Abstract
Background. Kidney disease is commonly accompanied by hyperuricemia. However, the contribution of serum uric acid (SUA) to kidney injury is debated. Our objective was to assess the long-term prediction of renal failure by SUA.
Methods. Visit 2 participants in the Jerusalem Lipid Research Clinic cohort with normal baseline kidney function were followed for 24–28 years. SUA levels were assessed for associations with acute renal failure (ARF) and chronic renal failure (CRF) as defined by hospital discharge records, and mortality, ascertained through linkage with the national population registry.
Results. Among 2449 eligible participants (1470 men, 979 women aged 35–78 years in 1976–79), SUA was positively linked with male sex, serum creatinine and components of the metabolic syndrome but was lower in smokers and in diabetic subjects. The 22- to 25-year incidence of hospital-diagnosed kidney failure (145 first events, 67% CRF) and the 24- to 28-year mortality (587 events) were higher in subject with hyperuricemia (>6.5 mg/dL in men and >5.3 mg/dL in women, reflecting the upper quintiles), independent of baseline kidney function and covariates. Hyperuricemia conferred adjusted hazard ratios of 1.36 (P = 0.003), 2.14 (P < 0.001) and 2.87 (P = 0.003) for mortality, CRF and ARF, respectively.
Conclusions. SUA predicts renal failure incidence and all-cause mortality independently of demographic and clinical covariates. These results lend support to the undertaking of clinical trials to examine the effect of uric acid-lowering strategies on kidney outcomes.
Keywords: acute kidney injury, chronic kidney disease, cohort study, mortality, uric acid
Introduction
Uric acid was proposed long ago as a possible determinant of hypertension, diabetes and chronic kidney disease (CKD) in addition to rheumatism and gout [1]. Recent animal studies have convincingly established an association between serum uric acid (SUA) and CKD [2]. In rats, hyperuricemia causes slowly progressive glomerular and tubulointerstitial disease, which is prevented by a uricosuric agent [3]. In this and other animal models, kidney damage is in part mediated by hyperuricemia-induced hypertension [4] but has also a hypertension-independent component [5, 6].
Prospective epidemiological studies link hyperuricemia with risk of new onset or worsening kidney disease. Feig [2] found support for a role of elevated SUA in renal disease in 8 of 12 studies he recently reviewed. These included large studies from the USA [7, 8], Austria [9] and Japan [10], as well as smaller studies from Brazil [11], China [12], Taiwan [13] and South Korea [14]. Increased incidence of both CKD and end-stage renal disease (ESRD) was reported in relation to baseline SUA, as well accelerated CKD progression. Four other studies from Mexico [15], Taiwan [16], USA [17] and Germany/Austria/South Tyrol [18] reported associations that were either weak or did not withstand multivariable adjustment.
A major challenge in observational studies of the relationship between SUA and cardiovascular diseases is accounting for reverse causation and confounding effects. With regard to CKD, decreased glomerular filtration, which defines CKD, is itself a cause of increased SUA. Secondly, an association between SUA and CKD might be a reflection of the metabolic syndrome, which is associated with SUA levels (with increased SUA being both a cause and a consequence of the metabolic syndrome [19, 20]) and, through diabetes, leads to renal disease. Additionally, frank hyperuricemia sometimes leads to uric acid (gouty) nephropathy.
Interventional trials are needed to resolve the role of hyperuricemia in kidney disease. Several recently published small clinical studies collectively suggest a possible causative role [21–23]. Awaiting larger-scale clinical trials, the objective of this work is to evaluate whether SUA predicts the long-term occurrence of kidney disease independent of its associations with baseline kidney function and metabolic abnormalities. To do so, we analyzed data from the population-based Jerusalem Lipid Research Clinic (LRC) cohort study which was initiated in the 1970s as a cardiovascular prevalence study, using protocols of the North American LRC prevalence studies [24–26]. A subset of predominantly middle-aged adults with normal kidney function at baseline is the subject of this study.
Materials and methods
Participants
At Visit 2 of the Jerusalem LRC [24], information was gathered on 2544 middle-aged individuals, of whom 69.2% were randomly sampled from Visit 1 participants and 30.8% were additionally sampled from Visit 1 subjects with elevated lipid levels (for additional information about the sampling scheme see the supplementary data) [24]. Response rates were reported as 72.0% at Visit 1 and 79.7 and 83.0% for the Visit 2 random and high lipid samples, respectively [24]. Creatinine and SUA measurements were not available for 51 participants. An additional 44 had estimated glomerular-filtration rate (eGFR) <60 mL/min/1.73 m2 [‘calculated by the Modification of Diet in Renal Disease (MDRD) formula—see below], leaving 2449 for analysis.
Study variables
Analyses relate to the extensive sociodemographic, lifestyle, anthropometric and clinical chemistry measures collected at Visits 1 and 2 [24, 27, 28]. SUA was determined on a Technicon (USA) Autoanalyzer (AA-I) by a phosphotungstate method [29]. Creatinine clearance (milliliter per minute) was estimated (eCCr) using the Cockroft-Gault formula [30]. GFR (mL/min/1.73 m2) was estimated by the four-variable MDRD equation [31] or the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formulas, reported as more accurate in subjects with normal kidney function [32]. Variables introduced into models in this study include, unless otherwise specified: age, sex, dummy variable for sampling scheme (random recruitment versus high-lipid group enrichment), secular education (years), region of birth (Israel, Europe, Asia and North Africa; three dummy variables), smoking (yes/no), alcohol consumption (grams per week), protein consumption (grams per kilogram body weight), heart rate (1 per minute), diabetes medication status, systolic and diastolic blood pressure (BP: averages of 2 ordinary mercury sphygmomanometric and 2 random-zero measurements), triceps skinfold thickness (millimeters), body-mass index (BMI), hematocrit, SUA, creatinine, glucose, total-, low-density lipoprotein cholesterol (LDL-C) and high-density (HDL-C) lipoprotein cholesterol, triglycerides (ln-transformed), aspartate aminotransferase (AST), serum globulins, thyroxin, bilirubin and stick proteinuria [semiquantified from a casual urine sample as 0 (negative), 10 mg/dL (trace), 30 mg/dL (+1), 100 mg/dL (+2) or 300 mg/dL (+3)]. All laboratory tests were performed on fasting samples.
