Summary
Normally, cell free haemoglobin is bound by haptoglobin and efficiently cleared. However, the chronic haemolysis in sickle cell disease (SCD) overwhelms haptoglobin binding capacity and protein turnover, resulting in elevated cell free haemoglobin. Cell free haemoglobin acts as both a scavenger of vasoactive nitric oxide and a pro-oxidant. In addition, methaemoglobin (metHb) releases the haem moiety, which can bind to albumin to form methaemalbumin (metHSA). This study used electron paramagnetic resonance to detect metHSA in SCD plasma and demonstrated that haptoglobin prevents haem transfer from metHb to HSA. MetHSA may either provide a second line of defence against haemoglobin/haem-mediated oxidation or contribute to the pro-oxidant environment of SCD plasma. We demonstrated that HSA inhibited oxidative protein modification induced by metHb. Additionally, we showed that while metHb induced haem oxygenase 1 (HO-1), an indicator of oxidative stress, HSA attenuated metHb induction of this enzyme, thereby limiting the potential benefits of HO-1. Furthermore, HO-1 induction by metHSA was less than HO-1 induction by equimolar metHb not bound to albumin. Our findings confirm the presence of metHSA in SCD and suggest that haem transfer from metHb to HSA reduces the oxidative effects of free haemoglobin/haem on endothelium with both beneficial (reduced protein oxidation) and potentially harmful (reduced HO-1 induction) outcomes.
Keywords: methaemalbumin, methaemoglobin, haem, sickle cell disease, haem oxygenase 1
Sickle cell disease (SCD) occurs as a result of a single amino acid change (glutamate to valine) at position 6 of the beta subunit of haemoglobin (Reiter et al, 2002). This mutation facilitates the polymerization of sickle haemoglobin once it is deoxygenated. Cycles of polymerization and depolymerization that occur as red cells circulate through areas of differing oxygen tensions result in membrane fragility and accelerated red cell senescence. In normal individuals, senescent red cells are cleared by reticuloendothelial macrophages in the spleen and liver (Andrews, 2000), and the haemoglobin from any cells that lyse in the intravascular space is cleared by the plasma haptoglobin/haemopexin system (Ascenzi et al, 2005). Consequently, normal individuals have plasma haemoglobin levels less than 1 μM at steady-state, which is tightly bound to haptoglobin (Wang et al, 2004). However, in SCD, the level of plasma haemoglobin is significantly higher, in some cases as high as ~20 μM (Reiter et al, 2002), due both to the accelerated lysis of red cells in circulation and the fact that the haptoglobin/haemopexin system is saturated. Haptoglobin levels are low to undetectable in individuals with SCD (Hedo et al, 1993;Reiter et al, 2002).
While the role of plasma free haemoglobin in antagonizing the effect of nitric oxide (NO) has recently been a topic of great debate (Bunn et al, 2010;Gladwin et al, 2010), there is evidence that plasma haemoglobin limits NO function in SCD and appears to contribute to the vascular pathology associated with SCD (Yang et al, 2003). More recently, plasma haemoglobin level has been linked to pulmonary hypertension, a leading indicator of morbidity in SCD individuals (Jison & Gladwin, 2003). Importantly, haemoglobin is a pro-oxidant due to its peroxidase-like activity and is able to initiate lipid oxidation in low density lipoproteins (Grinshtein et al, 2003;Miller et al, 1997;Reeder & Wilson, 2005). In addition, haem, which readily dissociates from methaemoglobin, in which the haem iron is in the ferric oxidation state, is extremely lipophilic and easily intercalates into cellular membranes and lipids, initiating oxidative processes (Umbreit, 2007). Therefore, plasma free haemoglobin may significantly contribute to oxidative stress in SCD.
A well-recognized indication of oxidative stress is the induction of the protein haem oxygenase 1 (HO-1). This protein can be induced in most cell types in response to several factors including reactive oxygen species, oxidized lipids, free haemoglobin, as well as its substrate-free haem (Loboda et al, 2008;Takahashi et al, 2004). Importantly, HO-1 is upregulated in several disease states, including SCD (Jison et al, 2004;Lanaro et al, 2009;Nath et al, 2001). It is proposed that the cytoprotective effects of HO-1 are mediated through the diverse effects of its enzymatic products, such as the antioxidant effects of biliverdin being rapidly reduced to bilirubin (Loboda et al, 2008;Minetti et al, 1998;Sedlak et al, 2009), free iron upregulating the iron-chelator ferritin (Belcher et al, 2010;Loboda et al, 2008), and the anti-inflammatory and anti-apoptotic activities of carbon monoxide (Beckman et al, 2009;Loboda et al, 2008;Wagener et al, 2003). Therefore, while the induction of HO-1 may be generally beneficial, reduced HO-1 induction may indicate either a diminished protective response or be a favourable sign of reduced tissue oxidants stress.
Here, we examine differences in the fate of haemoglobin in plasma from normal and SCD individuals. We report that plasma from a subset of individuals with SCD exhibited an electron paramagnetic resonance (EPR) spectrum indicative of the formation of methaemalbumin (metHSA), oxidized haem bound to albumin, in this plasma. We then examined the role of haptoglobin deficiency in metHSA formation in SCD plasma. As it is not known whether metHSA contributes to the disease process or if metHSA is a second line of defence in SCD, we also investigated how metHSA affects the oxidative properties of haemoglobin and subsequent induction of endothelial HO-1. We demonstrated that haptoglobin prevents the transfer of haem to albumin, thus inhibiting metHSA formation. We also showed that metHSA formation reduces the oxidative effects of haemoglobin and inhibits HO-1 induction.
Materials and Methods
Materials
Chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise mentioned. Chemiluminescence reagents were from Pierce Biotechnology, Inc. (Rockford, IL). MetHb was obtained from human oxyferrous haemoglobin (2.3 mM) with addition of excess K3[Fe(CN)6], followed by separation on a Sephadex G-25 column equilibrated with 50 mM phosphate buffer (pH 7.4) containing diethylenetriamine pentaacetic acid (DTPA, 1 mM). Oxyhaemoglobin was prepared from fresh human blood according to a published method (Rossi-Fanelli et al, 1961).
Human subjects
The use of human subjects was approved by the Institutional Review Boards of Children’s Hospital of Wisconsin, Medical College of Wisconsin, and Blood Center of Wisconsin. Informed consent was obtained from healthy volunteers and individuals with homozygous haemoglobin SS disease and/or guardians for minor children, with assent when appropriate. Individuals with a history of red cell transfusion within 2 months prior to blood collection were excluded from this study. Blood samples were collected into 3.8% sodium citrate (vol. 1:9), plasma separated by centrifugation at 2,000 × g for 10 min and further clarified by centrifugation at 8,100 × g for 10 min, and then plasma was aliquoted and stored at −80°C until further use.
