Abstract
We have previously found that CHF1/Hey2 prevents the development of phenylephrine-induced cardiac hypertrophy. To determine the role of CHF1/Hey2 in pressure overload hypertrophy, we performed ascending aortic banding on wild-type and transgenic mice overexpressing CHF1/Hey2 in the myocardium. We found that both wild-type and transgenic mice developed increased ventricular weight to body weight ratios 1 week after aortic banding. Wild-type mice also developed decreased fractional shortening after 1 week when compared to preoperative echocardiograms and sham-operated controls. Transgenic mice, in comparison, demonstrated preserved fractional shortening. Histological examination of explanted heart tissue demonstrated extensive fibrosis in wild-type hearts, but minimal fibrosis in transgenic hearts. TUNEL staining demonstrated increased apoptosis in the wild-type hearts but not in the transgenic hearts. Exposure of cultured neonatal myocytes from wild-type and transgenic animals to hydrogen peroxide, a potent inducer of apoptosis, demonstrated increased apoptosis in the wild-type cells. Gene Set Analysis of microarray data from wild-type and transgenic hearts 1 week after banding revealed suppression and activation of multiple pathways involving apoptosis, cell signaling, and biosynthesis. These findings demonstrate that CHF1/Hey2 promotes physiological over pathological hypertrophy through suppression of apoptosis and regulation of multiple transcriptional pathways. These findings also suggest that CHF1/Hey2 and its downstream pathways provide a variety of targets for novel heart failure drug discovery, and that genetic polymorphisms in CHF1/Hey2 may affect susceptibility to hypertrophy and heart failure.
Introduction
Cardiac hypertrophy is both a physiologic process allowing for adaptation to hemodynamic load and a pathologic process ultimately resulting in chamber dilatation and progression to heart failure. Heart failure can be due to systolic dysfunction, where the heart is unable to contract with normal force, or diastolic dysfunction, in which the heart does not relax normally during diastole, leading to elevated filling pressures. Under conditions such as organismal growth or exercise training, the myocardium hypertrophies by increasing the number of sarcomeres, myofibrils, and by increasing myocyte width, resulting in an increased ventricular wall thickness. Under pathological loading conditions such as in chronic hypertension, the heart initially undergoes hypertrophy by the same mechanisms; however, the myocytes also elongate, eventually develop contractile dysfunction and undergo apoptotic cell death, resulting in systolic cardiac failure, fibrosis, and diastolic failure. Heart failure is a leading cause of morbidity and mortality in Western Society, with the incidence increasing every year. Although multiple signaling pathways have been implicated in the development of hypertrophy, the molecular mechanisms that regulate the transition from a normal, physiologic response to a maladaptive, pathological response are poorly understood. In addition, the molecular mechanisms responsible for the progression from hypertrophy to heart failure remain to be elucidated. Finally, factors that negatively regulate hypertrophy are even less well characterized, and are of tremendous interest due to their potential therapeutic value.
We have previously reported that the Hairy-related bHLH transcriptional repressor, CHF1 (also called Hey2, Hesr-2, Hrt2, HERP1, and gridlock), functions as a suppressor of cardiac hypertrophy induced by phenylephrine (Xiang et al., 2006). Suppression of cardiac hypertrophy is mediated, at least in part, by the binding of CHF1/Hey2 to the transcription factor GATA4, thereby inhibiting the ability of GATA4 to activate atrial naturietic factor (ANF) gene expression along with other genes associated with cardiac hypertrophy (Fischer et al., 2005; Kathiriya et al., 2004; Xiang et al., 2006). CHF1/Hey2 is normally expressed in the developing heart and vasculature, and plays a critical role in heart development, possibly as an effector of Notch signaling, as previously described (Chin et al., 2000; Donovan et al., 2002; Gessler et al., 2002; Iso et al., 2001; Kokubo et al., 1999; Leimeister et al., 1999; Nakagawa et al., 1999; Sakata et al., 2002; Zhong et al., 2000). Additional studies have underscored the importance of CHF1/Hey2 in the development of the myocardium, as loss of CHF1/Hey2 leads to a thin walled myocardium (Sakata et al., 2006), cardiomyopathy (Sakata et al., 2002), and ectopic expression of atrial genes in the ventricular myocardium (Koibuchi and Chin, 2007; Xin et al., 2007).
Although CHF1/Hey2 can suppress phenylephrine-induced cardiac hypertrophy through inhibition of GATA4 activity, and is essential for normal myocardial function, a more detailed analysis of its role in hypertrophy and progression to heart failure has not been reported. To assess further how CHF1/Hey2 affects hypertrophy and progression to heart failure we assessed the response of wild-type and CHF1/Hey2 transgenic mice to ascending aortic banding. In this study we report that myocardial overexpression of CHF1/Hey2 suppresses apoptosis, development of fibrosis, and prevents the development of heart failure, thereby promoting physiological, rather than pathological hypertrophy after aortic banding. Further global analysis of transcriptional pathways altered in the transgenic heart reveals profound alterations in a variety of cellular pathways associated with apoptosis, signaling, and biosynthetic pathways.
Materials and Methods
Transgenic mice, echocardiography, and aortic banding surgery
Transgenic mice overexpressing CHF1/Hey2 in the myocardium under the control of the mlc2v promoter have been previously described (Xiang et al., 2006). Echocardiography was performed 1 day prior to surgery and 1 day prior to harvest using a Visual Sonics VEVO 770 system equipped with a 707B scan head, as previously described (Liao et al., 2002). Wild-type and transgenic mice were subjected to ascending aortic constriction or sham operation for 1 week as previously described (Liao et al., 2002). Four wild-type mice were included in both the sham and banding groups. Five mice were in the transgenic sham group and seven mice were in the transgenic aortic banding group. All animal procedures were approved by the University of Washington Institutional Animal Care and Use Committee.
Gravimetry, histology, and apoptosis detection
One week after surgery, mice were euthanized by carbon dioxide inhalation followed by weighing, heart removal, and exsanguination. Hearts were rinsed in cold phosphate-buffered saline, trimmed of atrial and vascular tissue, dried briefly, and weighed. Heart tissue was then quick frozen in liquid nitrogen and either stored at −80° C or processed for histology or RNA as described below. For analysis of hypertrophy after aortic banding, four animals were included in the wild-type sham-operated group and the wild-type aortic banding groups, five in the transgenic sham-operated group, and seven in the transgenic aortic banding group.
For histology, hearts were fixed in 4% paraformaldehyde, embedded in paraffin and sectioned by standard methods. Sections were deparaffinized, rehydrated, and stained with Masson-trichrome by standard methods. Fibrosis was quantified using the NIH Image J program to measure fibrotic area as a percentage of total area. To detect apoptotic cells, sections were deparaffinized, stained with TUNEL reagent, and counterstained with methyl green with a commercially available kit according to the manufacturer's instructions (Roche Molecular Diagnostics, Pleasanton, CA). The number of apoptotic cells was normalized to the total number of nuclei to derive the percentage of apoptotic cells.