Outcome variables
Deaths were ascertained through linkage with the national population registry (linkage date: April 2004). Incidence diagnoses of acute renal failure (ARF) (ICD 9: 484), chronic renal failure (CRF) (ICD 9: 485) and unspecified (ICD 9: 486) renal failure were obtained from hospital discharge diagnosis lists by data linkage with the medical archives of all relevant Jerusalem hospitals (linkage date: October 2001).
Statistical analysis
We stratified most of our analyses by sex. To identify variables related to SUA, we grouped participants by sex-specific quintiles of SUA levels and evaluated co-variables using analysis of variance or chi-squared tests, as appropriate. Triglyceride levels were ln-transformed, but actual values are presented. Multivariable linear regression was used to compute the contribution to the variance of SUA through evaluation of the stepwise change in the model R2. Hierarchical clustering by squared Pearson coefficients using average linkage was applied to all baseline variables (standardized) to evaluate the location of SUA in the variable space. Additionally, principal components were extracted from baseline variables (supplementary data). The relationship of SUA with outcome (all-cause mortality, CRF, ARF and unspecified renal failure) was assessed using Kaplan–Meier (KM) curves, Cox proportional hazards and logistic regression models. Unless otherwise stated, SUA was introduced as the highest quintile versus the four lower quintiles grouped. In Cox and logistic regression, age, serum creatinine, the sampling scheme (Cox only) and sex (in sex-pooled models) were forced into the models, while all other variables were subjected to backward elimination (P for entry and removal 0.05 and 0.1, respectively). Introduction of a quadratic SUA term to exploratory Cox models to detect a possible U-shaped relationship with outcome did not improve the fit of the models. A separate analysis evaluated the occurrence of CRF in models censored on coronary heart disease (hospital discharge diagnosis) to address a possible detection bias related to comorbidity [33]. The proportional hazards assumption was validated by introducing the ln(time) × variable interaction, which for SUA was significant only in a non-adjusted Cox model for CRF in men. Weighted logistic regression that reflects the sampling scheme, i.e. the probability to have been sampled from Visit 1 subjects, based on age-specific lipid levels, was also used to evaluate CRF outcomes at 16, 20 and 24 years of follow-up. Analyses were conducted using PASW 17.0 software (SPSS Inc., Chicago, IL). Hierarchical clustering was performed using MeV software version 4.5 [34]. A nominal two-sided P-value <0.05 was considered significant. Data are displayed as mean ± SD unless stated otherwise.
Results
Baseline associations of SUA
SUA was normally distributed, with 30% higher values in men than women: 5.7 ± 1.1 versus 4.5 ± 1.0 mg/dL, respectively (supplementary Figure S1). Notable associations (Table 1 and supplementary Table S1) included lower SUA in diabetic subjects of both sexes [adjusted for age, sex and BMI, SUA was 0.8 mg/dL lower in subjects treated for diabetes (2.0% of participants), 4.3 versus 5.1 mg/dL, P < 0.001]; lower SUA in male smokers (0.3 mg/dL lower age- and BMI-adjusted SUA, P < 0.001); increasing SUA with various constituents of the metabolic syndrome; increasing SUA with education in men (+0.3 mg/dL in those with highest versus lowest education level, adjusted for age, BMI and country of birth, P = 0.005) but decreasing SUA with education in women (not significant after adjustments) and higher SUA in European-born men (5.9 mg/dL, adjusted for age, BMI and education) compared to Asian (5.6 mg/dL, P = 0.01 with Bonferroni correction) or North African-born men (5.7 mg/dL, P = 0.04). SUA levels increased with creatinine and decreased with eGFR but not with eCCr (Table 1). The variance of SUA explained by serum creatinine was 8% (both sexes), while that captured by eGFR was 8%/9% (men/women) using the eGFRMDRD and 6%/8% with the CKD-EPI equations. By multivariable stepwise linear regression, 23% of the SUA variance in men and 29% in women could be attributed to the measured variables, most notably BMI and creatinine (Table 2).
Table 1.
Sociodemographic, anthropometric and clinical characteristics of the study population, partitioned by sex-specific quintiles of SUA (weighed analysis and complete quintile data available as supplementary Table S1)
| Uric acid quintile | Men, n = 1470 |
