Abstract
Epidemiological studies indicate that stress, chronic depression and lack of social support might serve as risk factors for cancer development and progression. Recent cellular and molecular studies have identified biological processes that could potentially mediate such effects. This review integrates clinical, cellular and molecular studies to provide a mechanistic understanding of the interface between biological and behavioural influences in cancer, and identifies novel behavioural or pharmacological interventions that might help improve cancer outcomes.
Clinical studies indicate that stress, chronic depression, social support and other psychological factors might influence cancer onset and progression1–5. Recent mechanistic studies have identified biological signalling pathways that could contribute to such effects. Environmental and psycho-social processes initiate a cascade of information-processing pathways in the central nervous system (CNS) and periphery, which subsequently trigger fight-or-flight stress responses in the autonomic nervous system (ANS), or defeat/ withdrawal responses that are produced by the hypothalamic–pituitary–adrenal axis (HPA)6. FIGURE 1 shows the areas of the brain that are thought to be responsible for mediating stress responses and their effects on the adrenal glands and other target tissues. Cognitive and emotional feedback from cortical and limbic areas of the brain modulate the activity of hypothalamic and brain-stem structures that directly control HPA and ANS activity7.
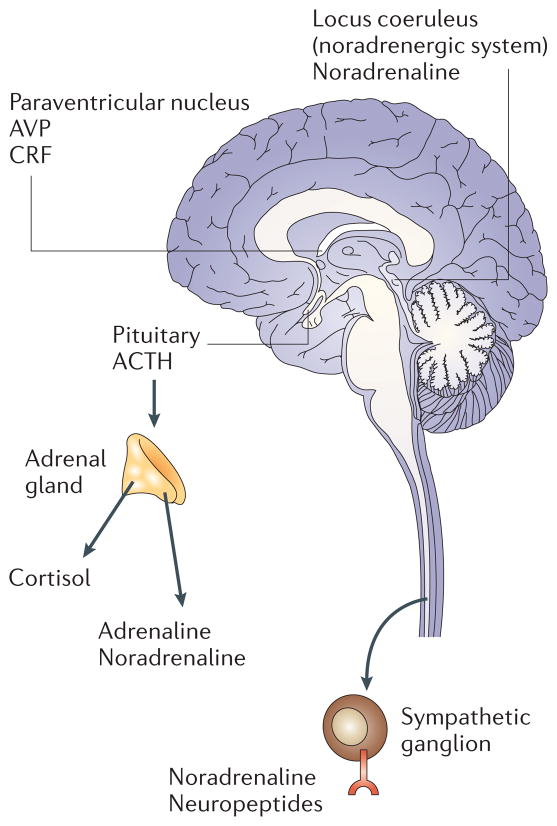
Figure 1. Important components of the central and peripheral stress systems.
Stressful experiences activate components of the limbic system, which includes the hypothalamus, the hippocampus, the amygdala, and other nearby areas. In response to neurosensory signals, the hypothalamus secretes corticotrophin-releasing factor (CRF) and arginine vasopressin (AVP), both of which activate the pituitary to produce hormones such as adrenocorticotropic hormone (ACTH). Circulating ACTH stimulates the production of glucocorticoids from the adrenal cortex. The sympathetic nervous system originates from the brainstem, and the pre-ganglionic neurons terminate in the ganglia near the spinal column. From these ganglia, post-ganglionic fibres run to the effector organs. The main neurotransmitter of the pre-ganglionic sympathetic fibres is acetylcholine and the typical neurotransmitter released by the post-ganglionic neurons is noradrenaline. The adrenal medulla contains chromaffin cells, which release mainly adrenaline.
HPA responses are mediated by hypo-thalamic production of corticotrophin-releasing factor and arginine vasopressin, both of which activate the secretion of pituitary hormones such as adrenocortico-tropic hormone (ACTH), enkephalins and endorphins. ACTH induces downstream release of glucocorticoids such as cortisol from the adrenal cortex. Glucocorticoids control growth, metabolism and immune function, and have a pivotal role in regulating basal function and stress reactivity across a wide variety of organ systems8. ANS responses to stress are mediated primarily by activation of the sympathetic nervous system (SNS) and subsequent release of catecholamines (principally noradrenaline and adrenaline) from sympathetic neurons and the adrenal medulla. Levels of catecholamines are increased in individuals who experience acute or chronic stress, and are responsible for ANS effects on cardiac, respiratory, vascular and other organ systems8. Examples of stressors associated with alterations in the HPA and/or ANS include marital disruption, bereavement, depression, chronic sleep disruption, severe trauma and post-traumatic stress disorder9,10.
The activation of these pathways prepares an individual to survive a threat, and the physiological stress responses are therefore generally considered adaptive. However, under chronic stress most physiological systems are negatively affected by prolonged exposure to glucocorticoids and catecholamines11. These changes are manifested by deleterious health consequences such as increased risk for cardiac disease, slower wound healing and increased risk from infections11. In the past decade, it has become increasingly clear that chronic alterations in neuroendocrine dynamics can also alter multiple physiological processes involved in tumour pathogenesis12–15.
In this article, we review the clinical and experimental evidence regarding the effects of stress on tumour development, growth and progression. Special emphasis is placed on neuroendocrine influences on the tumour microenvironment, viral oncogenesis and the immune system (FIG. 2). Although the mechanisms and clinical relevance of these pathways are described separately, there are numerous interactions between them, reflecting the complexity of cancer pathogenesis. These pathways might provide additional clues about factors that regulate the course of disease in cancer patients and might offer new opportunities for therapeutic interventions.
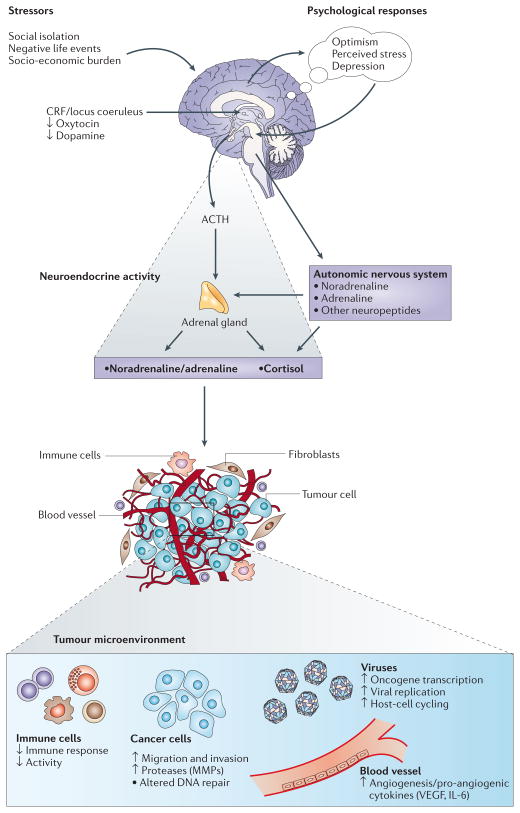
Figure 2. Effects of stress-associated factors on the tumour microenvironment.
The responses to stressors involve central nervous system (CNS) perceptions of threat and subsequent activation of the autonomic nervous system (ANS) and the hypothalamic–pituitary–adrenal (HPA) axis. Catecholamines, glucocorticoids and other stress hormones are subsequently released from the adrenal gland, brain and sympathetic nerve terminals and can modulate the activity of multiple components of the tumour microenvironment. Effects include the promotion of tumour-cell growth, migration and invasive capacity, and stimulation of angiogenesis by inducing production of pro-angiogenic cytokines. Stress hormones can also activate oncogenic viruses and alter several aspects of immune function, including antibody production, cytokine production profiles and cell trafficking (see REF. 6 for a comprehensive review of immune effects). Collectively, these downstream effects create a permissive environment for tumour initiation, growth and progression. CRF, corticotrophin-releasing factor; IL-6, interleukin-6; MMP, matrix metalloproteinase; VEGF, vascular endothelial growth factor.
Endocrine stress response and cancer
There is evidence linking stress, concomitant behavioural response patterns and resultant neurohormonal and neurotransmitter changes (all of which are referred to collectively within this paper as bio-behavioural factors) to cancer development and progression. Epidemiological data show that psychological and social characteristics might be associated with differential cancer onset, progression and mortality. For example, a twofold increase in breast cancer risk is evident after disruption of marriage owing to divorce, separation or death of a spouse5. Data from 3 eastern and midwestern states in the United States indicate that cancer risk increases after chronic depression that has lasted for at least 6 years16. A third study found that the combination of extreme stress and low social support was related to a ninefold increase in breast cancer incidence4. However, findings have been inconsistent. In general, stronger relationships have been observed between psycho-social factors and cancer progression than between psychosocial factors and cancer incidence (see REF. 3 for a discussion of the strengths and weaknesses of this literature). Data from patients with existing tumours show that cancer diagnosis and treatment cause substantial distress, and that those who tend toward depressive coping methods, such as hopelessness and helplessness, might experience accelerated disease progression2. By contrast, positive factors such as social support and optimism have predicted longer survival17,18. Additionally, there are important interactions between behavioural stress factors and health behaviours — including smoking, insomnia, alcohol abuse and obesity — that might have a further impact on cancer risk19. Recent experimental studies have begun to elucidate the mechanisms underlying these observations.