Neonatal mouse myocyte culture
Neonatal mouse cardiac myocytes were harvested and cultured as previously described (Xiang et al., 2006). To induce apoptosis, neonatal myocytes in serum-free medium were exposed to hydrogen peroxide at varying concentrations for 24 h. Apoptotic cells were detected by TUNEL staining with a commercially available kit (Roche Molecular Diagnostics) while the remaining cells were counterstained with hematoxylin or methyl green by standard methods. The percent apoptotic cells was derived by dividing the number of apoptotic cells by the total number of cells and multiplying by 100.
RNA isolation and microarray analysis
RNA was isolated from frozen heart tissue by homogenization with a Polytron homogenizer and then extraction with a commercially available kit (Qiagen, Chatsworth, CA) according to the manufacturer's instructions. For microarray hybridization, RNA was processed for hybridization to Affymetrix Mouse 430 2.0 arrays through a Core Facility at the Harvard Partners Center for Genetics and Genomics (Cambridge, MA), as previously described (Shirvani et al., 2007) and according to the manufacturer's instructions. RNA was isolated from four biological replicates in each group of mice (WT and TG) subjected to aortic banding.
Microarray data was analyzed by Gene Set Analysis (Efron and Tibshirani, 2007), a modification of Gene Set Enrichment Analysis (Subramanian et al., 2005). A full description of the method is available online at http://www-stat.stanford.edu/∼tibs/GSA/. Gene sets for analysis were obtained from the Gene Ontology Project (www.geneontology.org) and the Broad Institute (www.broad.mit.edu). The specific gene set collections focused on were Biological Process (BP; Gene Ontology) and Curated Gene Sets (C2; Broad). Gene sets were considered to be significantly altered when their statistical p-values and false discovery rates as determined by the software were <0.001 (see below). All microarray data have been deposited in the Gene Expression Omnibus Database under accession number GSE13031.
Statistical analysis
Echocardiographic and gravimetric data were compared between groups by a Student t-test, with error bars indicating standard error of the mean. Statistical significance was defined by a value of p < 0.05.
Raw microarray data was processed and analyzed with Bioconductor (Gentleman et al., 2004) and normalized with the Bioconductor GC-RMA package (Wu et al., 2004). From the normalized data, genes with significant evidence for differential expression were identified using the limma package (Smyth, 2004) in Bioconductor. The p-values were calculated with a modified t-test with in conjunction with an empirical Bayes method to moderate the standard errors of the estimated log-fold changes. The p-values were adjusted for multiplicity with the Bioconductor package qvalue (Tusher et al., 2001), which allows for selecting statistically significant genes while controlling the estimated false discovery rate.
We used Gene Set Analysis (GSA) to investigate categories of genes where the constituent genes show coordinated changes in expression over the experimental conditions (Efron and Tibshirani, 2007; Gentleman et al., 2005; Subramanian et al., 2005). GSA considers all the genes in the experiment and allows for the identification of gene sets with strong crosscorrelation, which makes it possible to detect gene sets based on modest changes in gene expression. We used two gene set collections for the GSA, one from the Gene Ontology Project (Camon et al., 2004), called Biological Process, and one set from the Molecular Signature Database (Subramanian et al., 2005), called functional C2 (http://www.broad.mit.edu/gsea/msigdb/msigdb_index.html).
Results
Mice overexpressing CHF1/Hey2 in the myocardium develop hypertrophy after aortic banding but demonstrate preserved ventricular function
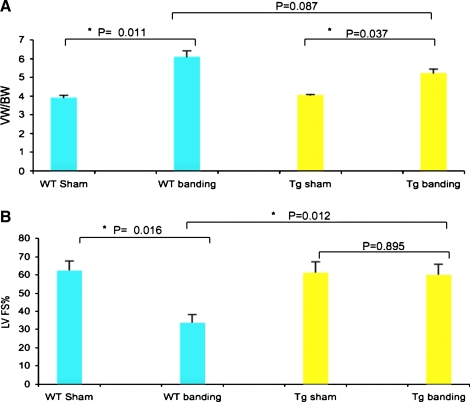
To assess whether CHF1/Hey2 overexpress affects the development of pressure-overload induced hypertrophy, we performed ascending aortic banding and sham operations on wild-type and transgenic mice as previously reported (Liao et al., 2002). As shown in Figure 1, both wild-type and transgenic mouse hearts demonstrate an increase in ventricular weight to body weight ratio 1 week after aortic banding when compared to sham-operated animals. Body weight did not change significantly during the study and was not different between groups (data not shown). Echocardiographic analysis, however, showed a marked difference in left ventricular function in wild-type and transgenic mice 1 week after banding. Wild-type mice showed a marked decrement in left ventricular fractional shortening after aortic banding when compared with sham-operated controls (33.3 ± 4.5% vs. 62.3 ± 5.3%, p = 0.016). Transgenic mice, in contrast, showed no significant decrement in left ventricular function when compared with sham-operated animals (60.0 ± 5.9% vs. 61.2 ± 6.0%, p = 0.895). Comparison of wild-type mice and transgenic mice after banding demonstrated a significant protective effect of CHF1/Hey2 overexpression on left ventricular fractional shortening (33.3 ± 4.5% vs. 60.0 ± 5.9%, p = 0.012). These findings demonstrate that CHF1/Hey2 overexpression prevents the progression to systolic heart failure that normally develops in response to pressure overload.
FIG. 1.
CHF1/Hey2 overexpression in myocardium prevents the progression of hypertrophy to heart failure after aortic banding. (A) Ventricle weight to body weight ratio of the WT or TG hearts at 1 week after sham operation or aortic banding are shown. (B) Left ventricular fractional shorting assessed by echocardiography in the WT and TG mice at 1 week after sham operation or aortic banding are shown. p-Values are listed between the groups, and asterisks indicate significant differences.