Electron Paramagnetic Resonance (EPR) detection of metHb
EPR studies were performed at 3.65 K on a Bruker Elexys X-band EPR system (Billerica, MA) equipped with a liquid helium cryostat and a liquid nitrogen-based variable temperature unit. MetHb/plasma mixtures were incubated at 37°C, aliquots were withdrawn at regular time intervals, placed in a 3-mm diameter quartz EPR tube, and instantly frozen in liquid nitrogen for EPR analysis. Samples were stored at −80 °C before EPR spectra were taken. EPR spectra were recorded under the following conditions: microwave power, 1 mW; modulation amplitude, 10 G; accumulation of 5 scans.
On-Gel detection of haem proteins
MetHb (100μM) was co-incubated with either human serum albumin (HSA, 600 μM), human haptoglobin 1-1 (HP, 200μM), or both for 4 h at 37°C. Samples were taken at 0 min, 2 h and 4 h, and subjected to sodium docecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE). The gel was then treated with 10 ml (NH4)2S2O8 (0.02 %, w/v) for 10 min, followed by 16 μl/cm2 H2O2 for 15 s and 80 μl/cm2 luminol for 50 s, respectively, as previously reported (Huang et al, 2004). The enhanced chemiluminescence (ECL) signal was detected with a BioSpectrum ImagingSystem (UVP, Upland, CA) equipped with a CCD camera (Shimadzu, Columbia, MA). Afterwards the gel was stained with SimplyBlue SafeStain (Invitrogen, Carlsbad, CA). The intensities of the chemiluminescent metHb and metHb + HSA bands, and the stained protein bands were measured by densitometry.
Immunoprecipitation experiments
Immunoprecipitation was performed by Seize Primary Immunoprecipitation Kits from Pierce Biotechnology, Inc. First, antibodies were covalently coupled to the beaded agarose resin. Human plasma was subject to immunoprecipitation using non-specific control antibodies or antibodies raised against human haemoglobin (Bethyl Laboratories, Inc., Montgomery, TX) or HSA. The immunoprecipitate was washed three times and the level of ferric haem was determined by EPR spectroscopy at 3.65 K as described above.
Methaemalbumin (MetHSA) synthesis
To synthesize MetHSA, 2 mM HSA in phosphate buffer and 1 mM haemin in dimethyl sulphoxide were incubated together, with rocking, overnight at room temperature and protected from light. MetHSA was then dialysed for 2 h in phosphate buffer followed by additional dialysis in phosphate buffer overnight at 4°C. MetHSA was separated from free haemin using a G25 Sephadex column and concentrated used a 50 kDa Centricon (Millipore, Billerica, MA). MetHSA concentration was determined spectrophometrically reading absorbance at 403 nm, ε = 83/mM/cm(Beaven et al, 1974).
Cell Culture
Bovine aortic endothelial cells (BAECs) were cultured using Dulbecco’s Modified Eagle Medium (DMEM, Invitrogen) containing 4 mM L-glutamine and supplemented with 10% fetal bovine serum, penicillin (200 u/ml), and streptomycin (200 μg/ml) in humidified incubators at 37°C in room air with 5% CO2. For each experiment, cells were seeded into 6-well plates and grown overnight to reach 70–80% confluence. Cells were washed twice with phosphate-buffered saline (PBS) before treatments were added in serum-free medium. Cells used in this study were between passages 6–10.
Induction and Detection of HO-1 in BAEC
MetHb (100 μM) was co-incubated with HSA (600 μM) for 4 h at 37°C and then diluted 10-fold with serum-free medium. Cells were then treated with MetHb in the presence or absence of HSA or presynthesized MetHSA for 2 or 4 h in serum-free medium. At the end of treatment, cells were washed twice with PBS, and complete medium was added to cells for 24 h. BAECS were then washed twice with ice-cold PBS, harvested by scraping, and lysed in radioimmunoprecipitation assay buffer (50 mM Tris-base, 150 mM NaCl, 0.5% Nonidet-P40, 0.5% deoxycholate, 0.1% SDS) containing 1% protease inhibitor cocktail (Sigma). Laemlli Sample Buffer (Bio-Rad, Hercules, CA) containing 5% β-mercaptoethanol was added to cell lysate. Samples were boiled, loaded onto a pre-cast 10% gel, and subjected to electrophoresis at 120 V for 1 h 50 min prior to transfer to a nitrocellulose membrane (100 V for 30 min) in buffer containing 25 mM Tris-base, 150 mM glycine, and 10% methanol. Membranes were blocked for 2 h at room temperature with 5% non-fat dry milk in TBST (Tris-Buffered Saline and Tween 20), immunoblotted with a primary antibody in 1% non-fat dry milk directed against HO-1 (SPA-894, Enzo Life Sciences, Plymouth Meeting, PA) overnight at 4°C, followed by incubation with rabbit secondary antibody in 1% non-fat dry milk for 2 h at room temperature, and visualized using enhanced chemiluminescence. Protein expression was normalized to β-actin.
Detection of Protein Modification Using Biotinylated Arachidonic Acid (Bt-AA)
MetHb (100 μM) was co-incubated with HSA (600 μM) for 4 h at 37°C and then diluted 10-fold with serum-free medium. In the mean time, BAECs cultured as described were preloaded with 10 μM Bt-AA (Cayman, Ann Arbor, MI) for 1 h in serum-free medium. Cells were washed with serum-free medium and treatments were added for 4 h. At the end of treatment, cells were collected and prepared for immunoblot analysis as described above. Samples were loaded onto a pre-cast 10% gel, subjected to electrophoresis at 120 V for 1 h 50 min and transferred to a nitrocellulose membrane (100 V for 30 min) in buffer containing 25 mM Tris-base, 150 mM glycine, and 10% methanol. Membranes were blocked for 1 h at room temperature with 5% non-fat dry milk in TBST, washed thoroughly to remove all milk, and incubated with streptavidin-horseradish peroxidase (HRP; 1:10,000) in TBST for 1 h at room temperature. Membranes were then washed 3 times with TBST and visualized using ECL (Higdon et al, 2009).
Data Analysis
Statistical significance was determined using a T-test. Results were reported as means the standard error of the means (SEM).
