Abstract
G protein-coupled receptors (GPCRs) represent the largest family of membrane receptors and are responsible for regulating a wide variety of physiological processes. This is accomplished via ligand binding to GPCRs, activating associated heterotrimeric G proteins and intracellular signaling pathways. G protein-coupled receptor kinases (GRKs), in concert with β-arrestins, classically desensitize receptor signal transduction, thus preventing hyperactivation of GPCR second messenger cascades. As changes in GRK expression have featured prominently in many cardiovascular pathologies, including heart failure, myocardial infarction, hypertension, and cardiac hypertrophy, GRKs have been intensively studied as potential diagnostic or therapeutic targets. Herein, we review our evolving understanding of the role of GRKs in cardiovascular pathophysiology.
Keywords: G protein-coupled receptors, G protein-coupled receptor kinases, heart failure, adrenergic receptors, cardiovascular disease
Introduction
Diseases of the heart, including heart failure (HF), are the leading cause of death for both men and women in the United States, accounting for more than one in four deaths in 20061. Roughly 5.8 million Americans have HF and 670,000 new cases are diagnosed annually, with associated health care and loss of productivity costs estimated at $39.2 billion for 20102, 3. Though significant improvements in patient care have been realized with β-adrenergic receptor (β-AR) blockers, angiotensin receptor blockers, angiotensin converting enzyme (ACE) inhibitors, aldosterone inhibitors, and diuretics, these standard HF treatments remain insufficient. Increasing our understanding of the molecular and cellular processes that contribute to HF pathogenesis, therefore, is of critical importance to developing improved therapeutic strategies.
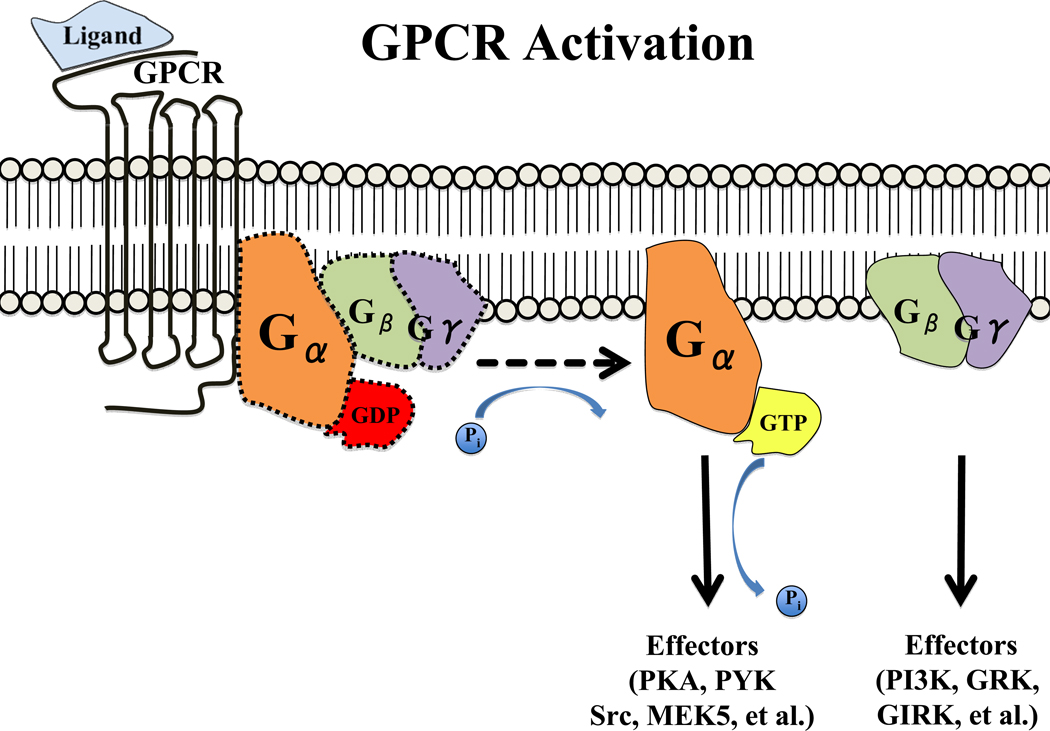
It has long been appreciated that in response to the reduced cardiac output of the failing heart, the sympathetic nervous system (SNS) releases neurohormones to both stimulate the heart and retain salt and water4, 5. Postganglionic and systemic release of catecholamines stimulate guanine nucleotide (G)-protein-coupled β-ARs of the myocardium, increasing heart rate, enhancing contraction, and improving cardiac performance6. At the receptor level, agonist binding promotes dissociation of the heterotrimeric G protein into α and βγ subunits (see Figure 1), stimulating adenylyl cyclase to increase cAMP production, and activating protein kinase A (PKA)7. Sustained β-AR stimulation is deleterious over time, however, causing receptor desensitization and downregulation, loss of responsiveness to catecholamines, and further contractile dysfunction.6, 8, 9
Figure 1. G Protein-Coupled Receptor (GPCR) Activation.
Generalized schematic for GPCR activation. Upon ligand binding to the GPCR, the receptor undergoes a conformational change whereby the α subunit of its associated G protein is activated by exchanging bound GDP for GTP. The α and βγ subunits of the G protein subsequently dissociate to activate their respective downstream signaling cascades. For a more comprehensive overview of the complex variety of GPCR signaling cascades, please refer to Neves et al.148.
Abbreviations: PKA, protein kinase A; PYK, protein-rich tyrosine kinase; MEK5, Mitogen/extracellular signal regulated kinase kinase-5; PI3K, phosphatidylinositol-3 kinase; GRK, G protein-coupled receptor kinase; GIRK, G protein-activated inward rectifying K+ channel).
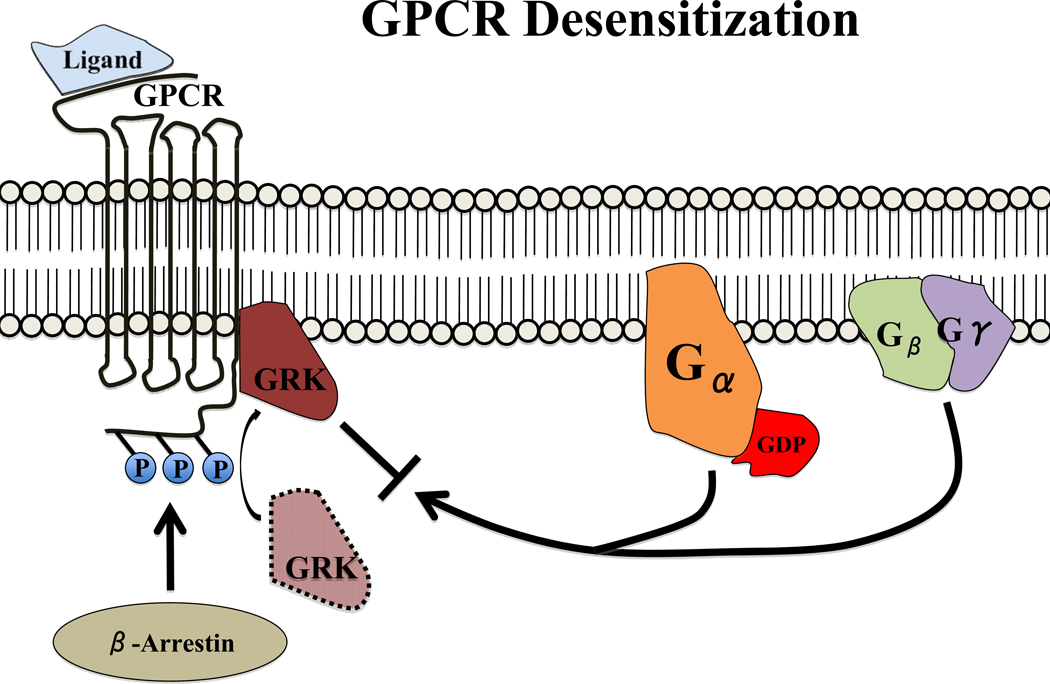
The harmful effects of chronic G-protein coupled receptor (GPCR) stimulation are initially mitigated by negative feedback via G-protein coupled receptor kinases (GRKs), originally named β-adrenergic receptor kinases. In a process termed homologous desensitization, GRKs phosphorylate agonist-bound receptors10, 11, leading to β-arrestin recruitment to the receptor12 (see Figure 2). Consequently, dissociated G-proteins are sterically inhibited from coupling to the receptor/β-arrestin complex and further downstream signaling is inhibited13. Furthermore, β-arrestins target the receptor for clathrin-coated pits in the cell membrane that are internalized and either recycled back to the cell surface or degraded14.
Figure 2. GPCR Desensitization.
GRKs are recruited to and phosphorylate ligand-occupied GPCRs on the cytoplasmic carboxyl-terminal tail. β-arrestins bind phosphorylated GPCRs with enhanced affinity, thereby creating a platform for: blocking recoupling of the dissociated G-protein subunits to the GPCR, thereby preventing further receptor activation (i.e. desensitization); coordination of GRK and arrestin in assembly of macromolecular signaling complexes; recruitment of endocytotic machinery as a precursor to receptor internalization (i.e. downregulation), whence the receptor may be dephosphorylated and recycled back to the membrane or targeted for lysosomal degradation.
This classic mechanism of regulating GPCR signaling and the primary role of GRKs in initiating this process have been extensively studied over the last 30 years15–19. Even so, evolving appreciation of the role of GRKs in both cardiac and non-cardiac tissue, as well as the complex variety of non-GPCR substrates that make up the GRK “interactome”20, suggest GRK functions beyond GPCR desensitization and downregulation may provide novel insights. In this review, we highlight our understanding of GRK physiology, with particular emphasis on recent findings with relevance to cardiovascular disease.
GRK Family Members
Seven genes are known to encode the mammalian GRKs (1–7)15, 21, a family of serine/threonine kinases sharing common structural and functional features. All GRKs possess a central catalytic domain, flanked by an amino terminus containing a regulator of G-protein signaling (RGS) homology domain and a variable length carboxyl end22. The N-terminal region seems critical for receptor recognition and intracellular membrane anchoring23, 24 while the C-terminal domain dictates subcellular localization and membrane association or translocation25, 26.
Based on sequence homology and tissue expression, GRKs are further separated into three subfamilies: rhodopsin kinases (GRKs 1 and 7); β-adrenergic receptor kinases (GRKs 2 and 3); and the GRK4 subfamily (GRKs 4, 5, and 6). Rhodopsin kinases and GRK4 are generally restricted to retina and testes, respectively, even as the remaining GRKs are ubiquitously expressed, though to varying degrees depending on the tissue16, 18, 20. Essentially, only GRKs 2, 3, and 5 are appreciably expressed in the human heart, with GRKs 2 and 5 the most abundant in the myocardium16, 27, 28.
