Abstract
Articular cartilage repair and regeneration continue to be largely intractable due to the poor regenerative properties of this tissue. The field of articular cartilage tissue engineering, which aims to repair, regenerate, and/or improve injured or diseased articular cartilage functionality, has evoked intense interest and holds great potential for improving articular cartilage therapy. This review provides an overall description of the current state and progress in articular cartilage repair and regeneration. Traditional therapies and related problems are introduced. More importantly, a variety of promising cell sources, biocompatible tissue engineered scaffolds, scaffoldless techniques, growth factors, and mechanical stimuli used in current articular cartilage tissue engineering are reviewed. Finally, the technical and regulatory challenges of articular cartilage tissue engineering and possible future directions are discussed.
Keywords: Articular cartilage, tissue engineering, regeneration, repair, scaffolds, cells, stem cells, self-assembly, bioactive factors, regulatory affairs
I. INTRODUCTION
Joint and articular cartilage injuries are frequent occurrences; over 6 million people visit hospitals in the U.S. each year for various knee, wrist, and ankle problems.1 Progressive wear and tear on articular cartilage can lead to a progressive cartilage tissue loss, further exposing the bony ends, leaving them without protection. This finally deteriorates into the most common arthritis—osteoarthritis (or degenerative joint disease).2 It has been reported that osteoarthritis affects 33.6% (12.4 million) of adults age 65 and older in the U.S.3,4 The American Academy of Orthopaedic Surgeons (AAOS) reports that osteoarthritis is a primary diagnosis accounting for 67% of short-stay and nonfatal hospitalizations in 2004.5 Considering the increasing population, especially in the elderly with longer life expectancies, occurrences of injuries and osteoarthritis will undoubtedly increase, not only in the U.S., but world-wide.
Unlike other self-repairing tissues, such as bone, cartilage has a low regenerative capacity. Consequently, once injured, cartilage is much more difficult to self-heal. Three types of cartilage exist in the human body: hyaline cartilage (e.g., within diarthrodial joints), fibrocartilage (e.g., knee meniscus and TMJ disc), and elastic cartilage (e.g., ear).2,6 Specifically, articular cartilage covering bone surfaces is a soft and specialized hyaline cartilage that exhibits superior lubrication, wear, and low friction properties; it also reduces stresses in the joint.7,8 Articular cartilage is composed of a small percentage of chondrocytes, but a dense extracellular matrix (ECM) prevents chondrocyte mobility. In addition, articular cartilage lacks vascular, neural, and lymphatic networks, as well as various local progenitor cells. It has also been described as having high levels of protease inhibitors, which may inhibit efficient tissue repair.9,10 For these reasons, currently it is challenging to restore full tissue function in damaged or diseased articular cartilage.
Although traditional methods like autografts and allografts have been clinically employed to treat articular cartilage lesions, there still exist many shortcomings associated with these therapies. Autografts, which require the transplantation of a small portion of low-weight-bearing cartilage from the patient into defect sites, have disadvantages such as donor site morbidity and limited cartilage tissue availability.11–13 Allografts, cartilage pieces obtained from tissue banks, may potentially induce immune responses.13 For patients with severe joint damage and osteoarthritis, total joint replacement surgery is needed. However, many complications such as inflammation, infection, and implant loosening frequently occur after joint replacement and may lead to implant failure, necessitating future revision surgery.14,15 In fact, nearly 36,000 revisions for 328,000 hip replacements (11%) and 33,000 revisions for 418,000 knee replacements (8%) were performed in the U.S. in 2003 due to failed hip and knee replacements.5 Therefore, it is desirable to develop an efficient and simple method to successfully repair and regenerate articular cartilage tissues.
As a rapidly expanding field, tissue engineering may provide alternative solutions for articular cartilage repair and regeneration through developing biomimetic tissue substitutes. This review describes the anatomy of articular cartilage, traditional strategies and related problems, the current progress of articular cartilage tissue engineering, and future directions of articular cartilage repair and regeneration. In this context, the term “repair” is used to denote the restoration of normal function of cartilage regardless of the composition of new tissue that fills the defect sites. On the other hand, “regeneration” is defined as a process, which not only restores the normal functions of injured articular cartilage, but also results in the formation of new tissue that is indistinguishable from the native cartilage.
II. ARTICULAR CARTILAGE COMPOSITION AND STRUCTURE
II.A. Composition of Articular Cartilage
Articular cartilage is a thin connective tissue covering the surfaces in diarthrodial joints. For example, the thickness of articular cartilage in a normal human adult knee is roughly 1.5–3 mm.16,17 It is composed of two phases – solid and liquid. Table 1 summarizes its components, contents in two phases, and their corresponding functions. Generally, 60–80% of total wet weight of articular cartilage is fluid (e.g., interstitial water and electrolytes), which contributes to many important physical and physiological characteristics of articular cartilage.18,19 The remaining 20–40% of the tissue is mainly solid ECM and chondrocytes.20
Table 1.
The composition of articular cartilage
| Articular Cartilage | % wet weight19,20 | % dry weight21 | Functions | |
|---|---|---|---|---|
| Solid Phase (ECM) | Collagen | Type II collagen is 15–20% All other collagens are < 2% | 50–75% | Contributes to tensile properties and macromolecule entrapment19,11 |
| Proteoglycan | 10% | 20–30% | Contributes to compressive and flow-dependent viscoelastic properties283 | |
| Other glycoprotein, fibronectin etc. | Small amount | Small amount | Contributes to cell-ECM interaction and the stability of ECM | |
| Solid Phase (Cells) | Chondrocytes | < 5–10% of total tissue volume | Modify ECM and maintain suitable tissue size | |
| Fluid Phase | Interstitial water and electrolytes | 60–80% | __ | Exchanges nutrients with synovial fluid, lubricates the joint, and contributes to compressive resistance and deformation19 |
Chondrocytes, the only cell type existing in articular cartilage, account for less than 5–10% of the total tissue volume.21 Although chondrocytes do not directly contribute to the mechanical properties of cartilage,20 they can sense and respond to various mechanical stimuli within their individual microenvironments.22 In addition, chondrocytes from different zones of articular cartilage may respond to forces differently22 and exhibit diverse morphologies (see section II.B for details). Mature chondrocytes are completely encapsulated in the dense cartilage ECM and are not able to migrate or proliferate in a significant manner, unlike cells in bone,11,19 thus potentially limiting the regenerative capacities of cartilage after injuries.