Animal models have provided compelling evidence regarding the effects of behavioural stress on tumorigenesis and the biological mechanisms involved (TABLE 1). For example, immobilization stress in rats that were given a carcinogen, diethylnitrosamine, increased both the incidence and rate of tumour growth20. Experimental stressors have also been found to increase the pathogenesis of various virally mediated tumours in animal models (see below). Swim stress, surgical stress, social confrontation and hypothermia resulted in increased lung metastasis from injected breast cancer cells21–24. Swim stress, laparotomy (opening the abdomen) and social confrontation caused a 2- to 5-fold increase in the number of rat MADB106 breast tumour metastases present in the lung24,25 and a similar increase in the number of lung metastases counted 3 weeks later24–26. β-Adrenergic agonists (which simulate activation of the SNS) such as metaproterenol show dose-dependent increases in lung tumour metastases. Similarly, adrenaline injections promoted mammary tumour metastasis21–24. Perhaps most importantly, pre-treatment of animals with β-adrenergic antagonists (to block the activity of SNS activation) and indomethacin (to block inflammation) synergistically blocked the effects of behavioural stress on lung tumour metastasis27.
Table 1.
Effects of stress and stress-associated hormones on cancer
| Experimental manipulation | Animal | Biological effect | Tumour type | Effect on tumour growth | References |
|---|---|---|---|---|---|
| Confrontation | Rats | NA | Breast | Increased metastasis of tumour cells to the lung | 25 |
| Restraint stress | Rats | Decreased numbers of T cells | Mammary | Increased growth during stress | 144 |
| Forced swim | Rats | Decreased natural-killer-cell activity | Leukaemia | Increased mortality | 22 |
| Abdominal surgery | Rats | Decreased natural-killer-cell activity | Mammary | Increased metastasis of tumour cells to the lung | 22 |
| High versus low dopaminergic reactivity | Rats | Decreased angiogenesis with high dopaminergic reactivity | Mammary | Fewer lung metastasis with increased dopaminergic reactivity | 145 |
| Dopamine administration | Mice | Decreased angiogenesis; decreased VEGF– VEGFR2 binding and phosphorylation | Ovarian | Decreased ascites formation | 56 |
| Dopamine administration | Mice | Decreased angiogenesis | Gastric | Decreased growth | 55 |
| Social isolation | Mice | Decreased macrophage activity | Ehrlich | Increased growth | 146 |
| Immobilization stress | Mice | Increased angiogenesis | Ovarian | Increased growth | 30 |
| Restraint stress | Mice | Decreased IL-12, IFNγ, CCL27 (also known as CTACK) and numbers of infiltrating T cells; increased numbers of suppressor cells | Skin and squamous cell carcinoma | Increased incidence, number, size and density | 110 |
CTACK, cutaneous T-cell attracting chemokine; IL-12, interleukin-12; IFNγ, interferon-γ; NA, not available; VEGF, vascular endothelial growth factor; VEGFR2, VEGF receptor 2.
Cellular and molecular events that promote cancer growth are also affected by stress. Swim stress in rodents results in induction of chromosomal aberrations and sister chromatid exchanges28 as well as lower activity of metaphase nucleolar organizer regions in bone marrow cells29. These findings indicate that stress might compromise DNA repair mechanisms. Stress can also influence the expression of viral oncogenes and replication of tumorigenic viruses (see below). In an orthotopic murine model of ovarian carcinoma, immobilization stress increased tumour burden and enhanced angiogenesis and tumour production of vascular endothelial growth factor (VEGF)30, indicating that stress might promote tumour growth by facilitating development of a blood supply. VEGF is a pro-angiogenic molecule that stimulates endothelial cell migration, proliferation and proteolytic activity31. VEGF also interferes with the development of T cells and the functional maturation of dendritic cells32,33, indicating possible effects on anti-tumour immune responses (see below). In line with these findings, recent clinical studies have shown links between higher levels of social support and lower serum levels of VEGF in patients with ovarian cancer34. Furthermore, social support has also been linked to lower levels of interleukin-6 (IL-6), another pro-angiogenic factor, both in peripheral blood and in ascites from patients with ovarian cancer35.
Understanding the mechanisms responsible for mediating the effects of stress on human tumour tissues is crucial for determining the full impact of stress on tumorigenesis and for devising effective interventions. Experimental evidence indicates that stress hormones have multiple effects on human tumour biology. Hormones that are associated with SNS activation might favour angiogenesis in human tumours. Noradrenaline has been shown to upregulate VEGF in adipose tissue and two ovarian cancer cell lines through the β-adrenergic receptor (βAR)–cyclic AMP (cAMP)–protein kinase A (PKA) pathway36,37. This effect was abolished by a β-blocker, propranolol, and was mimicked by isopro-terenol (a synthetic drug that mimics the effects of SNS stimulation), and was therefore thought to be mediated through βARs36,37. Noradrenaline also promotes various steps that are essential to tumour metastasis, including invasion and migration. In in vitro experimental models, noradrenaline increased colon cancer cell migration, an effect that was inhibited by β-blockers38. Both adrenaline and noradrenaline promoted in vitro invasion of ovarian cancer cells by increasing the expression levels of matrix metalloproteinase 2 (MMP2) and MMP9 12.
βARs, which mediate most of the effects of catecholamines, have been identified on breast and ovarian cancer cells12,13. In both of these studies, β2AR was the dominant adrenergic receptor present. β ARs are G-protein-coupled receptors whose primary function is the transmission of information from the extracellular environment to the interior of the cell, leading to activation of adenylyl cyclase and accumulation of the second messenger cAMP39. In mammary tumours, activation of βARs has been linked to accelerated tumour growth13–15. The cAMP-responsive-element-binding (CREB) protein is an important transcription factor that is activated by multiple signal-transduction pathways in response to external stimuli, including stress hormones40,41. Several studies have shown a role for the CREB family of proteins in tumour cell proliferation, migration, angiogenesis and inhibition of apoptosis40–42, as well as the expression of viral oncogenes (see below). An additional cAMP target, EPAC (also known as Rap guanine-nucleotide-exchange factor 3 (RAPGEF3)) is an exchange protein that is directly activated by cAMP. EPAC was recently shown to control a number of cellular processes that were previously attributed to PKA43. For example, βAR-mediated activation of cAMP promotes ovarian cancer cell adhesion through the EPAC–RAP1 pathway44. Collectively, these studies demonstrate the growing evidence that mediators of SNS activate cellular pathways within tumours that contribute to growth and progression. However, the clinical relevance in human studies of the bio-behavioural stress mechanisms described above remains to be demonstrated.
Glucocorticoids and other mediators
Glucocorticoids regulate a wide variety of cellular processes through glucocorticoid-receptor-mediated activation or repression of target genes. Recent studies have demonstrated that whereas glucocorticoid hormones induce apoptosis in lymphocytes45, these hormones activate survival genes that protect cancer cells from the effects of chemotherapy in both in vitro and in vivo experimental models46,47. Glucocorticoids can also activate oncogenic viruses and inhibit anti-tumour and antiviral cellular immune responses (see below). Glucocorticoids such as cortisol might function in a synergistic fashion with catecholamines to facilitate cancer growth. For example, in lung carcinoma cells cortisol increased βAR density and potentiated the isoproterenol-induced increase in cAMP accumulation48. So, it is plausible that stressful situations characterized by both increased catecholamine and cortisol concentrations (for example, uncontrollable stress) might have the greatest impact on cancer-related processes.
The expression levels of other hormones affected by stress include prolactin, which increases with stress49,50, and oxytocin and dopamine, which decrease with stress51. Prolactin can promote cell growth and survival in breast tumour and other tumour cells52. Oxytocin inhibits the growth of epithelial cell (such as breast and endometrial) tumours and those of neuronal or bone origin, but the hormone has a growth-stimulating effect in trophoblast and endothelium tumours53. For example, exogenous oxytocin has a dose-dependent mitogenic effect on human small-cell lung cancer cell lines, which is blocked by an oxytocin receptor antagonist54. Dopamine, which is known to inhibit the growth of several types of malignant tumours55, blocks VEGF-induced angiogenesis both in vitro and in vivo, primarily by inducing endocytosis of VEGF receptor 2 in endothelial cells56.
Effect of circadian deregulation on cancer
Evidence indicates that circadian deregulation influences the secretion of some stress-associated hormones, and this might explain the associations between stress and cancer57,58. Data from separate lines of investigation show that stress can disrupt circadian glucocorticoid rhythms57,59 and favour tumour initiation and progression57,58,60. Night-time shift work, a condition that is known to disrupt endocrine rhythms, is a risk factor for breast and colorectal cancer61. Mice with circadian disruption owing to Per1 (period 1) or Per2 gene mutations are prone to tumour development and early death62,63. Tumour-bearing animals and cancer patients have disrupted endocrine, metabolic and immunological cycles, with greater disruption in cases where the tumour is advanced or fast-growing64. In murine studies, tumour progression and mortality are dramatically accelerated after elimination of circadian rhythms by manipulation of light–dark cycles (imposed ‘jet-lag’) and by the use of bilateral electrolytic lesions to destroy the suprachiasmatic nuclei (SCN), which eliminates circadian rhythms60. Two clinical studies have also shown that the status of circadian cycles, such as cortisol or the 24-hour-rest–activity cycle, can predict long-term cancer survival58,65.