Results
MetHb in SCD plasma
EPR spectroscopy is a sensitive and convenient technique to dectect metHb. EPR capitalizes on the presence of unpaired electrons in the ferric form of iron in metHb, which produces a unique signal in the EPR spectrum that is distinct from other signals found in plasma (e.g. signals from copper(II) ions and free radicals). We have previously demonstrated that increased metHb is directly detected by EPR spectroscopy in whole plasma from individuals with SCD, and that the level of metHb dramatically increases upon addition of NO (Reiter et al, 2002). This latter observation is due to the fact that the majority of circulating haemoglobin is in the oxygenated ferrous form (oxyHb) that is oxidized to metHb by reaction with NO (Equation 1).
| [1] |
In the current study, we observed that while the EPR spectrum of plasma from some individuals with SCD showed a single peak, indicating the presence of metHb, the plasma from the many individuals with SCD had a distinctly different spectral feature, i.e., double peak. To determine if the haemoglobin mutation in SCD accounts for the variation in spectra, we examined the EPR spectra of metHb prepared from both normal (HbA) and mutant (HbS) haemoglobin. In both cases the spectrum for metHb was identical and consisted of a single peak that is typical of high-spin ferric haemoglobin (Fig 1A). The fact that HbA and HbS are identical indicates that differences seen in SCD plasma are not caused by intrinsic differences in the mutant haemoglobin itself.
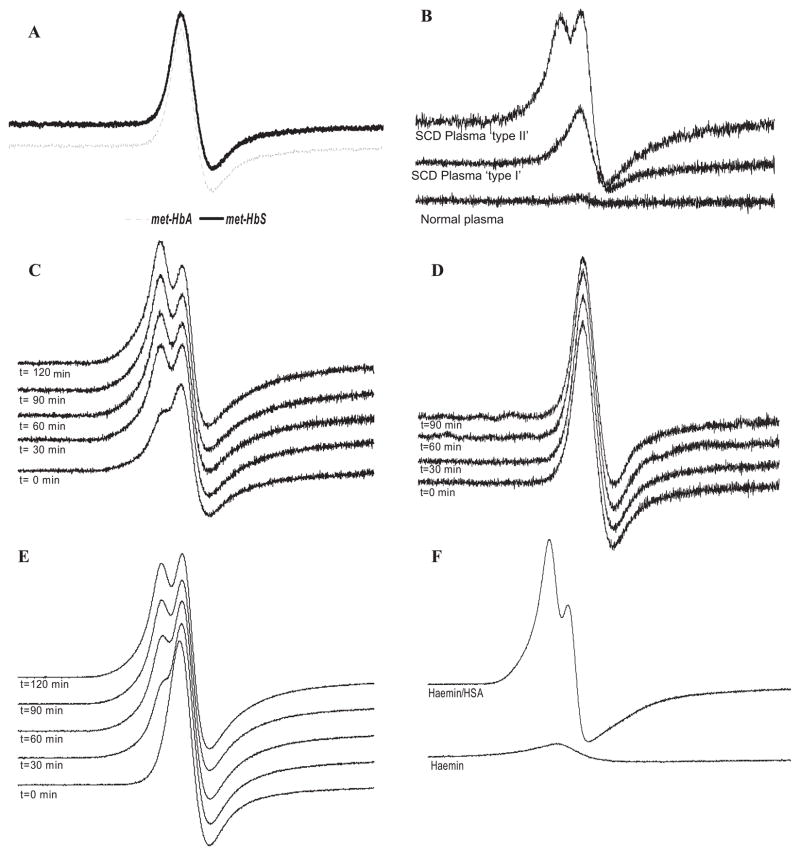
Fig. 1.
The ‘split’ EPR spectrum in the g=6 region in sickle cell plasma. A) EPR spectrum of metHb from HbA (metHbA) and HbS (metHbS). B) EPR spectra of plasma from 2 representative individuals with SCD compared to plasma from a normal individual. C) MetHb (10 μM) added to plasma from an individual with SCD and incubated at 37°C for the indicated times. D) MetHb (10 μM) added to normal plasma and incubated at 37°C for the indicated times. E) MetHb (100 μM) added to normal plasma and incubated at 37°C for the indicated times. F) EPR spectra of haemin (100 μM) and a mixture of haemin (100 μM) and HSA (30 mg/ml). All EPR spectra were obtained at 3.65 K.
Fig 1B shows the EPR spectrum of normal plasma compared to that of two SCD plasma samples. Both SCD plasma samples contained much more EPR-detectable material than the normal sample. Whereas one sample (“type I”) appeared to have an identical spectrum to that of purified metHb (Fig 1A), the other sample (“type II”) exhibited a splitting of the EPR line into a double peak. From a total of 29 SCD plasma samples tested, 10 (34%) exhibited the “type 1” EPR spectrum expected for metHb whereas 19 (66%) exhibited the split “type II” spectrum. To understand the origin of this split peak, both HbA (Figs 1C and D) and HbS (supporting information) were added to plasma from both normal individuals and those with SCD, and incubated at 37 °C. Incubation of HbA with SCD plasma resulted in a slow conversion from the type I to the type II spectral pattern (Fig 1C). In contrast, the addition of either HbA or HbS to normal plasma caused no change in the EPR spectrum (Fig 1D, for HbA, see Supporting Information for HbS). This indicated that the type II spectrum observed in most of the SCD samples, but not control samples, derives from a plasma component rather than intrinsic differences in the type of haemoglobin. Interestingly, when a 10-fold greater amount of metHb was added to normal plasma, the split/type II peak was generated (Fig 1E). This suggested that a factor in normal plasma, which could be limiting the formation of the type II peak, may be overwhelmed at very high metHb levels.
Methaem-albumin (metHSA) formation in SCD plasma
Albumin contains a haem binding site (Adams & Berman, 1980), and haem can transfer between haemoglobin and albumin (Adachi & Asakura, 1976). To examine if the type II EPR peak corresponded to the formation of metHSA, haemin (haem containing iron in the oxidized ferric state) was added directly to HSA. As shown in Fig 1F, addition of haemin to HSA results in a spectrum similar to that observed in SCD (Fig 1B). Addition of haemin to low density lipoprotein (LDL), a plasma lipoprotein, resulted in a single line spectrum (data not shown), suggesting that the type II spectrum may be specific to the binding of ferric haem to the HSA binding site and not simply a reflection of haem in a more hydrophobic environment. To confirm the formation of metHSA in vivo, SCD plasma was immunoprecipitated with an antibody that recognizes HSA or a non-specific control antibody (Supporting Information). A weak ferric EPR signal was associated with the anti-HSA immunoprecipitates (Supporting Information). This suggests that ferric haem is associated with HSA in SCD plasma in vivo and that HSA may have an important role in haem trafficking in SCD.