Articular cartilage ECM, which includes various organic constituents like collagen, proteoglycans, and other noncollagenous proteins, accounts for most of the dry weight of the tissue (Table 1) since chondrocytes occupy but a small fraction of the tissue. As will be described below, the ECM and its interplay with the interstitial fluid play a critical role in cartilage biomechanics. A variety of collagens such as collagen II, VI, IX, X, and XI are the main components of articular cartilage ECM and contribute to the tensile properties of articular cartilage.7 With maturation, the proportion of collagen II to other collagens increases from 75% in fetal cartilage to over 90% in adult cartilage.7,23 Conversely, the proportion of collagen XI to all collagens decreases from 10% of fetal cartilage to 3% of adult cartilage.23 The organization of collagens also changes from random distribution and uniform size in immature articular cartilage into oriented distribution and non-uniform size in mature tissues.7
Moreover, collagens IX and XI can crosslink with collagen II to form larger fibrils. These fibrils then interconnect into a mesh network, which is the main contributor to the tensile properties of cartilage.7 Small amounts of collagen VI in the pericellular matrix surrounding chondrocytes24 have been shown to play a role in mechanotransduction via cell-collagen interactions.25 Moreover, by balancing proteoglycan swelling, the collagen fibers affect the degree of tissue hydration, thereby contributing to tissue compressive properties.26–28
A special class of glycoproteins, proteoglycans, is another main component in hyaline cartilage. Its biomechanical role is to provide compressive properties to the tissue. The majority of proteoglycans found in cartilage are associated in aggregates (aggrecan). Aggrecan is a large proteoglycan with long and unbranched glycosaminoglycan (GAG) chains that spread out like tubular brushes. These brush-like structures are chondroitin sulfate and keratin sulfate molecules attached to a high molecular weight protein core.29 The aggregating structure is stabilized by aggrecan molecules being connected to hyaluronic acid GAG chains via link protein. Aggrecan is highly negatively charged due to abundant carboxyl (COO−) and sulfate (SO3−) groups on chondroitin sulfate or hyaluronic acid GAG chains.30 Since the collagen fibers prevent aggrecan from escaping from cartilage, the fixed negative charges associated with aggrecan attract freely mobile cations in the fluid phase into the tissue. The resultant high density of ions within the tissue creates what is termed the “Donnan osmotic pressure.” This osmotic pressure causes cartilage to swell and also manages water amounts within the tissue.8,31 Aside from aggrecan, smaller proteoglycans like biglycan, fibromodulin and decorin also occur in minute amounts; many of these have been shown to contribute to matrix organization.11,32
II.B. Structure of Articular Cartilage
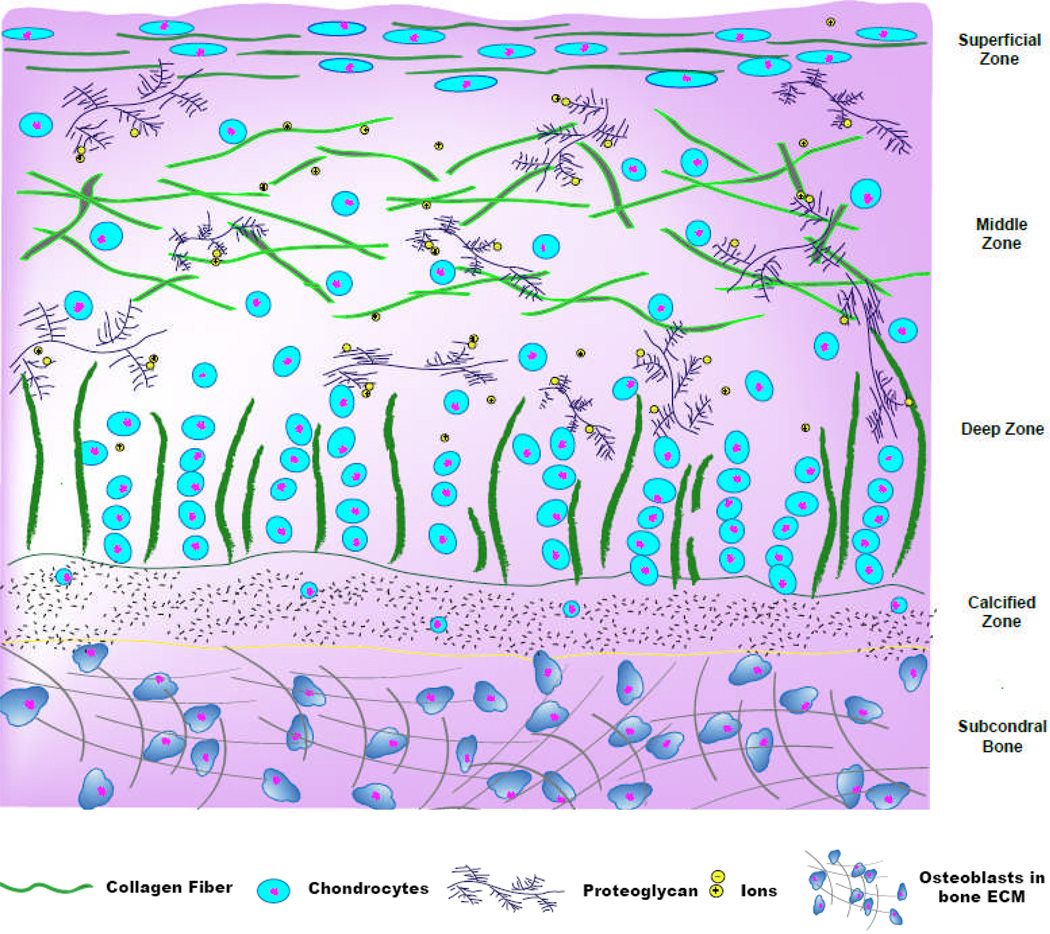
Articular cartilage is divided into four different zones, each with varying matrix composition, morphology, cellular, mechanical, and metabolic properties.22 These are termed the superficial, middle (or transitional), deep (or radial), and calcified zones. Figure 1 illustrates the non-homogeneous distribution of cells and ECM in zones of mature articular cartilage. Each zone plays a different role in contributing to the functional properties of articular cartilage.
Fig 1.
Schematic illustration of composition and structure of articular cartilage lining the bone (not drawn to scale). There are four zones with different structures in articular cartilage: superficial, middle, deep, and calcified.