Women, n = 979 |
||||
| Q1 | Q5 | P-valuea | Q1 | Q5 | P-valuea | |
| SUA, mg/dL | 4.3 ± 0.6 | 7.3 ± 0.7 | <0.001 | 3.1 ± 0.4 | 6.1 ± 0.6 | <0.001 |
| Age, years | 50 ± 6 | 50 ± 6 | 0.8 | 45 ± 5 | 47 ± 5 | <0.001 |
| Place of birth: Israel | 29.9% | 32.8% | 35.6% | 27.9% | ||
| Place of birth: Europe | 14.6% | 30.1% | 22.1% | 24.0% | ||
| Place of birth: Asia | 33.8% | 22.4% | 25.5% | 29.0% | ||
| Place of birth: North Africa | 21.7% | 14.7% | 0.002b | 16.8% | 19.1% | 0.9b |
| Education, years | 8.3 ± 5.2 | 10.3 ± 5.7 | <0.001 | 10.0 ± 5.9 | 8.4 ± 5.5 | 0.003 |
| Alcohol, g/week | 33 ± 30 | 29 ± 22 | 0.2 | 15 ± 13 | 14 ± 16 | 0.2 |
| Smoking | 52.3% | 34.1% | <0.001 | 30.3% | 19.1% | 0.04 |
| BP medication | 7.9% | 29.3% | <0.001 | 6.8% | 29.5% | <0.001 |
| Hypoglycemics | 5.7% | 0.3% | <0.001 | 2.4% | 1.6% | 0.2 |
| Height, cm | 167 ± 7 | 169 ± 7 | <0.001 | 157 ± 6 | 157 ± 6 | 0.3 |
| Weight, kg | 72 ± 11 | 80 ± 12 | <0.001 | 63 ± 8 | 73 ± 12 | <0.001 |
| BMI, kg/m2 | 25.6 ± 3.3 | 28.0 ± 3.5 | <0.001 | 25.5 ± 3.5 | 29.9 ± 5 | <0.001 |
| Triceps skinfold, mm | 22 ± 10 | 24 ± 10 | 0.002 | 27 ± 8 | 33 ± 9 | <0.001 |
| Systolic BP, mmHg | 119 ± 16 | 124 ± 18 | 0.001 | 115 ± 17 | 125 ± 20 | <0.001 |
| Diastolic BP, mmHg | 80 ± 11 | 82 ± 11 | 0.001 | 75 ± 10 | 81 ± 12 | <0.001 |
| Hematocrit, % | 46 ± 5 | 45 ± 4 | 0.3 | 41 ± 5 | 42 ± 4 | 0.04 |
| Glucose, mg/dL | 116 ± 51 | 106 ± 16 | 0.02 | 102 ± 42 | 104 ± 18 | 0.004 |
| Creatinine, mg/dL | 0.83 ± 0.13 | 0.94 ± 0.14 | <0.001 | 0.64 ± 0.12 | 0.74 ± 0.12 | <0.001 |
| eCCr, mL/min | 111 ± 25 | 110 ± 27 | 0.7 | 115 ± 29 | 112 ± 28 | 0.3 |
| eGFR, mL/min/1.73 m2 | 108 ± 20 | 93 ± 18 | <0.001 | 113 ± 26 | 93 ± 18 | <0.001 |
| CKD-EPI, mL/min/1.73 m2 | 102 ± 10 | 93 ± 13 | <0.001 | 107 ± 12 | 96 ± 14 | <0.001 |
| Cholesterol, mg/dL | 209 ± 43 | 213 ± 41 | 0.3 | 207 ± 41 | 225 ± 45 | <0.001 |
| LDL-C, mg/dL | 135 ± 36 | 132 ± 36 | 0.3 | 130 ± 37 | 143 ± 39 | <0.001 |
| HDL-C, mg/dL | 41 ± 10 | 39 ± 9 | 0.001 | 54 ± 13 | 46 ± 12 | <0.001 |
| VLDL-C, mg/dL | 33 ± 28 | 43 ± 26 | <0.001 | 23 ± 15 | 36 ± 25 | <0.001 |
| Triglycerides, mg/dL | 160 ± 130 | 216 ± 121 | <0.001 | 110 ± 59 | 178 ± 108 | <0.001 |
| Globulins, g/L | 31 ± 4 | 32 ± 4 | <0.001 | 33 ± 3 | 33 ± 3 | 0.03 |
| Bilirubin, mg/L | 5.6 ± 2.4 | 6.5 ± 2.7 | <0.001 | 4.6 ± 1.8 | 5.5 ± 2.8 | 0.001 |
| AST, IU/L | 30 ± 17 | 33 ± 12 | 0.01 | 25 ± 6 | 30 ± 11 | <0.001 |
| Thyroxin, μg/dL | 44 ± 7 | 44 ± 8 | 0.7 | 46 ± 8 | 45 ± 8 | 0.1 |
Shown are P-values for linear trends derived from analysis of variance or chi-squared (linear-by-linear) tests, as appropriate.
Pearson chi-square with 3 degrees of freedom. VLDL-C, very low-density lipoprotein cholesterol.
Table 2.
Determinants (correlates) of SUAa
| Variable | Men |
Women |
||
| R2 change (%) | Cumulative R2 (%) | R2 change (%) | Cumulative R2 (%) | |
| Creatinine | 7.6 | 7.6 | 10.5 | 10.5 |
| BMI | 5.8 | 13.4 | 10.3 | 20.8 |
| DM med | 2.0 | 15.4 | 0.6 | 21.4 |
| Triglycerides | 2.0 | 17.4 | 4.8 | 26.2 |
| Smoking | 1.4 | 18.8 | ||
| AST | 1.1 | 27.3 | ||
| Globulins | 1.0 | 19.8 | ||
| Bilirubin | 0.9 | 20.7 | 1.6 | 28.9 |
| Education | 0.9 | 21.6 | ||
| Glucose | 0.7 | 22.3 | ||
| Asian origin | 0.4 | 22.7 | ||
| Cholesterol | 0.3 | 23.0 | ||
Derived by stepwise linear regression models (with Pin 0.05 and Pout 0.1). The following were introduced into the models: age, secular education level (years), birth origin (three dummy variables), protein and alcohol consumption, smoking, diabetes medication status (DM med), BMI, triceps skinfold thickness, systolic and diastolic blood pressure, hematocrit, creatinine, globulins, serum AST, thyroxine, bilirubin, fasting glucose (ln), total cholesterol, triglycerides (ln), LDL-C, HDL-C and very low-density lipoprotein cholesterol, urine protein (stick) and sampling scheme dummy variable.
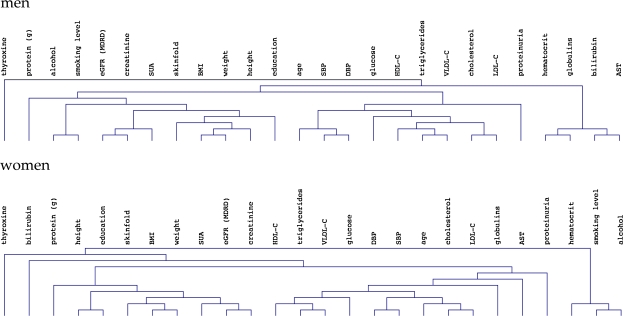
To further evaluate relationships between variables, and to specifically search for those closely associated with SUA, we subjected the baseline data to unsupervised hierarchical clustering (Figure 1), which placed SUA in the vicinity of creatinine, adiposity indices, height and education in both sexes. Analysis of principal components is presented in the supplementary material.
Fig. 1.
Hierarchical clustering dendrograms of baseline variables (using average linkage on squared Pearson coefficients of standardized variables) in men (top) and women (bottom). ‘Protein (g)’ denotes dietary protein consumption in grams per kg body weight.