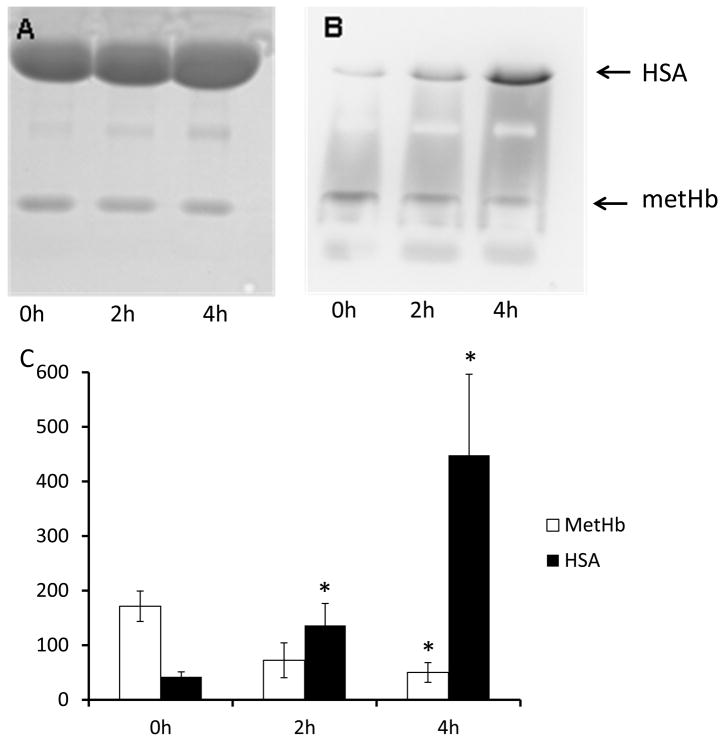
To confirm haem transfer from metHb to HSA, we used an on-gel detection method. This system allows the direct detection of haem-containing proteins within a gel by utilizing the inherent peroxidase activity of haem to elicit luminol-dependent chemiluminescence. As shown in Figure 2, the peroxidase activity of the haemoglobin band diminished and that of the HSA band increased as a function of time (Figs 2B and C), strongly suggesting that haem is transferred from haemoglobin to HSA during this time period. Interestingly, metHSA appeared substantially more active as a peroxidase in this system because the chemiluminescence of HSA at 4 h was more than twice that of the original metHb sample.
Fig. 2.
On-gel detection of haem transfer. MetHb (100μM) was co-incubated with HSA (600 μM) for 4 h at 37°C. Samples taken at 0h, 2h and 4h were subjected to SDS-PAGE. A) The protein bands after staining the gel with Coomassie Blue. B) Gel was treated with (NH4)2S2O8 (0.02 %, w/v) for 10 min, then with H2O2/luminol chemiluminescence reagent, and the signal emitted by the haem was detected. C) Chemiluminescence intensity of metHb and metHb+HSA bands (means+SE, n=3) measured by densitometry. p<0.05; *different from same treatment at time 0.
The role of haptoglobin in metHSA formation
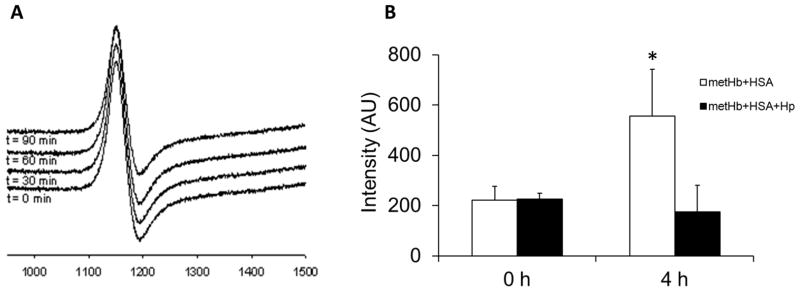
The above results demonstrate that the haem group from metHb can transfer to albumin in plasma from SCD individuals and in mixtures of HSA and metHb, but not in plasma from normal individuals. This suggests that SCD plasma is deficient in a factor that may inhibit this transfer. Given that SCD plasma is deficient in haptoglobin (Reiter et al, 2002), we examined whether haptoglobin prevents haem transfer to HSA. As shown in Fig 3A, when metHb was added to SCD plasma in the presence of excess haptoglobin, the formation of the type II EPR peak associated with metHSA formation was prevented (compare with Fig 1C). We also confirmed the inhibitory effect of haptoglobin on haem transfer using the gel-based system described above, as quantified in Fig 3B, the chemiluminescence signal associated with the HSA protein increased over 4 h in the absence of haptoglobin, but not in the presence of haptoglobin. These data indicate that the binding of haptoglobin to metHb prevents haem release and transfer to albumin suggesting an additional function for this haemoglobin-scavenging protein.
Fig. 3.
Haptoglobin prevents the migration of haem from metHb to HSA in sickle cell plasma. A) MetHb (10 μM) and haptoglobin (20 μM) were added to sickle plasma and incubated at 37°C for 90 min. EPR spectra were taken at the indicated time points. All EPR spectra were obtained at 3.65 K. B) metHb (100 μM) was incubated with HSA (600 μM), or human haptoglobin 1-1 (Hp, 200 μM) and HSA for 4 h at 37oC. Samples taken at 0h and 4h were subjected to SDS-PAGE. Bands of haem-containing proteins were detected by chemiluminescence imaging. The intensities (means+SE, n=3) of HSA-haem bands were measured by densitometry. p<0.05; * different from all other values.
Haem transfer from MetHb to HSA inhibits protein oxidative modification
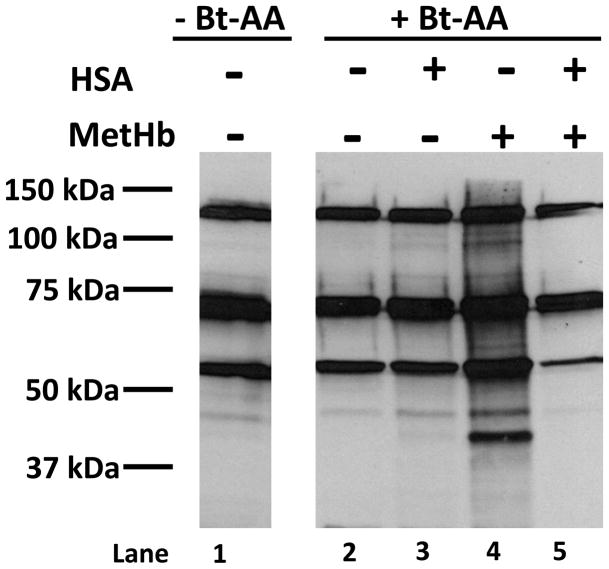
Ferric haem, as found in metHb, readily dissociates from haemoglobin (Umbreit, 2007). As free haem is extremely lipophilic, it easily intercalates into cell membranes and can initiate lipid peroxidation (Belcher et al, 2010). Using biotinylated arachidonic acid (Bt-AA), we investigated if the formation of metHSA-inhibited lipid peroxidation by metHb. Upon oxidation, Bt-AA forms multiple lipid peroxidation products that are electrophilic and able to modify nucleophilic cellular protein targets, which can be readily detected as lipid-protein adducts by immunoblot analyses (Higdon et al, 2009). The biotin tag allows these modified proteins to be visualized by streptavidin-HRP. To determine if the haem transfer from metHb to HSA impacts oxidative effects of metHb, BAECs were pretreated with Bt-AA and then exposed to metHb in the absence or presence of HSA. Figure 4, lane 1 demonstrates endogenous biotin-containing cellular proteins (e.g. carboxylases) in control lysates as detected by streptavidin-HRP. Treatment with either Bt-AA or HSA did not result in increased lipid-protein adduct formation (lanes 2 and 3, respectively). However, in lane 4, the presence of metHb, which catalyses the peroxidation of Bt-AA, significantly potentiated the streptavidin-HRP signal indicative of increased oxidative protein modification. Importantly, the presence of HSA inhibits oxidative protein modification by metHb (lane 5). These data suggest that the formation of metHSA inhibits oxidative stress induced by metHb/haem in the circulation.