Starting from the articulating surface, the superficial zone only accounts for 10–20% of the total articular cartilage thickness, but contains the highest density of collagen within the tissue (Table 2). When compared to other zones, the collagen fibers here are the thinnest and most densely packed to form an oriented lamina splendens that covers the joint. Similarly, a relatively small number of fibroblast-like chondrocytes with few organelles33 are flattened in the superficial zone and are oriented parallel to the surface and the direction of shear stress. The ECM in this zone has fewer proteoglycans compared to other zones. It is believed that the composition and organization of this zone contributes to tensile strength, resists shear during articulation, and adjusts fluid permeability.29
Table 2.
The different morphology, size and distribution of components in four articular cartilage zones
| Name | Superficial Zone |
Middle Zone | Deep Zone | Calcified Zone |
|
|---|---|---|---|---|---|
| Chondrocytes | morphology | flattened | rounded | rounded or ellipsoid284 | small and inert |
| Collagen fibrils | % dry weight7 | 86% | between | 67% | ND |
| diameter7,285,286 | 30–35 nm | between | 40–80 nm | ND | |
| Proteoglycan | % dry weight29 | 15% | 25% | 20% | ND |
| Water | % wet weight284 | 84% | between | 40–60% | ND |
| Total thickness | % total tissue21 | 10–20% | 40–60% | 20–30% | ND |
The middle zone is a transitional zone between the superficial and deep zones. This zone has the highest proteoglycan content in the tissue. When examined from the superficial to the deep zone, the collagen and water contents gradually decrease, and the collagen fiber size increases in this zone (Table 2). Unlike the superficial zone, chondrocytes in the middle zone exhibit a rounded morphology and have synthetic organelles. In addition, the collagen fibrils transition from a tangential orientation in the superficial zone to a random orientation here, to finally reach a perpendicular orientation in the deep zone (Figure 1).
The collagen fibrils in the deep zone are the largest in diameter. They are organized in radial directions (perpendicular to the articulating surface) and are inserted across the tidemark (a visible basophilic line that separates deep and calcified zones). The functional role of these collagens is to strengthen the bond between cartilage and bone.19,32 The chondrocytes are packed in columns parallel to the organized collagen fibers (Figure 1). Moreover, cells in the deep zone show 10-fold higher synthetic activities although they only have twice as much surface area and volume than cells in the superficial zone.34 It was observed that cells from the deep zone attach and spread faster on tissue culture plastic (TCP) and synthesize more keratin sulfate than cells from the upper zones.35
The transitional zone from articular cartilage to subchondral bone is the calcified zone, which contains few inert chondrocytes embedded in a calcified ECM. It is the only zone having collagen type X, which helps cartilage mineralization and provides structure integrity.36
From a matrix point of view, articular cartilage is classified into three regions including territorial, interterritorial, and pericellular matrices based on their distances from the cells. The thin pericellular matrix is composed of proteoglycans, collagen type VI, and other non-collagenous proteins. This matrix closely surrounds individual or a column of chondrocytes and protects the cells from various mechanical loads.36,37 The interterritorial matrix is farther from the cells and is made of organized collagen fibrils that are the largest in diameter when compared across the three types of matrices.7,37 This matrix accounts for a large percent of the total matrix volume.36 Finally, the territorial matrix is the farthest matrix from cells, and it consists of collagen fibrils that may be less organized than the other two matrices.7
II.C. Mechanical Properties of Articular Cartilage
Due to the small volume of articular cartilage, the amount of shock and energy that can be absorbed by cartilage during normal activities are far less than those taken up by surrounding muscles, tendons, ligaments and the underlying bones.29 However, the fiber-reinforced, permeable articular cartilage plays a unique role in repeatedly dissipating compressive loads, redistributing loading forces, and lowering joint frictions.38 According to the biphasic cartilage model,39,40 cartilage is composed of liquid and solid phases, and the interactions between these two phases characterize the viscoelastic properties of this tissue. The incompressible interstitial fluid phase of cartilage encounters friction as it flows out of the porous collagen-proteoglycan solid matrix. This frictional drag counterbalances the compressive forces applied onto the tissue. Fluid exudation from the tissue also serves to lubricate the joint during loading.41
Aside from structure, cartilage composition is also important in determining the tissue’s biomechanical properties (e.g., tensile, compressive, and shear). As mentioned above, collagen fibrils are the main contributors to the tensile properties of articular cartilage. Since different zones have different collagen diameters and organization, the tensile properties vary significantly among zones. For example, Akizuki and associates42 measured tensile moduli of human knee joint cartilages and found that the equilibrium tensile modulus value was higher in the superficial zone (10.1 MPa) as compared to the other zones (e.g., 5.4 MPa in the middle zone). This can be attributed to fact that collagen is the most abundant and organized in the superficial zone.20 Within the same study, it was also shown that high weight-bearing areas have lower tensile modulus values than low weight-bearing areas.
Compressive properties of articular cartilage are important because the cartilage tissue is frequently compressively loaded during physiological use. Through confined compression, unconfined compression, or indentation methods,20 the compressive properties of articular cartilage have been evaluated. Generally, compressive moduli change with the depth and location.43,44 It was reported that the compressive modulus increased nearly 27-fold from the superficial zone (0.079±0.039 MPa) to the deepest zone (2.10±2.69 MPa) in bovine articular cartilage.44 In addition, human articular cartilage’s aggregate equilibrium compressive moduli, a measure of the solid ECM stiffness, may range from 0.1 to 2 MPa depending on location.17,21,45–47
III. TRADITIONAL STRATEGIES AND PROBLEMS FOR ARTICULAR CARTILAGE REPAIR
III.A. Articular Cartilage Injuries
Articular cartilage defects, which are caused by traumatic destruction or degenerative joint diseases, are primarily divided into two categories: partial-thickness and full-thickness cartilage defects.48,49 The partial-thickness defects only damage the zonal articular cartilage but do not penetrate into the underlying subchondral bone, rendering the defect site inaccessible to blood cells, bone cells, and progenitor cells in bone morrow.49 Thus, the defect site lacks fibrin clots and other self-healing responses. Although some metabolic and enzymatic activities occur and chondrocytes may begin to proliferate and synthesize ECM right after the creation of a partial-thickness defect, there are still not enough new chondrocytes to migrate into the injured sites to effectively repair the injury. Furthermore, the reparative activities of chondrocytes typically cease before the cartilage defect is healed, thus resulting in a lasting defect that reduces tissue function and can serve as a starting point for tissue degeneration.29,49
Full-thickness (or osteochondral) defects penetrate the entire thickness of articular cartilage, beyond the calcified zone, and into the subchondral bone. Unlike partial-thickness defects, full-thickness defects are accessible to mesenchymal progenitor cells, macrophages, and blood cells,49 all of which are involved in a spontaneous immune response and a healing process after injuries as described elsewhere.48–50 Briefly, immediately following injury, the defect void is filled with a fibrin clot and an inflammatory response is activated. Next, mesenchymal stem cells from bone marrow migrate into the defect, gradually replacing the fibrin clot and completely filling the defect after one week.49 Many of these mesenchymal stem cells can differentiate into chondrocytes later, which secrete a proteoglycan-rich ECM and repair the damaged cartilage tissue. However, it has consistently been observed that fibrous, not hyaline, tissues with weaker mechanical properties and higher permeability are formed in defect sites.51,52 Consequently, the spontaneous repair process in full-thickness defects is only transient and imperfect, and tissue degeneration eventually occurs several months later and proceeds continuously.49,50,53 After this point, the cartilage tissue often becomes hypertrophic and is finally replaced by the progressive deposition of subchondral bone.48,49 At this point, while chondrogenesis may still occur sporadically, complete resurfacing is rarely observed, leading to bone to bone articulation, inflammation, significant pain, and disability.