All-cause mortality
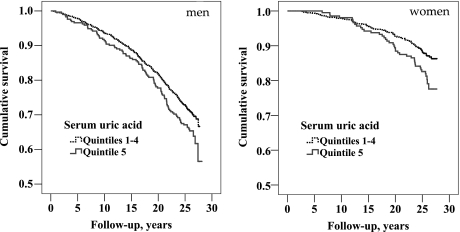
Data linkage identified 587 deaths during follow-up, for mortality rates of 12.5 and 6.2 per 1000 person-years in men (448 events) and women (139 events), respectively. SUA was associated with mortality in both sexes (Figure 2), with a stronger effect apparent in women. Cox proportional hazards model indicated a multivariable adjusted 36% [95% confidence interval (CI) 11–66%] increase in all-cause mortality in subjects with hyperuricemia (highest sex-specific quintile). Table 3 displays additional predictors. Notably, serum creatinine was not a significant predictor and neither were eGFRMDRD nor the CKD-EPI eGFR in analogous models (both P-values = 0.1).
Fig. 2.
KM curves plotting overall survival by SUA threshold in men (left), log-rank P-value = 0.02 (n = 1470; 448 events) and women (right), log-rank P-value = 0.001 (n = 979; 139 events).
Table 3.
Cox models predicting all-cause mortality (n = 2449, 587 events)a
| Variable, unit | HR (95% CI) | P-value |
| Hyperuricemia, Q5 versus Q1–Q4 | 1.36 (1.11–1.66) | 0.003 |
| Age, year | 1.11 (1.09–1.12) | <0.001 |
| Glucose, per 2.7-fold increase | 4.10 (2.90–5.78) | <0.001 |
| Smoking, Y versus N | 1.66 (1.39–1.98) | <0.001 |
| Sex, F versus M | 0.61 (0.46–0.79) | <0.001 |
| Education, year | 0.97 (0.96–0.99) | <0.001 |
| Systolic BP, 10 mmHg | 1.09 (1.04–1.14) | <0.001 |
| LDL-C, 10 mg/dL | 1.03 (1.01–1.06) | 0.003 |
| Triceps skinfold, 5 mm | 0.94 (0.90–0.99) | 0.02b |
| Globulins, g/L | 1.03 (1.00–1.06) | 0.02 |
| AST, 10 IU/L | 1.06 (1.01–1.12) | 0.02 |
| BMI, 5 kg·m−2 | 1.14 (1.02–1.29) | 0.03 |
| Creatinine, mg/dL | 0.65 (0.34–1.23) | 0.2 |
Cox proportional hazards were computed with backward elimination of variables (SUA, age and creatinine and were forced into the model). BP, blood pressure.
Borderline interaction was noted with sex (P = 0.07); the HR being <1 only in men.
Hospital diagnosis of CRF
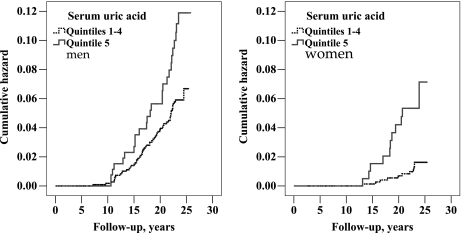
CRF (ICD 9: 585) was reported in hospital discharge summaries of 87 men (2.8 per 1000 subject-years) and 22 women (1.0 per 1000 subject-years). Leading comorbid conditions were ischemic heart disease (49%), diabetes mellitus (38%) and heart failure (25%) (comorbid diagnoses are presented in supplementary Table S2). KM curves (Figure 3) showed that a steeper relationship between SUA and CRF might exist in women compared to men. Cox models were thus constructed to adjust for co-variables and to assess interactions with SUA (entered as the upper fifth versus the lower four quintiles grouped together). The gender interaction with SUA was borderline, both in an age-adjusted model (P = 0.07) and in the fully adjusted model (P = 0.05). Further models were thus either stratified by sex or included the interaction term. The highest SUA quintile was associated with CRF in both men and women; hazards ratio (HR) 1.94 (95% CI 1.20–3.14, P = 0.007) in men and HR 5.20 (95% CI 1.90–14.2, P = 0.001) in women. SUA predicted CRF similarly in subjects with either normal eGFRMDRD (≥90 mL/min/1.73 m2, n = 1683, 65 events) or subnormal eGFRMDRD (<90 mL/min/1.73 m2, n = 766, 44 events); adjusted HR 2.21 (95% CI 1.24–3.96, P = 0.007) and 2.37 (95% CI 1.20–4.66, P = 0.01), respectively, for the highest quintile subjects. Other predictor variables are depicted in Table 4.
Fig. 3.
KM curves plotting cumulative hazards of CRF in men (left), log-rank P-value = 0.001 (n = 1470; 87 events) and women (right), log-rank P-value = 0.001 (n = 979; 22 events) by SUA threshold.
Table 4.
Cox models predicting CRFa
| Variable | Men (n = 1470, 87 events) |
Women (n = 979, 22 events) |
||
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Hyperuricemia, Q5 versus Q1–4 | 1.94 (1.20–3.14) | 0.007 | 5.20 (1.90–14.2) | 0.001 |
| Glucose, per 2.7-fold increase | 13.7 (6.61–28.4) | 0.001 | N/A | |
| Age, year | 1.09 (1.05–1.12) | 0.001 | 1.10 (1.00–1.21) | 0.05 |
| Smoking, Y versus N | 2.89 (1.82–4.59) | 0.001 | 4.48 (1.48–13.6) | 0.008 |
| Globulins, g/L | 1.12 (1.05–1.19) | 0.001 | N/A | |
| Cholesterol, 10 mg/dL | 1.09 (1.03–1.15) | 0.002 | N/A | |
| Origin: Israel | N/A | 1 | 0.05b | |
| Origin: Europe | N/A | 0.32 (0.06–1.71) | 0.2 | |
| Origin: Asia | N/A | 0.91 (0.27–3.11) | 0.9 | |
| Origin: North Africa | N/A | 3.11 (0.88–11.1) | 0.08 | |
| Stick proteinuria, 10 mg/dL | 1.04 (1.01–1.08) | 0.008 | 1.12 (1.05–1.19) | 0.001 |
| LDL-C, 10 mg/dL | N/A | 1.51 (1.11–2.07) | 0.01 | |
| DM med, Y versus N | N/A | 20.0 (1.94–205) | 0.01 | |
| Alcohol, g/week | N/A | 0.95 (0.91–0.99) | 0.03 | |
| Education, years | 0.95 (0.92–1.00) | 0.03 | N/A | |
| Systolic BP, 10 mmHg | N/A | 1.25 (1.00–1.57) | 0.05 | |
| Creatinine, mg/dL | 2.27 (0.45–11.4) | 0.3 | 1.36 (0.03–65.3) | 0.9 |
Cox proportional hazards were computed with backward elimination of variables (SUA, age and creatinine were forced into the model). BP, blood pressure; DM med, diabetes medication status. As separate models were run for men and women, some variables entered only one of the gender models and are depicted with ‘N/A’ in the opposite gender.