These three primary cardiac GRK isoforms display distinct structural and functional characteristics that likely shape their impact on cardiovascular disease. For example, the C-terminus of GRK5 binds phospholipids, promoting preferential membrane localization29, whereas GRK2 and GRK3 are primarily cytoplasmic. Unique to GRK2 and GRK3 is a C-terminal pleckstrin homology (PH) domain that binds Gβγ subunits, thereby greatly enhancing GPCR phosphorylation through GRK plasma membrane translocation30. It should also be noted that the GPCR serine or threonine residue(s) phosphorylated by individual GRKs may influence which downstream signaling pathway is activated. For example, GRK2 or 3 phosphorylation was required for Angiotensin II receptor endocytosis, whereas the kinase activity of GRK5 or 6 directed extracellular signal-regulated kinase (ERK) activation in HEK293 cells31. It has been speculated that the GPCR phosphorylation pattern, or “barcode,” may dictate the structural conformation assumed by bound β-arrestins, or recruit variable arrestin isoforms, either one of which may influence the functional outcome31, 32.
Conventional dogma is that GRK activation in cardiac pathologies is mostly attributed to increased SNS stimulation, yet multiple molecular mechanisms of GRK regulation have been proposed. In HEK293 cells, PKA directly phosphorylates GRK2 on serine 685, enhancing Gβγ subunit binding and promoting membrane translocation33. Other in vitro work has found that Protein Kinase C (PKC) phosphorylation inhibits GRK534, yet activates GRK2 via disinhibition of tonic calmodulin regulation35, 36. It has been recently reported that GRK2 activity can also be enhanced independently of circulating catecholamines. Equi-biaxial mechanical stretch of neonatal rat ventricular myocytes activated GRK2 through Angiotensin II type 1 receptor, Gαq, and PKC37. Furthermore, cardiac-specific PKCα activation in transgenic mice impaired ex vivo left ventricle (LV) systolic and diastolic function in response to β-AR agonism through enhanced GRK2 activity37. The method of producing mechanical stretch is apparently critical to deciphering the pathway(s) of GRK activation, as the use of an inflatable balloon in explanted hearts activated GRK5 and GRK6, but not GRK238. This ligand-independent pathway necessarily entailed GRK-mediated angiotensin II receptor internalization and β-arrestin directed prosurvival signaling through ERK38.
In vivo function of individual GRKs has been clarified by gene knockout studies. Such work defined the role of the rhodopsin kinases in terminating phototransduction in the retina39–41, as well as GRK3, GRK5, and GRK6 in regulating olfactory senses42, multiple cholinergic responses including airway smooth muscle tone43, 44, and central nervous system psychostimulant responses45, respectively. Despite modest physiological alterations, animals featuring germline deletion of each GRK develop normally into adulthood, with the notable exception of GRK2. GRK2−/− mouse embryos develop myocardial hypoplasia and none survive past gestational day 15.5, suggesting that GRK2 may be critical in heart development46. Interestingly, GRK effects on mitogenic signaling are not without precedent, as GRK2 overexpression in smooth muscle cells attenuated cell proliferation induced by several GPCR agonists47. Systematic analysis of the developmental role of GRK2 was made possible by mating floxed GRK2 mice with mice expressing Cre recombinase under the control of the Nkx2.5 promoter, to specifically delete GRK2 in embryonic cardiomyocytes48. The fact that these animals developed normally with essentially no adult basal cardiac phenotype, implied that embryonic lethality on global GRK2 deletion might entail extracardiac or non-cardiomyocyte effects.
Utilizing the α-myosin heavy chain promoter49 to drive cardiac-specific expression in adult mice has proven invaluable in characterizing the in vivo function of GRKs in the heart. Indeed, this technique yielded the important discovery that GRKs 2 and 5 attenuate cardiac contractile responses to β-AR stimulation, but only GRK2 affects angiotensin II receptor-mediated contraction50, 51. On the other hand, GRK3 selectively targets myocardial thrombin and α1b-adrenergic receptors52, 53, buttressing the notion that GRKs expressed in the heart are not functionally redundant, but rather serve distinct physiological purposes which coincide with their unique structural and substrate properties.
GRKs in Cardiac Pathologies
Diseases of the cardiovascular system, particularly HF, myocardial ischemia (MI), and hypertension are unified by the persistent strain placed upon the heart muscle in such conditions. Whether faced with increased afterload (e.g. HF, hypertension) or cardiac muscle damage (e.g. MI), heart performance must adjust to the altered homeostasis in order to meet the body’s energetic demands. This entails SNS activation to increase heart rate and contractility through catecholaminergic stimulation of cardiac β-ARs. In human myocardium, the β1 and β2 subtypes are the primary mediators of positive chronotropic and inotropic adrenergic effects through Gs coupling. Furthermore, the β1 subtype accounts for about three quarters of myocardial β-ARs in the non-failing human heart, a distribution pattern that is fairly consistent in atrial and ventricular tissue54. Interestingly, chronic stimulation of β1-ARs appears to be deleterious, whereas β2-AR agonism may be cardioprotective55. Though historically thought to exist only in adipose tissue, functional cardiac β3-ARs56, which signal through Gi proteins and are upregulated in failing myocardium57, 58, have also been reported.
Consistent upregulation of the SNS leads to a number of biochemical and molecular alterations in GPCR pathways that have been extensively evaluated over the past 30 years. Perhaps most familiar is that failing human hearts demonstrate reduced β-AR density and responsiveness, primarily through downregulation of β1-ARs59–63. Dampened β-AR signaling is generally believed to be an early adaptation to protect the heart against cardiotoxicity from catecholamine overstimulation. Pathological β-AR downregulation and desensitization, on chronic catecholamine exposure, prevents the desired increases in cardiac output from SNS stimulation. Thus, further SNS activation follows, leading to a self-reinforcing, pernicious cycle of progressively deteriorating heart function. For this reason, extended use of β-AR agonists such as dobutamine in HF is generally contraindicated, as clinical trials have associated their chronic (but not acute) use with increased mortality64.
In light of GRKs primary function of modulating GPCR signaling, it stands to reason that alterations in GRKs may be observed in cardiovascular diseases55. Of particular note, increased cardiac expression and activity of GRK2 and GRK5, the predominant GRK isoforms in the heart, have been manifestly associated with human and numerous experimental models of HF27, 62, 63, 65–74. Enhanced GRK2 expression and activity are also linked to hypertension75, cardiac hypertrophy76, and MI65. Perhaps most intriguingly, GRK2 levels often increase prior to overt clinical HF66, 71, and normalize in concert with improved β-AR signaling and ventricular function77, 78, as seen in patients using left ventricular assist devices (LVADs)79–81. Such findings have been interpreted to cast GRK2 as an alluring therapeutic target and potential biomarker of cardiac function77.
The SNS regulates glucose homeostasis in body fat, liver, and skeletal muscle, thus GRK2 has been implicated in insulin receptor regulation and the development of insulin resistance (IRES) associated with metabolic disorders like diabetes. In vitro studies using adipocytes have shown GRK2-mediated dampening of insulin sensitivity (glucose transporter 4 membrane translocation) through Gαq/11 interference82, 83. GRK2 also promotes enhanced insulin desensitization on chronic endothelin-1 treatment by phosphorylation-dependent insulin receptor substrate-1 (IRS1) degradation83. In HEK293 cells, chronic isoproterenol treatment or β2-AR overexpression led to insulin resistance through GRK2-mediated IRS1 serine/threonine phosphorylation and inhibition of IRS1 tyrosine phosphorylation84. Concordant in vivo work showed that intravenous administration of a peptide containing the carboxyl terminus amino acid sequence of the antennapedia protein and modified from an existing GRK2 inhibitor85 to promote intracellular localization, ameliorated glucose homeostasis and IRES in a hypertensive rat model84. Together, these findings point to a causal relationship between GRK2 activity and IRES, independent of canonical receptor phosphorylation and desensitization.
The nuclear localization of GRK5, as well as divergent receptor specificity, and more gradual expression changes in HF compared to GRK2, hint at unique cardiac regulatory functions for GRK5. For one, GRK5 seems to be directly relevant to myocardial hypertrophic gene transcription through its role as a histone deacetylase kinase86. Subcellular distribution of GRK5 is apparently critical to this function, as overexpression of a nuclear excluded GRK5 construct does not produce the exaggerated hypertrophy observed with wild type GRK5 overexpression86. Furthermore, intracardiac injection of adenovirus encoding the amino terminus of GRK5 (AdGRK5-NT) reduced cardiac hypertrophy, cardiomyocyte size, and apoptosis, and improved cardiac ejection fraction and fractional shortening in SHRs87. Mechanistically, the amino terminus of GRK5 contains the RGS homology domain that, through its interaction with IκBα, and stabilization of IκBα/nFκB complex, reduces nFκB transcriptional activity87. In contrast to GRK2, which appears devoid of any nonsynonymous nucleotide polymorphisms, four such GRK5 polymorphisms have been discovered, the most common of which is a leucine substitution for glutamine at position 4188, 89. This mutation is most abundant in African-Americans and mimics the effects of β-blocker therapy, thereby improving survival in individuals with the leucine 41 polymorphism, yet also rendering β-blockers as less efficacious in such populations89. These findings provide a clear example of how pharmacogenomics may be harnessed to optimize treatment results. Finally, GRK5 has been found to regulate the signaling of vascular endothelial growth factor receptor, a non G protein-coupled substrate, in coronary artery endothelial cells, indicating that GRK functions beyond GPCRs also may be important90. Overall, our understanding of a central role for GRKs in a variety of cardiac diseases is amply supported by the extant literature.
GRK2 Inhibition
Since the seminal finding that transgenic cardiac overexpression of GRK2 or a GRK2 inhibitor in mouse hearts reciprocally modulate myocardial β-AR signaling in vivo50, substantial work has been performed to investigate the effects of GRK inhibition in cardiac maladies. GRK2 inhibition is commonly achieved by utilizing βARKct, a peptide composed of 194-amino acids of the carboxyl-terminal of GRK2 and containing the Gβγ binding site30, 50, 91. Whether the observed effects of βARKct are due to GRK2 inhibition followed by resensitization of β-ARs, or by blocking Gβγ signaling92, or both, remains unresolved. Regardless, reports of functional cardiac improvement with βARKct are legion93.
The βARKct peptide has demonstrated efficacy improving cardiac performance in failing myocytes94, 95, HF models67, 68, 96, and post-MI97–99, and in preventing myocardial dysfunction in the settings of cardioplegic arrest100 and acute coronary ischemia101. Moreover, βARKct normalized fetal gene expression changes in two murine models of HF102. The precise mechanism of action of βARKct remains elusive, however. A recent report has proposed that reduced infarct size in βARKct-expressing mice subjected to acute MI is attributed to activation of the prosurvival Akt and eNOS pathway, through enhanced β2-AR signaling in cardiac myocytes103. Whether NO-mediated S-nitrosylation and inhibition of GRK2104 figures in this observation, independent of βARKct, remains to be determined. Another study found that viral delivery of βARKct enhanced ICa in β-AR-stimulated normal and failing cardiomyoycytes, presumably through sequestration of Gβγ, which binds the α1c subunit of L-type Ca2+ channels and modulates current105, 106. More than likely, βARKct has multiple actions that become more or less apparent depending on the model used and assay performed. Nevertheless, cardiac function was remarkably preserved and remodeling reduced on conditional cardiac GRK2 knockout either before or after MI107, confirming the beneficial effects of GRK2 inhibition.