III.B. Traditional Therapies and Problems for Articular Cartilage Repair
1. Microfracture
Microfracture surgery is one quick and common method to treat smaller articular cartilage defects. Inspired by the spontaneous repair process of full-thickness cartilage defects, this method aims to create microfractures in the underlying subchondral bone via drilling, shaving, or abrasion.48 Microfracture causes the subchondral bone to release bone marrow progenitor cells and, as expected, repair occurs similar to full-thickness defects. This treatment is effective especially for small articular cartilage defects (e.g., < 2 cm2), and is attractive due to its relatively minimally invasive nature, short surgery and recovery time, and low morbidity.12 However, it should be noted that articular cartilage repair results using microfracture has high inter-patient variability. Younger patients, earlier treatment of defects,12 or smaller lesions may yield better cartilage repair, particularly since mesenchymal stem cells, the cell type responsible for repair, are more abundant and active in younger patients.54,55 In some cases, little or no hyaline cartilage is regenerated, and the generated hyaline cartilage may turn over into weaker fibrocartilage, thus resulting in high failure rates and limiting microfracture surgery effectiveness.11,48,56
2. Autologous Chondrocyte Implantation
As the first generation of cell transplantation techniques for cartilage repair,57,58 autologous chondrocyte implantation (ACI, also known as the Carticel® procedure by Genzyme corporation, MA) has been accepted and used widely. It has been recommended for patients who have cartilage lesions between 1 cm2 and 12 cm2, or have had previously failed microfracture surgeries.59 There are two surgeries involved in this technique. In the first surgery, a small piece of healthy cartilage is harvested from the low weight-bearing area of a patient’s knee. Then, chondrocytes are retrieved from the cartilage tissue and further expanded in vitro for 3–5 weeks on monolayer to obtain sufficient numbers for reimplantation (approximately 12 × 106 cells).60 A second surgery then occurs to inject the cells into the trimmed and prepped lesion, and a periosteal patch from the patient’s shin bone is sutured as a cover to secure chondrocytes within the injured site.60 Although many satisfactory results have been reported, this technique still has some limitations and disadvantages. For example, the invasive ACI procedure has a long recovery time and requires multiple surgeries to harvest healthy cartilage, to harvest a periosteal patch, and to re-implant the healthy cells.61 In addition, the possibility of periosteal hypertrophy, dedifferentiation of patients’ chondrocytes during in vitro culture, and decreased human chondrocyte number or cellularity with aging may impair or even result in the failure of repair using ACI.12,62,63
3. Autografts and Allografts
Autografts and allografts are two other popular therapies for repairing small cartilage lesions. For an osteochondral autograft, healthy, cylindrical cartilage tissue plugs are harvested from a patient’s low weight-bearing area and are then implanted into defect sites to restore function.12 Encouraging clinical results and excellent tissue integration associated with autografts have been reported.64 However, there are some limitations related to autografts including insufficient donor tissues (both in quantity and quality), donor site morbidity, surface mismatch of the graft and implant sites, graft instability, and long-term survival of the implant at its new high weight-bearing location considering that it was harvested from a low weight-bearing region.12,48 Using the autologous mosaicplasty technique that implants many small osteochondral autografts into one defect site, a smoother contour can be created for small or medium defects.64,65 Smaller donor tissues have three significant advantages. First, smaller donor site defects are produced, and donor site morbidity is reduced. Second, more sites can serve to provide donor tissue as compared to only sites that are as large or larger than the defect to be filled. Third, the smaller plugs address the surface congruity and contour problems seen with only one large plug. The technique has shown promising results for treating 1–4 cm2 articular cartilage lesions at short, middle, and long term follow-ups.66,67
Osteochondral allografts adopt cartilage tissues from tissue banks, thus avoiding donor site morbidity, and alleviate the insufficient donor tissue supply. Allografts also circumvent the multiple step surgeries required in autograft procedures. However, it has similar limitations to autografts, such as contour matching and load-bearing capacity (which is typically reduced during processing). The use of allografts may also induce immune reactions such as inflammation or rejection. Finally, allografts contain dead cells that cannot maintain the articular surface. Whereas cartilage has been shown to secrete proteins to lower the friction of its articulating surface, the dead cells of allografts do not replace this function.