This P-value reflects the overall effect, with 3 degrees of freedom.
Weighted logistic regression that accounted for the sampling scheme of the Visit-2 cohort (see Materials and methods) was consistent with the Cox findings. Odds ratios (OR) for CRF in subjects with hyperuricemia (fifth SUA quintile) were 4.6 (1.1–19.9) after 12 years (n = 2299, 15 events), 3.21 (1.1–9.4) after 16 years (n = 2223; 38 events), 1.8 (0.9–3.8) at 20 years (n = 2124; 70 events) and 3.2 (1.4–7.2) at 24 years (n = 730; 108 events) (see supplementary Table S3).
Addressing a possible detection bias related to comorbidity
In this study, renal failure outcomes were extracted from hospital discharge diagnoses. This might have introduced a detection bias, wherein exposure to high SUA levels would favor the detection of CRF through a direct relationship of hyperuricemia with the disease-causing hospitalization. Indeed, in >9500 hospitalizations during follow-up of our cohort, several common conditions that have been previously linked with increased uric acid levels were found to be closely associated with CRF. For example, CRF was more likely to occur with a concomitant diagnosis of heart failure (OR = 26), chronic ischemic heart disease (OR = 12), diabetes mellitus (OR = 10) and aortic aneurysm (OR = 52). We thus reanalyzed CRF outcome after censoring (at the date of hospital diagnosis) for acute and chronic forms of ischemic heart disease (ICD 9: 410–414), the most frequent comorbid diagnoses of CRF and thus possibly a source of detection bias. CHD-‘free’ hospital diagnoses of CRF were found in only 32 men (1.2 per 1000 subject-years) and 7 women (0.3 per 1000 subject-years). KM curves (with both sexes combined, due to the small number of events) showed that highest SUA quintile subjects were prone to CRF (P = 0.003, supplementary Figure S2); the HR was 2.51 (95% CI 1.28–4.92, P = 0.007) in a CHD-censored Cox model with adjustment for age (P < 0.001), sex (P = 0.6), smoking (P = 0.09), ln-glucose (P < 0.001) and creatinine [P = 0.006 with HR 19.7 (95% CI 2.4–163)].
Acute renal failure
ARF (ICD 9: 584) was reported in hospital summaries of 34 men (1.1 per 1000 subject-years) and 10 women (0.44 per 1000 subject-years). Comorbid diagnoses are presented in supplementary Table S2. More than half had CRF. The small number of events allowed only limited analyses. Subjects in the upper sex-specific quintile of SUA were diagnosed with ARF more often than others as determined by a KM analysis (log-rank P-value = 0.003, supplementary Figure S3) and by an adjusted Cox model (HR 2.87; 95% CI 1.45–5.69, P = 0.003). As opposed to CRF, KM analysis did not suggest a specific cutoff for prediction of ARF by SUA (supplementary Figure S4). Thus, SUA was introduced as an interval variable and was found to be a strong predictor of ARF (second only to age), with a 74% (95% CI 33–127%) increase in hazards per 1 mg/dL of SUA (P < 0.001). Other independent predictors of ARF were smoking [HR 2.40 (1.23–4.67, P = 0.01)], diabetes [HR 6.16 (1.43–26.5), P = 0.02] and stick proteinuria [HR 1.06 (1.02–1.10) per 10 mg/dL, P = 0.007]. Creatinine (P = 0.2) and sex (P = 0.7) were not significantly predictive.
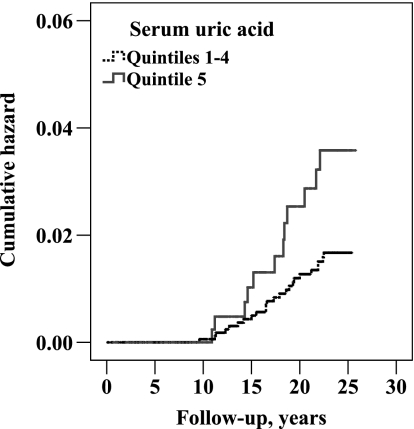
In this study, elevated SUA conferred increased risks of ARF and CRF. We thus concluded with additional analyses combining both forms of renal failure, as well as unspecified renal failure (ICD 9: 586). A total of 145 individuals were diagnosed with renal failure (the first diagnosis being CRF in 97, ARF in 22 and unspecified RF in 26). KM curves plotting the cumulative hazard of a first renal event were similar for subjects in SUA quintiles 1–4 but were significantly steeper in the upper quintile of SUA (Figure 4, P < 0.001). In sex-stratified Cox backward elimination multivariable models, HRs of SUA were 2.25 (1.50–3.38, P < 0.001) in men and 2.70 (1.10–6.31, P = 0.03) in women, for the highest versus lower four pooled quintiles. Other significant renal hazard predictors were age, smoking, education (inverse, men only), ln-glucose, cholesterol (men only), serum globulins (in men and borderline in women) and stick proteinuria. Alcohol consumption (inverse, P = 0.05), triceps skinfold thickness (P = 0.09) and systolic BP (P = 0.06) showed borderline prediction in women.
Fig. 4.
KM curves plotting the cumulative hazards of any renal event (ARF, CRF or unspecified) by SUA quintiles, log-rank P-value <0.001 (n = 2449; 145 events).
Discussion
In this study, we extend previous findings regarding the relationship of uric acid with renal failure and its determinants. We address reverse causation and confounding.