Fig. 4.
Haem transfer from MetHb to HSA inhibits protein oxidation. 100 μM MetHb was incubated in the presence or absence of 600 μM HSA for 4 h at 37 °C. These treatments were diluted 10-fold with serum-free medium and added to BAECs that had been pretreated with 10 μM Bt-AA for 1 h. After 4 h, BAECs were collected and the labelling of proteins modified by Bt-AA peroxidation products were visualized (n=3). Lane 1: Control lysate in the absence of Bt-AA. Lane 2: Control lysate in the presence of 10 μM Bt-AA. Lane 3: Cell lysate treated with 60 μM HSA in the presence of 10 μM Bt-AA. Lane 4: Cell lysate treated with10 μM metHb in the presence of 10 μM Bt-AA. Lane 5: Cell lysate treated with10 μM metHb + 60 μM HSA in the presence of 10 μM Bt-AA.
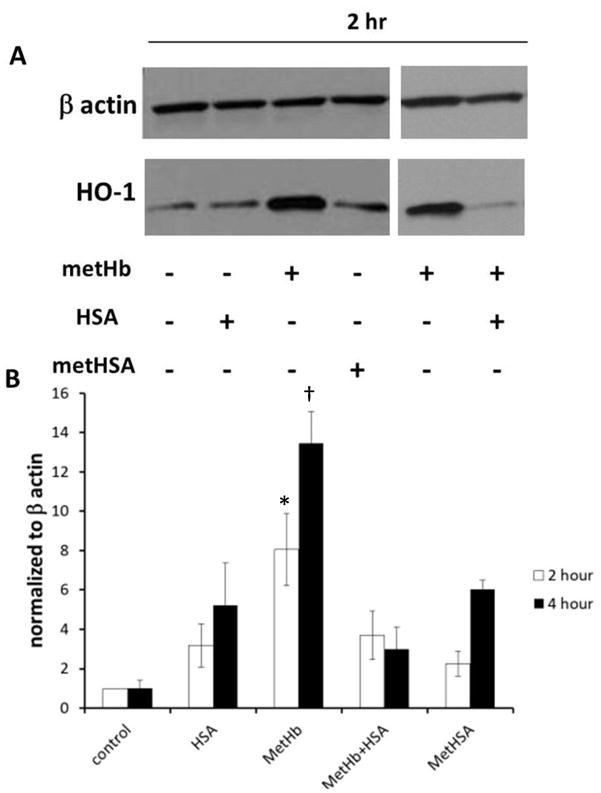
The Effect of MetHSA formation on Haem Oxygenase 1(HO-1) Induction
HO-1 expression is induced by several factors including reactive oxygen species, oxidized lipids, Hb, as well as its substrate - free haem (Loboda et al, 2008). Although the expression of HO-1 results from the presence of oxidative stress, HO-1 induction is probably a cellular defence mechanism as HO-1 enzymatic products possess protective anti-oxidant, anti-inflammatory, anti-apoptotic and vasoactive properties (Balla et al, 1993;Beckman et al, 2009;Belcher et al, 2006;Loboda et al, 2008;Takahashi et al, 2004;Wagener et al, 2003). To determine how HSA affects the induction of HO-1 by metHb, cultured BAECs were treated with metHb in the absence or presence of HSA. Consistent with previous reports, metHb was a potent inducer of HO-1 (Fig 5) (Foresti et al, 2006;Loboda et al, 2008;Takahashi et al, 2004). However, the addition of HSA greatly inhibited HO-1 induction by metHb.
Fig 5.
MetHSA formation inhibits haem oxygenase 1 (HO-1) induction. 100 μM MetHb was incubated in the presence or absence of 600 μM HSA for 4 h at 37 °C. These treatments were diluted 10-fold with serum-free medium and added to BAECs for 2 h or 4 h or cells were treated with synthesized metHSA for 2 h or 4 h. At the end of treatment, complete medium replaced treatment media and cells were collected 24 h later. A) Western blot analysis demonstrating HO-1 expression in BAECs after 2 h treatment. B) HO-1 expression in BAECs after 2h or 4h treatment normalized to β actin (n= 3–11). p<0.05; * metHb+HSA and metHSA at 2 h, †different from all other values at 4 h.
MetHSA possesses peroxidase activity and can induce HO-1 protein expression and activity (Appleton et al, 2003). However, the extent of HO-1 induction by metHSA is probably dependent on the ratio of albumin to haemin during metHSA synthesis (Beaven et al, 1974;Kamal & Behere, 2002). Albumin has a single high affinity binding site for haemin in addition to approximately 10 low-affinity haemin binding sites (Beaven et al, 1974). Synthesizing metHSA with a high haemin to albumin ratio increases the probability of the release of haemin from low-affinity HSA binding sites, resulting in a robust upregulation of HO-1. Using a 2:1 ratio of albumin to haemin reduces the amount of free haemin in the metHSA preparation (Beaven et al, 1974;Kamal & Behere, 2002), ensuring that the HO-1 induction observed is indeed due to metHSA and not free, or loosely bound, haemin. Using a 2:1 albumin:haemin metHSA preparation, we compared the induction of HO-1 by metHb to synthesized metHSA. We found that HO-1 induction by synthesized metHSA was attenuated compared to an equimolar amount of metHb (Figure 5). Altogether, these data support the idea that the formation of metHSA in the plasma of individuals with SCD inhibits HO-1 induction, representing another line of defence against Hb/haem-mediated oxidative stress in this disease.
Discussion
This study examined differences in the fate of haemoglobin/haem in plasma from control and SCD individuals. We provide evidence that metHb in SCD plasma releases its haem group which, in the majority of samples analysed, bound primarily to plasma albumin forming methaemalbumin (metHSA) detectable by a change in the EPR spectrum. The ease of haem transfer appears to be unrelated to the underlying haemoglobin mutation in that haem transfer occurs with both HbS and HbA (Supporting Information). Bunn & Jandl (1968) demonstrated that haptoglobin will prevent the transfer of haem between haemoglobins. Here, we demonstrated that haptoglobin, when added to SCD plasma, inhibits the transfer of haem from metHb to albumin preventing metHSA formation. These data suggest that haptoglobin is playing a dual role, both binding free haemoglobin and preventing haem release from metHb. Therefore, the metHSA observed in SCD plasma probably results from the lack of haptoglobin in the plasma of individuals suffering from SCD.