4. Total and Partial Joint Replacements
For severe joint injuries, disease, or advanced osteoarthritis, articular cartilage cannot be recovered by any of the above discussed treatments. In these cases, total or partial joint replacements are performed to help patients restore normal function. In joint replacement therapies, the damaged osteochondral tissue is partially or totally removed and resurfaced. An artificial implant composed of a metal shell (such as titanium, stainless steel, or alloys), a polymer piece (such as polyethylene in order to glide smoothly), and a metal stem is implanted to replace the damaged joint.14 As the average age of the population increases, there is a potentially large market for total knee and hip replacements. However, due to frequently reported complications including infection, implant loosening, osteolysis, implant wear and tear, and relatively short life spans of current implants, revision surgeries are often a necessity which burdens the patient with increased pain and health insurance costs.14
IV. PROMISE OF TISSUE ENGINEERING FOR ARTICULAR CARTILAGE REPAIR AND REGENERATION
IV.A. The Concept of Tissue Engineering
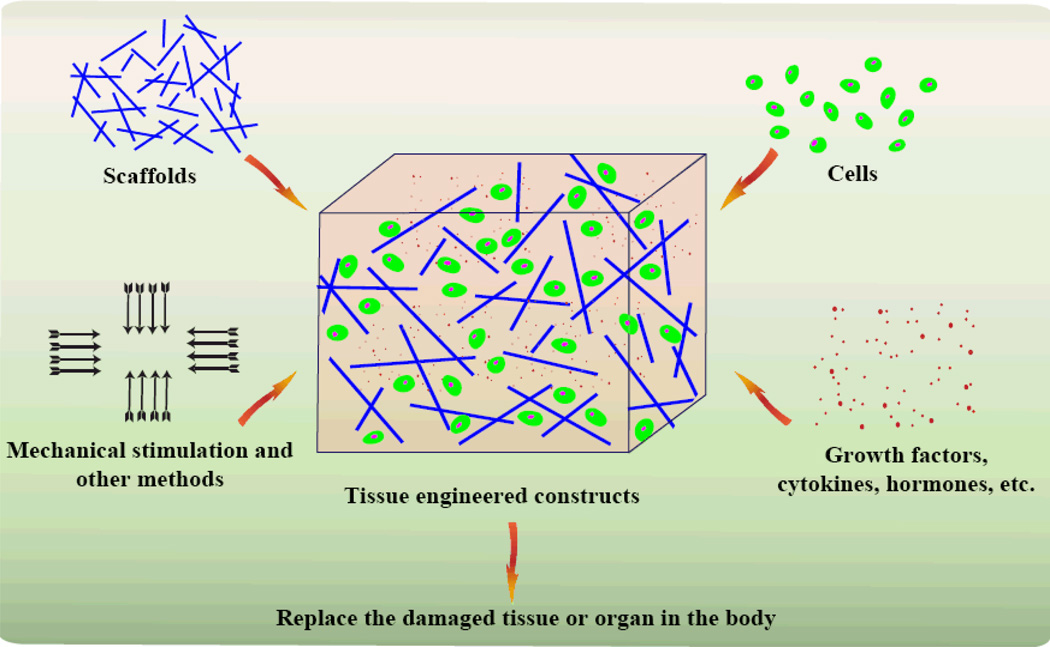
Tissue engineering (sometimes called regenerative medicine, though the latter refers primarily to the use of stem cells) is an emerging interdisciplinary research field initially defined in the early 1990s.68–70 It uses principles and methods in engineering, material science, biology, and chemistry to develop biological substitutes that restore, maintain, or improve functionality of damaged tissues and organs. In the ensuing years, this discipline quickly developed to encompass a variety of cell types (e.g., stem cell, chondrocytes, osteoblasts, endothelial cells, fibroblasts, and smooth muscle cells), scaffolds (e.g., biodegradable, natural or synthetic materials, polymers, and nanocomposites), bioactive factors (e.g., various growth factors and cytokines), and physical stimuli (mechanical, electrical, etc.) to form biomimetic tissues (Figure 2). In the following sections, cells, scaffolds, bioactive factors, and mechanical stimuli for articular cartilage tissue engineering will be discussed in detail.
Fig 2.
The concept of tissue engineering. Tissue engineering incorporates many critical factors including cells, scaffolds, bioactive factors, and physical stimuli to assemble biomimetic tissue engineered constructs for replacing damaged tissues in humans.
IV.B. Cell Sources for Articular Cartilage Repair and Regeneration
1. Chondrocytes
Chondrocytes are the sole cell type in articular cartilage and are 10–13 µm in diameter.71 Clinically, these have served as the only cell source for articular cartilage repair. Autologous chondrocytes have been extensively used in articular cartilage repair and regeneration; however, there are some limitations. For example, autologous chondrocyte availability is limited and cannot satisfy the high cellular demand of articular cartilage repair. Although in vitro cell expansion methods, such as those used in ACI, have been adopted to increase cell numbers for transplantation, chondrocytes may dedifferentiate during in vitro culture.72
Other readily available alternative chondrocyte sources (such as allogeneic or xenogeneic chondrocytes) have also been widely studied. However, these chondrocytes can potentially induce immune responses and transmit diseases. Thus, the field of allogeneic and xenogeneic chondrocyte sourcing requires further investigations to mitigate such concerns. Another area of investigation is the use of separately seeded zonal chondrocytes toward regenerating biomimetic functional cartilage tissue,29,73 since chondrocytes from each of the four zones have been shown to exhibit different properties.74,75 To more efficiently form different sizes of cartilage tissues with suitable mechanical properties, the various cell sources described above have also been grown in numerous biocompatible scaffolds and treated with growth factors for articular cartilage tissue engineering applications, which will be discussed later.
2. Stem Cells
Due to the many aforementioned limitations related to chondrocyte sources, there is much effort to explore better alternative cell sources. Desirable characteristics for such sources include accessibility, availability, and chondrogenic capacity. Consequently, stem cells such as adult mesenchymal stem cells (MSCs) and embryonic stem cells (ESCs) have emerged as promising cell sources for articular cartilage tissue engineering.