The well-known difference in baseline SUA between men and women (supplementary Figure 1) invited sex-stratified analyses, as performed in some, but not all previous studied. Furthermore, the determinants of SUA levels were in part gender specific. Unadjusted, age and LDL-C were associated with SUA in women but not in men and measures of adiposity were more important in women than in men, while education, smoking, height and fasting glucose were associated with SUA only in men (Table 1). Most of these differences persisted upon multivariable adjustment (Table 2). The inverse association of SUA and glucose was prominent but was limited to subjects with high fasting glucose levels (upper quintile; data not shown). We suspect that this represents the hyperglycemic effect of glomerular hyperfiltration [35, 36], but hypouricemia linked to overproduction of nitric oxide due to hyperglycemia-induced oxidative stress has also been suggested [37]. Likewise, decreased SUA levels in smokers [38] has been linked to antioxidant neutralization [39]. As expected, SUA levels correlated with creatinine, suggesting similar renal handling since this relationship withstood adjustment for age, sex and BMI, although only 7.6% of the variance of SUA in men and 10% in women could be attributed to creatinine. Thus, either production of uric acid is in general a stronger determinant of SUA levels or more likely elimination is not in simple relation with kidney function [40].
The relationship of baseline SUA with all-cause mortality (36% increased risk associated with hyperuricemia, defined in our study as SUA levels in the top 20%) is in line with that of most large population studies (summarized by Ioachimescu et al. [41]). Independence from baseline kidney function was suggested by exclusion of participants with eGFR < 60 mL/min/1.73 m2 and by including serum creatinine in the models (with similar findings when eGFR replaced creatinine). Of note, in the National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study, mortality was related to baseline SUA only in women [42]. In our study, similar gender differences existed but were abolished by multivariable adjustment and/or introduction of SUA as dichotomized variable (data not shown).
SUA consistently proved predictive of CRF, the major outcome of interest in this study. The nature of the association of SUA with CRF was not linear. A clear increase in incident CRF was observed only with SUA in the upper fifth. These levels were >6.5 mg/dL (mean ± SE: 7.3 ± 0.7) in men and >5.3 mg/dL (mean ± SE: 6.1 ± 0.6) in women, close to the standard clinical definition of hyperuricemia (7 mg/dL in men and 6 mg/dL in women) [43]. In both sexes, hyperuricemia predicted outcome independent of serum creatinine in multivariable-adjusted analyses, and did so in subjects with either normal or subnormal baseline eGFR.
Among 13 prior observational studies on the association of SUA with kidney outcome of which 12 were reviewed by Feig [2], the largest was reported from Northern California using the US Renal Data System registry [7]. The adjusted HR for ESRD was 2.14 (1.65–2.77) for the highest versus lowest quartile of SUA. In an Okinawa study population with normal baseline kidney function, the 2-year risk of developing abnormal renal function was 2.9-fold and 10-fold in men and women with SUA levels >8 mg/dL, respectively [10]. Pooled Atherosclerosis Risk in Communities and Cardiovascular Health Study (CHS) data revealed that 1 mg/dL increase in SUA was associated with a multivariable-adjusted OR of 1.11 (1.02–1.21) for incident kidney disease [8]. In healthy Viennese, the OR for new onset kidney disease was 1.30 (1.26–1.34) per 1 mg/dL increase in SUA [9]. Very recently, a linear association was reported in a cohort of Italian blood donors between SUA and kidney function decline at 5 years [44]. Studies reporting weak or absent independent associations include a separate analysis of the CHS (in which SUA predicted CKD progression but not incident renal disease [17]; Weiner et al. [8] suggested that the finding in this elderly population may be biased toward the null) and the small-sized Mild-to-Moderate Kidney Disease study [18]. Dissimilarities and inconsistencies between the studies reinforce the need for additional study.
Hyperuricemia has seldom been addressed as a possible predictor of (or contributor to) ARF [45, 46], aside from acute urate nephropathy that is characteristic of tumor lysis. Despite the small number of ARF incidence events, we found that SUA strongly and independently predicted ARF, second only to age, conferring a 74% increased risk per 1 mg/dL. Ejaz et al. [46] proposed several mechanisms by which uric acid may contribute to ARF, including renal vasoconstriction (reduction of nitrous oxide and renin-angiotensin system stimulation), alteration of autoregulation (preglomerular arteriolar disease), antiangiogenic effects and proinflammatory and prooxidative properties. Uricase treatment provided subtle renal benefits in a hyperuricemia rat model of cisplatin toxicity [47]. Combining ARF, CRF and unspecified renal failure events, hyperuricemia (highest quintile) conferred HR of 2.2 in men and 2.7 in women, independent of other potential predictors, including serum creatinine.
A weakness of our study is the modest number of events, which limited the possibilities for sensitivity/subgroup analyses. In women, who contributed fewer events than men, outcome-related estimates are less precise. For ARF, the number of events did not allow stratification by sex, which may be important in light of findings from the data reduction procedures (see principal component analysis in the supplement data). Another potential limitation relates to the mode of ascertainment of outcome events. For this study, relevant laboratory data (i.e. creatinine) from follow-up visits of the Visit 2 adult cohort were not available. Instead, renal outcomes were derived from hospital discharge and death notification diagnoses. Firstly, these may not be complete due to admission to hospitals outside Jerusalem (although 97% still had a recorded Jerusalem address at the end of the mortality follow-up). Secondly, while diagnosis of ARF typically leads to hospitalization, and is thus a good candidate outcome for our design, CRF is predominantly included in hospital summaries as a secondary diagnosis, related to medical history or current laboratory findings. Conceivably, some subjects with CRF were not identified because they were never hospitalized. In those identified, the censoring time is inevitably overestimated, as CRF must have existed prior to the admission date (a possible advantage of CRF diagnosis by hospital records might be higher specificity [8]). Thirdly, hospitalized CRF patients differ from never-hospitalized individuals with regard to comorbidities and complications. This may have led to overestimation of the link between SUA and CRF caused by a detection bias. For example, hospitalization due to a manifestation of SUA-linked coronary disease will provide an opportunity to diagnose CRF and also create an indirect association between SUA and CRF. Our CHD-censored analysis addressed this potential source of bias. Although the number of outcome events remaining in these analyses was small, the point estimate was similar to the uncensored estimate. Lastly, we cannot exclude the possibility that subtle kidney dysfunction, not detectable by creatinine-based baseline estimates in subjects with eGFR ≥60 mL/min/1.73 m2, led to high SUA levels (i.e. reverse causation).