Overexpression of CHF1/Hey2 in the myocardium suppresses fibrosis and apoptosis after aortic banding
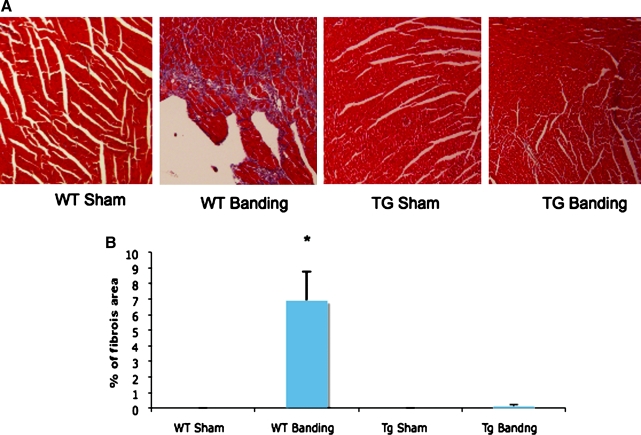
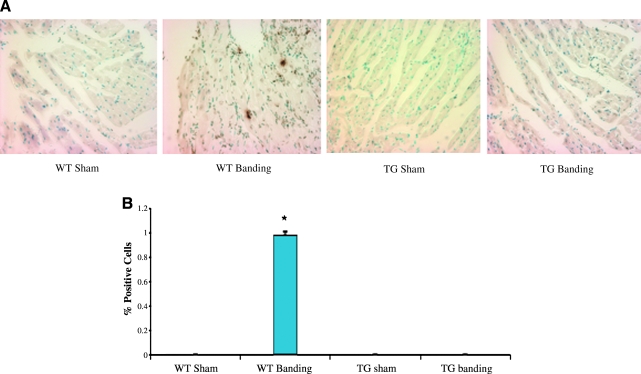
To assess for the development of fibrosis, hearts were explanted from banded and sham-operated wild-type and transgenic animals, paraffin-embedded, sectioned, and stained with Masson-Trichrome, which detects the presence of collagen fibers and delineates tissue fibrosis. As shown in Figure 2, wild-type hearts demonstrate diffuse fibrosis throughout the myocardium after banding, while transgenic hearts do not demonstrate any significant fibrosis. Quantification of the fibrotic area as a percentage of total area confirmed the significance of these results (Fig. 2B). To assess whether programmed cell death could contribute to cell loss and heart failure, tissue sections were analyzed by TUNEL staining. As shown in Figure 3, TUNEL positive cells are detected in wild-type hearts after aortic constriction, while none are detected in transgenic hearts, although the exact identity of these cells has not been determined. Quantification of TUNEL-positive cells as a percentage of total nuclei demonstrated a significant decrease in transgenic hearts compared to wild-type hearts (0.0 ± 0% vs. 0.98 ± 0.03%, p = 0.0009). These findings demonstrate that overexpression of CHF1/Hey2 in the myocardium prevents the development of fibrosis and apoptosis under conditions of pressure overload, two features of pathological hypertrophy and progression to heart failure.
FIG. 2.
Overexpression of CHF1/Hey2 in the myocardium suppresses fibrosis after aortic banding. (A) Representative images of the Masson-Trichrome stained sections are shown. (B) Quantitative assessment of the area of fibrosis in mouse heart sections. The percentage of fibrosis area was quantified using the NIH Image J program. A Student t-test was used for data analysis, and asterisks indicate significant differences.
FIG. 3.
Overexpression of CHF1/Hey2 in the myocardium suppresses apoptosis after aortic banding. (A) Representative images of TUNEL stained heart sections are shown. Brown spots are positively stained cells. (B) Quantitative assessment of TUNEL-positive stained cells. The percentage of apoptotic cells after normalizing for total nuclei are graphed. Each group has three replicates. The asterisk indicates significant differences.
Isolated neonatal cardiac myocytes that overexpress CHF1/Hey2 are resistant to apoptosis
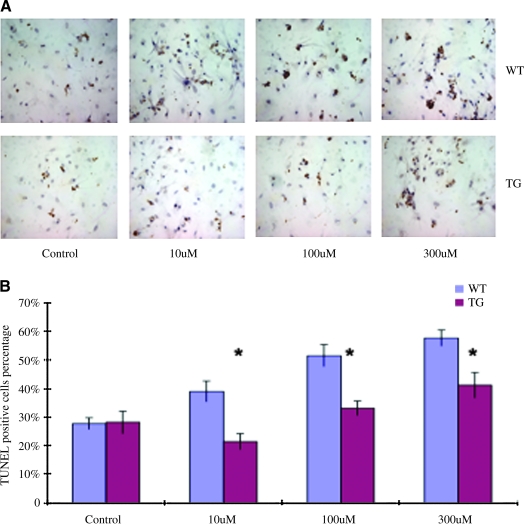
To determine whether CHF1/Hey2 overexpression affects the susceptibility of cardiac myocytes to apoptosis, we cultured neonatal myocytes from wild-type and transgenic mice as previously described (Xiang et al., 2006) and treated them with hydrogen peroxide, a potent inducer of oxidative stress and subsequent apoptosis, as previously described (Aikawa et al., 1997). As shown in Figure 4, wild-type and transgenic myocytes showed comparable rates of apoptosis in culture in the absence of hydrogen peroxide. After treatment, however, the transgenic myocytes demonstrated decreased apoptosis when compared to wild-type cells at all concentrations studied. These findings demonstrate that CHF1/Hey2 overexpression in cardiac myocytes confers resistance to oxidative stress-induced apoptosis.
FIG. 4.
Neonatal cardiac myocytes that overexpress CHF1/Hey2 are resistant to apoptosis. (A) Representative TUNEL staining of neonatal cardiac myocytes from WT or TG mice treated with different concentrations of hydrogen peroxide. (B) Quantitative assessment of TUNEL staining positive cells in neonatal cardiac myocytes from WT29or TG mice with or without hydrogen peroxide treatment. The experiments were repeated more than three times. *p < 0.05.
Transcriptional profiling of wild-type and transgenic hearts after banding reveals alterations in multiple gene sets
To identify the potential downstream targets of CHF1/Hey2 that may play a role in the conversion of a pathological to a physiological hypertrophic response, we analyzed transcriptional profiles from four wild-type hearts and four transgenic hearts after aortic banding. Using Gene Set Analysis, we examined gene sets organized by Gene Ontology Biological Process (BP) or as curated in the C2 set from the Broad Institute. As shown in Table 1, multiple BP gene sets involved in development, protein posttranslational modification, DNA binding, protein targeting, and interferon gamma biosynthesis/innate immunity were suppressed by expression of the transgene during pressure overload. A similar analysis of C2 gene sets (http://www.broad.mit.edu/gsea/msigdb/index.jsp) demonstrates suppression of multiple important signaling pathways such as the Protein Kinase A-G protein-coupled receptor pathway, the phosphoinositide 3-kinase (PI3K) pathway and the Sonic Hedgehog (SHH) signaling pathway (Table 2). Other important pathways implicated suggest that apoptosis (BAD, BCL2, Mitochondrial Death Pathway) and cell growth (Cell Growth an/or Maintenance, Tumor Suppressor, and Cell Cycle Checkpoint) are suppressed in the transgenic hearts. Analysis of BP gene sets that were enriched in the transgenic hearts relative to wild type under conditions of pressure overload revealed a striking increase in pathways involved in cellular biosynthesis and metabolism, as well as neuron and glial cell maturation (Table 1). Analysis of C2 gene sets that were upregulated revealed multiple pathways, although a clear theme of gene expression was not apparent (Table 2).