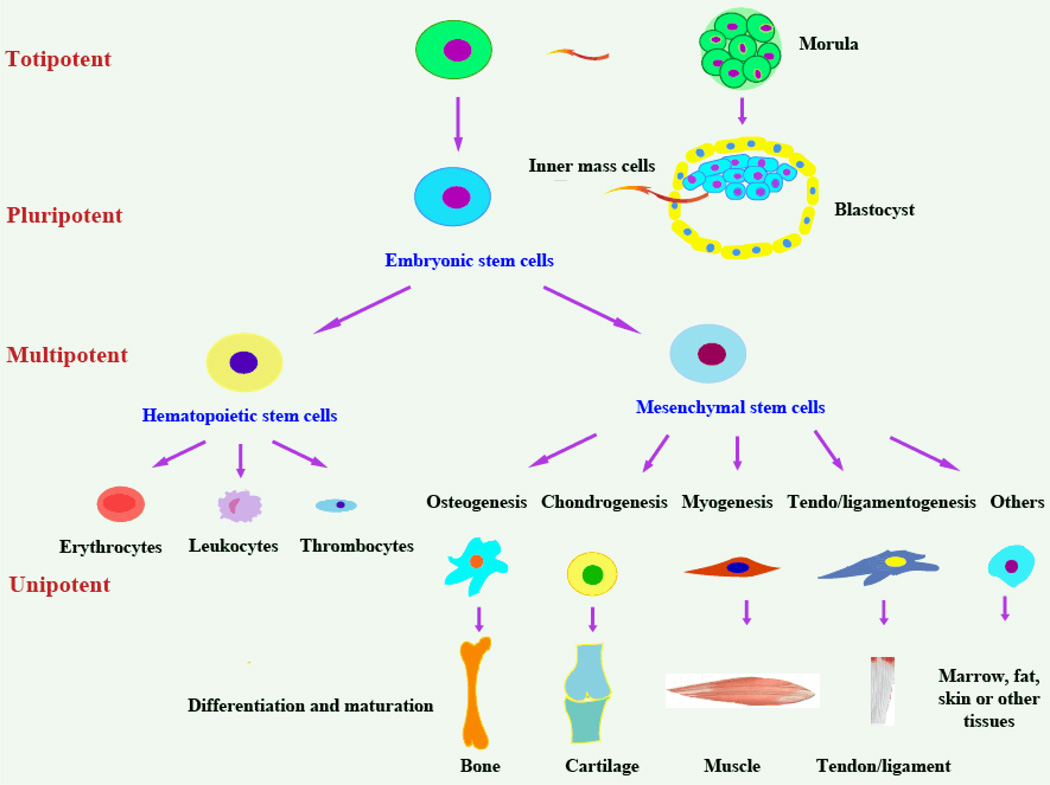
Functionally, the broad definition of stem cells originates from two unique properties: the self-renewal capabilities that can generate numerous descendant cells identical to the mother cells while maintaining an undifferentiated state throughout, and the potent ability to differentiate into multiple types of specialized cells. Figure 3 illustrates the hierarchical structure of stem cells. According to the number of cell types that can be differentiated from them, human stem cells can be classified into four types: totipotent, pluripotent, multipotent, and unipotent stem cells.76 Morula cells are totipotent stem cells, which have the ability to differentiate into any tissue in human body, while ESCs, usually harvested from the inner cell mass in a 5–6 day old blastocyst from artificial in vitro fertilization, are pluripotent stem cells. Pluripotent stem cells are almost totipotent; however, they cannot differentiate into placental cells. Multipotent stem cells including adult stem cells derived from many parts of the body have the ability to differentiate into multiple closely-related cell types only. For example, MSCs, as shown in Figure 3, are able to differentiate into cartilage, bone, muscle, etc., while hematopoietic stem cells can create all blood cell types such as red and white blood cells, but not cartilage, bone, and muscle. Lastly, unipotent cells are the non-strictly defined stem cells that only differentiate into one cell type but have self-renewal capabilities.
Fig 3.
The totipotent, pluripotent, multipotent, and unipotent stem cells.
a. Mesenchymal Stem Cells
Autologous MSCs from a variety of human tissues including bone marrow, fat, synovium, periosteum, skeletal muscle, skin, etc., have been widely investigated in regenerative medicine for small and large cartilage defect repair.77–79 These autologous MSCs have a high enough proliferative capacity to expand to enough cell numbers without losing their MSC phenotype, and they do not induce immune responses as allografts and xenografts do. In addition, they can be easily isolated from many mesenchymal tissues (especially the minimally invasive procedure to isolate MSCs from adipose and skin tissues), which may decrease donor site morbidity and patient pain compared to autograft and ACI therapies.71
Many methods have been studied to induce chondrogenesis of MSCs (typically marked by GAG and collagen type II production).71 Various transforming growth factors (e.g., TGF-β1 and TGF-β3), insulin-like growth factors (e.g., IGF-I), dexamethasone, bone morphogenetic proteins (e.g., BMP-2 or BMP-6), and fibroblast factors are supplemented in media,77,80–85 and mechanical stimuli such as hydrostatic pressure86 and cyclic compression87 have all been reported to improve the chondrogenic differentiation of MSCs.
Chondrogenic potentials of MSCs from different tissues have also been investigated and compared.88–91 Specifically, MSCs from bone marrow (Figure 4) are the most popular considering they are easily harvested (via the iliac crest) and have good chondrogenic potential. Many in vitro and in vivo studies have revealed promising results of marrow-derived MSCs combined with various biomaterials or growth factors for repairing cartilage defects.92–96 For example, Koga and associates88 embedded MSCs isolated from bone marrow, synovium, adipose tissue, and muscle of adult rabbits in collagen gels and then implanted them into full-thickness cartilage defects in rabbits. Their results demonstrated that MSCs from bone marrow and synovium had greater chondrogenic capability in vivo than those from other mesenchymal tissues. Another study showed that synovium-derived MSCs proliferated faster than bone marrow-derived MSCs when cultured in autologous human serum, thus serving as another promising cell source for cartilage regeneration.97
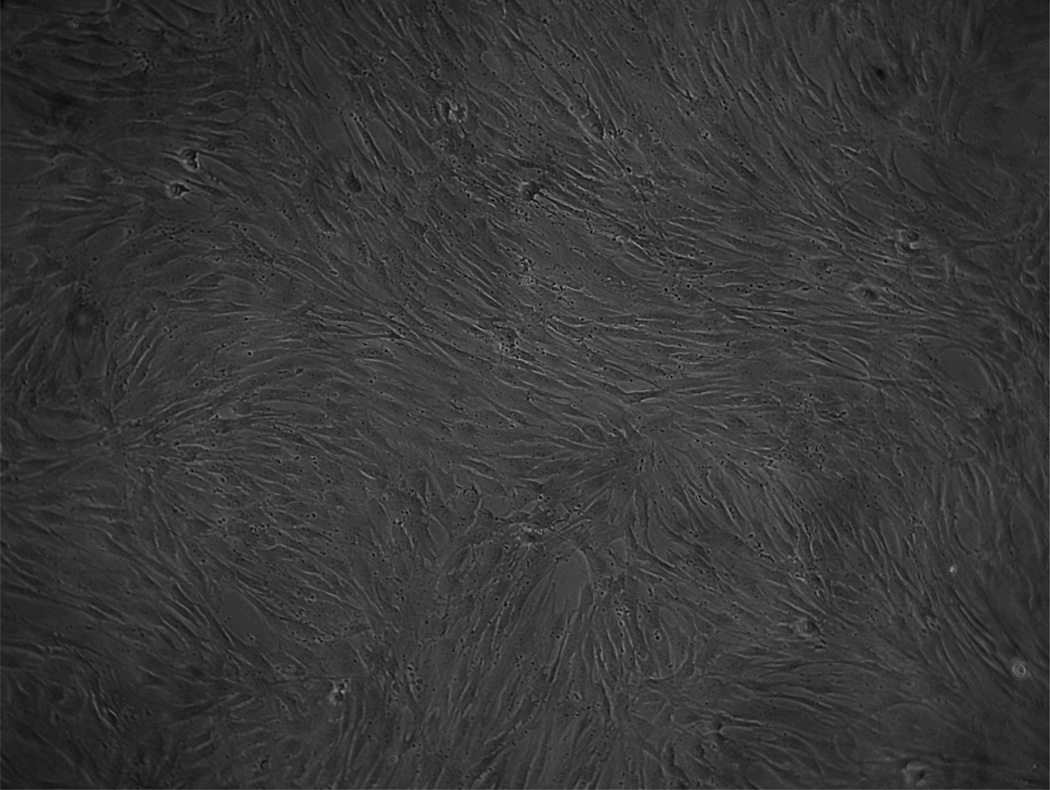
Fig 4.