Stress-related disruption of circadian cycles might impair cancer-defence mechanisms through genetic and/or gluco-corticoid and immune pathways. Animal studies show that behavioural factors such as imposed chronic jet-lag can alter Per1 expression in the SCN60, and circadian genes, including Per1, regulate tumour suppression, cellular response to DNA damage, and apoptosis63. Glucocorticoid rhythms that are driven by the SCN62 are linked to both enumerative and functional immunity66. Sleep disruption can increase the release of cortisol as well as increase the expression of pro-inflammatory cytokines (for example, IL-6 and tumour-necrosis factor-α (TNFα)) in cancer patients67. Pro-inflammatory cytokines might promote tumorigenesis by inducing DNA damage or inhibiting DNA repair through the generation of reactive oxygen species. Pro-inflammatory cytokines can also lead to the inactivation of tumour-suppressor genes, the promotion of autocrine or paracrine growth and survival of tumour cells, the stimulation of angiogenesis, or the subversion of the immune response (which leads to the activation of B cells rather than T cells in the tumour microenvironment)68. Conversely, agents that are capable of re-establishing circadian regulation (for example, melatonin) might have anti-tumour effects. Research on oestrogen-receptor-positive MCF-7 human breast cancer cells has shown that melatonin reversibly inhibits cell proliferation, increases p53 expression, modulates the cell cycle, and reduces metastatic capacity by increasing the expression of cell-surface adhesion proteins69,70. Taken together, these data indicate a potentially important role of circadian regulation in cancer defence and treatment62.
Influences on viral oncogenesis
The first experimental demonstration that bio-behavioural factors could promote cancer came from animal studies of tumour viruses71. Many studies have demonstrated the accelerated growth of virally induced tumours in stressed animals, as well as the more surprising protective effects of handling, fighting and crowding72,73. Neuroendocrine function has a central role in these processes because it can modulate viral replication, activate viral oncogenes, increase tumour metabolism and regulate the immune response74–76. The evidence for a viral contribution to human cancer has grown77 (BOX 1), and stress hormones have been found to influence the activity of various human tumour viruses (BOX 2; TABLE 2).
Box 1. Physiological pathways, bio-behavioural processes and oncogenesis.
Environmental and social processes activate interpretive processes in the central nervous system (CNS) that can subsequently trigger fight-or-flight stress responses in the autonomic nervous system (ANS) or defeat/withdrawal responses through the activation of the hypothalamic–pituitary–adrenal axis (HPA)141.
Individual differences in perception and evaluation of external events (coping) creates variability in individual ANS and HPA activity levels.
Over long periods of time, these neuroendocrine dynamics can alter various physiological processes involved in tumorigenesis, including oxidative metabolism, DNA repair, oncogene expression by viruses and somatic cells, and production of growth factors and other regulators of cell growth.
Once a tumour is initiated, neuroendocrine factors can also regulate the activity of proteases, angiogenic factors, chemokines and adhesion molecules involved in invasion, metastasis and other aspects of tumour progression.
CNS processes can also shape behavioural processes that govern cancer risk (for example, smoking, transmission of oncogenic viruses or exposure to genotoxic compounds).
Box 2. Viral oncology.
Viral infections contribute to approximately 15% of human cancers worldwide77
Pathogenic mechanisms include expression of viral oncogenes (for example, human T-cell lymphotropic virus Tax, and Epstein–Barr virus nuclear antigens and latent membrane protein 1), inhibition of host-cell tumour-suppressors (for example, human papillomavirus E6, which targets p53 and E7, which targets RB), and genomic damage stemming from immune-mediated cell turnover (for example, hepatitis B and C viruses)77,142,143.
All major human tumour viruses are sensitive to the intracellular signalling pathways activated by the hypothalamic–pituitary–adrenal axis and autonomic nervous system. These mediators can reactivate latent tumour viruses, stimulate oncogene expression and inhibit host-cell antiviral responses.
Table 2.
Neuroendocrine influences on tumour viruses
| Human tumour virus | Malignancy | Sensitivity* |
|---|---|---|
| Human papilloma viruses 16 and 33 | Cervical and head/neck cancer | HPA |
| Hepatitis B virus | Hepatocellular carcinoma | HPA |
| Hepatitis C virus | Hepatocellular carcinoma | HPA |
| Epstein–Barr virus | Lymphoma, and nasopharygeal carcinoma | HPA |
| Human T-cell lymphotropic viruses 1 and 2 | Adult T-cell leukaemia/lymphoma | ANS |
| Kaposi sarcoma-associated herpesvirus | Kaposi sarcoma, and primary effusion lymphoma | ANS |
Presumptive, based on in vitro studies. ANS, autonomic nervous system; HPA, hypothalamic–pituitary–adrenal axis. Vaccination is an important primary prevention strategy against viral tumours, and behavioural factors can influence the efficacy of this approach by modulating vaccine-induced immune responses102,103.
Epstein–Barr virus (EBV) is reactivated in healthy people who experience prolonged psychological stress78,79. In these studies HPA activity increased in parallel with reactivation of EBV79,80, and gluco-corticoid hormones were subsequently found to increase EBV gene expression in vitro80,81. High-risk human papilloma viruses (HPVs), which contribute to cervical and rectal carcinomas, also respond to glucocorticoids by activating gene expression82–84, interacting with cellular proto-oncogenes such as HRAS85, and evading cellular immune responses by downregulating the expression of tumour MHC-I (major histocompatibility complex class I) molecules86. Clinical studies have identified stressful life events as a risk factor for increased progression of cervical dysplasia in HPV-positive women87,88. Furthermore, glucocorticoid antagonists can inhibit HPV activity in vitro89–91, providing a molecular rationale for clinical interventions that target HPA activity. Although hepatitis B and C viruses come from different viral lineages, glucocorticoids increase gene expression in and replication of both viruses90,92–94. These dynamics are so pronounced that glucocorticoids are employed clinically to activate hepatitis B and C viruses for eradication by replication-dependent antiviral drugs93,95.
Cancer-related viruses are also sensitive to catecholamines and the PKA signalling pathway. Molecular mechanisms are especially well defined for AIDS-associated malignancies. Catecholamines can accelerate human immunodeficiency virus 1 (HIV1) replication by increasing cellular susceptibility to infection96,97, activating viral gene transcription96 and suppressing antiviral cytokines98. People with heightened ANS activity show an increased viral load in the plasma and an impaired response to antiretroviral therapy96, placing them at increased risk for AIDS-associated B-cell lymphomas99. Catecholamines can also activate the Kaposi sarcoma-associated herpesvirus (KSHV) through PKA induction of the viral transcription factor Rta100. Human T-cell lymphotropic viruses 1 and 2 (HTLV1 and HTLV2, respectively) are sensitive to PKA-mediated induction of the oncogenic Tax transcription factor101. Hormonal regulation of viral replication represents an important pathway by which bio-behavioural factors might influence malignant processes, but it also indicates novel therapeutic approaches such as β-adrenergic priming of viral genomes for clearance by replication-dependent nucleoside analogue drugs.
In addition to direct effects on viral gene expression, bio-behavioural factors can also indirectly affect tumour viruses by modulating host immune responses (see below). Antiviral vaccines will have an increasing role in the primary prevention of virally mediated cancers, and bio-behavioural influences on vaccine-induced immune responses will become especially relevant102,103. Neuroendocrine influences on the immune response might also explain why oncogenic viruses so consistently acquire hormone-responsive replication dynamics. Viruses that coordinate their gene expression with periods of hormone-induced immunosuppression should enjoy a significant survival advantage. Similar selective pressures might also shape the evolution of non-viral malignancies104 such that genomic alterations are selected based on their ability to evade immune clearance or to synergize with endocrine dynamics to optimize tumour growth and metastasis.
Influences on immune mechanisms
Chronic stress has been shown to suppress different facets of immune function2 such as antigen presentation, T-cell proliferation, and humoral and cell-mediated immunity, mainly through the release of catecholamine and/or glucocorticoid hormones105–107. Relevant neuroendocrine and immune system interactions include direct synapse-like connections between sympathetic nerves and lymphocytes in lymphoid organs108, neural and endocrine modulation of lymphocyte trafficking109, and modulation of leukocyte function through glucocorticoid receptors and other receptors70. Tumour incidence and progression based on modulation of the immune response by chronic stress has been demonstrated in many animal models (see above). Recent studies have shown that chronic stress experienced during exposure to non-blistering ultraviolet radiation significantly increases susceptibility to squamous cell carcinoma by suppressing type 1 cytokines and the infiltration of protective T cells. Regulatory or suppressor T-cell numbers within the tumours and in the circulation were also increased110. Studies in mice of the immune response to transplanted syngeneic tumours showed that noradrenaline111 and adrenaline112,113 directly inhibited the generation of anti-tumour cytotoxic T cells through β-adrenergic signalling mechanisms. Chronic stress has been shown to modulate lymphocyte apoptosis through an increase in FAS (also known as CD95 or APO1) expression. It has been hypothesized that such lymphocyte reduction might result in an increase in the incidence of oncogenic viral infections and DNA damage114.
Compromised natural killer (NK)-cell function has been shown in both animal and clinical studies of surgical stress22,115. High levels of psychological distress have been linked to reduced cellular immunity in patients with breast116 and ovarian cancer117. More specifically, distress measured by self-report was correlated with low NK-cell cyto-toxicity in tumour-infiltrating lymphocytes from human ovarian cancers117. Low peripheral NK-cell counts are prognostic for early breast cancer mortality, and reduced NK-cell cytotoxicity is predictive of a poor clinical outcome in patients with breast carcinoma58. Positive psycho-social factors such as social support have been associated with increased levels of NK-cell cytotoxicity in patients with breast118 and ovarian cancer117. The relationship of increased NK-cell cytotoxicity with social support was not limited to the periphery; it was also seen in tumour-infiltrating lymphocytes isolated from human ovarian cancers, reflecting possible psycho-social influences on the tumour microenvironment117. Patients with breast cancer who reported increased psychological growth through participation in a cognitive behavioural intervention programme demonstrated increased levels of cellular immune function119. Preliminary studies have found that the expression of spirituality was related to increased numbers of circulating T cells in patients with breast cancer120, and that the use of humour as a coping mechanism was associated with increased NK-cell activity in cancer patients121.