Table 1.
Gene Ontology Biological Process (BP) Gene Set Changes in CHF1 Transgenic Hearts versus Wild-type Hearts after Banding
| GO ID | Biological process | p-Value | FDR | Size | Score |
|---|---|---|---|---|---|
| Downregulation | |||||
| GO:0019058 | viral infectious cycle | 0 | 0 | 54 | −0.4175225 |
| GO:0048547 | gut morphogenesis | 0 | 0 | 19 | −0.450444 |
| GO:0048660 | regulation of smooth muscle cell proliferation | 0 | 0 | 39 | −0.5640157 |
| GO:0018345 | Protein palmitoylation | 0 | 0 | 13 | −0.7775406 |
| GO:0051093 | negative regulation of developmental process | 0 | 0 | 699 | −0.1313092 |
| GO:0051099 | positive regulation of binding | 0 | 0 | 78 | −0.5377002 |
| GO:0031214 | biomineral formation | 0 | 0 | 212 | −0.3777978 |
| GO:0006612 | protein targeting to membrane | 0 | 0 | 43 | −1.1217363 |
| GO:0006613 | cotranslational protein targeting to membrane | 0 | 0 | 24 | −1.4767097 |
| GO:0051173 | positive regulation of nitrogen compound metabolic process | 0 | 0 | 19 | −0.5543403 |
| GO:0042228 | interleukin-8 biosynthetic process | 0 | 0 | 13 | −0.5613182 |
| GO:0045088 | regulation of innate immune response | 0 | 0 | 35 | −0.3498941 |
| GO:0045089 | positive regulation of innate immune response | 0 | 0 | 31 | −0.4118965 |
| GO:0045078 | positive regulation of interferon-gamma biosynthetic process | 0 | 0 | 15 | −0.8523111 |
| GO:0045047 | protein targeting to ER | 0 | 0 | 16 | −1.184931 |
| GO:0007369 | gastrulation | 0 | 0 | 84 | −0.2704839 |
| GO:0045410 | positive regulation of interleukin-6 biosynthetic process | 0 | 0 | 11 | −1.1335204 |
| GO:0045416 | positive regulation of interleukin-8 biosynthetic process | 0 | 0 | 9 | −1.0549346 |
| GO:0007281 | germ cell development | 0 | 0 | 51 | −0.3836074 |
| GO:0032845 | negative regulation of homeostatic process | 0 | 0 | 14 | −0.4267299 |
| GO:0060193 | positive regulation of lipase activity | 0 | 0 | 9 | −0.5739478 |
| GO:0043388 | positive regulation of DNA binding | 0 | 0 | 72 | −0.5384653 |
| GO:0033044 | regulation of chromosome organization and biogenesis | 0 | 0 | 19 | −0.8839245 |
| GO:0043543 | protein amino acid acylation | 0 | 0 | 52 | −0.7305637 |
| GO:0050792 | regulation of viral reproduction | 0 | 0 | 21 | −0.8001997 |
| GO:0001704 | formation of primary germ layer | 0 | 0 | 47 | −0.4741349 |
| GO:0001707 | mesoderm formation | 0 | 0 | 43 | −0.4592077 |
| GO:0001503 | ossification | 0 | 0 | 211 | −0.3821917 |
| Upregulation | |||||
| GO:0014015 | positive regulation of gliogenesis | 0 | 0 | 11 | 1.3162319 |
| GO:0000096 | sulfur amino acid metabolic process | 0 | 0 | 41 | 0.887549 |
| GO:0000097 | sulfur amino acid biosynthetic process | 0 | 0 | 26 | 0.995851 |
| GO:0048709 | oligodendrocyte differentiation | 0 | 0 | 29 | 0.6032096 |
| GO:0019751 | polyol metabolic process | 0 | 0 | 39 | 0.7205886 |
| GO:0006071 | glycerol metabolic process | 0 | 0 | 36 | 0.7961914 |
| GO:0006183 | GTP biosynthetic process | 0 | 0 | 22 | 0.820716 |
| GO:0006171 | cAMP biosynthetic process | 0 | 0 | 37 | 0.6533879 |
| GO:0006220 | pyrimidine nucleotide metabolic process | 0 | 0 | 45 | 0.5972666 |
| GO:0006221 | pyrimidine nucleotide biosynthetic process | 0 | 0 | 35 | 0.6099049 |
| GO:0006662 | glycerol ether metabolic process | 0 | 0 | 66 | 0.6323004 |
| GO:0006638 | neutral lipid metabolic process | 0 | 0 | 62 | 0.553058 |
| GO:0006639 | acylglycerol metabolic process | 0 | 0 | 62 | 0.553058 |
| GO:0006997 | nuclear organization and biogenesis | 0 | 0 | 74 | 0.3104331 |
| GO:0009147 | pyrimidine nucleoside triphosphate metabolic process | 0 | 0 | 25 | 0.7655897 |
| GO:0009251 | glucan catabolic process | 0 | 0 | 15 | 0.9068106 |
| GO:0009220 | pyrimidine ribonucleotide biosynthetic process | 0 | 0 | 26 | 0.8260659 |
| GO:0009218 | pyrimidine ribonucleotide metabolic process | 0 | 0 | 27 | 0.9118403 |
| GO:0042769 | DNA damage response, detection of DNA damage | 0 | 0 | 8 | 1.6054863 |
| GO:0045017 | glycerolipid biosynthetic process | 0 | 0 | 18 | 0.6567305 |
| GO:0032147 | activation of protein kinase activity | 0 | 0 | 108 | 0.3184162 |
| GO:0045687 | positive regulation of glial cell differentiation | 0 | 0 | 11 | 1.3162319 |
| GO:0030148 | sphingolipid biosynthetic process | 0 | 0 | 37 | 0.3515467 |
| GO:0046039 | GTP metabolic process | 0 | 0 | 22 | 0.820716 |
| GO:0046486 | glycerolipid metabolic process | 0 | 0 | 65 | 0.6103246 |
| GO:0010001 | glial cell differentiation | 0 | 0 | 85 | 0.3705212 |
| GO:0046504 | glycerol ether biosynthetic process | 0 | 0 | 18 | 0.6567305 |
| GO:0015872 | dopamine transport | 0 | 0 | 10 | 1.0127539 |
| GO:0001522 | pseudouridine synthesis | 0 | 0 | 20 | 0.8010556 |
Table 2.