In summary, our study adds a layer to the growing body of observational data that argue for a causal relationship in which hyperuricemia precedes and begets renal dysfunction, ARF and CRF, independent of baseline kidney function [2, 48]. The accumulating evidence supports large-scale interventional trials to test the hypothesis that lowering SUA can prevent or halt CKD [21–23].
Supplementary data
Supplementary data, Figures S1–S3 and, Tables S1–S3 are available online at http://ndt.oxfordjournals.org.
Acknowledgments
The authors gratefully acknowledge Professors Y. Stein and (the late) S. Eisenberg who established the Jerusalem LRC Prevalence Study.
Sources of support: IZB is supported by Grant Award Number UL1RR024143 from the National Center for Research Resources, a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research. IZB is also supported in part by a fellowship from the American Physicians Fellowship for Medicine in Israel. The Jerusalem LRC follow-up component was supported by grants to JDK from the US-Israel Binational Science Foundation and the Israel Science Foundation. The Jerusalem LRC Prevalence Study was supported by the US National Heart, Lung and Blood Institute Contract No NO1-HV-5-3015-L.
Conflict of interest statement. None declared.
The results presented in this paper have not been published previously in whole or part, except in abstract format.
References
- 1.Mahomed FA. On chronic Bright's disease, and its essential symptoms. Lancet. 1879;114:399–401. [Google Scholar]
- 2.Feig DI. Uric acid: a novel mediator and marker of risk in chronic kidney disease? Curr Opin Nephrol Hypertens. 2009;18:526–530. doi: 10.1097/MNH.0b013e328330d9d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mazzali M, Kanellis J, Han L, et al. Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. Am J Physiol Renal Physiol. 2002;282:F991–F997. doi: 10.1152/ajprenal.00283.2001. [DOI] [PubMed] [Google Scholar]
- 4.Mazzali M, Hughes J, Kim YG, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38:1101–1106. doi: 10.1161/hy1101.092839. [DOI] [PubMed] [Google Scholar]
- 5.Sanchez-Lozada LG, Tapia E, Soto V, et al. Effect of febuxostat on the progression of renal disease in 5/6 nephrectomy rats with and without hyperuricemia. Nephron Physiol. 2008;108:p69–p78. doi: 10.1159/000127837. [DOI] [PubMed] [Google Scholar]
- 6.Corry DB, Eslami P, Yamamoto K, et al. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin-angiotensin system. J Hypertens. 2008;26:269–275. doi: 10.1097/HJH.0b013e3282f240bf. [DOI] [PubMed] [Google Scholar]
- 7.Hsu C-Y, Iribarren C, McCulloch CE, et al. Risk factors for end-stage renal disease: 25-year follow-up. Arch Intern Med. 2009;169:342–350. doi: 10.1001/archinternmed.2008.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiner DE, Tighiouart H, Elsayed EF, et al. Uric acid and incident kidney disease in the community. J Am Soc Nephrol. 2008;19:1204–1211. doi: 10.1681/ASN.2007101075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Obermayr RP, Temml C, Knechtelsdorfer M, et al. Predictors of new-onset decline in kidney function in a general middle-European population. Nephrol Dial Transplant. 2008;23:1265–1273. doi: 10.1093/ndt/gfm790. [DOI] [PubMed] [Google Scholar]
- 10.Iseki K, Oshiro S, Tozawa M, et al. Significance of hyperuricemia on the early detection of renal failure in a cohort of screened subjects. Hypertens Res. 2001;24:691–697. doi: 10.1291/hypres.24.691. [DOI] [PubMed] [Google Scholar]
- 11.Borges RL, Hirota AH, Quinto BM, et al. Uric acid as a marker for renal dysfunction in hypertensive women on diuretic and nondiuretic therapy. J Clin Hypertens (Greenwich) 2009;11:253–259. doi: 10.1111/j.1751-7176.2009.00101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen N, Wang W, Huang Y, et al. Community-based study on CKD subjects and the associated risk factors. Nephrol Dial Transplant. 2009;24:2117–2123. doi: 10.1093/ndt/gfn767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen YC, Su CT, Wang ST, et al. A preliminary investigation of the association between serum uric acid and impaired renal function. Chang Gung Med J. 2009;32:66–71. [PubMed] [Google Scholar]
- 14.Park JT, Kim DK, Chang TI, et al. Uric acid is associated with the rate of residual renal function decline in peritoneal dialysis patients. Nephrol Dial Transplant. 2009;24:3520–3525. doi: 10.1093/ndt/gfp272. [DOI] [PubMed] [Google Scholar]
- 15.Magdalena M, Mark JS, Xuelei W, et al. Uric acid and long-term outcomes in CKD. Am J Kidney Dis. 2009;53:796–803. doi: 10.1053/j.ajkd.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.See L-C, Kuo C-F, Chuang F-H, et al. Serum uric acid is independently associated with metabolic syndrome in subjects with and without a low estimated glomerular filtration rate. J Rheumatol. 2009;36:1691–1698. doi: 10.3899/jrheum.081199. [DOI] [PubMed] [Google Scholar]
- 17.Chonchol M, Shlipak MG, Katz R, et al. Relationship of uric acid with progression of kidney disease. Am J Kidney Dis. 2007;50:239–247. doi: 10.1053/j.ajkd.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Sturm G, Kollerits B, Neyer U, et al. Uric acid as a risk factor for progression of non-diabetic chronic kidney disease? The Mild to Moderate Kidney Disease (MMKD) Study. Exp Gerontol. 2008;43:347–352. doi: 10.1016/j.exger.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Ford ES, Li C, Cook S, et al. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation. 2007;115:2526–2532. doi: 10.1161/CIRCULATIONAHA.106.657627. [DOI] [PubMed] [Google Scholar]
- 20.Borges RL, Ribeiro AB, Zanella MT, et al. Uric acid as a factor in the metabolic syndrome. Curr Hypertens Rep. 2010;12:113–119. doi: 10.1007/s11906-010-0098-2. [DOI] [PubMed] [Google Scholar]