Clinical opportunities and challenges
Our understanding of the biological and clinical significance of psycho-social and bio-behavioural influences on cancer pathogenesis is expanding. As described in this review, factors such as chronic stress, depression and social support have been linked to tumour biology, viral oncogenesis and cell-mediated immunity (FIG. 3). Although the molecular pathways have not been completely delineated, observations to date indicate a need for novel therapeutic paradigms that integrate a bio-behavioural perspective.
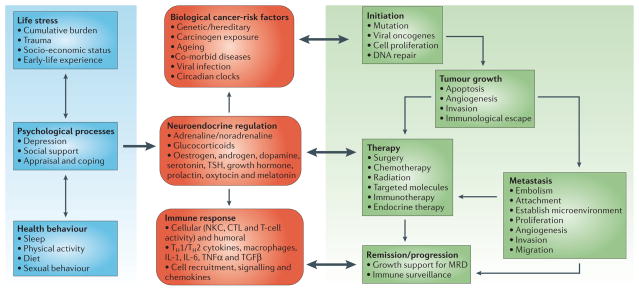
Figure 3. Integrated model of bio-behavioural influences on cancer pathogenesis through neuroendocrine pathways.
In this model, bio-behavioural factors such as life stress, psychological processes and health behaviours (blue panel) influence tumour-related processes (green panel) through the neuroendocrine regulation of hormones, including adrenaline, noradrenaline and glucocorticoids (red panel). Central control of peripheral endocrine function also allows social, environmental and behavioural processes to interact with biological risk factors such as genetic background, carcinogens and viral infections to systemically modulate malignant potential (red panel). Direct pathways of influence include effects of catecholamines and glucocorticoids on tumour-cell expression of genes that control cell proliferation, invasion, angiogenesis, metastasis and immune evasion (green panel). Stress-responsive neuroendocrine mediators can also influence malignant potential indirectly through their effects on oncogenic viruses and the cellular immune system (red panel). These pleiotropic hormonal influences induce a mutually reinforcing system of cellular signals that collectively support the initiation and progression of malignant cell growth (green panel). Furthermore, neuroendocrine deregulation can influence the response to conventional therapies such as surgery, chemotherapy and immunotherapy (green panel). In addition to explaining bio-behavioural risk factors for cancer, this model suggests novel targets for pharmacological or behavioural intervention. CTL, cytotoxic T lymphocytes; IL, interleukin; MRD, minimal residual disease; NKC, natural killer cell; TGFβ, transforming growth factor-β; TNFα, tumour-necrosis factor-α; TSH, thyroid-stimulating hormone.
It is plausible that successful management of factors such as stress and negative mood might have a salubrious effect on the neuroendocrine regulation of oncogenesis, tumour growth and metastasis, and cancer immunoediting processes. Psycho-social interventions such as relaxation and cognitive behavioural techniques that alter negative mood seem to modulate ANS and HPA hormonal activity122–124. Moreover, such interventions can potentially be used in conjunction with conventional therapies to maximize treatment efficacy125,126. Stress-management interventions that dampen chronic-stress-related physiological changes might facilitate immune system ‘recovery’ and thereby increase immune surveillance during the active treatment of cancer119,124. Group-based psycho-social interventions that combine relaxation with cognitive behavioural techniques, such as cognitive behavioural stress management (CBSM), have been shown to increase indicators of immune responses against potentially oncogenic viral infections, such as EBV127. Such alterations are paralleled by decreased expression levels of cortisol in the serum, a reduced depressive mood, increased social support and enhanced relaxation skills122.
In HIV-infected individuals, who as a group are at risk for multiple opportunistic cancers, CBSM seems to accelerate reconstitution of naive T-lymphocytes, increase CD8+ cytotoxic T-cell numbers and decrease the viral load of HIV over time122,128. These changes are pre-dated by decreases in negative mood and decreases in urinary cortisol and noradrenaline output122,129. It is plausible that CBSM might also help decrease the replication and function of other oncogenic viruses such as HPV and improve immune defences against them. Psycho-social interventions in cancer patients have resulted in alterations in neuroendocrine regulation and immunological functions124,130,131 that are relevant for monitoring neoplastic cell changes. For example, two recent randomized clinical trials have documented increases in lymphocyte proliferation in patients with breast cancer following psycho-social interventions119,124, and post-intervention changes in NK-cell activity have also been shown in patients with malignant melanoma131. Collectively, this work indicates that stress management can modify neuroendocrine deregulation and immunological functions that potentially have implications for tumour progression. This might be particularly important among vulnerable populations such as older adults because ageing is associated with a suppression of the immune response.
Clinical studies of psycho-social interventions with cancer survival as an outcome have been methodologically flawed or have failed to confirm a survival advantage in the treatment group1,126,132–134. Similar to most medical interventions for cancer, the effectiveness of psycho-social interventions is likely to vary with the type and stage of cancer, characteristics of the patient (for example, age, gender, education, co-morbid medical conditions, and health behaviours such as tobacco use, alcohol consumption and physical activity) and the type and delivery of the intervention. Nevertheless, epidemiological evidence correlating psychological and social factors (for example, chronic depression, hopelessness, marital disruption and social support) with cancer incidence, progression and survival give credence to examining the biological signalling pathways and mechanisms that underlie these observations.
Pharmacological interventions can potentially be used to ameliorate stress-associated influences on cancer development and progression. As discussed above, β-blockers have been shown to block many of the deleterious effects of stress. In a large case–control study of patients with prostate cancer who were taking anti-hypertensive medication, only β-blockers were associated with a reduction of cancer risk135. A cohort study of cardiovascular patients showed that the use of β-blockers, relative to never-using, resulted in a 49% decrease in cancer risk, with a 6% decrease in risk for every year of use136. Large population-based case–control studies have not confirmed alterations in risk for invasive breast carcinoma with β-blocker use137,138. The use of antidepressant medications might be promising, owing to a concomitant suppression of an inflammatory response that has been associated with certain types of cancer139. For example, lithium inhibits prostaglandin E1, and tricyclic antidepressants antagonize thromboxanes140. Some monoamine oxidase inhibitors exert a more potent anti-prostaglandin effect than indomethacin140. Whether these agents can be used to reduce cancer risk through bio-behavioural-related mechanisms remains to be determined, but these studies indicate that further inquiry is warranted.
Conclusion
Despite significant progress in the past decade, further research is needed to define the mechanisms underlying the complex circuits involving the HPA and ANS axes and their effects on the processes involved in cancer development and progression. The body of data outlined above supports a model in which bio-behavioural factors influence multiple aspects of tumorigenesis through their impact on neuroendocrine function (FIG. 3). These effects include direct promotion of tumour growth by affecting steps in the metastatic cascade and viral oncogenesis. Furthermore, the interplay between behavioural processes and cellular immune factors also supports a favourable physiological environment for tumour establishment and growth. In the context of this ‘systems biology’ perspective, pharmacological and behavioural interventions that address neuroendocrine dysfunction could have a clinically significant role in avoiding these deleterious effects on tumour growth. Although stress per se does not cause cancer, the clinical and experimental data outlined above indicate that factors such as mood, coping mechanisms and social support can significantly influence the underlying cellular and molecular processes that facilitate malignant cell growth. As cancer treatment evolves towards a more patient-specific approach, consideration of the influence of bio-behavioural factors provides a novel perspective for mechanistic studies and new therapeutic targets.
Acknowledgments
The authors gratefully acknowledge the support of several Institutes and Centers of the National Institutes of Health; National Cancer Institute (M.H.A., S.K.L., F.S.D., and A.K.S.), National Center for Complementary and Alternative Medicine (S.K.L.), National Institute of Allergy and Infectious Diseases (S.W.C. and F.S.D.) and National Institute of Mental Health (M.H.A.). The authors also acknowledge support received from the Dana Foundation (F.S.D.), Jonssen Comprehensive Cancer Center (S.W.C.) and Norman Cousins Center at the University of California, Los Angeles (S.W.C.). Preparation of this perspective was facilitated by support from the Division of Cancer Control and Populations Sciences at the National Cancer Institute. We are indebted to Wendy Nelson for her editorial review of the manuscript.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
DATABASES
The following terms in this article are linked online to:
Entrez Gene:http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gene
ACTH | β2AR | EPAC | VEGF
National Cancer Institute:http://www.cancer.gov breast cancer | lung tumour | ovarian cancer
FURTHER INFORMATION
Anil K. Sood’s web page:http://www.mdanderson.org/departments/gynonc/display.cfm?id=ff562a10-edb5-4561-b95d6ac0618b5184&method=displayfull&pn=AFADFC5A-36B0-48EA-B11B71824784D641
NCI Cancer Control and Population Sciences web site:http://www.cancercontrol.cancer.gov/bimped/
Steven Cole’s web page:http://www.cancer.mednet.ucla.edu/institution/personnel?personnel%5fid=45359
Susan Lutgendorf’s web page:http://www.psychology.uiowa.edu/Faculty/Lutgendorf/Lutgendorf.html
Access to this interactive links box is free online.