Human bone marrow-derived mesenchymal stem cells after 6 days of culture, exhibiting a fibroblastic morphology.
Although in vitro studies have shown that adipose-derived stem cells (ASCs) may have lower chondrogenic potentials than bone marrow-derived MSCs,89–91 ASCs still attract increasing attention for cartilage tissue engineering because of their abundance and ease of procurement (e.g., high yields of ASCs obtained from waste adipose tissues via liposuction).98 In addition, the minimally invasive acquisition of ASCs from subcutaneous adipose tissue circumvents donor site morbidity and pain. Chondrogenic growth of human ASCs has also been reported to occur on different biomaterial scaffolds including agarose, alginate, and biologically active gelatin to create tissue engineered cartilage constructs.99
It is important to note that most of the current MSC transplantation studies are still in preclinical trials. Only a few results are available from clinical trials of MSC-based articular cartilage repair on patients.100–103 Wakitani and colleagues102 evaluated clinical results of autologous bone marrow-derived MSC transplantation into the knees of three patients. The bone marrow-derived MSCs were harvested from the iliac crests of the patients, expanded in vitro, embedded into collagen gels, and then reimplanted into 9 full-thickness articular cartilage defects of the patients’ patello-femoral joints. The patients’ clinical symptoms were improved after 6-month transplantation and maintained satisfactory performance during 17–27 months. In addition, as a leading stem cell company, Osiris Therapeutics Inc. has developed a manufacturing process to expand human bone marrow-derived MSCs for clinical use. Their stem products, such as Chondrogen, have shown significant therapeutic potential for preventing osteoarthritis during Phase I or II clinical trials.
Based on the results from preclinical and clinical studies, MSC transplantation exhibits tremendous promise for promoting articular cartilage repair and regeneration. Thus, more work is needed to optimize MSC culture conditions, understand underlying chondrogenic differentiation mechanisms, regenerate biomimetic MSC-based cartilages, and explore clinical therapies for successful human cartilage regeneration.
b. Embryonic Stem Cells
When compared with multipotent adult MSCs, ESCs have features of unlimited proliferation (seemingly immortal) and almost universal differentiation potential into any somatic cell type.71 These features make them promising for tissue regeneration demanding large numbers of cells (e.g., traumatic cartilage defects). To date, in vitro and in vivo studies have provided some evidence of direct chondrogenic differentiation of ESCs via growth factors such as BMP-2, BMP-4, TGF-β1104 and TGF-β3,105 or via co-culture with primary chondrocytes,106 embryonic limb bud cells107. For human ESCs, Koay and associates108 developed a novel scaffold-free, modular approach that first differentiates human ESCs in serum-free, chemically-defined conditions and then assemble them into neocartilage constructs for cartilage tissue engineering applications (Figure 5). This scaffold-free, modular approach has also been applied to fibrocartilage tissue engineering.109 In addition, Hwang and colleagues derived MSCs from human ESCs and demonstrated in vivo commitment and cartilaginous tissue formation from the MSCs by using chondrocyte-secreted morphogenetic factors.110 ESCs have also been seeded into various biocompatible scaffolds such as polycaprolactone,111 3D fiber-deposited scaffolds,112 and poly(ethylene glycol)-based (PEG) hydrogels113 to induce their chondrogenic differentiation. One of the current challenges in scaffold development is in fabricating materials that can improve differentiation efficiency via controlled release of growth factors, linked peptides, and other biochemical methods. As ESCs may also respond to mechanical forces by shifting their lineage, scaffold load-shielding effects should be considered, but are yet to be investigated, in the area of scaffold development for stem cell use. In short, there is a plethora of biochemical methods being pursued in conjunction with scaffold effects on differentiation, but few investigators are examining the effects of scaffold mechanics on differentiation.
Fig 5.
A scaffold-free, modular approach for the engineering of articular cartilage from stem cells.
Since ESC research is still in its infancy stages, there are many unexplored areas and ethical concerns related to their clinical applications. For example, we still do not know the best method to selectively differentiate ESCs into desirable cell lineages at injury sites to regenerate desirable tissues. It is possible that multiple tissues are formed out of ESC-differentiation, resulting in an undesirable teratoma.114 Due to the allogeneic nature of ESCs, potential immunogenicity problems also exist for clinical transplants. Additionally, since a layer of feeder cells like mouse embryonic fibroblasts are normally adopted to culture ESCs, animal pathogens may potentially be introduced.115 It is, however, possible to use human fetal and adult fibroblasts as safer alternative feeders to support human ESC growth.116 More importantly, there are concerns about the sources of blastocysts, the safety of ESCs, and so on115 for ESC research and clinical applications. Obviously, these issues require more investigations to fully explore the medical potential of this flexible cell source.