The presence of metHSA has been previously observed in SCD (Lathem & Jensen, 1959), and also in haemorrhagic shock (Friedman-Mor et al, 1978) and acute pancreatitis (Lankisch et al, 1978), as it has been used as a diagnostic marker for intravascular haemolysis (Ali & Vanderlinder, 1977). Albumin will bind ferric haem relatively rapidly, with a rate constant of 1.7 × 105/M/s at 24 °C, and strongly with an equilibrium constant of 1.1 × 108/M(Adams & Berman, 1980). A high resolution crystal structure of metHSA revealed that haem bound to a hydroxyl oxygen of tyrosine161 in a hydrophobic cleft of albumin (Wardell et al, 2002). Our EPR evidence indicates that the ferric iron is high spin (five unpaired electrons) and is found in a more rhombic geometry than observed with metHb, as indicated by the splitting of the perpendicular components of the g tensor (Fig 1). While this rhombocity was not observed in a previous EPR study of metHSA (Bearden et al, 1974), other investigators have observed this signal in serum from individuals with β-thalassemia and identified it as metHSA (Cannistraro et al, 1980). It is possible that the haem geometry is altered by the binding of additional ligands to metHSA, such as fatty acids or metals, allowing for spectral differences between preparations.
It is not clear whether the sequestration of haem by albumin represents a second line of defence against the oxidative reactions of free haem or, conversely, if metHSA contributes to the oxidative pathology of SCD. Although there are relatively few studies defining the oxidative reactions of metHSA, it does possess peroxidase activity, albeit lower than a ‘professional’ peroxidase such as horseradish peroxidase (Kamal & Behere, 2002). Albumin inhibits haem-mediated lipid peroxidation though with much less efficacy than haemopexin and with concomitant protein oxidation (Vincent et al, 1988). Therefore, we investigated how metHSA formation affected protein oxidation induced by metHb.
Using biotinylated arachidonic acid (Bt-AA), the transfer of haem from metHb to albumin was found to inhibit the formation protein-reactive lipid products triggered by metHb (Fig. 4) (Higdon et al, 2009). This suggests that the formation of metHSA is protective against oxidative stress imposed by metHb and/or free haem.
To further confirm that metHSA formation is protective against oxidative stress, we evaluated the level of HO-1 expression in response to treatment of cells with metHb versus metHSA. HO-1 expression is considered a general indicator of oxidative stress as transcription of this protein is initiated by several factors including reactive oxygen species, oxidized lipids, free haemoglobin, as well as its substrate - free haem (Loboda et al, 2008;Takahashi et al, 2004). While metHb is a potent inducer of HO-1 (Foresti et al, 2006), the pre-incubation of metHb with HSA attenuated HO-1 induction compared to metHb alone (Fig 5). Furthermore, induction of HO-1 by synthesized metHSA was significantly less than induction by metHb at 4 h. Together, these data support the hypothesis that the formation of metHSA in SCD plasma inhibits HO-1 induction.
While HO-1 indicates cellular stress, it has been proposed that HO-1 induction is protective due to the antioxidant properties of its enzymatic products (Wagener et al, 2003). HO-1 is upregulated in SCD, however the level of HO-1 expression appears inadequate to aid in combating the severe free haem overload and oxidative stress present in this disease. Importantly, it has been shown that further potentiation of HO-1 expression or administration of HO-1 products is protective in murine models of SCD (Beckman et al, 2009;Belcher et al, 2010;Loboda et al, 2008). These reports suggest that impacting HO-1 expression in SCD could potentially affect the severity of this disease. Thus, while metHSA formation inhibits HO-1 induction, its unclear if this inhibition prevents the compensation needed for the amount of oxidative stress present in SCD or if it reflects a general reduction in oxidative stress. Further studies need to be done to address this question.
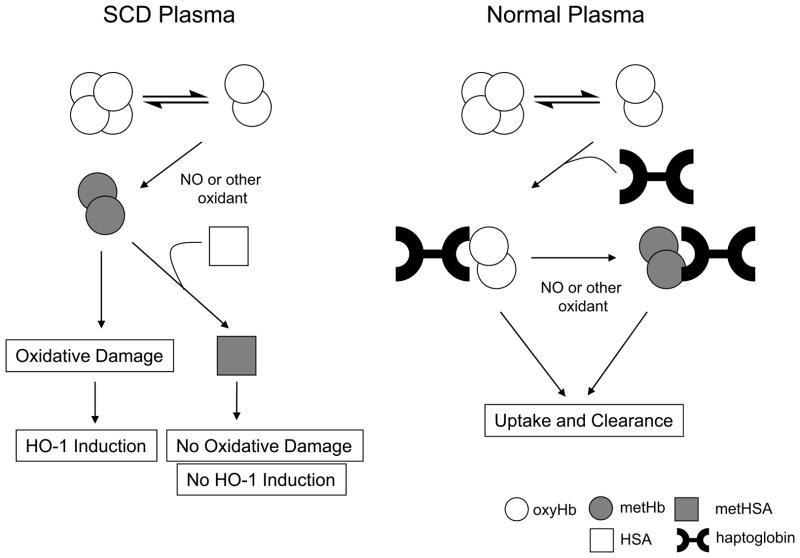
Fig 6 illustrates the proposed differences for the clearance of free haemoglobin in normal versus SCD plasma. According to this model, cell-free haemoglobin in plasma from a normal individual will dissociate into dimers, bind to haptoglobin and be cleared from circulation (Belcher et al, 2010). Dimer formation occurs due to the fact that the tetramer-dimer equilibrium favours dimer formation at the low micromolar levels of Hb found in plasma. If this haemoglobin is indeed oxidized to metHb by reacting with NO or other oxidants, it will remain bound to haptoglobin, and importantly, the haem will remain associated with haemoglobin and be cleared. In contrast, haemoglobin in SCD plasma will dimerize, and if oxidized to the metHb form; the ferric haem can escape the haem pocket and directly contribute to oxidative stress present in SCD or bind to a hydrophobic binding site in HSA, forming metHSA. Once formed, metHSA can inhibit metHb-induced oxidative stress along with subsequent increase in HO-1 expression. Therefore, free haemoglobin in SCD plasma may not only contribute to dysfunction in NO-dependent vascular signalling (Reiter et al, 2002), but may ultimately increase the oxidative stress in the vascular system through haem transfer to HSA that contributes to vascular dysfunction.
Fig 6.
Proposed model for the differences in haem and haemoglobin clearance in normal and SCD plasma.
In conclusion, this study demonstrated that the transfer of ferric haem from metHb to HSA prevents the direct oxidative effects of free haem, including inhibition of oxidative protein modification and HO-1 induction. In turn, haptoglobin, which is present in negligible amounts in SCD, inhibits the transfer of haem to HSA, preventing the formation and subsequent effects of metHSA.