C2 Gene Sets Changes in CHF1 Transgenic Hearts versus Wild-type Hearts after Banding
| Name | p-Value | FDR | Size | Score |
|---|---|---|---|---|
| Downregulation | ||||
| MCALPAINPATHWAY | 0 | 0 | 23 | −0.5048976 |
| AGPCRPATHWAY | 0 | 0 | 12 | −0.6601133 |
| ST_PHOSPHOINOSITIDE_3_KINASE_PATHWAY | 0 | 0 | 32 | −0.3123639 |
| BADPATHWAY | 0 | 0 | 19 | −0.6192746 |
| YAMA_RECURRENT_HCC_UP | 0 | 0 | 10 | −0.8839298 |
| SHHPATHWAY | 0 | 0 | 13 | −0.7455815 |
| CELL_GROWTH_AND_OR_MAINTENANCE | 0 | 0 | 59 | −0.274771 |
| SIG_CHEMOTAXIS | 0 | 0 | 44 | −0.3581732 |
| BCL2_FAMILY_AND_REG_NETWORK | 0 | 0 | 21 | −0.4430197 |
| BECKER_CANCER_ASSOCIATED_SUBSET_2 | 0 | 0 | 12 | −0.6853539 |
| TUMOR_SUPRESSOR | 0 | 0 | 20 | −0.5595032 |
| MITOCHONDRIAPATHWAY | 0 | 0 | 20 | −0.3626908 |
| CELL_CYCLE_CHECKPOINT_II | 0 | 0 | 8 | −0.7899912 |
| CHREBPPATHWAY | 0 | 0 | 16 | −0.5171871 |
| LINDSTEDT_DEND_8H_VS_48H_DN | 0 | 0 | 58 | −0.3669714 |
| HDACI_COLON_TSABUT_DN | 0 | 0 | 16 | −0.6385193 |
| UVC_HIGH_D7_DN | 0 | 0 | 29 | −0.469932 |
| HDACI_COLON_SUL2HRS_UP | 0 | 0 | 11 | −0.8317397 |
| FSH_OVARY_MCV152_DN | 0 | 0 | 41 | −0.6036231 |
| Upregulation | ||||
| RNA_POLYMERASE | 0 | 0 | 12 | 1.0778809 |
| AGUIRRE_PANCREAS_CHR22 | 0 | 0 | 55 | 0.7075367 |
| SMITH_HTERT_DN | 0 | 0 | 61 | 0.2820412 |
| SELENOAMINO_ACID_METABOLISM | 0 | 0 | 12 | 0.6277189 |
| ASTON_DEPRESSION_DN | 0 | 0 | 133 | 0.21018 |
| ASTON_OLIGODENDROGLIA_MYELINATION_SUBSET | 0 | 0 | 14 | 0.5149096 |
| YAO_P4_KO_VS_WT_UP | 0 | 0 | 80 | 0.3353383 |
| CMV-UV_HCMV_6HRS_DN | 0 | 0 | 91 | 0.2841596 |
| GAMMA-UV_FIBRO_DN | 0 | 0 | 37 | 0.2858421 |
| CREB_BRAIN_8WKS_DN | 0 | 0 | 42 | 0.5487613 |
| IGF_VS_PDGF_UP | 0 | 0 | 64 | 0.4450024 |
| TSA_CD4_DN | 0 | 0 | 17 | 0.6375096 |
| ET743_HELA_DN | 0 | 0 | 16 | 0.65793 |
| OLDONLY_FIBRO_UP | 0 | 0 | 34 | 0.3175369 |
| 4NQO_UNIQUE_FIBRO_DN | 0 | 0 | 8 | 0.9573262 |
| ZMPSTE24_KO_DN | 0 | 0 | 26 | 0.6011035 |
| IFN_GAMMA_UP | 0 | 0 | 37 | 0.5207427 |
Analysis of individual components from various BP gene sets suppressed by CHF1/Hey2 provided multiple potential targets that are known to play a role in hypertrophy, such as EGFR, GNAQ, and HBEGF (GO:0048661). Numerous other potentially suppressed targets are known to play a role in development, such as SLIT2, PRKAR1A, TBX6, BMP4, KLF4, and SHH (GO:0007369, GO:0001704), while other potential targets, such as TLR3, TLR4, TLR7, TLR8, and IL18 (GO:0042095, GO:0045088, GO:0045089, GO:0045078, GO:0032069), play a role in innate immunity (Table 3). Although none of these potential target genes demonstrated significant differential expression when assessed individually, these aggregate findings raise the interesting possibility that CHF1/Hey2 may function by limiting growth factor pathway activation and suppressing an innate immunity response that results in inflammation, apoptosis and fibrosis and progression to heart failure.
Table 3.
Core Signaling and Processes Alterted in CHF1 Transgenic Hearts after Banding
| Regulatory process or pathway | Representative altered genes |
|---|---|
| GO:0048661 (regulation of smooth muscle cell proliferation) | EGFR, GNAQ, HBEGF |
| GO:0007369 (gastrulation) | SLIT2, PRKAR1A, TBX6, BMP4, KLF4, SHH |
| GO:0001704 (formation of primary germ layer) | SLIT2, PKAR1A, TBX6, BMP4, KLF4, SHH |
| GO:0042095 (IFN-gamma biosynthesis) | TLR3, TLR8, TLR7,IL18 |
| GO:0045088 (regulation of innate immune response) | TLR8, TLR4, TLR3 |
| GO:0045089 (positive regulation of innate immune response) | TLR8, TLR4, TLR3 |
| GO:0045078 (positive regulation of interferon-gamma biosynthetic process) | TLR8, TLR3, TLR7 |
| GO:0032069 (negative regulation of response to food) | CCK, PYY, CRHR1, CARTPT |
| MCALPAINPATHWAY | PRKAR1A, PRKAR2B, PRKAR2A, PRKAR1A, PRKACB |
| AGPCRPATHWAY | PRKAR1A, PRKAR2B, PRKAR2A, PRKAR1A, PRKACB |
| BADPATHWAY | PRKAR1A, PRKAR2B, PRKAR2A, PRKAR1A, PRKACB |
| SHHPATHWAY | PRKAR1A, PRKAR2B, PRKAR2A, PRKAR1A, PRKACB |
| CHREBPPATHWAY | PRKAR1A, PRKAR2B, PRKAR2A, PRKAR1A, PRKACB |
| ST_PHOSPHOINOSITIDE_3_KINASE_PATHWAY | PTEN |
| SIG_CHEMOTAXIS | PTEN |
| TUMOR_SUPRESSOR | PTEN |
| BCL2_FAMILY_AND_REG_NETWORK | APAF1, CASP8 |
| MITOCHONDRIAPATHWAY | APAF1, CASP8 |
A similar analysis of individual components from the various C2 gene sets suppressed by CHF1/Hey2 suggested an effect on molecules implicated in protein kinase A signaling such as PRKAR1A, PRKAR2B, PRKAR2A, and PRKACB in (MCALPAIN, AGPCR, BAD, SHH, CHREB). Other molecules suppressed and affecting more than one pathway include PTEN (ST_PHOSPHOINOSITIDE_3_KINASE_PATHWAY, SIG_CHEMOTAXIS, TUMOR SUPPRESSOR) and the proapoptotic molecules APAF1 and CASP8, (BCL2, MITOCHONDRIA) (Table 3). These findings suggest that CHF1/Hey2 may also function by suppressing protein kinase A activity, PTEN activity and apoptosis during pressure overload, thereby facilitating myocyte survival.