Cardioprotection conferred by GRK inhibition in the setting of myocardial injury may seem paradoxical given the overwhelming evidence that suggests that continuous SNS stimulation of heart muscle is detrimental108, and the classical function of GRKs is to mitigate GPCR signaling. In agreement with this premise, Matkovich et al. found that cardiac-specific GRK2 deletion actually hastens the progression of cardiomyopathy on chronic isoproterenol infusion in mice48. The miniosmotic pumps that deliver isoproterenol in this model are incapable of responding to afferent nerve input, and thus will continue to deliver the drug regardless of cardiac performance. This scenario contrasts with genetic or myocardial injury models, in which SNS activation is presumably lessened upon improvement of heart function. Such negative feedback prevents incessant β-AR stimulation, and the deleterious effects on cardiac muscle, perhaps explaining the apparently discrepant results observed on GRK2 inhibition or deletion.
Because it is a large peptide, βARKct application requires a vehicle (typically a virus) for delivery to the target organ, which is not optimal. An alternative approach is to find small molecules that can be administered systemically, thus precluding immune response and cytotoxicity often associated with viral use. By screening a small molecule library, promising compounds that bind to the Gβγ site modulating protein-protein interactions were identified109. Our group recently assessed two of these compounds, M119 and gallein, for efficacy in treating two murine HF models110. Small molecule Gβγ inhibition mitigated cardiac dysfunction and enhanced β-AR signaling, at least in part because of reduced GRK2 expression and membrane recruitment, in either new onset or pre-existing heart failure110 (recently reviewed111). Whether these compounds exert significant extracardiac effects, or regulate other Gβγ signaling pathways, such as hypertrophic ERK1/2 activation112, remains to be clarified.
Vascular GRKs
Numerous GPCR agonists, including angiotensin, endothelin, norepinephrine, and epinephrine provide the neurohormonal inputs that modulate blood pressure. More specifically, vasoconstriction through angiotensin II, endothelin, and α-adrenergic receptor activation is counteracted by β-AR-mediated vasodilation, fine-tuning vascular tone. As approximately 1/3 of adults in the U.S. have hypertension113, increasing heart disease risk, and >70% of HF patients have antecedent hypertension2, GPCR dysregulation in the vasculature can have a profound impact on cardiovascular health.
Impairment of β2-AR-mediated vasodilator response in vascular smooth muscle cells (VSMCs) has been described in hypertensive patients114 and in animal models of hypertension115, 116. Assessment of β-AR functionality in humans is made possible by the use of human lymphocytes, in which β-AR properties mirror changes in β-ARs from less accessible tissues117, 118. Using lymphocytes from hypertensive and normotensive subjects, Gros. et al. established a link between defective β-AR responsiveness in hypertension and altered GRK2 activity75. Hypertensive patients displayed elevated GRK2 activity and protein expression without concomitant changes in GRK5, GRK6, PKA, β-arrestin 1 and 2, suggesting selective variation of GRK2 in this condition75.
Support for the hypothesis that increased GRK activity may underlie reduced β-AR responsiveness characteristic of the hypertensive state was supplied by the generation of transgenic mice with VSMC-targeted GRK2 overexpression driven by a portion of the SM22α promoter119. These animals display elevated resting blood pressure, reduced isoproterenol-mediated drop in diastolic blood pressure, vascular wall thickening, and myocardial hypertrophy119. It should be noted that VSMC GRK2 overexpression curiously attenuated blood pressure rise upon vasoconstricting Angiotensin II challenge. Enhanced GRK2-mediated phosphorylation and desensitization of Angiotensin II receptor signaling, as has been observed in vivo in the heart51, 53, 120 was the authors’ proposition, yet the apparent primacy of β-AR signaling defects in regulating blood pressure highlights the need for continued investigation into GRK substrate specificity.
In vivo analysis of GRK substrate selectivity using hybrid transgenic mice with myocardium-targeted overexpression of GRK2, 3, or 5, and constitutively activated mutant or wild type α1b-ARs, revealed that GRK2 has no effect on cardiac α1b adrenergic signaling, as assessed by diacylglycerol production, myocardial hypertrophy, and atrial natriuretic factor (ANF) expression53. Alone among these isoforms, GRK3 reduced myocardial diacylglycerol and either GRK3 or GRK5 reduced hypertrophy and ANF expression53. These results indicate differential substrate targeting by various GRK isoforms, proof of which had been limited in various in vitro cell culture system studies121–124. More recently, GRK3 was found to be highly selective for endothelin receptors and α1-ARs of adult rat cardiac myocytes, more so than GRK2, which displayed greater potency and efficacy at β-ARs125. Cardiac-specific expression of a GRK3 inhibitor in mice raised blood pressure and cardiac output through overactive α1-AR signaling, substantiating the concept of preferential regulation of this receptor subtype by GRK3126.
In a murine renal artery stenosis model of hypertension, in which plasma norepinephrine and VSMC GRK2 expression is increased, inhibition of VSMC GRK2 via genetic ablation or peptide (βARKct) failed to reduce hypertension, even though β-AR mediated vasodilation was functionally improved127. The authors attributed this finding to increased α1D-AR vasoconstricting activity upon GRK2 antagonism, although concerns about the pharmacological specificity of agonists used preclude discounting a role for α1a-AR signaling127. Blocking α1b-AR signaling, moreover, did not affect enhanced α1-AR constriction of GRK2-inhibited vessels, suggesting this receptor subtype is not involved127. In rat mesenteric arterial smooth muscle cells (resistance arteries), inhibition of GRK2 (but not GRK3, 5, and 6) with siRNA or dominant negative mutants reduced desensitization of endothelin-induced Ca2+ and IP3 signaling128. GRK2 has also been implicated in disrupting non-adrenergic (endothelial cell nitric oxide synthase (eNOS)) vasodilation via Akt inhibition in portal hypertensive rats129. GRK2 knockout in this animal model restored NO production and normalized portal pressure129. Together, these findings implicate GRK2 in vascular adaptations of the hypertensive state, yet the antagonistic effects on constriction and relaxation merit further investigation to effectuate therapeutic benefit.
A large cohort study of 133 black Americans reported GRK2 mRNA expression and activity, but not that of GRK5, correlated with blood pressure and plasma norepinephrine levels130. Furthermore, GRK2 protein expression doubled and GRK2 activity rose more than 40% in hypertensive subjects130. In contrast, one group has reported that lymphocyte mRNA levels for both GRK2 and GRK5 increase on isoproterenol injection in a rat HF model131. The discrepancy in results can likely be attributed to the obvious physiological differences between humans and rodents, as well as the respective conditions studied.
Another clinical study identified a negative correlation between GRK3 mRNA and systolic and diastolic blood pressure, although corresponding GRK3 protein levels were not assessed132. Oliver et al. have shed new mechanistic light on hypertension etiology with a systematic analysis of α- and β-AR subtype expression, as well as ex vivo contraction of aortic rings from spontaneously hypertensive rats (SHR)133. To wit, α1D- and β3-ARs are the subtypes most resistant to GRK2-mediated desensitization, thereby enhancing their functional importance in the setting of hypertension133. Coupled with the observation that α1D-ARs are most sensitive and β3-ARs least sensitive to agonists, greater vasoconstrictor tone prevails in the hypertensive state.
Whether increased GRK2 contributes to a rise in peripheral resistance through desensitization of β-AR-mediated vasodilation remains to be definitively resolved in the clinical setting. An alternative hypothesis is that GRK2 upregulation merely reflects overactive SNS activity on the vasculature, since the GRK2 promoter activity is stimulated by Gq and α1-AR signaling134. It must also be emphasized that cardiovascular disorders involve the complex interplay of many tissues and systems, with multiple potential etiologies. Although initially thought to be confined to the testes, mRNA for each of the four GRK4 isoforms135, 136 have been identified in the renal proximal tubule137. Transgenic expression of a naturally occurring single nucleotide polymorphism of GRK4γ in mice enhances GRK4-mediated phosphorylation of D1 dopamine receptors in the kidney, thus dampening urinary sodium excretion and producing hypertension137. Such findings highlight the still incompletely understood pathophysiological role of GRKs in cardiovascular diseases.
Adrenal GRK2
Modulating GRKs in myocardial tissue has produced many exciting findings with the potential to improve human health. However, the compensatory SNS response to diminished cardiac output involves the coordination of GPCR activity in various cell types and tissues beyond the heart. Systemic release of catecholamines, primarily epinephrine from the adrenal gland and norepinephrine from presynaptic nerve terminals, provides the initial stimulus to enhance cardiac contractility and maintain adequate perfusion of blood to the body’s tissues138. As noted previously, however, continued sympathetic activation damages the heart, partially explaining the beneficial effects of myocardial β-AR antagonism in HF. Indeed, a classic prognostic indicator of heart failure is increased plasma norepinephrine, which is highly correlated with mortality139, 140.
Another approach to alleviate excessive SNS burden is to inhibit adrenal catecholamine release. Under normal circumstances, catecholamine release by chromaffin cells of the adrenal medulla is under feedback inhibition by α2-ARs expressed on the membranes of these cells. In two different animal models of HF, calsequestrin-overexpressing mice and rats subjected to MI, Lymperopoulos et al. showed significant downregulation and desensitization of adrenal α2-ARs, correlated with increased adrenal GRK2 expression and catecholamine secretion141. Furthermore, GRK2-Gβγ inhibition via adenoviral-mediated delivery of βARKct to adrenal glands of HF rats restored α2-AR signaling, resulting in lowered plasma catecholamine levels, and improved βAR-mediated cardiac contractility and relaxation after 7 days141.
In a separate study, adrenal-specific transgene expression of GRK2 in rats produced enhanced plasma catecholamine levels compared to control animals, whereas βARKct effected the opposite result142. In vitro results revealed that α2-ARs from GRK2-infected chromaffin cells failed to inhibit catecholamine secretion, in contrast to βARKct-infected cells, providing proof of principle that catecholamine secretion from the adrenal gland can be manipulated through adrenal GRK2 regulation of α2-AR signaling142.
These findings were extended by utilizing adrenal-specific genetic knockdown of GRK2 in mice. 50% reduction of adrenal GRK2 protein precipitated a significant, though modest, reduction in circulating catecholamines at 4 weeks post-MI in these animals143. Moreover, GRK2 knockdown was associated with increased adrenal membrane α2-AR density, reduced adrenal gland size, and diminished catecholamine biosynthetic capacity143. Interestingly, cardiac GRK2 protein also went down, improving ejection fraction and isoproterenol-induced contractility in the failing hearts143. Though it is not clear why adrenal GRK2 expression increases in HF, nevertheless adrenal GRK2 inhibition highlights the potential therapeutic benefit of a comprehensive approach to regulating catecholamines. Further, concomitant inhibition of cardiac and adrenal Gβγ-GRK2 with systemic inhibitors, such as those described recently110, 111, may provide dual clinical efficacy in heart failure.
