43.Juang YT, et al. PP2A dephosphorylates Elf-1 and determines the expression of CD3zeta and FcRgamma in human systemic lupus erythematosus T cells. J Immunol. 2008;181:3658–3664. doi: 10.4049/jimmunol.181.5.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Juang YT, et al. Transcriptional activation of the cAMP-responsive modulator promoter in human T cells is regulated by protein phosphatase 2A-mediated dephosphorylation of SP-1 and reflects disease activity in patients with systemic lupus erythematosus. J Biol Chem. 2011;286:1795–1801. doi: 10.1074/jbc.M110.166785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Götz J, Probst A, Ehler E, Hemmings B, Kues W. Delayed embryonic lethality in mice lacking protein phosphatase 2A catalytic subunit Calpha. Proc Natl Acad Sci USA. 1998;95:12370–12375. doi: 10.1073/pnas.95.21.12370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tan EM, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.