Supplementary Material
Acknowledgments
This work was supported by National Heart, Lung, and Blood Institute grants HL-090503 (NH, CAH), HL-081139 (CAH), HL-44612 (CAH), HL-098032 (MTG), HL-096973 (MTG), and a National Institute of Diabetes and Digestive and Kidney Diseases grant RC1DK0850852 (MTG) as well as the National Biomedical EPR Center funded by NIH grant P41 EB001980-34 and the Hemophilia Center of Western Pennsylvania (MTG). The collection of human samples was supported by Clinical and Translational Science Award (CTSA) 1-UL1-RR031973 from the NIH.
References
- Adachi K, Asakura T. Interaction of serum albumin with normal and sickle hemoglobins. Biochimica et Biophysica Acta. 1976;427:536–548. doi: 10.1016/0005-2795(76)90196-3. [DOI] [PubMed] [Google Scholar]
- Adams PA, Berman MC. Kinetics and mechanism of the interaction between human serum albumin and monomeric haemin. Biochemical Journal. 1980;191:95–102. doi: 10.1042/bj1910095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali MA, Vanderlinder B. Diagnosis of intravascular haemolysis using starch gel electrophoresis. Scandinavian Journal of Haematology. 1977;19:343–346. doi: 10.1111/j.1600-0609.1977.tb01484.x. [DOI] [PubMed] [Google Scholar]
- Andrews NC. Iron homeostasis: insights from genetics and animal models. Nature Reviews Genetics. 2000;1:208–217. doi: 10.1038/35042073. [DOI] [PubMed] [Google Scholar]
- Appleton SD, Marks GS, Nakatsu K, Brien JF, Smith GN, Graham CH, Lash GE. Effects of hypoxia on heme oxygenase expression in human chorionic villi explants and immortalized trophoblast cells. American Journal of Physiology-Heart and Circulatory Physiology. 2003;284:H853–H858. doi: 10.1152/ajpheart.00655.2002. [DOI] [PubMed] [Google Scholar]
- Ascenzi P, Bocedi A, Visca P, Altruda F, Tolosano E, Beringhelli T, Fasano M. Hemoglobin and heme scavenging. IUBMB Life. 2005;57:749–759. doi: 10.1080/15216540500380871. [DOI] [PubMed] [Google Scholar]
- Balla J, Jacob HS, Balla G, Nath K, Eaton JW, Vercellotti GM. Endothelial-cell heme uptake from heme proteins: induction of sensitization and desensitization to oxidant damage. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:9285–9289. doi: 10.1073/pnas.90.20.9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden AJ, Morgan WT, Muller-Eberhard U. Heme complexes of rabbit hemopexin, human hemopexin and human serum albumin: electron spin resonance and Mssbauer spectroscopic studies. Biochemical and Biophysical Research Communications. 1974;61:265–272. doi: 10.1016/0006-291x(74)90562-2. [DOI] [PubMed] [Google Scholar]
- Beaven GH, Chen SH, d’ AA, Gratzer WB. A spectroscopic study of the haemin--human-serum-albumin system. European Journal of Biochemistry. 1974;41:539–546. doi: 10.1111/j.1432-1033.1974.tb03295.x. [DOI] [PubMed] [Google Scholar]
- Beckman JD, Belcher JD, Vineyard JV, Chen C, Nguyen J, Nwaneri MO, O’Sullivan MG, Gulbahce E, Hebbel RP, Vercellotti GM. Inhaled carbon monoxide reduces leukocytosis in a murine model of sickle cell disease. American Journal of Physiology-Heart and Circulatory Physiology. 2009;297:H1243–H1253. doi: 10.1152/ajpheart.00327.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcher JD, Mahaseth H, Welch TE, Otterbein LE, Hebbel RP, Vercellotti GM. Heme oxygenase-1 is a modulator of inflammation and vaso-occlusion in transgenic sickle mice. Journal of Clinical Investigation. 2006;116:808–816. doi: 10.1172/JCI26857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcher JD, Beckman JD, Balla G, Balla J, Vercellotti G. Heme degradation and vascular injury. Antioxidants and Redox Signaling. 2010;12:233–248. doi: 10.1089/ars.2009.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn HF, Jandl JH. Exchange of heme among hemoglobins and between hemoglobin and albumin. Journal of Biological Chemistry. 1968;243:465–475. [PubMed] [Google Scholar]
- Bunn HF, Nathan DG, Dover GJ, Hebbel RP, Platt OS, Rosse WF, Ware RE. Pulmonary hypertension and nitric oxide depletion in sickle cell disease. Blood. 2010;116:687–692. doi: 10.1182/blood-2010-02-268193. [DOI] [PubMed] [Google Scholar]
- Cannistraro S, Indovina PL, Sportelli L. Paramagnetic species in beta-thalassemic sera: an ESR study. Zeitschrift fur Naturforschung C. 1980;35:193–196. doi: 10.1515/znc-1980-3-403. [DOI] [PubMed] [Google Scholar]
- Foresti R, Bains S, Sulc F, Farmer PJ, Green CJ, Motterlini R. The interaction of nitric oxide with distinct hemoglobins differentially amplifies endothelial heme uptake and heme oxygenase-1 expression. Journal of Pharmacology and Experimental Therapeutics. 2006;317:1125–1133. doi: 10.1124/jpet.105.097907. [DOI] [PubMed] [Google Scholar]
- Friedman-Mor Z, Chalon J, Gorstein F, Turndorf H, Chuba JV, Orkin LR. Abnormal heme-protein patterns in hemorrhagic shock. Journal of Trauma. 1978;18:104–107. doi: 10.1097/00005373-197802000-00005. [DOI] [PubMed] [Google Scholar]
- Gladwin MT, Barst RJ, Castro OL, Gordeuk VR, Hillery CA, Kato GJ, Kim-Shapiro DB, Machado R, Morris CR, Steinberg MH, Vichinsky EP. Pulmonary hypertension and NO in sickle cell. Blood. 2010;116:852–854. doi: 10.1182/blood-2010-04-282095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinshtein N, Bamm VV, Tsemakhovich VA, Shaklai N. Mechanism of low-density lipoprotein oxidation by hemoglobin-derived iron. Biochemistry. 2003;42:6977–6985. doi: 10.1021/bi020647r. [DOI] [PubMed] [Google Scholar]
- Hedo CC, Aken’ova YA, Okpala IE, Durojaiye AO, Salimonu LS. Acute phase reactants and severity of homozygous sickle cell disease. Journal of Internal Medicine. 1993;233:467–470. doi: 10.1111/j.1365-2796.1993.tb01000.x. [DOI] [PubMed] [Google Scholar]