Discussion
We have found that CHF1/Hey2 overexpression in the myocardium is protective against apoptosis, fibrosis, and development of systolic heart failure under conditions of pressure overload but do not prevent the development of hypertrophy. These findings suggest that CHF1/Hey2 promotes physiological hypertrophy and suppresses pathological hypertrophy. Furthermore, we have performed a comprehensive bioinformatics analysis of gene sets that are transcriptionally altered in CHF1/Hey2 transgenic mouse heart under conditions of pressure overload, and have identified a number of important affected pathways. To the best of our knowledge, our study is the first to demonstrate that CHF1/Hey2 expression in myocardial cells can convert a pathological hypertrophic response to a physiological hypertrophic response during pressure overload, and is also the first to delineate extensively the cellular pathways that are altered under these conditions.
One limitation of our study is that we did not assess for diastolic dysfunction, which is an important cause of heart failure. Hypertrophy in general predisposes to diastolic dysfunction, as does cardiac fibrosis. We cannot exclude the presence of diastolic dysfunction in our CHF1/Hey2 transgenic mice, but given the greater degree of fibrosis and similar degrees of hypertrophy in the wild-type mice, we are confident that the transgenic mice are likely to have better diastolic function than their wild-type counterparts after aortic banding.
Our previous report suggested that CHF1/Hey2 functions as an “antihypertrophic” factor (Xiang et al., 2006) in transgenic mice treated with subpressor doses of phenylephrine, a weak hypertrophic stimulus. In the current study we have found that wild-type and transgenic mice display similar hypertrophy after ascending aortic banding, a powerful hypertrophic stimulus, although the transgenic mice are protected from fibrosis, apoptosis, and systolic dysfunction. The difference in response is most likely due to the strength of the hypertrophic stimulus in each model. Both studies are consistent with a cardioprotective effect of CHF1/Hey2 that suppresses the pathologic hypertrophic response but allows a physiologic response. Although CHF1/Hey2 can no longer be considered a strictly “antihypertrophic” factor, it is still a potent suppressor of the pathological hypertrophic response, and may provide an important target for therapy of hypertrophic heart disease and heart failure.
At present, little is known about the transcriptional and signaling pathways that determine a pathological hypertrophic response or a physiological response to a specific stimulus, although the physiological and histological differences between these two responses are well described (Heineke and Molkentin, 2006). Numerous microarray studies examining the transcriptional profiles in various models of hypertrophy have been performed, but consensus findings have been limited (reviewed in Dorn, 2007). No specific molecular signature for hypertrophy has been described, as transcriptional profiles are divergent in different models of hypertrophy (Aronow et al., 2001). In multiple studies, various groups have attempted to compare specific models of pathological and physiological hypertrophy, with interesting but variable findings (Dorn and Force, 2005; Iemitsu et al., 2001; Kong et al., 2005; Strom et al., 2005). Despite these limitations, a consensus has emerged that physiological hypertrophy is associated with increased fatty acid oxidation and increased mitochondrial biogenesis, while pathological hypertrophy is associated with increased inflammation, apoptosis, glycolysis, and expression of fetal contractile protein isoforms. In addition, physiological hypertrophy has been associated with stimulation of polypeptide growth factor signaling pathways such as IGF1 signaling via PI3 kinase, while pathological hypertrophy has been associated with activation of G-protein coupled receptors signaling through Gq.
Our study circumvents a major caveat of previous studies by using a single hypertrophic stimulus (pressure overload) and demonstrates how overexpression of a single transcription factor can affect the hypertrophic response through alterations in diverse but specific transcriptional expression patterns that affect multiple biological pathways. Furthermore, our study utilizes Gene Set Analysis (Efron and Tibshirani, 2007), a modification of Gene Set Enrichment Analysis (Subramanian et al., 2005), which is superior in identifying differentially expressed groups of genes. In our system, we specifically observed suppression of innate immunity pathways, protein kinase A, PI3K, SHH, and apoptotic pathways. The diversity of the identified gene sets clearly demonstrates that this process is not driven by changes in a small set of genes. Our study is consistent with previous studies in that it demonstrates increased expression of immune-related and apoptotic molecules in hypertrophic, failing hearts subjected to pressure overload, and also demonstrates increased biosynthetic activity in hypertrophied, nonfailing hearts in which CHF1/Hey2 is overexpressed, although the specific genes and pathways involved are not identical to those previously reported. Our findings indicate that CHF1/Hey2 can function as a cellular switch that promotes a physiological response over a pathological response to pressure overload.
The identification of specific pressure overload-induced biological processes altered by CHF1/Hey2 through Gene Set Analysis reveals previously unappreciated pathways that likely affect the development of a physiological rather than a pathological response. The advantage of Gene Set Analysis and its relative, Gene Set Enrichment Analysis, is that it more readily detects small concordant changes in the expression of genes acting in concert, which may alter the flux through a pathway more readily than a more dramatic change in a single gene (Subramanian et al., 2005). Using this method, we have identified potential roles for protein kinase A-dependent signaling and Sonic Hedgehog signaling in development of pathological hypertrophy, although the exact mechanisms will require further study. We have also found that the PI3K pathway is suppressed by CHF1/Hey2, and that this suppression correlates with development of physiological rather than pathological hypertrophy. This finding is surprising, and initially seemed contradictory, as others have reported that PI3K signaling determines cell size and promotes physiological rather than pathological hypertrophy (Condorelli et al., 2002; Matsui et al., 2002; Shioi et al., 2002). These prior studies have focused on AKT, and have found that overexpression of constitutively active AKT results in hypertrophy but not heart failure, while overexpression of a dominant negative form of AKT inhibits normal growth and exercise induced hypertrophy, but not pathological hypertrophy induced by pressure overload. Although these published reports seem at variance with our findings, it is important to note that our study examines multiple components of the PI3K pathway in addition to AKT, and therefore is a better reflection of overall pathway activity. Furthermore, examination of expression of AKT within the pathway demonstrates that AKT itself is increased in transgenic hearts, even though the overall GSA enrichment score for the pathway is negative, that is, the pathway is suppressed in the transgenic relative to the wild type. Accordingly, our data indicates that both a decrease in overall PI3K signaling pathway component gene expression and an increase in AKT expression correlate with the development of a physiological hypertrophic response to pressure overload when CHF1/Hey2 is overexpressed. Concurrent effects such as the suppression of PTEN may partially explain these results, as PTEN is known to inhibit the activity of AKT and cause apoptosis in cultured cardiac myocytes (Schwartzbauer and Robbins, 2001), and therefore can potentially contribute to a pathological hypertrophic response.