Adrenal GRK2 levels and activity may, in part, provide the molecular mechanism underlying the observed benefits of moderate exercise training in ameliorating cardiotoxic SNS hyperactivity in chronic HF. Indeed, rats that began a treadmill exercise regimen for 10 weeks at 4 weeks post-MI demonstrated significantly reduced circulating catecholamines and gene markers of cardiac remodeling (ANF, collagen type 1, and transforming growth factor-β1 mRNA levels in heart) compared to sedentary animals144. Importantly, adrenal and cardiac GRK2 protein expression, as well as adrenal α2AR membrane expression, were also normalized in the exercise group144. Despite improved LV contractile response to β-AR stimulation, consistent with increased cardiac β-AR density, the post-MI exercise-trained group showed no functional improvement in ejection fraction, suggesting the primary advantage of physical activity may be inhibition of adverse cardiac remodeling.
Conclusions
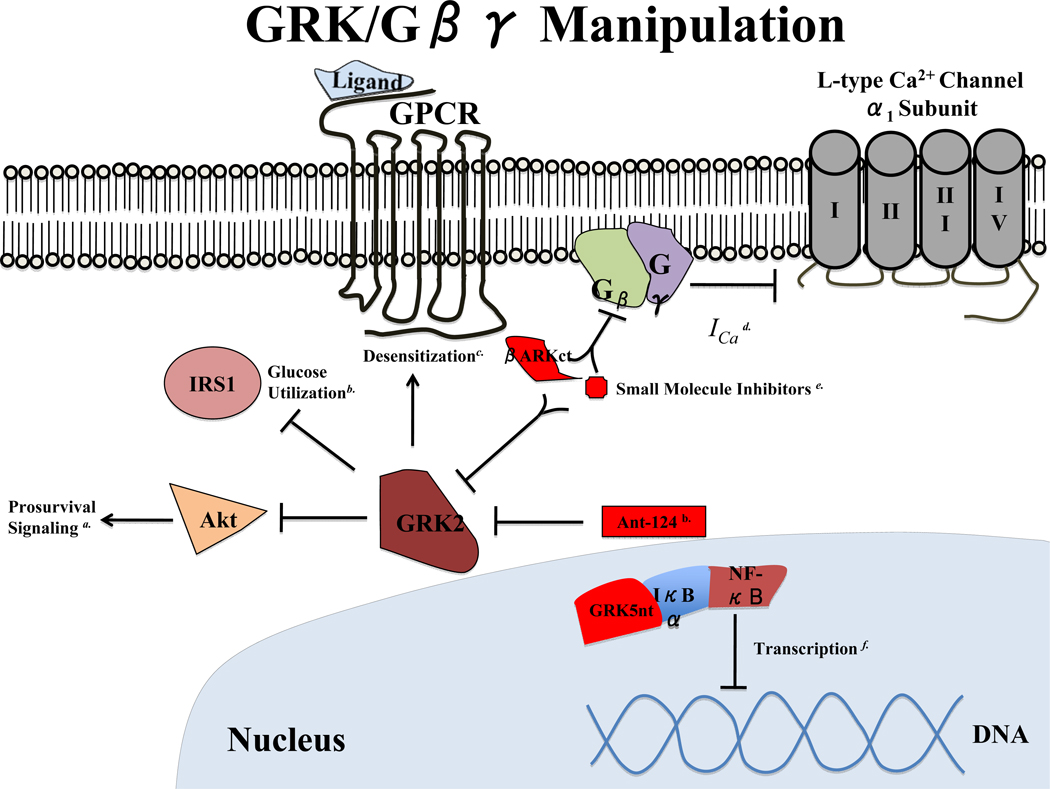
GPCR signaling is a ubiquitous means of effecting physiological processes throughout the body, hence GPCRs are the most common target of pharmacotherapy today. Investigation of GRK function is, therefore, a logical extension of efforts to uncover improved treatments for heart diseases afflicting Western societies. Modulation of GRK activity has yielded promising results in alleviating cardiovascular dysfunction in a wide variety of animal models and cell culture systems, the most recent of which are depicted in Figure 3. The concept of “functional selectivity”, that divergent downstream signaling pathways can be activated by a single ligand-receptor interaction147, highlights the emerging notion that long-held principles regarding GPCR signaling will no longer be sufficient to generate the next generation of therapeutic drugs (see other articles in this special review series for Circulation Research). Nevertheless, the overwhelming data implicating GRKs in cardiovascular diseases suggest that GRK regulation will continue to be an important target of investigation in multiple aspects of not only cardiovascular disease, but also of its comorbidities (e.g. diabetes), new diagnostics (e.g. elevated GRK2) and novel therapeutics (e.g. small molecules, stem cells, etc.).
Figure 3. GRK/Gβγ Manipulation.
Table 1.
Summary of Mammalian GRKs
| GRK | Tissue Expression |
Primary Target GPCR(s) |
GRK Modification |
Functional Effect | Reference |
|---|---|---|---|---|---|
| 1 | Retina | Rhodopsin | Gene ablation | Prolonged photon response and rod apoptosis | 39 |
| 2 | All, heart | β-AR, Angiotensin II type 1 | Gene ablation | Embryonic lethality | 46 |
| Conditional myocardial gene ablation | Enhanced inotropic β-AR sensitivity, blunted inotropic and lusitropic tachyphylaxis | 48 | |||
| Myocardial overexpression | Enhanced desensitization to β-AR- or Angiotensin II-mediated effects on contractility and heart rate | 50, 51 | |||
| Vascular overexpression | Impaired β-AR-mediated vasodilation | 119 | |||
| 3 | All, olfactory epithelium | α1-AR, thrombin, M2 and M3 muscarinic | Gene ablation | Loss of odorant-receptor mediated desensitization; enhanced airway smooth muscle constriction | 42, 45 |
| Myocardial overexpression | Reduced α1B-AR signaling and mitogen-activated protein kinase (MAPK) activation | 53 | |||
| 4 | Testis, kidney, brain | Dopamine-1 | Overexpression | No effect with WT GRK4γ but A142V polymorphism yields impaired natriuresis, hypertension | 137 |
| 5 | All, heart | β-AR, Angiotensin II type 1, M2 muscarinic | Gene ablation | Heightened response to cholinergic stimulation (e.g. hypothermia, salivation, tremor, antinociception); Diminished airway smooth muscle relaxation | 43, 44 |
| Myocardial overexpression | Enhanced desensitization to β-AR chronotropic and inotropic effects | 51 | |||
| 6 | All | Chemokine receptor 4, Dopamine-2 | Gene ablation | Impaired T-cell chemotaxis; Enhanced sensitivity to locomotor-stimulating effects of cocaine, amphetamine | 45, 46 |
| 7 | Retina | Cone opsin | Inhibition (antibody) | Reduced termination of phototransduction | 40, 41 |
Acknowledgments
Sources of Funding
This work was supported by American Heart Association (AHA) Postdoctoral Fellowship 09POST2190063 (SLB); R01-HL89885, 3R01-HL089885-02S1, and R01-HL091475 (BCB).
Non-standard Abbreviations and Acronyms
- AR
adrenergic receptor
- ACE
angiotensin converting enzyme
- ANF
atrial natriuretic factor
- βARKct
β adrenergic receptor carboxyl terminus peptide
- ICa
Calcium current
- eNOS
endothelial nitric oxide synthase
- ERK
extracellular signal-regulated kinase
- Gβγ
G protein βγ subunit
- GPCR
G protein-coupled receptor
- GRK
G protein-coupled receptor kinase
- HF
heart failure
- IRS
insulin receptor substrate
- IRES
insulin resistance
- LV
left ventricle
- LVAD
left ventricular assist device
- MI
myocardial ischemia
- PH
pleckstrin homology
- PKA
protein kinase A
- PKC
protein kinase C
- RGS
regulator of G protein signaling
- SNS
sympathetic nervous system
- VSMC
vascular smooth muscle cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Heron M, Hoyert DL, Murphy SL, Xu J, Kochanek KD, Tejada-Vera B. Deaths: final data for 2006. Natl Vital Stat Rep. 2009;57:1–134. [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 3.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 4.Foody JM, Farrell MH, Krumholz HM. beta-Blocker therapy in heart failure: scientific review. JAMA. 2002;287:883–889. doi: 10.1001/jama.287.7.883. [DOI] [PubMed] [Google Scholar]
- 5.Mudd JO, Kass DA. Tackling heart failure in the twenty-first century. Nature. 2008;451:919–928. doi: 10.1038/nature06798. [DOI] [PubMed] [Google Scholar]
- 6.Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- 7.Salazar NC, Chen J, Rockman HA. Cardiac GPCRs: GPCR signaling in healthy and failing hearts. Biochim Biophys Acta. 2007;1768:1006–1018. doi: 10.1016/j.bbamem.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tilley DG, Rockman HA. Role of beta-adrenergic receptor signaling and desensitization in heart failure: new concepts and prospects for treatment. Expert Rev Cardiovasc Ther. 2006;4:417–432. doi: 10.1586/14779072.4.3.417. [DOI] [PubMed] [Google Scholar]
- 9.Mann DL. Mechanisms and models in heart failure: A combinatorial approach. Circulation. 1999;100:999–1008. doi: 10.1161/01.cir.100.9.999. [DOI] [PubMed] [Google Scholar]
- 10.Benovic JL, Strasser RH, Caron MG, Lefkowitz RJ. Beta-adrenergic receptor kinase: identification of a novel protein kinase that phosphorylates the agonist-occupied form of the receptor. Proc Natl Acad Sci U S A. 1986;83:2797–2801. doi: 10.1073/pnas.83.9.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benovic JL, Mayor F, Jr, Staniszewski C, Lefkowitz RJ, Caron MG. Purification and characterization of the beta-adrenergic receptor kinase. J Biol Chem. 1987;262:9026–9032. [PubMed] [Google Scholar]
- 12.Lohse MJ, Benovic JL, Codina J, Caron MG, Lefkowitz RJ. beta-Arrestin: a protein that regulates beta-adrenergic receptor function. Science. 1990;248:1547–1550. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- 13.Hausdorff WP, Caron MG, Lefkowitz RJ. Turning off the signal: desensitization of beta-adrenergic receptor function. FASEB J. 1990;4:2881–2889. [PubMed] [Google Scholar]
- 14.Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3:639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 15.Pitcher JA, Freedman NJ, Lefkowitz RJ. G protein-coupled receptor kinases. Annu Rev Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- 16.Dorn GW., 2nd GRK mythology: G-protein receptor kinases in cardiovascular disease. J Mol Med. 2009;87:455–463. doi: 10.1007/s00109-009-0450-7. [DOI] [PubMed] [Google Scholar]
- 17.Hata JA, Williams ML, Koch WJ. Genetic manipulation of myocardial beta-adrenergic receptor activation and desensitization. J Mol Cell Cardiol. 2004;37:11–21. doi: 10.1016/j.yjmcc.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 18.Metaye T, Gibelin H, Perdrisot R, Kraimps JL. Pathophysiological roles of G-protein-coupled receptor kinases. Cell Signal. 2005;17:917–928. doi: 10.1016/j.cellsig.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Penela P, Murga C, Ribas C, Tutor AS, Peregrin S, Mayor F., Jr Mechanisms of regulation of G protein-coupled receptor kinases (GRKs) and cardiovascular disease. Cardiovasc Res. 2006;69:46–56. doi: 10.1016/j.cardiores.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 20.Penela P, Murga C, Ribas C, Lafarga V, Mayor F., Jr The complex G protein-coupled receptor kinase 2 (GRK2) interactome unveils new physiopathological targets. Br J Pharmacol. 2010;160:821–832. doi: 10.1111/j.1476-5381.2010.00727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohout TA, Lefkowitz RJ. Regulation of G protein-coupled receptor kinases and arrestins during receptor desensitization. Mol Pharmacol. 2003;63:9–18. doi: 10.1124/mol.63.1.9. [DOI] [PubMed] [Google Scholar]