Contributor Information
Michael H. Antoni, Departments of Psychology, Psychiatry, and Behavioural Sciences and the Sylvestor Cancer Center, University of Miami, P.O. Box 248185, Coral Gables, Florida 33124, USA
Susan K. Lutgendorf, Departments of Psychology and Obstetrics and Gynecology and The Holden Comprehensive Cancer Center, University of Iowa, E11 Seashore Hall, Iowa City, Iowa 52242, USA
Steven W. Cole, Division of Hematology–Oncology, University of California, Los Angeles School of Medicine 11-934 Factor Building, Los Angeles, California 90095-1678, USA
Firdaus S. Dhabhar, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, 401 Quarry Road, Office 2325, Stanford, California 94305, USA
Sandra E. Sephton, Department of Psychological and Brain Sciences, James Graham Brown Cancer Center, University of Louisville, 2301 South 3rd Street, Room 317, Louisville, Kentucky 40202, USA
Paige Green McDonald, Basic and Biobehavioural Research Branch, Behavioural Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute, National Institutes of Health, 6130 Executive Boulevard, MSC 7363, Bethesda, Maryland 20892, USA.
Michael Stefanek, Basic and Biobehavioural Research Branch, Behavioural Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute, National Institutes of Health, 6130 Executive Boulevard, MSC 7363, Bethesda, Maryland 20892, USA.
Anil K. Sood, Departments of Gynecologic Oncology and Cancer Biology, University of Texas M. D. Anderson Cancer Center, Unit 1362, P.O. Box 301439, Houston, Texas 77230-1439, USA
References
- 1.Stefanek M, McDonald PG. In: Handbook of Behavioral Science and Cancer. Miller SM, Bowen DJ, Croyle RT, Rowland J, editors. American Psychological Association; Washington DC: in the press. [Google Scholar]
- 2.Reiche EM, Nunes SO, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol. 2004;5:617–625. doi: 10.1016/S1470-2045(04)01597-9. [DOI] [PubMed] [Google Scholar]
- 3.Spiegel D, Giese-Davis J. Depression and cancer: mechanisms and disease progression. Biol Psychiatry. 2003;54 :269–282. doi: 10.1016/s0006-3223(03)00566-3. [DOI] [PubMed] [Google Scholar]
- 4.Price MA, et al. The role of psychosocial factors in the development of breast carcinoma: part I. The cancer prone personality. Cancer. 2001;91:679–685. [PubMed] [Google Scholar]
- 5.Lillberg K, et al. Stressful life events and risk of breast cancer in 10,808 women: a cohort study. Am J Epidemiol. 2003;157:415–423. doi: 10.1093/aje/kwg002. [DOI] [PubMed] [Google Scholar]
- 6.Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nature Rev Immunol. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- 7.Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–1252. [PubMed] [Google Scholar]
- 8.Charmandari E, Tsigos C, Chrousos GP. Endocrinology of the stress response. Annu Rev Physiol. 2005;67:259–284. doi: 10.1146/annurev.physiol.67.040403.120816. [DOI] [PubMed] [Google Scholar]
- 9.McEwen BS. Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology. 2000;22:108–124. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- 10.Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Emotions, morbidity, and mortality: new perspectives from psychoneuroimmunology. Annu Rev Psychol. 2002;53:83–107. doi: 10.1146/annurev.psych.53.100901.135217. [DOI] [PubMed] [Google Scholar]
- 11.McEwen BS. Sex, stress and the hippocampus: allostasis, allostatic load and the aging process. Neurobiol Aging. 2002;23:921–939. doi: 10.1016/s0197-4580(02)00027-1. [DOI] [PubMed] [Google Scholar]
- 12.Sood AK, et al. Stress hormone mediated invasion of ovarian cancer cells. Clin Cancer Res. 2006;12:369–375. doi: 10.1158/1078-0432.CCR-05-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Badino GR, Novelli A, Girardi C, Di Carlo F. Evidence for functional β-adrenoceptor subtypes in CG-5 breast cancer cell. Pharm Res. 1996;33:255–260. doi: 10.1006/phrs.1996.0036. [DOI] [PubMed] [Google Scholar]
- 14.Vandewalle B, Revillion F, Lefebvre J. Functional β-adrenergic receptors in breast cancer cells. J Cancer Res Clin Oncol. 1990;116:303–306. doi: 10.1007/BF01612908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marchetti B, Spinola PG, Pelletier G, Labrie F. A potential role for catecholamines in the development and progression of carcinogen-induced mammary tumors: hormonal control of β-adrenergic receptors and correlation with tumor growth. J Steroid Biochem Mol Biol. 1991;38:307–320. doi: 10.1016/0960-0760(91)90102-b. [DOI] [PubMed] [Google Scholar]
- 16.Penninx BW, et al. Chronically depressed mood and cancer risk in older persons. J Natl Cancer Inst. 1998;90:1888–1893. doi: 10.1093/jnci/90.24.1888. [DOI] [PubMed] [Google Scholar]
- 17.Reynolds P, Kaplan GA. Social connections and risk for cancer: prospective evidence from the Alameda County Study. Behav Med. 1990;16:101–110. doi: 10.1080/08964289.1990.9934597. [DOI] [PubMed] [Google Scholar]
- 18.Allison PJ, Guichard C, Fung K, Gilain L. Dispositional optimism predicts survival status 1 year after diagnosis in head and neck cancer patients. J Clin Oncol. 2003;21:543–548. doi: 10.1200/JCO.2003.10.092. [DOI] [PubMed] [Google Scholar]
- 19.Gotay CC. Behavior and cancer prevention. J Clin Oncol. 2005;23:301–310. doi: 10.1200/JCO.2005.06.069. [DOI] [PubMed] [Google Scholar]
- 20.Laconi E, et al. Early exposure to restraint stress enhances chemical carcinogenesis in rat liver. Cancer Lett. 2000;161:215–220. doi: 10.1016/s0304-3835(00)00621-2. [DOI] [PubMed] [Google Scholar]
- 21.Ben-Eliyahu S, Yirmiya R, Liebeskind JC, Taylor AN, Gale RP. Stress increases metastatic spread of a mammary tumor in rats: evidence for mediation by the immune system. Brain Behav Immun. 1991;5:193–205. doi: 10.1016/0889-1591(91)90016-4. [DOI] [PubMed] [Google Scholar]
- 22.Ben-Eliyahu S, Page GG, Yirmiya R, Shakhar G. Evidence that stress and surgical interventions promote tumor development by suppressing natural killer cell activity. Int J Cancer. 1999;80:880–888. doi: 10.1002/(sici)1097-0215(19990315)80:6<880::aid-ijc14>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 23.Page GG, Ben-Eliyahu S. A role for NK cells in greater susceptibility of young rats to metastatic formation. Dev Comp Immunol. 1999;23:87–96. doi: 10.1016/s0145-305x(98)00040-8. [DOI] [PubMed] [Google Scholar]
- 24.Page GG, Ben-Eliyahu S, Yirmiya R, Liebeskind JC. Morphine attenuates surgery-induced enhancement of metastatic colonization in rats. Pain. 1993;54 :21–28. doi: 10.1016/0304-3959(93)90095-7. [DOI] [PubMed] [Google Scholar]
- 25.Stefanski V, Ben-Eliyahu S. Social confrontation and tumor metastasis in rats: defeat and β-adrenergic mechanisms. Physiol Behav. 1996;60:277–282. doi: 10.1016/0031-9384(96)00014-5. [DOI] [PubMed] [Google Scholar]
- 26.Ben-Eliyahu S, et al. The NMDA receptor antagonist MK-801 blocks nonopioid stress induced analgesia and decreases tumor metastasis in the rat. Proc West Pharmacol Soc. 1993;36:293–298. [PubMed] [Google Scholar]
- 27.Melamed R, et al. Marginating pulmonary-NK activity and resistance to experimental tumor metastasis: suppression by surgery and the prophylactic use of a β-adrenergic antagonist and a prostaglandin synthesis inhibitor. Brain Behav Immun. 2005;19:114–126. doi: 10.1016/j.bbi.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Fischman HK, Pero RW, Kelly DD. Psychogenic stress induces chromosomal and DNA damage. Int J Neurosci. 1996;84:219–227. doi: 10.3109/00207459608987267. [DOI] [PubMed] [Google Scholar]
- 29.Sacharczuk M, Jaszczak K, Sadowski B. Chromosomal NOR activity in mice selected for high and low swim stress-induced analgesia. Behav Genet. 2003;33 :435–441. doi: 10.1023/a:1025373626833. [DOI] [PubMed] [Google Scholar]
- 30.Thaker PH, et al. The 36th Annual Meeting of the Society of Gynecologic Oncologists; Miami, Florida. 30, 2005. [Google Scholar]
- 31.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 32.Ohm JE, et al. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood. 2003;101:4878–4886. doi: 10.1182/blood-2002-07-1956. [DOI] [PubMed] [Google Scholar]
- 33.Gabrilovich DI, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nature Med. 1996;2:1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 34.Lutgendorf SK, et al. Vascular endothelial growth factor and social support in patients with ovarian carcinoma. Cancer. 2002;95:808–815. doi: 10.1002/cncr.10739. [DOI] [PubMed] [Google Scholar]