The attenuation of the SHH signaling pathway in conjunction with alteration in other biological processes associated with embryonic development is also a novel finding, and suggests that these developmental processes may play an important role in the pathological hypertrophic response. Alternatively, they may also indicate that CHF1/Hey2 overexpression affects fundamental aspects of cardiac myocyte development in utero that later promote the onset of physiological rather than pathological hypertrophy in response to pressure overload, although no gross developmental effects are observed in our transgenic mice. We and others have previously demonstrated, through loss of function studies, that CHF1/Hey2 is an important regulator of cardiac myocyte development, rendering such an argument plausible (Fischer et al., 2005; Gessler et al., 2002; Koibuchi and Chin, 2007; Sakata et al., 2002, 2006; Xiang et al., 2006; Xin et al., 2007). In addition, CHF1/Hey2 is widely considered to be a downstream effector of Notch signaling, raising the interesting possibility that crosstalk between Notch, SHH, and other developmental pathways may determine the nature of the hypertrophic response in pressure overload. Further studies are required to address these important questions.
Conclusions
We have previously reported that CHF1/Hey2 regulates smooth muscle responsiveness to growth factors through effects on diverse transcriptional pathways (Sakata et al., 2004; Shirvani et al., 2007). We have also previously reported that CHF1/Hey2 can blunt the hypertrophic response to phenylephrine in vivo and in vitro (Xiang et al., 2006). Our current study demonstrates that CHF1/Hey2 is an essential regulator of the cardiac myocyte response to pressure overload, and promotes the development of a physiological rather than a pathological response. Novel drug therapies that activate CHF1/Hey2 or its downstream pathways may provide additional therapeutic modalities for heart failure, hypertension, and hypertrophic cardiomyopathies. In addition, genetic polymorphisms in CHF1/Hey2 may predict risk for cardiac hypertrophy and heart failure. These aggregate findings underscore the importance of CHF1/Hey2 in cardiovascular development and disease, and suggest that future therapies targeted toward gain of function in cardiac myocytes may have beneficial effects in hypertension and heart failure.
Acknowledgments
This work was supported by NIH Grant HL081088 to M.T.C. This work was also supported in part by the UW NIEHS sponsored Center for Ecogenetics and Environmental Health, Grant Number NIEHS P30ES07033.
Author Disclosure Statement
The authors declare that no conflicting financial interests exist.
References
- Aikawa R. Komuro I. Yamazaki T. Zou Y. Kudoh S. Tanaka M., et al. Oxidative stress activates extracellular signal-regulated kinases through Src and Ras in cultured cardiac myocytes of neonatal rats. J Clin Invest. 1997;100:1813–1821. doi: 10.1172/JCI119709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronow B.J. Toyokawa T. Canning A. Haghighi K. Delling U. Kranias E., et al. Divergent transcriptional responses to independent genetic causes of cardiac hypertrophy. Physiol Genomics. 2001;6:19–28. doi: 10.1152/physiolgenomics.2001.6.1.19. [DOI] [PubMed] [Google Scholar]
- Camon E. Magrane M. Barrell D. Lee V. Dimmer E. Maslen J., et al. The Gene Ontology Annotation (GOA) Database: sharing knowledge in Uniprot with Gene Ontology. Nucleic Acids Res. 2004;32:D262–D266. doi: 10.1093/nar/gkh021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin M.T. Maemura K. Fukumoto S. Jain M.K. Layne M.D. Watanabe M., et al. Cardiovascular basic helix loop helix factor 1, a novel transcriptional repressor expressed preferentially in the developing and adult cardiovascular system. J Biol Chem. 2000;275:6381–6387. doi: 10.1074/jbc.275.9.6381. [DOI] [PubMed] [Google Scholar]
- Condorelli G. Drusco A. Stassi G. Bellacosa A. Roncarati R. Iaccarino G., et al. Akt induces enhanced myocardial contractility and cell size in vivo in transgenic mice. Proc Natl Acad Sci USA. 2002;99:12333–12338. doi: 10.1073/pnas.172376399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan J. Kordylewska A. Jan Y.N. Utset M.F. Tetralogy of Fallot and other congenital heart defects in Hey2 mutant mice. Curr Biol. 2002;12:1605–1610. doi: 10.1016/s0960-9822(02)01149-1. [DOI] [PubMed] [Google Scholar]
- Dorn G.W., 2nd The fuzzy logic of physiological cardiac hypertrophy. Hypertension. 2007;49:962–970. doi: 10.1161/HYPERTENSIONAHA.106.079426. [DOI] [PubMed] [Google Scholar]
- Dorn G.W., 2nd Force T. Protein kinase cascades in the regulation of cardiac hypertrophy. J Clin Invest. 2005;115:527–537. doi: 10.1172/JCI24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B. Tibshirani R. On testing the significance of sets of genes. Ann Appl Stat. 2007;1:107–129. [Google Scholar]
- Fischer A. Klattig J. Kneitz B. Diez H. Maier M. Holtmann B., et al. Hey basic helix-loop-helix transcription factors are repressors of GATA4 and GATA6 and restrict expression of the GATA target gene ANF in fetal hearts. Mol Cell Biol. 2005;25:8960–8970. doi: 10.1128/MCB.25.20.8960-8970.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentleman R.C. Carey V.J. Bates D.M. Bolstad B. Dettling M. Dudoit S., et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentleman R., editor; Carey V., editor; Huber W., editor; Irizarry R., editor; Dudoit S., editor. Bioinformatics and Computatiional Biology Solutions Using R and Bioconductor. Springer; New York: 2005. [Google Scholar]
- Gessler M. Knobeloch K.-P. Helisch A. Amann K. Schumacher N. Rohde E., et al. Mouse gridlock: no aortic coarctation or deficiency, but fatal cardiac defects in Hey2−/−mice. Curr Biol. 2002;12:1601–1604. doi: 10.1016/s0960-9822(02)01150-8. [DOI] [PubMed] [Google Scholar]
- Heineke J. Molkentin J.D. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- Iemitsu M. Miyauchi T. Maeda S. Sakai S. Kobayashi T. Fujii N., et al. Physiological and pathological cardiac hypertrophy induce different molecular phenotypes in the rat. Am J Physiol. 2001;281:R2029–R2036. doi: 10.1152/ajpregu.2001.281.6.R2029. [DOI] [PubMed] [Google Scholar]