- 21.Kanbay M, Ozkara A, Selcoki Y, et al. Effect of treatment of hyperuricemia with allopurinol on blood pressure, creatinine clearance, and proteinuria in patients with normal renal functions. Int Urol Nephrol. 2007;39:1227–1233. doi: 10.1007/s11255-007-9253-3. [DOI] [PubMed] [Google Scholar]
- 22.Siu YP, Leung KT, Tong MK, et al. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis. 2006;47:51–59. doi: 10.1053/j.ajkd.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Goicoechea M, de Vinuesa SG, Verdalles U, et al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol. 2010;5:1388–1393. doi: 10.2215/CJN.01580210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slater PE, Friedlander Y, Baras M, et al. The Jerusalem Lipid Research Clinic: sampling, response and selected methodological issues. Isr J Med Sci. 1982;18:1106–1112. [PubMed] [Google Scholar]
- 25.The Lipid Research Clinics Program. Protocol of the Lipid Research Program Prevalence study. Chapel Hill, NC: University of North Carolina at Chapel Hill; 1974. [Google Scholar]
- 26.Manual of Laboratory Operations. Lipid and Lipoprotein Analysis. Vol. 1. Bethesda, MD: DHEW Publication; 1974. Lipid research cinics program. [Google Scholar]
- 27.Kark JD, Slater PE, Stein Y. Cardiovascular risk factors in Jerusalem: The Lipid Research Clinic Prevalence Study. I and II. Isr J Med Sci. 1982;18 pp. 1106–1252. [PubMed] [Google Scholar]
- 28.Slater PE, Kark JD, Friedlander Y, et al. Correlates of biochemical variables in the Jerusalem Lipid Research Clinic population. Isr J Med Sci. 1982;18:1223–1232. [PubMed] [Google Scholar]
- 29.Hawk PB, Oser BL, Summerson WH. Practical Physiologic Chemistry. 13th edn. Philadelphia: Blackiston; 1954. [Google Scholar]
- 30.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 31.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 32.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sackett DL. Bias in analytic research. J Chronic Dis. 1979;32:51–63. doi: 10.1016/0021-9681(79)90012-2. [DOI] [PubMed] [Google Scholar]
- 34.Saeed AI, Sharov V, White J, et al. TM4: a free, open-source system for microarray data management and analysis. Biotechniques. 2003;34:374–378. doi: 10.2144/03342mt01. [DOI] [PubMed] [Google Scholar]
- 35.Bo S, Cavallo-Perin P, Gentile L, et al. Hypouricemia and hyperuricemia in type 2 diabetes: two different phenotypes. Eur J Clin Invest. 2001;31:318–321. doi: 10.1046/j.1365-2362.2001.00812.x. [DOI] [PubMed] [Google Scholar]
- 36.Edyta G, Kazimierz C, Krzysztof S, et al. Renal handling of uric acid in patients with type 1 diabetes in relation to glycemic control. Arch Med Res. 2005;36:32–35. doi: 10.1016/j.arcmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Pitocco D, Stasio ED, Romitelli F, et al. Hypouricemia linked to an overproduction of nitric oxide is an early marker of oxidative stress in female subjects with type 1 diabetes. Diabetes Metab Res Rev. 2008;24:318–323. doi: 10.1002/dmrr.814. [DOI] [PubMed] [Google Scholar]
- 38.Goldbourt U, Medalie JH. Characteristics of smokers, non-smokers and ex-smokers among 10,000 adult males in Israel: II. Physiologic, biochemical and genetic characteristics. Am J Epidemiol. 1977;105:75–86. doi: 10.1093/oxfordjournals.aje.a112358. [DOI] [PubMed] [Google Scholar]
- 39.Waring WS, McKnight JA, Webb DJ, et al. Uric acid restores endothelial function in patients with type 1 diabetes and regular smokers. Diabetes. 2006;55:3127–3132. doi: 10.2337/db06-0283. [DOI] [PubMed] [Google Scholar]
- 40.So A, Thorens B. Uric acid transport and disease. J Clin Invest. 2010;120:1791–1799. doi: 10.1172/JCI42344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ioachimescu AG, Brennan DM, Hoar BM, et al. Serum uric acid is an independent predictor of all-cause mortality in patients at high risk of cardiovascular disease: a preventive cardiology information system (PreCIS) database cohort study. Arthritis Rheum. 2008;58:623–630. doi: 10.1002/art.23121. [DOI] [PubMed] [Google Scholar]
- 42.Freedman DS, Williamson DF, Gunter EW, et al. Relation of serum uric acid to mortality and ischemic heart disease. The NHANES I Epidemiologic Follow-up Study. Am J Epidemiol. 1995;141:637–644. doi: 10.1093/oxfordjournals.aje.a117479. [DOI] [PubMed] [Google Scholar]
- 43.Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359:1811–1821. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bellomo G, Venanzi S, Verdura C, et al. Association of uric acid with change in kidney function in healthy normotensive individuals. Am J Kidney Dis. 2010;56:264–272. doi: 10.1053/j.ajkd.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 45.Nanji AA, Stewart DJ, Mikhael NZ. Hyperuricemia and hypoalbuminemia predispose to cisplatin-induced nephrotoxicity. Cancer Chemother Pharmacol. 1986;17:274–276. doi: 10.1007/BF00256698. [DOI] [PubMed] [Google Scholar]
- 46.Ejaz AA, Mu W, Kang D-H, et al. Could uric acid have a role in acute renal failure? Clin J Am Soc Nephrol. 2007;2:16–21. doi: 10.2215/CJN.00350106. [DOI] [PubMed] [Google Scholar]
- 47.Roncal CA, Mu W, Croker B, et al. Effect of elevated serum uric acid on cisplatin-induced acute renal failure. Am J Physiol Renal Physiol. 2007;292:F116–F122. doi: 10.1152/ajprenal.00160.2006. [DOI] [PubMed] [Google Scholar]
- 48.Berbari AE. European Society of Hypertension Scientific Newsletter: Update on Hypertension Management. The role of uric acid in hypertension, cardiovascular events, and chronic kidney disease. Vol. 11. Via Medica, www.viamedica.pl. (2010, date last accessed) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.