- 35.Costanzo ES, et al. Psychosocial factors and interleukin-6 among women with advanced ovarian cancer. Cancer. 2005;104:305–313. doi: 10.1002/cncr.21147. [DOI] [PubMed] [Google Scholar]
- 36.Fredriksson JM, Lindquist JM, Bronnikov GE, Nedergaard J. Norepinephrine induces vascular endothelial growth factor gene expression in brown adipocytes through a β-adrenoreceptor–cAMP–protein kinase A pathway involving Src but independently of Erk1/2. J Biol Chem. 2000;275:13802–13811. doi: 10.1074/jbc.275.18.13802. [DOI] [PubMed] [Google Scholar]
- 37.Lutgendorf SK, et al. Stress-related mediators stimulate vascular endothelial growth factor secretion by two ovarian cancer cell lines. Clin Cancer Res. 2003;9:4514–4521. [PubMed] [Google Scholar]
- 38.Masur K, Niggemann B, Zanker KS, Entschladen F. Norepinephrine-induced migration of SW 480 colon carcinoma cells is inhibited by β-blockers. Cancer Res. 2001;61:2866–2869. [PubMed] [Google Scholar]
- 39.McDonald PH, Lefkowitz RJ. β-Arrestins: new roles in regulating heptahelical receptors’ functions. Cell Signal. 2001;13:683–689. doi: 10.1016/s0898-6568(01)00203-0. [DOI] [PubMed] [Google Scholar]
- 40.Abramovitch R, et al. A pivotal role of cyclic AMP-responsive element binding protein in tumor progression. Cancer Res. 2004;64:1338–1346. doi: 10.1158/0008-5472.can-03-2089. [DOI] [PubMed] [Google Scholar]
- 41.Lang K, et al. Induction of a metastatogenic tumor cell type by neurotransmitters and its pharmacological inhibition by established drugs. Int J Cancer. 2004;112:231–238. doi: 10.1002/ijc.20410. [DOI] [PubMed] [Google Scholar]
- 42.Jean D, Bar-Eli M. Regulation of tumor growth and metastasis of human melanoma by the CREB transcription factor family. Mol Cell Biochem. 2000;212:19–28. [PubMed] [Google Scholar]
- 43.de Rooij J, et al. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- 44.Enserink JM, et al. The cAMP–Epac–Rap1 pathway regulates cell spreading and cell adhesion to laminin-5 through the α3β1 integrin but not the α6β4 integrin. J Biol Chem. 2004;279:44889–44896. doi: 10.1074/jbc.M404599200. [DOI] [PubMed] [Google Scholar]
- 45.Distelhorst CW. Recent insights into the mechanism of glucocorticosteroid-induced apoptosis. Cell Death Differ. 2002;9:6–19. doi: 10.1038/sj.cdd.4400969. [DOI] [PubMed] [Google Scholar]
- 46.Herr I, et al. Glucocorticoid cotreatment induces apoptosis resistance toward cancer therapy in carcinomas. Cancer Res. 2003;63:3112–3120. [PubMed] [Google Scholar]
- 47.Wu W, et al. Microarray analysis reveals glucocorticoid-regulated survival genes that are associated with inhibition of apoptosis in breast epithelial cells. Cancer Res. 2004;64:1757–1764. doi: 10.1158/0008-5472.can-03-2546. [DOI] [PubMed] [Google Scholar]
- 48.Nakane T, et al. Effects of IL-1 and cortisol on β-adrenergic receptors, cell proliferation, and differentiation in cultured human A549 lung tumor cells. J Immunol. 1990;145:260–266. [PubMed] [Google Scholar]
- 49.Dave JR, et al. Chronic sustained stress increases levels of anterior pituitary prolactin mRNA. Pharmacol Biochem Behav. 2000;67:423–431. doi: 10.1016/s0091-3057(00)00388-9. [DOI] [PubMed] [Google Scholar]
- 50.Almeida SA, Petenusci SO, Franci JA, Rosa e Silva AA, Carvalho TL. Chronic immobilization-induced stress increases plasma testosterone and delays testicular maturation in pubertal rats. Andrologia. 2000;32:7–11. [PubMed] [Google Scholar]
- 51.Young WS, 3rd, Lightman SL. Chronic stress elevates enkephalin expression in the rat paraventricular and supraoptic nuclei. Brain Res Mol Brain Res. 1992;13:111–117. doi: 10.1016/0169-328x(92)90050-l. [DOI] [PubMed] [Google Scholar]
- 52.Clevenger CV, Furth PA, Hankinson SE, Schuler LA. The role of prolactin in mammary carcinoma. Endocr Rev. 2003;24:1–27. doi: 10.1210/er.2001-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pequeux C, et al. Oxytocin- and vasopressin-induced growth of human small-cell lung cancer is mediated by the mitogen-activated protein kinase pathway. Endocr Relat Cancer. 2004;11:871–885. doi: 10.1677/erc.1.00803. [DOI] [PubMed] [Google Scholar]
- 54.Pequeux C, et al. Oxytocin synthesis and oxytocin receptor expression by cell lines of human small cell carcinoma of the lung stimulate tumor growth through autocrine/paracrine signaling. Cancer Res. 2002;62:4623–4629. [PubMed] [Google Scholar]
- 55.Chakroborty D, et al. Depleted dopamine in gastric cancer tissues: dopamine treatment retards growth of gastric cancer by inhibiting angiogenesis. Clin Cancer Res. 2004;10:4349–4356. doi: 10.1158/1078-0432.CCR-04-0059. [DOI] [PubMed] [Google Scholar]
- 56.Basu S, et al. The neurotransmitter dopamine inhibits angiogenesis induced by vascular permeability factor/ vascular endothelial growth factor. Nature Med. 2001;7:569–574. doi: 10.1038/87895. [DOI] [PubMed] [Google Scholar]
- 57.Sephton S, Spiegel D. Circadian disruption in cancer: a neuroendocrine-immune pathway from stress to disease? Brain Behav Immun. 2003;17:321–328. doi: 10.1016/s0889-1591(03)00078-3. [DOI] [PubMed] [Google Scholar]
- 58.Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst. 2000;92:994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- 59.Chrousos GP, Gold PW. A healthy body in a healthy mind — and vice versa — the damaging power of ‘‘uncontrollable’’ stress. J Clin Endocrinol Metab. 1998;83 :1842–1845. doi: 10.1210/jcem.83.6.4908. [DOI] [PubMed] [Google Scholar]
- 60.Filipski E, et al. Host circadian clock as a control point in tumor progression. J Natl Cancer Inst. 2002;94:690–697. doi: 10.1093/jnci/94.9.690. [DOI] [PubMed] [Google Scholar]
- 61.Schernhammer ES, et al. Night-shift work and risk of colorectal cancer in the nurses’ health study. J Natl Cancer Inst. 2003;95:825–828. doi: 10.1093/jnci/95.11.825. [DOI] [PubMed] [Google Scholar]
- 62.Fu L, Lee CC. The circadian clock: pacemaker and tumour suppressor. Nature Rev Cancer. 2003;3:350–361. doi: 10.1038/nrc1072. [DOI] [PubMed] [Google Scholar]
- 63.Fu L, Pelicano H, Liu J, Huang P, Lee C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002;111:41–50. doi: 10.1016/s0092-8674(02)00961-3. [DOI] [PubMed] [Google Scholar]
- 64.Mormont MC, Levi F. Circadian-system alterations during cancer processes: a review. Int J Cancer. 1997;70:241–247. doi: 10.1002/(sici)1097-0215(19970117)70:2<241::aid-ijc16>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 65.Mormont MC, et al. Marked 24-h-rest–activity rhythms are associated with better quality of life, better response, and longer survival in patients with metastatic colorectal cancer and good performance status. Clin Cancer Res. 2000;6:3038–3045. [PubMed] [Google Scholar]
- 66.Kronfol Z, Nair M, Zhang Q, Hill EE, Brown MB. Circadian immune measures in healthy volunteers: relationship to hypothalamic–pituitary–adrenal axis hormones and sympathetic neurotransmitters. Psychosom Med. 1997;59:42–50. doi: 10.1097/00006842-199701000-00006. [DOI] [PubMed] [Google Scholar]
- 67.Rich T, et al. Elevated serum cytokines correlated with altered behavior, serum cortisol rhythm, and dampened 24-hour rest–activity patterns in patients with metastatic colorectal cancer. Clin Cancer Res. 2005;11:1757–1764. doi: 10.1158/1078-0432.CCR-04-2000. [DOI] [PubMed] [Google Scholar]
- 68.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 69.Blask DE, Sauer LA, Dauchy RT. Melatonin as a chronobiotic/anticancer agent: cellular, biochemical, and molecular mechanisms of action and their implications for circadian-based cancer therapy. Curr Top Med Chem. 2002;2:113–132. doi: 10.2174/1568026023394407. [DOI] [PubMed] [Google Scholar]
- 70.Sanchez-Barcelo EJ, Cos S, Fernandez R, Mediavilla MD. Melatonin and mammary cancer: a short review. Endocr Relat Cancer. 2003;10:153–159. doi: 10.1677/erc.0.0100153. [DOI] [PubMed] [Google Scholar]
- 71.Jensen MM. The influence of stress on murine leukemia virus infection. Proc Soc Exp Biol Med. 1968;127:610–614. doi: 10.3181/00379727-127-32754. [DOI] [PubMed] [Google Scholar]
- 72.Justice A. Review of the effects of stress on cancer in laboratory animals: importance of time of stress application and type of tumor. Psychol Bull. 1985;98:108–138. [PubMed] [Google Scholar]
- 73.Riley V. Psychoneuroendocrine influences on immunocompetence and neoplasia. Science. 1981;212:1100–1109. doi: 10.1126/science.7233204. [DOI] [PubMed] [Google Scholar]