- Iso T. Sartorelli V. Chung G. Shichinohe T. Kedes L. Hamamori Y. HERP, a new primary target of Notch regulated by ligand binding. Mol Cell Biol. 2001;21:6071–6079. doi: 10.1128/MCB.21.17.6071-6079.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathiriya I.S. King I.N. Murakami M. Nakagawa M. Astle J.M. Gardner K.A., et al. Hairy-related transcription factors inhibit GATA-dependent cardiac gene expression through a signal-responsive mechanism. J Biol Chem. 2004;279:54937–54943. doi: 10.1074/jbc.M409879200. [DOI] [PubMed] [Google Scholar]
- Koibuchi N. Chin M.T. CHF1/Hey2 plays a pivotal role in left ventricular maturation through suppression of ectopic atrial gene expression. Circ Res. 2007;100:850–855. doi: 10.1161/01.RES.0000261693.13269.bf. [DOI] [PubMed] [Google Scholar]
- Kokubo H. Lun Y. Johnson R.L. Identification and expression of a novel family of bHLH cDNAs related to drosophila hairy and enhancer of split. Biochem Biophys Res Commun. 1999;260:459–465. doi: 10.1006/bbrc.1999.0880. [DOI] [PubMed] [Google Scholar]
- Kong S.W. Bodyak N. Yue P. Liu Z. Brown J. Izumo S., et al. Genetic expression profiles during physiological and pathological cardiac hypertrophy and heart failure in rats. Physiol Genomics. 2005;21:34–42. doi: 10.1152/physiolgenomics.00226.2004. [DOI] [PubMed] [Google Scholar]
- Leimeister C. Externbrink A. Klamt B. Gessler M. Hey genes: a novel subfamily of hairy- and Enhancer of split related genes specifically expressed during mouse embryogenesis. Mech Dev. 1999;85:173–177. doi: 10.1016/s0925-4773(99)00080-5. [DOI] [PubMed] [Google Scholar]
- Liao R. Jain M. Cui L. D'Agostino J. Aiello F. Luptak I., et al. Cardiac-specific overexpression of GLUT1 prevents the development of heart failure attributable to pressure overload in mice. Circulation. 2002;106:2125–2131. doi: 10.1161/01.cir.0000034049.61181.f3. [DOI] [PubMed] [Google Scholar]
- Matsui T. Li L. Wu J.C. Cook S.A. Nagoshi T. Picard M.H., et al. Phenotypic spectrum caused by transgenic overexpression of activated Akt in the heart. J Biol Chem. 2002;277:22896–22901. doi: 10.1074/jbc.M200347200. [DOI] [PubMed] [Google Scholar]
- Nakagawa O. Nakagawa M. Richardson J.A. Olson E.N. Srivastava D. HRT1, HRT2, and HRT3: a new subclass of bHLH transcription factors marking specific cardiac, somitic, and pharyngeal arch segments. Dev Biol (Orlando) 1999;216:72–84. doi: 10.1006/dbio.1999.9454. [DOI] [PubMed] [Google Scholar]
- Sakata Y. Kamei C.N. Nakagami H. Bronson R. Liao J.K. Chin M.T. Ventricular septal defect and cardiomyopathy in mice lacking the transcription factor CHF1/Hey2. Proc Natl Acad Sci USA. 2002;99:16197–16202. doi: 10.1073/pnas.252648999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata Y. Koibuchi N. Xiang F. Youngblood J.M. Kamei C.N. Chin M.T. The spectrum of cardiovascular anomalies in CHF1/Hey2 deficient mice reveals roles in endocardial cushion, myocardial and vascular maturation. J Mol Cell Cardiol. 2006;40:267–273. doi: 10.1016/j.yjmcc.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Sakata Y. Xiang F. Chen Z. Kiriyama Y. Kamei C.N. Simon D.I., et al. Transcription factor CHF1/Hey2 regulates neointimal formation in vivo and vascular smooth muscle proliferation and migration in vitro. Arterioscler Thromb Vasc Biol. 2004;24:2069–2074. doi: 10.1161/01.ATV.0000143936.77094.a4. [DOI] [PubMed] [Google Scholar]
- Schwartzbauer G. Robbins J. The tumor suppressor gene PTEN can regulate cardiac hypertrophy and survival. J Biol Chem. 2001;276:35786–35793. doi: 10.1074/jbc.M102479200. [DOI] [PubMed] [Google Scholar]
- Shioi T. McMullen J.R. Kang P.M. Douglas P.S. Obata T. Franke T.F., et al. Akt/protein kinase B promotes organ growth in transgenic mice. Mol Cell Biol. 2002;22:2799–2809. doi: 10.1128/MCB.22.8.2799-2809.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirvani S.M. Mookanamparambil L. Ramoni M.F. Chin M.T. Transcription factor CHF1/Hey2 regulates the global transcriptional response to platelet-derived growth factor in vascular smooth muscle cells. Physiol Genomics. 2007;30:61–68. doi: 10.1152/physiolgenomics.00277.2006. [DOI] [PubMed] [Google Scholar]
- Smyth G.K. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3 doi: 10.2202/1544-6115.1027. Article3. [DOI] [PubMed] [Google Scholar]
- Strom C.C. Aplin M. Ploug T. Christoffersen T.E. Langfort J. Viese M., et al. Expression profiling reveals differences in metabolic gene expression between exercise-induced cardiac effects and maladaptive cardiac hypertrophy. FEBS J. 2005;272:2684–2695. doi: 10.1111/j.1742-4658.2005.04684.x. [DOI] [PubMed] [Google Scholar]
- Subramanian A. Tamayo P. Mootha V.K. Mukherjee S. Ebert B.L. Gillette M.A., et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusher V.G. Tibshirani R. Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z. Irizzary R.A. Gentleman R.C. Martinez Murillo F. Spencer F. A model based background adjustment for oligonucleotide expression arrays. J Am Stat Assoc. 2004;99:909–917. [Google Scholar]
- Xiang F. Sakata Y. Cui L. Youngblood J.M. Nakagami H. Liao J.K., et al. Transcription factor CHF1/Hey2 suppresses cardiac hypertrophy through an inhibitory interaction with GATA4. Am J Physiol Heart Circ Physiol. 2006;290:H1997–H2006. doi: 10.1152/ajpheart.01106.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin M. Small E.M. van Rooij E. Qi X. Richardson J.A. Srivastava D., et al. Essential roles of the bHLH transcription factor Hrt2 in repression of atrial gene expression and maintenance of postnatal cardiac function. Proc Natl Acad Sci USA. 2007;104:7975–7980. doi: 10.1073/pnas.0702447104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong T.P. Rosenberg M. Mohideen M.-A.P.K. Weinstein B. Fishman M.C. gridlock, an HLH gene required for assembly of the aorta in zebrafish. Science. 2000;287:1820–1824. doi: 10.1126/science.287.5459.1820. [DOI] [PubMed] [Google Scholar]