- 22.Penela P, Ribas C, Mayor F., Jr Mechanisms of regulation of the expression and function of G protein-coupled receptor kinases. Cell Signal. 2003;15:973–981. doi: 10.1016/s0898-6568(03)00099-8. [DOI] [PubMed] [Google Scholar]
- 23.Palczewski K, Buczylko J, Lebioda L, Crabb JW, Polans AS. Identification of the N-terminal region in rhodopsin kinase involved in its interaction with rhodopsin. J Biol Chem. 1993;268:6004–6013. [PubMed] [Google Scholar]
- 24.Penn RB, Pronin AN, Benovic JL. Regulation of G protein-coupled receptor kinases. Trends Cardiovasc Med. 2000;10:81–89. doi: 10.1016/s1050-1738(00)00053-0. [DOI] [PubMed] [Google Scholar]
- 25.Pitcher JA, Inglese J, Higgins JB, Arriza JL, Casey PJ, Kim C, Benovic JL, Kwatra MM, Caron MG, Lefkowitz RJ. Role of beta gamma subunits of G proteins in targeting the beta-adrenergic receptor kinase to membrane-bound receptors. Science. 1992;257:1264–1267. doi: 10.1126/science.1325672. [DOI] [PubMed] [Google Scholar]
- 26.Inglese J, Koch WJ, Caron MG, Lefkowitz RJ. Isoprenylation in regulation of signal transduction by G-protein-coupled receptor kinases. Nature. 1992;359:147–150. doi: 10.1038/359147a0. [DOI] [PubMed] [Google Scholar]
- 27.Dzimiri N, Muiya P, Andres E, Al-Halees Z. Differential functional expression of human myocardial G protein receptor kinases in left ventricular cardiac diseases. Eur J Pharmacol. 2004;489:167–177. doi: 10.1016/j.ejphar.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 28.Kunapuli P, Benovic JL. Cloning and expression of GRK5: a member of the G protein-coupled receptor kinase family. Proc Natl Acad Sci U S A. 1993;90:5588–5592. doi: 10.1073/pnas.90.12.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thiyagarajan MM, Stracquatanio RP, Pronin AN, Evanko DS, Benovic JL, Wedegaertner PB. A predicted amphipathic helix mediates plasma membrane localization of GRK5. J Biol Chem. 2004;279:17989–17995. doi: 10.1074/jbc.M310738200. [DOI] [PubMed] [Google Scholar]
- 30.Lodowski DT, Pitcher JA, Capel WD, Lefkowitz RJ, Tesmer JJ. Keeping G proteins at bay: a complex between G protein-coupled receptor kinase 2 and Gbetagamma. Science. 2003;300:1256–1262. doi: 10.1126/science.1082348. [DOI] [PubMed] [Google Scholar]
- 31.Kim J, Ahn S, Ren XR, Whalen EJ, Reiter E, Wei H, Lefkowitz RJ. Functional antagonism of different G protein-coupled receptor kinases for beta-arrestin-mediated angiotensin II receptor signaling. Proc Natl Acad Sci U S A. 2005;102:1442–1447. doi: 10.1073/pnas.0409532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patwardhan P, Miller WT. Processive phosphorylation: mechanism and biological importance. Cell Signal. 2007;19:2218–2226. doi: 10.1016/j.cellsig.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cong M, Perry SJ, Lin FT, Fraser ID, Hu LA, Chen W, Pitcher JA, Scott JD, Lefkowitz RJ. Regulation of membrane targeting of the G protein-coupled receptor kinase 2 by protein kinase A and its anchoring protein AKAP79. J Biol Chem. 2001;276:15192–15199. doi: 10.1074/jbc.M009130200. [DOI] [PubMed] [Google Scholar]
- 34.Pronin AN, Benovic JL. Regulation of the G protein-coupled receptor kinase GRK5 by protein kinase C. J Biol Chem. 1997;272:3806–3812. doi: 10.1074/jbc.272.6.3806. [DOI] [PubMed] [Google Scholar]
- 35.Chuang TT, LeVine H, 3rd, De Blasi A. Phosphorylation and activation of beta-adrenergic receptor kinase by protein kinase C. J Biol Chem. 1995;270:18660–18665. doi: 10.1074/jbc.270.31.18660. [DOI] [PubMed] [Google Scholar]
- 36.Krasel C, Dammeier S, Winstel R, Brockmann J, Mischak H, Lohse MJ. Phosphorylation of GRK2 by protein kinase C abolishes its inhibition by calmodulin. J Biol Chem. 2001;276:1911–1915. doi: 10.1074/jbc.M008773200. [DOI] [PubMed] [Google Scholar]
- 37.Malhotra R, D'Souza KM, Staron ML, Birukov KG, Bodi I, Akhter SA. G alpha(q)-mediated activation of GRK2 by mechanical stretch in cardiac myocytes: the role of protein kinase C. J Biol Chem. 2010;285:13748–13760. doi: 10.1074/jbc.M110.109272. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Rakesh K, Yoo B, Kim IM, Salazar N, Kim KS, Rockman HA. beta-Arrestin-biased agonism of the angiotensin receptor induced by mechanical stress. Sci Signal. 2010;3:ra46. doi: 10.1126/scisignal.2000769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen CK, Burns ME, Spencer M, Niemi GA, Chen J, Hurley JB, Baylor DA, Simon MI. Abnormal photoresponses and light-induced apoptosis in rods lacking rhodopsin kinase. Proc Natl Acad Sci U S A. 1999;96:3718–3722. doi: 10.1073/pnas.96.7.3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu P, Osawa S, Weiss ER. M opsin phosphorylation in intact mammalian retinas. J Neurochem. 2005;93:135–144. doi: 10.1111/j.1471-4159.2004.03003.x. [DOI] [PubMed] [Google Scholar]
- 41.Weiss ER, Ducceschi MH, Horner TJ, Li A, Craft CM, Osawa S. Species-specific differences in expression of G-protein-coupled receptor kinase (GRK) 7 and GRK1 in mammalian cone photoreceptor cells: implications for cone cell phototransduction. J Neurosci. 2001;21:9175–9184. doi: 10.1523/JNEUROSCI.21-23-09175.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peppel K, Boekhoff I, McDonald P, Breer H, Caron MG, Lefkowitz RJ. G protein-coupled receptor kinase 3 (GRK3) gene disruption leads to loss of odorant receptor desensitization. J Biol Chem. 1997;272:25425–25428. doi: 10.1074/jbc.272.41.25425. [DOI] [PubMed] [Google Scholar]
- 43.Gainetdinov RR, Bohn LM, Walker JK, Laporte SA, Macrae AD, Caron MG, Lefkowitz RJ, Premont RT. Muscarinic supersensitivity and impaired receptor desensitization in G protein-coupled receptor kinase 5-deficient mice. Neuron. 1999;24:1029–1036. doi: 10.1016/s0896-6273(00)81048-x. [DOI] [PubMed] [Google Scholar]
- 44.Walker JK, Gainetdinov RR, Feldman DS, McFawn PK, Caron MG, Lefkowitz RJ, Premont RT, Fisher JT. G protein-coupled receptor kinase 5 regulates airway responses induced by muscarinic receptor activation. Am J Physiol Lung Cell Mol Physiol. 2004;286:L312–L319. doi: 10.1152/ajplung.00255.2003. [DOI] [PubMed] [Google Scholar]
- 45.Gainetdinov RR, Bohn LM, Sotnikova TD, Cyr M, Laakso A, Macrae AD, Torres GE, Kim KM, Lefkowitz RJ, Caron MG, Premont RT. Dopaminergic supersensitivity in G protein-coupled receptor kinase 6-deficient mice. Neuron. 2003;38:291–303. doi: 10.1016/s0896-6273(03)00192-2. [DOI] [PubMed] [Google Scholar]
- 46.Jaber M, Koch WJ, Rockman H, Smith B, Bond RA, Sulik KK, Ross J, Jr, Lefkowitz RJ, Caron MG, Giros B. Essential role of beta-adrenergic receptor kinase 1 in cardiac development and function. Proc Natl Acad Sci U S A. 1996;93:12974–12979. doi: 10.1073/pnas.93.23.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peppel K, Jacobson A, Huang X, Murray JP, Oppermann M, Freedman NJ. Overexpression of G protein-coupled receptor kinase-2 in smooth muscle cells attenuates mitogenic signaling via G protein-coupled and platelet-derived growth factor receptors. Circulation. 2000;102:793–799. doi: 10.1161/01.cir.102.7.793. [DOI] [PubMed] [Google Scholar]
- 48.Matkovich SJ, Diwan A, Klanke JL, Hammer DJ, Marreez Y, Odley AM, Brunskill EW, Koch WJ, Schwartz RJ, Dorn GW., 2nd Cardiac-specific ablation of G-protein receptor kinase 2 redefines its roles in heart development and beta-adrenergic signaling. Circ Res. 2006;99:996–1003. doi: 10.1161/01.RES.0000247932.71270.2c. [DOI] [PubMed] [Google Scholar]
- 49.Ng WA, Grupp IL, Subramaniam A, Robbins J. Cardiac myosin heavy chain mRNA expression and myocardial function in the mouse heart. Circ Res. 1991;68:1742–1750. doi: 10.1161/01.res.68.6.1742. [DOI] [PubMed] [Google Scholar]
- 50.Koch WJ, Rockman HA, Samama P, Hamilton RA, Bond RA, Milano CA, Lefkowitz RJ. Cardiac function in mice overexpressing the beta-adrenergic receptor kinase or a beta ARK inhibitor. Science. 1995;268:1350–1353. doi: 10.1126/science.7761854. [DOI] [PubMed] [Google Scholar]
- 51.Rockman HA, Choi DJ, Rahman NU, Akhter SA, Lefkowitz RJ, Koch WJ. Receptor-specific in vivo desensitization by the G protein-coupled receptor kinase-5 in transgenic mice. Proc Natl Acad Sci U S A. 1996;93:9954–9959. doi: 10.1073/pnas.93.18.9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iaccarino G, Rockman HA, Shotwell KF, Tomhave ED, Koch WJ. Myocardial overexpression of GRK3 in transgenic mice: evidence for in vivo selectivity of GRKs. Am J Physiol. 1998;275:H1298–H1306. doi: 10.1152/ajpheart.1998.275.4.H1298. [DOI] [PubMed] [Google Scholar]
- 53.Eckhart AD, Duncan SJ, Penn RB, Benovic JL, Lefkowitz RJ, Koch WJ. Hybrid transgenic mice reveal in vivo specificity of G protein-coupled receptor kinases in the heart. Circ Res. 2000;86:43–50. doi: 10.1161/01.res.86.1.43. [DOI] [PubMed] [Google Scholar]
- 54.Brodde OE. Beta-adrenoceptors in cardiac disease. Pharmacol Ther. 1993;60(3):405–430. doi: 10.1016/0163-7258(93)90030-h. [DOI] [PubMed] [Google Scholar]