- Higdon AN, Dranka BP, Hill BG, Oh JY, Johnson MS, Landar A, rley-Usmar VM. Methods for imaging and detecting modification of proteins by reactive lipid species. Free Radical Biology and Medicine. 2009;47:201–212. doi: 10.1016/j.freeradbiomed.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Ouyang J, Delanghe JR, Baeyens WR, Dai Z. Chemiluminescent image detection of haptoglobin phenotyping after polyacrylamide gel electrophoresis. Analytical Chemistry. 2004;76:2997–3004. doi: 10.1021/ac035109e. [DOI] [PubMed] [Google Scholar]
- Jison ML, Gladwin MT. Hemolytic anemia-associated pulmonary hypertension of sickle cell disease and the nitric oxide/arginine pathway. American Journal of Respiratory and Critical Care Medicine. 2003;168:3–4. doi: 10.1164/rccm.2304002. [DOI] [PubMed] [Google Scholar]
- Jison ML, Munson PJ, Barb JJ, Suffredini AF, Talwar S, Logun C, Raghavachari N, Beigel JH, Shelhamer JH, Danner RL, Gladwin MT. Blood mononuclear cell gene expression profiles characterize the oxidant, hemolytic, and inflammatory stress of sickle cell disease. Blood. 2004;104:270–280. doi: 10.1182/blood-2003-08-2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal JK, Behere DV. Spectroscopic studies on human serum albumin and methemalbumin: optical, steady-state, and picosecond time-resolved fluorescence studies, and kinetics of substrate oxidation by methemalbumin. Journal of Biological Inorganic Chemistry. 2002;7:273–283. doi: 10.1007/s007750100294. [DOI] [PubMed] [Google Scholar]
- Lanaro C, Franco-Penteado CF, Albuqueque DM, Saad ST, Conran N, Costa FF. Altered levels of cytokines and inflammatory mediators in plasma and leukocytes of sickle cell anemia patients and effects of hydroxyurea therapy. Journal of Leukocyte Biology. 2009;85:235–242. doi: 10.1189/jlb.0708445. [DOI] [PubMed] [Google Scholar]
- Lankisch PG, Koop H, Otto J, Oberdieck U. Evaluation of methaemalbumin in acute pancreatitis. Scandinavian Journal of Gastroenterology. 1978;13:975–978. doi: 10.3109/00365527809181378. [DOI] [PubMed] [Google Scholar]
- Lathem W, Jensen WN. Plasma hemoglobin-binding capacity in sickle cell disease. Blood. 1959;14:1047–1056. [PubMed] [Google Scholar]
- Loboda A, Jazwa A, Grochot-Przeczek A, Rutkowski AJ, Cisowski J, Agarwal A, Jozkowicz A, Dulak J. Heme oxygenase-1 and the vascular bed: from molecular mechanisms to therapeutic opportunities. Antioxidant and Redox Signaling. 2008;10:1767–1812. doi: 10.1089/ars.2008.2043. [DOI] [PubMed] [Google Scholar]
- Miller YI, Altamentova SM, Shaklai N. Oxidation of low-density lipoprotein by hemoglobin stems from a heme-initiated globin radical: antioxidant role of haptoglobin. Biochemistry. 1997;36:12189–12198. doi: 10.1021/bi970258a. [DOI] [PubMed] [Google Scholar]
- Minetti M, Mallozzi C, Di Stasi AM, Pietraforte D. Bilirubin is an effective antioxidant of peroxynitrite-mediated protein oxidation in human blood plasma. Archives of Biochemistry and Biophysics. 1998;352:165–174. doi: 10.1006/abbi.1998.0584. [DOI] [PubMed] [Google Scholar]
- Nath KA, Grande JP, Haggard JJ, Croatt AJ, Katusic ZS, Solovey A, Hebbel RP. Oxidative stress and induction of heme oxygenase-1 in the kidney in sickle cell disease. American Journal of Pathology. 2001;158:893–903. doi: 10.1016/S0002-9440(10)64037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeder BJ, Wilson MT. Hemoglobin and myoglobin associated oxidative stress: from molecular mechanisms to disease States. Current Medicinal Chemistry. 2005;12:2741–2751. doi: 10.2174/092986705774463021. [DOI] [PubMed] [Google Scholar]
- Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, III, Schechter AN, Gladwin MT. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nature Medicine. 2002;8:1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- Rossi-Fanelli A, Antonini E, Caputo A. Studies on the relations between molecular and functional properties of hemoglobin. II. The effect of salts on the oxygen equilibrium of human hemoglobin. Journal of Biological Chemistry. 1961;236:397–400. [PubMed] [Google Scholar]
- Sedlak TW, Saleh M, Higginson DS, Paul BD, Juluri KR, Snyder SH. Bilirubin and glutathione have complementary antioxidant and cytoprotective roles. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:5171–5176. doi: 10.1073/pnas.0813132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Morita K, Akagi R, Sassa S. Heme oxygenase-1: a novel therapeutic target in oxidative tissue injuries. Current Medicinal Chemistry. 2004;11:1545–1561. doi: 10.2174/0929867043365080. [DOI] [PubMed] [Google Scholar]
- Umbreit J. Methemoglobin--it’s not just blue: a concise review. American Journal of Hematology. 2007;82:134–144. doi: 10.1002/ajh.20738. [DOI] [PubMed] [Google Scholar]
- Vincent SH, Grady RW, Shaklai N, Snider JM, Muller-Eberhard U. The influence of heme-binding proteins in heme-catalyzed oxidations. Archives of Biochemistry and Biophysics. 1988;265:539–550. doi: 10.1016/0003-9861(88)90159-2. [DOI] [PubMed] [Google Scholar]
- Wagener FA, Volk HD, Willis D, Abraham NG, Soares MP, Adema GJ, Figdor CG. Different faces of the heme-heme oxygenase system in inflammation. Pharmacological Reviews. 2003;55:551–571. doi: 10.1124/pr.55.3.5. [DOI] [PubMed] [Google Scholar]
- Wang X, Tanus-Santos JE, Reiter CD, Dejam A, Shiva S, Smith RD, Hogg N, Gladwin MT. Biological activity of nitric oxide in the plasmatic compartment. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:11477–11482. doi: 10.1073/pnas.0402201101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardell M, Wang Z, Ho JX, Robert J, Ruker F, Ruble J, Carter DC. The atomic structure of human methemalbumin at 1.9 A. Biochemical and Biophysical Research Communications. 2002;291:813–819. doi: 10.1006/bbrc.2002.6540. [DOI] [PubMed] [Google Scholar]
- Yang BK, Vivas EX, Reiter CD, Gladwin MT. Methodologies for the sensitive and specific measurement of S-nitrosothiols, iron-nitrosyls, and nitrite in biological samples. Free Radical Research. 2003;37:1–10. doi: 10.1080/1071576021000033112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.