- 74.Rowse GL, Weinberg J, Bellward GD, Emerman JT. Endocrine mediation of psychosocial stressor effects on mouse mammary tumor growth. Cancer Lett. 1992;65:85–93. doi: 10.1016/0304-3835(92)90217-j. [DOI] [PubMed] [Google Scholar]
- 75.Romero L, et al. A possible mechanism by which stress accelerates growth of virally-derived tumors. Proc Natl Acad Sci USA. 1992;89:11084–11087. doi: 10.1073/pnas.89.22.11084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McGrath CM, Prass WA, Maloney TM, Jones RF. Induction of endogenous mammary tumor virus expression during hormonal induction of mammary adenoacanthomas and carcinomas of BALB/c female mice. J Natl Cancer Inst. 1981;67:841–852. [PubMed] [Google Scholar]
- 77.zur Hausen H. Viruses in human cancers. Science. 1991;254:1167–1173. doi: 10.1126/science.1659743. [DOI] [PubMed] [Google Scholar]
- 78.Glaser R, et al. Stress-related activation of Epstein–Barr virus. Brain Behav Immun. 1991;5:219–232. doi: 10.1016/0889-1591(91)90018-6. [DOI] [PubMed] [Google Scholar]
- 79.Stowe RP, Pierson DL, Barrett DT. Elevated stress hormone levels relate to Epstein–Barr Virus reactivation in astronauts. Psychosom Med. 2001;63:891–895. doi: 10.1097/00006842-200111000-00007. [DOI] [PubMed] [Google Scholar]
- 80.Cacioppo JT, et al. Autonomic and glucocorticoid associations with the steady-state expression of latent Epstein–Barr Virus. Horm Behav. 2002;42:32–41. doi: 10.1006/hbeh.2002.1801. [DOI] [PubMed] [Google Scholar]
- 81.Glaser R, Kutz LA, MacCallum RC, Malarkey WB. Hormonal modulation of Epstein–Barr virus replication. Neuroendocrinology. 1995;62:356–361. doi: 10.1159/000127025. [DOI] [PubMed] [Google Scholar]
- 82.Bromberg-White JL, Meyers C. Comparison of the basal and glucocorticoid-inducible activities of the upstream regulatory regions of HPV18 and HPV31 in multiple epithelial cell lines. Virology. 2003;306:197–202. doi: 10.1016/s0042-6822(02)00041-7. [DOI] [PubMed] [Google Scholar]
- 83.Mittal R, Pater A, Pater MM. Multiple human papillomavirus type 16 glucocorticoid response elements functional for transformation, transient expression, and DNA–protein interactions. J Virol. 1993;67 :5656–5659. doi: 10.1128/jvi.67.9.5656-5659.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gloss B, et al. The upstream regulatory region of the human papilloma virus-16 contains an E2 protein-independent enhancer which is specific for cervical carcinoma cells and regulated by glucocorticoid hormones. EMBO J. 1987;6:3735–3743. doi: 10.1002/j.1460-2075.1987.tb02708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pater MM, Hughes GA, Hyslop DE, Nakshatri H, Pater A. Glucocorticoid-dependent oncogenic transformation by type 16 but not type 11 human papilloma virus DNA. Nature. 1988;335:832–835. doi: 10.1038/335832a0. [DOI] [PubMed] [Google Scholar]
- 86.Bartholomew JS, et al. Integration of high-risk human papillomavirus DNA is linked to the down-regulation of class I human leukocyte antigens by steroid hormones in cervical tumor cells. Cancer Res. 1997;57 :937–942. [PubMed] [Google Scholar]
- 87.Coker AL, Bond S, Madeleine MM, Luchok K, Pirisi L. Psychosocial stress and cervical neoplasia risk. Psychosom Med. 2003;65:644–651. doi: 10.1097/01.psy.0000041471.57895.08. [DOI] [PubMed] [Google Scholar]
- 88.Pereira DB, et al. Life stress and cervical squamous intraepithelial lesions in women with human papillomavirus and human immunodeficiency virus. Psychosom Med. 2003;65:427–434. doi: 10.1097/01.psy.0000041620.37866.89. [DOI] [PubMed] [Google Scholar]
- 89.Pater MM, Pater A. RU486 inhibits glucocorticoid hormone-dependent oncogenesis by human papillomavirus type 16 DNA. Virology. 1991;183:799–802. doi: 10.1016/0042-6822(91)91014-8. [DOI] [PubMed] [Google Scholar]
- 90.Chou CK, Wang LH, Lin HM, Chi CW. Glucocorticoid stimulates hepatitis B viral gene expression in cultured human hepatoma cells. Hepatology. 1992;16:13–18. doi: 10.1002/hep.1840160104. [DOI] [PubMed] [Google Scholar]
- 91.Kamradt MC, Mohideen N, Vaughan AT. RU486 increases radiosensitivity and restores apoptosis through modulation of HPV E6/E7 in dexamethasone-treated cervical carcinoma cells. Gynecol Oncol. 2000;77:177–182. doi: 10.1006/gyno.1999.5724. [DOI] [PubMed] [Google Scholar]
- 92.Tur-Kaspa R, Burk RD, Shaul Y, Shafritz DA. Hepatitis B virus DNA contains a glucocorticoid-responsive element. Proc Natl Acad Sci USA. 1986;83:1627–1631. doi: 10.1073/pnas.83.6.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chayama K, et al. A pilot study of corticosteroid priming for lymphoblastoid interferon α in patients with chronic hepatitis C. Hepatology. 1996;23:953–957. doi: 10.1053/jhep.1996.v23.pm0008621174. [DOI] [PubMed] [Google Scholar]
- 94.Magy N, et al. Effects of corticosteroids on HCV infection. Int J Immunopharmacol. 1999;21:253–261. doi: 10.1016/s0192-0561(99)00005-3. [DOI] [PubMed] [Google Scholar]
- 95.Yokosuka O. Role of steroid priming in the treatment of chronic hepatitis B. J Gastroenterol Hepatol. 2000;15:E41–E45. doi: 10.1046/j.1440-1746.2000.02109.x. [DOI] [PubMed] [Google Scholar]
- 96.Cole SW, et al. Impaired response to HAART in HIV-infected individuals with high autonomic nervous system activity. Proc Natl Acad Sci USA. 2001;98:12695–12700. doi: 10.1073/pnas.221134198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cole SW, Jamieson BD, Zack J. A cAMP externalizes lymphocyte CXCR4: implications for chemotaxis and HIV infection. J Immunol. 1999;162:1392–1400. [PubMed] [Google Scholar]
- 98.Cole SW, Korin YD, Fahey JL, Zack JA. Norepinephrine accelerates HIV replication via protein kinase A-dependent effects on cytokine production. J Immunol. 1998;161:610–616. [PubMed] [Google Scholar]
- 99.Killebrew D, Shiramizu B. Pathogenesis of HIV-associated non-Hodgkin lymphoma. Curr HIV Res. 2004;2:215–221. doi: 10.2174/1570162043351237. [DOI] [PubMed] [Google Scholar]
- 100.Chang M, et al. β Adrenoreceptors reactivate Kaposi’s Sarcoma-associated Herpesvirus lytic replication via PKA-dependent control of viral RTA. J Virol. 2005;79:13538–13547. doi: 10.1128/JVI.79.21.13538-13547.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Turgeman H, Aboud M. Evidence that protein kinase A activity is required for the basal and tax-stimulated transcriptional activity of human T-cell leukemia virus type-I-long terminal repeat. FEBS Lett. 1998;428:183–187. doi: 10.1016/s0014-5793(98)00513-4. [DOI] [PubMed] [Google Scholar]
- 102.Marsland AL, Cohen S, Rabin BS, Manuck SB. Associations between stress, trait negative affect, acute immune reactivity, and antibody response to hepatitis B injection in healthy young adults. Health Psychol. 2001;20:4–11. [PubMed] [Google Scholar]
- 103.Glaser R, et al. Stress-induced modulation of the immune response to recombinant hepatitis B vaccine. Psychosom Med. 1992;54:22–29. doi: 10.1097/00006842-199201000-00005. [DOI] [PubMed] [Google Scholar]
- 104.Kinzler KW, Vogelstein B. The Genetic Basis of Human Cancer. McGraw–Hill; Toronto: 1998. [Google Scholar]
- 105.Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav Immun. 1997;11:286–306. doi: 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- 106.Elenkov IJ. Systemic stress-induced Th2 shift and its clinical implications. Int Rev Neurobiol. 2002;52:163–186. doi: 10.1016/s0074-7742(02)52009-2. [DOI] [PubMed] [Google Scholar]
- 107.Glaser R, et al. Evidence for a shift in the Th-1 to Th-2 cytokine response associated with chronic stress and aging. J Gerontol A Biol Sci Med Sci. 2001;56:M477–M482. doi: 10.1093/gerona/56.8.m477. [DOI] [PubMed] [Google Scholar]
- 108.Felten S, Felten D. In: Psychoneuroimmunology. Ader R, Felten D, Cohen N, editors. St. Louis; Montana: 1991. pp. 27–71. [Google Scholar]
- 109.Dhabhar FS, McEwen BS. Stress-induced enhancement of antigen-specific cell-mediated immunity. J Immunol. 1996;156:2608–2615. [PubMed] [Google Scholar]
- 110.Saul AN, et al. Chronic stress and susceptibility to skin cancer. J Natl Cancer Inst. 2005;97:1760–1767. doi: 10.1093/jnci/dji401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kalinichenko VV, Mokyr MB, Graf LH, Jr, Cohen RL, Chambers DA. Norepinephrine-mediated inhibition of antitumor cytotoxic T lymphocyte generation involves a β-adrenergic receptor mechanism and decreased TNF-α gene expression. J Immunol. 1999;163:2492–2499. [PubMed] [Google Scholar]