- 55.Gauthier C, Tavernier G, Charpentier F, Langin D, Le Marec H. Functional beta3-adrenoceptor in the human heart. J Clin Invest. 1996;98:556–562. doi: 10.1172/JCI118823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng HJ, Zhang ZS, Onishi K, Ukai T, Sane DC, Cheng CP. Upregulation of functional beta(3)-adrenergic receptor in the failing canine myocardium. Circ Res. 2001;89:599–606. doi: 10.1161/hh1901.098042. [DOI] [PubMed] [Google Scholar]
- 57.Dessy C, Balligand JL. Beta3-adrenergic receptors in cardiac and vascular tissues emerging concepts and therapeutic perspectives. Adv Pharmacol. 2010;59:135–163. doi: 10.1016/S1054-3589(10)59005-7. [DOI] [PubMed] [Google Scholar]
- 58.Blaxall BC, Pellett AC, Wu SC, Pende A, Port JD. Purification and characterization of beta-adrenergic receptor mRNA-binding proteins. J Biol Chem. 2000;275:4290–4297. doi: 10.1074/jbc.275.6.4290. [DOI] [PubMed] [Google Scholar]
- 59.Bristow MR, Minobe WA, Raynolds MV, Port JD, Rasmussen R, Ray PE, Feldman AM. Reduced beta 1 receptor messenger RNA abundance in the failing human heart. J Clin Invest. 1993;92:2737–2745. doi: 10.1172/JCI116891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bristow MR, Ginsburg R, Minobe W, Cubicciotti RS, Sageman WS, Lurie K, Billingham ME, Harrison DC, Stinson EB. Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N Engl J Med. 1982;307:205–211. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- 61.Bristow MR, Hershberger RE, Port JD, Gilbert EM, Sandoval A, Rasmussen R, Cates AE, Feldman AM. Beta-adrenergic pathways in nonfailing and failing human ventricular myocardium. Circulation. 1990;82:I12–I25. [PubMed] [Google Scholar]
- 62.Ungerer M, Bohm M, Elce JS, Erdmann E, Lohse MJ. Altered expression of beta-adrenergic receptor kinase and beta 1-adrenergic receptors in the failing human heart. Circulation. 1993;87:454–463. doi: 10.1161/01.cir.87.2.454. [DOI] [PubMed] [Google Scholar]
- 63.Landmesser U, Drexler H. Update on inotropic therapy in the management of acute heart failure. Curr Treat Options Cardiovasc Med. 2007;9:443–449. doi: 10.1007/s11936-007-0039-9. [DOI] [PubMed] [Google Scholar]
- 64.Pleger ST, Boucher M, Most P, Koch WJ. Targeting myocardial beta-adrenergic receptor signaling and calcium cycling for heart failure gene therapy. J Card Fail. 2007;13:401–414. doi: 10.1016/j.cardfail.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 65.Ungerer M, Kessebohm K, Kronsbein K, Lohse MJ, Richardt G. Activation of beta-adrenergic receptor kinase during myocardial ischemia. Circ Res. 1996;79:455–460. doi: 10.1161/01.res.79.3.455. [DOI] [PubMed] [Google Scholar]
- 66.Iaccarino G, Keys JR, Rapacciuolo A, Shotwell KF, Lefkowitz RJ, Rockman HA, Koch WJ. Regulation of myocardial betaARK1 expression in catecholamine-induced cardiac hypertrophy in transgenic mice overexpressing alpha1B-adrenergic receptors. J Am Coll Cardiol. 2001;38:534–540. doi: 10.1016/s0735-1097(01)01396-1. [DOI] [PubMed] [Google Scholar]
- 67.Harding VB, Jones LR, Lefkowitz RJ, Koch WJ, Rockman HA. Cardiac beta ARK1 inhibition prolongs survival and augments beta blocker therapy in a mouse model of severe heart failure. Proc Natl Acad Sci U S A. 2001;98:5809–5814. doi: 10.1073/pnas.091102398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rockman HA, Chien KR, Choi DJ, Iaccarino G, Hunter JJ, Ross J, Jr, Lefkowitz RJ, Koch WJ. Expression of a beta-adrenergic receptor kinase 1 inhibitor prevents the development of myocardial failure in gene-targeted mice. Proc Natl Acad Sci U S A. 1998;95:7000–7005. doi: 10.1073/pnas.95.12.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brinks H, Koch WJ. betaARKct: a therapeutic approach for improved adrenergic signaling and function in heart disease. J Cardiovasc Transl Res. 2010;3:499–506. doi: 10.1007/s12265-010-9206-6. [DOI] [PubMed] [Google Scholar]
- 70.Petrofski JA, Koch WJ. The beta-adrenergic receptor kinase in heart failure. J Mol Cell Cardiol. 2003;35:1167–1174. doi: 10.1016/s0022-2828(03)00243-8. [DOI] [PubMed] [Google Scholar]
- 71.Anderson KM, Eckhart AD, Willette RN, Koch WJ. The myocardial beta-adrenergic system in spontaneously hypertensive heart failure (SHHF) rats. Hypertension. 1999;33:402–407. doi: 10.1161/01.hyp.33.1.402. [DOI] [PubMed] [Google Scholar]
- 72.Ping P, Anzai T, Gao M, Hammond HK. Adenylyl cyclase and G protein receptor kinase expression during development of heart failure. Am J Physiol. 1997;273:H707–H717. doi: 10.1152/ajpheart.1997.273.2.H707. [DOI] [PubMed] [Google Scholar]
- 73.Harris CA, Chuang TT, Scorer CA. Expression of GRK2 is increased in the left ventricles of cardiomyopathic hamsters. Basic Res Cardiol. 2001;96:364–368. doi: 10.1007/s003950170044. [DOI] [PubMed] [Google Scholar]
- 74.Vinge LE, Oie E, Andersson Y, Grogaard HK, Andersen G, Attramadal H. Myocardial distribution and regulation of GRK and beta-arrestin isoforms in congestive heart failure in rats. Am J Physiol Heart Circ Physiol. 2001;281:H2490–H2499. doi: 10.1152/ajpheart.2001.281.6.H2490. [DOI] [PubMed] [Google Scholar]
- 75.Gros R, Benovic JL, Tan CM, Feldman RD. G-protein-coupled receptor kinase activity is increased in hypertension. J Clin Invest. 1997;99:2087–2093. doi: 10.1172/JCI119381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Choi DJ, Koch WJ, Hunter JJ, Rockman HA. Mechanism of beta-adrenergic receptor desensitization in cardiac hypertrophy is increased beta-adrenergic receptor kinase. J Biol Chem. 1997;272:17223–17229. doi: 10.1074/jbc.272.27.17223. [DOI] [PubMed] [Google Scholar]
- 77.Bonita RE, Raake PW, Otis NJ, Chuprun JK, Spivack T, Dasgupta A, Whellan DJ, Mather PJ, Koch WJ. Dynamic changes in lymphocyte GRK2 levels in cardiac transplant patients: a biomarker for left ventricular function. Clin Transl Sci. 2010;3:14–18. doi: 10.1111/j.1752-8062.2010.00176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ogletree-Hughes ML, Stull LB, Sweet WE, Smedira NG, McCarthy PM, Moravec CS. Mechanical unloading restores beta-adrenergic responsiveness and reverses receptor downregulation in the failing human heart. Circulation. 2001;104:881–886. doi: 10.1161/hc3301.094911. [DOI] [PubMed] [Google Scholar]
- 79.Akhter SA, D'Souza KM, Malhotra R, Staron ML, Valeroso TB, Fedson SE, Anderson AS, Raman J, Jeevanandam V. Reversal of impaired myocardial beta-adrenergic receptor signaling by continuous-flow left ventricular assist device support. J Heart Lung Transplant. 2010;29:603–609. doi: 10.1016/j.healun.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hata JA, Williams ML, Schroder JN, Lima B, Keys JR, Blaxall BC, Petrofski JA, Jakoi A, Milano CA, Koch WJ. Lymphocyte levels of GRK2 (betaARK1) mirror changes in the LVAD-supported failing human heart: lower GRK2 associated with improved beta-adrenergic signaling after mechanical unloading. J Card Fail. 2006;12:360–368. doi: 10.1016/j.cardfail.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 81.Dipla K, Mattiello JA, Jeevanandam V, Houser SR, Margulies KB. Myocyte recovery after mechanical circulatory support in humans with end-stage heart failure. Circulation. 1998;97:2316–2322. doi: 10.1161/01.cir.97.23.2316. [DOI] [PubMed] [Google Scholar]
- 82.Usui I, Imamura T, Satoh H, Huang J, Babendure JL, Hupfeld CJ, Olefsky JM. GRK2 is an endogenous protein inhibitor of the insulin signaling pathway for glucose transport stimulation. EMBO J. 2004;23:2821–2829. doi: 10.1038/sj.emboj.7600297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Usui I, Imamura T, Babendure JL, Satoh H, Lu JC, Hupfeld CJ, Olefsky JM. G protein-coupled receptor kinase 2 mediates endothelin-1-induced insulin resistance via the inhibition of both Galphaq/11 and insulin receptor substrate-1 pathways in 3T3-L1 adipocytes. Mol Endocrinol. 2005;19:2760–2768. doi: 10.1210/me.2004-0429. [DOI] [PubMed] [Google Scholar]
- 84.Cipolletta E, Campanile A, Santulli G, Sanzari E, Leosco D, Campiglia P, Trimarco B, Iaccarino G. The G protein coupled receptor kinase 2 plays an essential role in beta-adrenergic receptor-induced insulin resistance. Cardiovasc Res. 2009;84:407–415. doi: 10.1093/cvr/cvp252. [DOI] [PubMed] [Google Scholar]
- 85.Anis Y, Leshem O, Reuveni H, Wexler I, Ben Sasson R, Yahalom B, Laster M, Raz I, Ben Sasson S, Shafrir E, Ziv E. Antidiabetic effect of novel modulating peptides of G-protein-coupled kinase in experimental models of diabetes. Diabetologia. 2004;47:1232–1244. doi: 10.1007/s00125-004-1444-1. [DOI] [PubMed] [Google Scholar]
- 86.Martini JS, Raake P, Vinge LE, DeGeorge B, Jr, Chuprun JK, Harris DM, Gao E, Eckhart AD, Pitcher JA, Koch WJ. Uncovering G protein-coupled receptor kinase-5 as a histone deacetylase kinase in the nucleus of cardiomyocytes. Proc Natl Acad Sci U S A. 2008;105:12457–12462. doi: 10.1073/pnas.0803153105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sorriento D, Santulli G, Fusco A, Anastasio A, Trimarco B, Iaccarino G. Intracardiac injection of AdGRK5-NT reduces left ventricular hypertrophy by inhibiting NF-kappaB-dependent hypertrophic gene expression. Hypertension. 2010;56:696–704. doi: 10.1161/HYPERTENSIONAHA.110.155960. [DOI] [PubMed] [Google Scholar]
- 88.Spinelli L, Trimarco V, Di Marino S, Marino M, Iaccarino G, Trimarco B. L41Q polymorphism of the G protein coupled receptor kinase 5 is associated with left ventricular apical ballooning syndrome. Eur J Heart Fail. 2010;12:13–16. doi: 10.1093/eurjhf/hfp173. [DOI] [PubMed] [Google Scholar]
- 89.Liggett SB, Cresci S, Kelly RJ, Syed FM, Matkovich SJ, Hahn HS, Diwan A, Martini JS, Sparks L, Parekh RR, Spertus JA, Koch WJ, Kardia SL, Dorn GW., 2nd A GRK5 polymorphism that inhibits beta-adrenergic receptor signaling is protective in heart failure. Nat Med. 2008;14:510–517. doi: 10.1038/nm1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou RH, Pesant S, Cohn HI, Soltys S, Koch WJ, Eckhart AD. Negative regulation of VEGF signaling in human coronary artery endothelial cells by G protein-coupled receptor kinase 5. Clin Transl Sci. 2009;2:57–61. doi: 10.1111/j.1752-8062.2008.00058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Koch WJ, Inglese J, Stone WC, Lefkowitz RJ. The binding site for the beta gamma subunits of heterotrimeric G proteins on the beta-adrenergic receptor kinase. J Biol Chem. 1993;268:8256–8260. [PubMed] [Google Scholar]
- 92.Li Z, Laugwitz KL, Pinkernell K, Pragst I, Baumgartner C, Hoffmann E, Rosport K, Munch G, Moretti A, Humrich J, Lohse MJ, Ungerer M. Effects of two Gbetagamma-binding proteins--N-terminally truncated phosducin and beta-adrenergic receptor kinase C terminus (betaARKct)--in heart failure. Gene Ther. 2003;10:1354–1361. doi: 10.1038/sj.gt.3301995. [DOI] [PubMed] [Google Scholar]
- 93.Brinks H, Koch WJ. Targeting G protein-coupled receptor kinases (GRKs) in Heart Failure. Drug Discov Today Dis Mech. 2010;7:e129–e134. doi: 10.1016/j.ddmec.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Williams ML, Hata JA, Schroder J, Rampersaud E, Petrofski J, Jakoi A, Milano CA, Koch WJ. Targeted beta-adrenergic receptor kinase (betaARK1) inhibition by gene transfer in failing human hearts. Circulation. 2004;109:1590–1593. doi: 10.1161/01.CIR.0000125521.40985.28. [DOI] [PubMed] [Google Scholar]
- 95.Eckhart AD, Koch WJ. Expression of a beta-adrenergic receptor kinase inhibitor reverses dysfunction in failing cardiomyocytes. Mol Ther. 2002;5:74–79. doi: 10.1006/mthe.2001.0508. [DOI] [PubMed] [Google Scholar]
- 96.Freeman K, Lerman I, Kranias EG, Bohlmeyer T, Bristow MR, Lefkowitz RJ, Iaccarino G, Koch WJ, Leinwand LA. Alterations in cardiac adrenergic signaling and calcium cycling differentially affect the progression of cardiomyopathy. J Clin Invest. 2001;107:967–974. doi: 10.1172/JCI12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.White DC, Hata JA, Shah AS, Glower DD, Lefkowitz RJ, Koch WJ. Preservation of myocardial beta-adrenergic receptor signaling delays the development of heart failure after myocardial infarction. Proc Natl Acad Sci U S A. 2000;97:5428–5433. doi: 10.1073/pnas.090091197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shah AS, White DC, Emani S, Kypson AP, Lilly RE, Wilson K, Glower DD, Lefkowitz RJ, Koch WJ. In vivo ventricular gene delivery of a beta-adrenergic receptor kinase inhibitor to the failing heart reverses cardiac dysfunction. Circulation. 2001;103:1311–1316. doi: 10.1161/01.cir.103.9.1311. [DOI] [PubMed] [Google Scholar]
- 99.Rengo G, Lymperopoulos A, Zincarelli C, Donniacuo M, Soltys S, Rabinowitz JE, Koch WJ. Myocardial adeno-associated virus serotype 6-betaARKct gene therapy improves cardiac function and normalizes the neurohormonal axis in chronic heart failure. Circulation. 2009;119:89–98. doi: 10.1161/CIRCULATIONAHA.108.803999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tevaearai HT, Eckhart AD, Shotwell KF, Wilson K, Koch WJ. Ventricular dysfunction after cardioplegic arrest is improved after myocardial gene transfer of a beta-adrenergic receptor kinase inhibitor. Circulation. 2001;104:2069–2074. doi: 10.1161/hc4201.097188. [DOI] [PubMed] [Google Scholar]
- 101.Tevaearai HT, Walton GB, Keys JR, Koch WJ, Eckhart AD. Acute ischemic cardiac dysfunction is attenuated via gene transfer of a peptide inhibitor of the beta-adrenergic receptor kinase (betaARK1) J Gene Med. 2005;7:1172–1177. doi: 10.1002/jgm.770. [DOI] [PubMed] [Google Scholar]
- 102.Blaxall BC, Spang R, Rockman HA, Koch WJ. Differential myocardial gene expression in the development and rescue of murine heart failure. Physiol Genomics. 2003;15:105–114. doi: 10.1152/physiolgenomics.00087.2003. [DOI] [PubMed] [Google Scholar]
- 103.Brinks H, Boucher M, Gao E, Chuprun JK, Pesant S, Raake PW, Huang ZM, Wang X, Qiu G, Gumpert A, Harris DM, Eckhart AD, Most P, Koch WJ. Level of G protein-coupled receptor kinase-2 determines myocardial ischemia/reperfusion injury via pro- and anti-apoptotic mechanisms. Circ Res. 2010;107:1140–1149. doi: 10.1161/CIRCRESAHA.110.221010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Whalen EJ, Foster MW, Matsumoto A, Ozawa K, Violin JD, Que LG, Nelson CD, Benhar M, Keys JR, Rockman HA, Koch WJ, Daaka Y, Lefkowitz RJ, Stamler JS. Regulation of beta-adrenergic receptor signaling by S-nitrosylation of G-protein-coupled receptor kinase 2. Cell. 2007;129:511–522. doi: 10.1016/j.cell.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 105.Ivanina T, Blumenstein Y, Shistik E, Barzilai R, Dascal N. Modulation of L-type Ca2+ channels by gbeta gamma and calmodulin via interactions with N and C termini of alpha 1C. J Biol Chem. 2000;275:39846–39854. doi: 10.1074/jbc.M005881200. [DOI] [PubMed] [Google Scholar]
- 106.Volkers M, Weidenhammer C, Herzog N, Qiu G, Spaich K, von Wegner F, Peppel K, Muller OJ, Schinkel S, Rabinowitz JE, Hippe HJ, Brinks H, Katus HA, Koch WJ, Eckhart AD, Friedrich O, Most P. The Inotropic Peptide {beta}ARKct Improves {beta}AR Responsiveness in Normal and Failing Cardiomyocytes Through G{beta}{gamma}-Mediated L-Type Calcium Current Disinhibition. Circ Res. 2010 doi: 10.1161/CIRCRESAHA.110.225201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Raake PW, Vinge LE, Gao E, Boucher M, Rengo G, Chen X, DeGeorge BR, Jr, Matkovich S, Houser SR, Most P, Eckhart AD, Dorn GW, 2nd, Koch WJ. G protein-coupled receptor kinase 2 ablation in cardiac myocytes before or after myocardial infarction prevents heart failure. Circ Res. 2008;103:413–422. doi: 10.1161/CIRCRESAHA.107.168336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev. 2010;90:513–557. doi: 10.1152/physrev.00007.2009. [DOI] [PubMed] [Google Scholar]
- 109.Bonacci TM, Mathews JL, Yuan C, Lehmann DM, Malik S, Wu D, Font JL, Bidlack JM, Smrcka AV. Differential targeting of Gbetagamma-subunit signaling with small molecules. Science. 2006;312:443–446. doi: 10.1126/science.1120378. [DOI] [PubMed] [Google Scholar]
- 110.Casey LM, Pistner AR, Belmonte SL, Migdalovich D, Stolpnik O, Nwakanma FE, Vorobiof G, Dunaevsky O, Matavel A, Lopes CM, Smrcka AV, Blaxall BC. Small Molecule Disruption of G{beta}{gamma} Signaling Inhibits the Progression of Heart Failure. Circ Res. 2010 doi: 10.1161/CIRCRESAHA.110.217075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kamal FA, Smrcka AV, Blaxall BC. Taking the heart failure battle inside the cell: Small molecule targeting of Gbetagamma subunits. J Mol Cell Cardiol. 2011 doi: 10.1016/j.yjmcc.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lorenz K, Schmitt JP, Schmitteckert EM, Lohse MJ. A new type of ERK1/2 autophosphorylation causes cardiac hypertrophy. Nat Med. 2009;15:75–83. doi: 10.1038/nm.1893. [DOI] [PubMed] [Google Scholar]
- 113.CDC. Health, United States, 2008. Hyattsville, MD: National Center for Health Statistics; 2008. [PubMed] [Google Scholar]
- 114.Feldman RD. Defective venous beta-adrenergic response in borderline hypertensive subjects is corrected by a low sodium diet. J Clin Invest. 1990;85:647–652. doi: 10.1172/JCI114487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Feldman RD. Beta-adrenergic receptor alterations in hypertension--physiological and molecular correlates. Can J Physiol Pharmacol. 1987;65:1666–1672. doi: 10.1139/y87-261. [DOI] [PubMed] [Google Scholar]
- 116.Feldman RD, Gros R. Impaired vasodilator function in hypertension: the role of alterations in receptor-G protein coupling. Trends Cardiovasc Med. 1998;8:297–305. doi: 10.1016/s1050-1738(98)00022-x. [DOI] [PubMed] [Google Scholar]
- 117.Feldman RD, Lawton WJ, McArdle WL. Low sodium diet corrects the defect in lymphocyte beta-adrenergic responsiveness in hypertensive subjects. J Clin Invest. 1987;79:290–294. doi: 10.1172/JCI112797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brodde OE, Kretsch R, Ikezono K, Zerkowski HR, Reidemeister JC. Human beta-adrenoceptors: relation of myocardial and lymphocyte beta-adrenoceptor density. Science. 1986;231:1584–1585. doi: 10.1126/science.3006250. [DOI] [PubMed] [Google Scholar]