- 112.Cook-Mills JM, Cohen RL, Perlman RL, Chambers DA. Inhibition of lymphocyte activation by catecholamines: evidence for a non-classical mechanism of catecholamine action. Immunology. 1995;85:544–549. [PMC free article] [PubMed] [Google Scholar]
- 113.Cook-Mills JM, Mokyr MB, Cohen RL, Perlman RL, Chambers DA. Neurotransmitter suppression of the in vitro generation of a cytotoxic T lymphocyte response against the syngeneic MOPC-315 plasmacytoma. Cancer Immunol Immunother. 1995;40:79–87. doi: 10.1007/BF01520288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shi Y, et al. Stressed to death: implication of lymphocyte apoptosis for psychoneuroimmunology. Brain Behav Immun. 2003;17 (Suppl):S18–S26. doi: 10.1016/s0889-1591(02)00062-4. [DOI] [PubMed] [Google Scholar]
- 115.Pollock RE, Lotzova E, Stanford SD. Mechanism of surgical stress impairment of human perioperative natural killer cell cytotoxicity. Arch Surg. 1991;126:338–342. doi: 10.1001/archsurg.1991.01410270082013. [DOI] [PubMed] [Google Scholar]
- 116.Andersen BL, et al. Stress and immune responses after surgical treatment for regional breast cancer. J Natl Cancer Inst. 1998;90:30–36. doi: 10.1093/jnci/90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lutgendorf SK, et al. Social support, distress, and natural killer cell activity in ovarian cancer patients. J Clin Oncol. 2005;23:7106–7113. doi: 10.1200/JCO.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 118.Levy SM, et al. Perceived social support and tumor estrogen/progesterone receptor status as predictors of natural killer cell activity in breast cancer patients. Psychosom Med. 1990;52:73–85. doi: 10.1097/00006842-199001000-00006. [DOI] [PubMed] [Google Scholar]
- 119.McGregor BA, et al. Cognitive-behavioral stress management increases benefit finding and immune function among women with early-stage breast cancer. J Psychosom Res. 2004;56:1–8. doi: 10.1016/S0022-3999(03)00036-9. [DOI] [PubMed] [Google Scholar]
- 120.Sephton S, Koopman C, Schaal M, Thoresen C, Spiegel D. Spiritual expression and immune status in women with metastatic breast cancer: an exploratory study. Breast J. 2001;7:345–353. doi: 10.1046/j.1524-4741.2001.20014.x. [DOI] [PubMed] [Google Scholar]
- 121.Christie W, Moore C. The impact of humor on patients with cancer. Clin J Oncol Nurs. 2005;9:211–218. doi: 10.1188/05.CJON.211-218. [DOI] [PubMed] [Google Scholar]
- 122.Antoni MH. Stress management effects on psychological, endocrinological, and immune functioning in men with HIV infection: empirical support for a psychoneuroimmunological model. Stress. 2003;6:173–188. doi: 10.1080/1025389031000156727. [DOI] [PubMed] [Google Scholar]
- 123.Carlson LE, Speca M, Patel KD, Goodey E. Mindfulness-based stress reduction in relation to quality of life, mood, symptoms of stress and levels of cortisol, dehydroepiandrosterone sulfate (DHEAS) and melatonin in breast and prostate cancer outpatients. Psychoneuroendocrinology. 2004;29:448–474. doi: 10.1016/s0306-4530(03)00054-4. [DOI] [PubMed] [Google Scholar]
- 124.Andersen BL, et al. Psychological, behavioral, and immune changes after a psychological intervention: a clinical trial. J Clin Oncol. 2004;22:3570–3580. doi: 10.1200/JCO.2004.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lekander M, Furst CJ, Rotstein S, Blomgren H, Fredrikson M. Social support and immune status during and after chemotherapy for breast cancer. Acta Oncol. 1996;35:31–37. doi: 10.3109/02841869609098476. [DOI] [PubMed] [Google Scholar]
- 126.Fawzy FI, Canada AL, Fawzy NW. Malignant melanoma: effects of a brief, structured psychiatric intervention on survival and recurrence at 10-year follow-up. Arch Gen Psychiatry. 2003;60:100–103. doi: 10.1001/archpsyc.60.1.100. [DOI] [PubMed] [Google Scholar]
- 127.Esterling BA, et al. Psychosocial modulation of antibody to Epstein–Barr viral capsid antigen and human herpesvirus type-6 in HIV-1-infected and at-risk gay men. Psychosom Med. 1992;54:354–371. doi: 10.1097/00006842-199205000-00011. [DOI] [PubMed] [Google Scholar]
- 128.Antoni MH, et al. Randomized clinical trial of cognitive behavioral stress management on HIV viral load in gay men treated with HAART. Psychosom Med. doi: 10.1097/01.psy.0000195749.60049.63. in the press. [DOI] [PubMed] [Google Scholar]
- 129.Antoni MH, et al. Increases in a marker of immune system reconstitution are predated by decreases in 24-hour urinary cortisol output and depressed mood during a 10-week stress management intervention in symptomatic gay men. J Psychosom Res. 2005;58:3–13. doi: 10.1016/j.jpsychores.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 130.Carlson LE, Speca M, Patel KD, Goodey E. Mindfulness-based stress reduction in relation to quality of life, mood, symptoms of stress, and immune parameters in breast and prostate cancer outpatients. Psychosom Med. 2003;65:571–581. doi: 10.1097/01.psy.0000074003.35911.41. [DOI] [PubMed] [Google Scholar]
- 131.Fawzy FI, et al. A structured psychiatric intervention for cancer patients. II Changes over time in immunological measures. Arch Gen Psychiatry. 1990;47:729–735. doi: 10.1001/archpsyc.1990.01810200037005. [DOI] [PubMed] [Google Scholar]
- 132.Spiegel D, Bloom JR, Kraemer HC, Gottheil E. Effect of psychosocial treatment on survival of patients with metastatic breast cancer. Lancet. 1989;2:888–891. doi: 10.1016/s0140-6736(89)91551-1. [DOI] [PubMed] [Google Scholar]
- 133.Goodwin PJ, et al. The effect of group psychosocial support on survival in metastatic breast cancer. N Engl J Med. 2001;345:1719–1726. doi: 10.1056/NEJMoa011871. [DOI] [PubMed] [Google Scholar]
- 134.Spiegel D. Effects of psychotherapy on cancer survival. Nature Rev Cancer. 2002;2:383–389. doi: 10.1038/nrc800. [DOI] [PubMed] [Google Scholar]
- 135.Perron L, Bairati I, Harel F, Meyer F. Antihypertensive drug use and the risk of prostate cancer (Canada) Cancer Causes Control. 2004;15:535–541. doi: 10.1023/B:CACO.0000036152.58271.5e. [DOI] [PubMed] [Google Scholar]
- 136.Algazi M, Plu-Bureau G, Flahault A, Dondon MG, Le MG. Could treatments with β-blockers be associated with a reduction in cancer risk? Rev Epidemiol Sante Publique. 2004;52:53–65. doi: 10.1016/s0398-7620(04)99022-0. (in French) [DOI] [PubMed] [Google Scholar]
- 137.Li CI, et al. Relation between use of antihypertensive medications and risk of breast carcinoma among women ages 65–79 years. Cancer. 2003;98:1504–1513. doi: 10.1002/cncr.11663. [DOI] [PubMed] [Google Scholar]
- 138.Meier CR, Derby LE, Jick SS, Jick H. Angiotensin-converting enzyme inhibitors, calcium channel blockers, and breast cancer. Arch Intern Med. 2000;160:349–353. doi: 10.1001/archinte.160.3.349. [DOI] [PubMed] [Google Scholar]
- 139.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lieb J. Antidepressants, eicosanoids and the prevention and treatment of cancer. A review. Prostaglandins Leukot Essent Fatty Acids. 2001;65:233–239. doi: 10.1054/plef.2001.0319. [DOI] [PubMed] [Google Scholar]
- 141.Sapolsky RM. Why Zebras Don’t Get Ulcers: A Guide to Stress, Stress-Related Diseases, and Coping. Freeman; New York: 1994. [Google Scholar]
- 142.Buchschacher GLJ, Wong-Staal F. In: Cancer: Principles and Practice of Oncology. DeVita VTJ, Hellman S, Rosenberg SA, editors. Lippincott Williams & Wilkins; Philadelphia: 2005. pp. 165–172. [Google Scholar]
- 143.Howley PM, Ganem D, Kieff E. In: Cancer: Principles and Practice of Oncology. DeVita VTJ, Hellman S, Rosenberg SA, editors. Lippincott Williams & Wilkins; Philadelphia: 2005. pp. 173–184. [Google Scholar]
- 144.Steplewski Z, Vogel WH, Ehya H, Poropatich C, Smith JM. Effects of restraint stress on inoculated tumor growth and immune response in rats. Cancer Res. 1985;45:5128–5133. [PubMed] [Google Scholar]
- 145.Teunis MA, et al. Reduced tumor growth, experimental metastasis formation, and angiogenesis in rats with a hyperreactive dopaminergic system. FASEB J. 2002;16:1465–1467. doi: 10.1096/fj.02-0145fje. [DOI] [PubMed] [Google Scholar]
- 146.Palermo-Neto J, de Oliveira Massoco C, Robespierre de Souza W. Effects of physical and psychological stressors on behavior, macrophage activity, and Ehrlich tumor growth. Brain Behav Immun. 2003;17:43–54. doi: 10.1016/s0889-1591(02)00057-0. [DOI] [PubMed] [Google Scholar]