- 119.Eckhart AD, Ozaki T, Tevaearai H, Rockman HA, Koch WJ. Vascular-targeted overexpression of G protein-coupled receptor kinase-2 in transgenic mice attenuates beta-adrenergic receptor signaling and increases resting blood pressure. Mol Pharmacol. 2002;61:749–758. doi: 10.1124/mol.61.4.749. [DOI] [PubMed] [Google Scholar]
- 120.Koch WJ, Lefkowitz RJ, Rockman HA. Functional consequences of altering myocardial adrenergic receptor signaling. Annu Rev Physiol. 2000;62:237–260. doi: 10.1146/annurev.physiol.62.1.237. [DOI] [PubMed] [Google Scholar]
- 121.Freedman NJ, Ament AS, Oppermann M, Stoffel RH, Exum ST, Lefkowitz RJ. Phosphorylation and desensitization of human endothelin A and B receptors. Evidence for G protein-coupled receptor kinase specificity. J Biol Chem. 1997;272:17734–17743. doi: 10.1074/jbc.272.28.17734. [DOI] [PubMed] [Google Scholar]
- 122.Oppermann M, Freedman NJ, Alexander RW, Lefkowitz RJ. Phosphorylation of the type 1A angiotensin II receptor by G protein-coupled receptor kinases and protein kinase C. J Biol Chem. 1996;271:13266–13272. doi: 10.1074/jbc.271.22.13266. [DOI] [PubMed] [Google Scholar]
- 123.Diviani D, Lattion AL, Larbi N, Kunapuli P, Pronin A, Benovic JL, Cotecchia S. Effect of different G protein-coupled receptor kinases on phosphorylation and desensitization of the alpha1B-adrenergic receptor. J Biol Chem. 1996;271:5049–5058. doi: 10.1074/jbc.271.9.5049. [DOI] [PubMed] [Google Scholar]
- 124.Freedman NJ, Liggett SB, Drachman DE, Pei G, Caron MG, Lefkowitz RJ. Phosphorylation and desensitization of the human beta 1-adrenergic receptor. Involvement of G protein-coupled receptor kinases and cAMP-dependent protein kinase. J Biol Chem. 1995;270:17953–17961. doi: 10.1074/jbc.270.30.17953. [DOI] [PubMed] [Google Scholar]
- 125.Vinge LE, Andressen KW, Attramadal T, Andersen GO, Ahmed MS, Peppel K, Koch WJ, Freedman NJ, Levy FO, Skomedal T, Osnes JB, Attramadal H. Substrate specificities of g protein-coupled receptor kinase-2 and -3 at cardiac myocyte receptors provide basis for distinct roles in regulation of myocardial function. Mol Pharmacol. 2007;72:582–591. doi: 10.1124/mol.107.035766. [DOI] [PubMed] [Google Scholar]
- 126.Vinge LE, von Lueder TG, Aasum E, Qvigstad E, Gravning JA, How OJ, Edvardsen T, Bjornerheim R, Ahmed MS, Mikkelsen BW, Oie E, Attramadal T, Skomedal T, Smiseth OA, Koch WJ, Larsen TS, Attramadal H. Cardiac-restricted expression of the carboxyl-terminal fragment of GRK3 Uncovers Distinct Functions of GRK3 in regulation of cardiac contractility and growth: GRK3 controls cardiac alpha1-adrenergic receptor responsiveness. J Biol Chem. 2008;283:10601–10610. doi: 10.1074/jbc.M708912200. [DOI] [PubMed] [Google Scholar]
- 127.Cohn HI, Harris DM, Pesant S, Pfeiffer M, Zhou RH, Koch WJ, Dorn GW, 2nd, Eckhart AD. Inhibition of vascular smooth muscle G protein-coupled receptor kinase 2 enhances alpha1D-adrenergic receptor constriction. Am J Physiol Heart Circ Physiol. 2008;295:H1695–H1704. doi: 10.1152/ajpheart.00564.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Morris GE, Nelson CP, Standen NB, Challiss RA, Willets JM. Endothelin signalling in arterial smooth muscle is tightly regulated by G protein-coupled receptor kinase 2. Cardiovasc Res. 2010;85:424–433. doi: 10.1093/cvr/cvp310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu S, Premont RT, Kontos CD, Zhu S, Rockey DC. A crucial role for GRK2 in regulation of endothelial cell nitric oxide synthase function in portal hypertension. Nat Med. 2005;11:952–958. doi: 10.1038/nm1289. [DOI] [PubMed] [Google Scholar]
- 130.Cohn HI, Xi Y, Pesant S, Harris DM, Hyslop T, Falkner B, Eckhart AD. G protein-coupled receptor kinase 2 expression and activity are associated with blood pressure in black Americans. Hypertension. 2009;54:71–76. doi: 10.1161/HYPERTENSIONAHA.108.125955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Oyama N, Urasawa K, Kaneta S, Sakai H, Saito T, Takagi C, Yoshida I, Kitabatake A, Tsutsui H. Chronic beta-adrenergic receptor stimulation enhances the expression of G-Protein coupled receptor kinases, GRK2 and GRK5, in both the heart and peripheral lymphocytes. Circ J. 2005;69:987–990. doi: 10.1253/circj.69.987. [DOI] [PubMed] [Google Scholar]
- 132.Oliver E, Rovira E, Monto F, Valldecabres C, Julve R, Muedra V, Ruiz N, Barettino D, D'Ocon P. beta-Adrenoceptor and GRK3 expression in human lymphocytes is related to blood pressure and urinary albumin excretion. J Hypertens. 2010;28:1281–1289. doi: 10.1097/HJH.0b013e3283383564. [DOI] [PubMed] [Google Scholar]
- 133.Oliver E, Marti D, Monto F, Flacco N, Moreno L, Barettino D, Ivorra MD, D'Ocon P. The impact of alpha1-adrenoceptors up-regulation accompanied by the impairment of beta-adrenergic vasodilatation in hypertension. J Pharmacol Exp Ther. 2009;328:982–990. doi: 10.1124/jpet.108.146043. [DOI] [PubMed] [Google Scholar]
- 134.Ramos-Ruiz R, Penela P, Penn RB, Mayor F., Jr Analysis of the human G protein-coupled receptor kinase 2 (GRK2) gene promoter: regulation by signal transduction systems in aortic smooth muscle cells. Circulation. 2000;101:2083–2089. doi: 10.1161/01.cir.101.17.2083. [DOI] [PubMed] [Google Scholar]
- 135.Sallese M, Mariggio S, Collodel G, Moretti E, Piomboni P, Baccetti B, De Blasi A. G protein-coupled receptor kinase GRK4. Molecular analysis of the four isoforms and ultrastructural localization in spermatozoa and germinal cells. J Biol Chem. 1997;272:10188–10195. doi: 10.1074/jbc.272.15.10188. [DOI] [PubMed] [Google Scholar]
- 136.Premont RT, Macrae AD, Stoffel RH, Chung N, Pitcher JA, Ambrose C, Inglese J, MacDonald ME, Lefkowitz RJ. Characterization of the G protein-coupled receptor kinase GRK4. Identification of four splice variants. J Biol Chem. 1996;271:6403–6410. doi: 10.1074/jbc.271.11.6403. [DOI] [PubMed] [Google Scholar]
- 137.Felder RA, Sanada H, Xu J, Yu PY, Wang Z, Watanabe H, Asico LD, Wang W, Zheng S, Yamaguchi I, Williams SM, Gainer J, Brown NJ, Hazen-Martin D, Wong LJ, Robillard JE, Carey RM, Eisner GM, Jose PA. G protein-coupled receptor kinase 4 gene variants in human essential hypertension. Proc Natl Acad Sci U S A. 2002;99:3872–3877. doi: 10.1073/pnas.062694599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liggett SB. Long-distance affair with adrenal GRK2 hangs up heart failure. Nat Med. 2007;13:246–248. doi: 10.1038/nm0307-246. [DOI] [PubMed] [Google Scholar]
- 139.Thomas JA, Marks BH. Plasma norepinephrine in congestive heart failure. Am J Cardiol. 1978;41:233–243. doi: 10.1016/0002-9149(78)90162-5. [DOI] [PubMed] [Google Scholar]
- 140.Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB, Rector T. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med. 1984;311:819–823. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- 141.Lymperopoulos A, Rengo G, Funakoshi H, Eckhart AD, Koch WJ. Adrenal GRK2 upregulation mediates sympathetic overdrive in heart failure. Nat Med. 2007;13:315–323. doi: 10.1038/nm1553. [DOI] [PubMed] [Google Scholar]
- 142.Lymperopoulos A, Rengo G, Zincarelli C, Soltys S, Koch WJ. Modulation of adrenal catecholamine secretion by in vivo gene transfer and manipulation of G protein-coupled receptor kinase-2 activity. Mol Ther. 2008;16:302–307. doi: 10.1038/sj.mt.6300371. [DOI] [PubMed] [Google Scholar]
- 143.Lymperopoulos A, Rengo G, Gao E, Ebert SN, Dorn GW, 2nd, Koch WJ. Reduction of sympathetic activity via adrenal-targeted GRK2 gene deletion attenuates heart failure progression and improves cardiac function after myocardial infarction. J Biol Chem. 2010;285:16378–16386. doi: 10.1074/jbc.M109.077859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rengo G, Leosco D, Zincarelli C, Marchese M, Corbi G, Liccardo D, Filippelli A, Ferrara N, Lisanti MP, Koch WJ, Lymperopoulos A. Adrenal GRK2 lowering is an underlying mechanism for the beneficial sympathetic effects of exercise training in heart failure. Am J Physiol Heart Circ Physiol. 2010;298:H2032–H2038. doi: 10.1152/ajpheart.00702.2009. [DOI] [PubMed] [Google Scholar]
- 145.Walker JK, Peppel K, Lefkowitz RJ, Caron MG, Fisher JT. Altered airway and cardiac responses in mice lacking G protein-coupled receptor kinase 3. Am J Physiol. 1999;276:R1214–R1221. doi: 10.1152/ajpregu.1999.276.4.R1214. [DOI] [PubMed] [Google Scholar]
- 146.Fong AM, Premont RT, Richardson RM, Yu YR, Lefkowitz RJ, Patel DD. Defective lymphocyte chemotaxis in beta-arrestin2- and GRK6-deficient mice. Proc Natl Acad Sci U S A. 2002;99:7478–7483. doi: 10.1073/pnas.112198299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Patel CB, Noor N, Rockman HA. Functional selectivity in adrenergic and angiotensin signaling systems. Mol Pharmacol. 2010;78:983–992. doi: 10.1124/mol.110.067066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Neves SR, Ram PT, Iyengar R. G protein pathways. Science. 2002;296:1636–1639. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]