3. Other Cell Sources
An equally exciting potential cell source is the dermis of the skin.117–122 Considering its relative abundance and accessibility, the dermis is considered one of the best autologous source organs to isolate stem/progenitor cells for future therapeutic applications. This is true not only in the replacement of skin,123–125 but also as an alternative cell source for several other organs. Human dermal fibroblasts cultured with demineralized bone powder have been shown to acquire a chondroblastic phenotype.117–121,126–132 Chondro-induction has also been shown for the human foreskin fibroblast cell line Hs27 and the adult rabbit dermal fibroblast cell line RAB-9 when cultured on aggrecan-coated surfaces.133 Several types of fibroblasts exist in the dermis,134–136 and not all dermis subpopulations may possess latent chondro-induction potentials. From these, a dermis-isolated, aggrecan-sensitive subpopulation has also been shown to yield engineered constructs containing cartilage specific matrix.122
IV.C. Tissue Engineering Scaffolds for Articular Cartilage Repair and Regeneration
For tissue engineering applications, biomaterial scaffolds play a critical role in providing a 3D environment to support cell growth, matrix deposition, and tissue regeneration. An ideal tissue engineering scaffold should satisfy several essential criteria: it should (1) be biocompatible to minimize local tissue response but maximize cell growth and tissue integration; (2) be biodegradable with a favorable resorption rate, which can provide structural support for the initial cell growth and then gradually degrade after new tissue formation; (3) have suitable porosity and interconnectivity to allow cell migration and efficient exchange of nutrients and wastes; (4) possess appropriate mechanical properties to support tissue growth under native mechanical loads.71,137 To date, a range of biomaterial scaffolds including natural polymers extracted from living organisms and synthetic materials obtained from various chemical processes have been widely investigated for tissue repair and regeneration.71 The most extensively used natural or synthetic scaffolds and the emerging nanostructured scaffolds in cartilage tissue engineering will be described in detail next.
1. Natural Scaffolds
Natural biomaterials are the popular scaffolds for cartilage repair and regeneration due to their good biocompatibility for cell attachment and differentiation. Specifically, natural scaffolds used in articular cartilage tissue engineering include carbohydrate-based hyaluronic acid, agarose, alginate, and chitosan, and protein-based collagen or fibrin glue.137
As a non-sulfated glycosaminoglycan derived from ECMs of many tissues, hyaluronic acid (or hyaluronan) has been used to support chondrocyte growth or stimulate MSC chondrogenesis.138,139 For example, a hyaluronan-based scaffold (Hyaff-11) seeded with autologous chondrocytes has shown to be effective in regenerating cartilage tissues in vivo.138 In addition, a minimally invasive surgical technique using hyaluronan as an injectable material has shown promise in healing cartilage defects.71
Agarose and alginate are polysaccharides derived from seaweed and used as biocompatible 3D scaffolds to encapsulate cells for cartilage tissue engineering. Agarose gel is obtained through changing temperatures, and a cross-linked alginate matrix can be formed via ionic bonding in the presence of Ca2+. Both of these scaffolds have exhibited excellent cytocompatibility for cell growth140–142: however, the poor degradation properties and the difficulty to modify the scaffolds’ life71 may hinder their clinical applications for tissue regeneration.
Collagens are main protein components in natural cartilage, bone, and other connective tissue ECMs. They contribute to cell adhesion, proliferation and differentiation,143 and, thus serve as one of the most common scaffold materials for cartilage tissue engineering. Many studies have demonstrated that a combination of collagens (such as type I and type II collagens) with chondrocytes and stem cells facilitated cartilage tissue growth in vitro and in vivo.51,144–146 Specifically, a clinical therapy named the Matrix-induced Autologous Chondrocyte Implant (MACI® implant, Genzyme) has been developed. In this method, chondrocytes are expanded in a collagen membrane and then reimplanted into articular cartilage defects without suturing. Moreover, other natural scaffold materials like the biodegradable fibrin, chitosan or composites thereof are widely studied and have shown potential to enhance cartilage tissue regeneration.71
2. Synthetic Scaffolds
Due to the ease of fabrication and chemical modification, good biocompatibility, high versatility, suitable mechanical properties, and controllable biodegradability, polymers currently elicit increasing interest from scientists who are investigating their potential as synthetic cartilage tissue engineering scaffolds.
The most popular synthetic polymers for cartilage tissue engineering scaffolds are poly lactic acid (PLA, which is present in both L and D forms), poly-glycolic acid (PGA), and their copolymer poly-lactic-co-glycolic acid (PLGA). These FDA approved biodegradable polymers can be fabricated into 3D matrices via particulate leaching, textile technologies, or three-dimensional (3D) printing techniques, etc.14,147 The fabricated polymer scaffolds have a controllable porosity and a suitable surface structure for cell attachment, proliferation, and differentiation. In particular, it has been shown that PGA improved proteoglycan synthesis when compared to collagen scaffolds.148 In addition, increasing chondrogenesis was observed in a chondrocyte/PGA/bioreactor system over 40 days of cultivation.149 PLLA has a slower degradation rate than PGA, and, thus is suitable for those applications requiring a longer duration of matrix structural supports. As a derivative copolymer of PLA and PGA, PLGA has high biocompatibility, an ability to degrade into harmless monomer units, a useful range of mechanical properties, and controllable degradation time depending on the copolymer ratio.14 Studies demonstrated that nonwoven PLGA scaffolds are suitable for the chondrogenesis of human adipose-derived stem cells.150 Additionally, PLGA scaffolds have been loaded with various chondrogenic factors like TGF-β1 and dexamethasone to improve chondrogenic differentiations of bone marrow-derived MSCs.151 Other polymers including poly(ε-caprolactone) (PCL) and PEG have also received substantial attention for articular cartilage tissue engineering.71
However, some disadvantages related to using synthetic polymers in cartilage engineering applications are still present. For example, although synthetic polymers have flexibility in design, they may lack the optimal cytocompatibility properties that natural materials possess for cell growth and may elicit a host response caused by the release of toxic byproducts during degradation. Therefore, there is a desire to design composite scaffolds combining the respective advantages of synthetic and natural materials to improve cartilage tissue repair and regeneration. For instance, fibrin glue, alginate, and hyaluronan have been used to modify various PLGA, PGA, PCL scaffolds,152–155 and the results revealed that these composite scaffolds can stimulate the chondrogenesis of different chondrocytes or progenitor cells, thus warranting further investigations.
3. Nanostructured Tissue Engineering Scaffolds
Conventional natural or synthetic scaffolds still require improvement to yield better biocompatibility and functional properties for cartilage regeneration. Since natural cartilage tissues are nanometers in dimension and chondrocytes directly interact with (and create) nanostructured ECMs, the biomimetic features and excellent physiochemical properties of nanomaterials play a key role in stimulating chondrocyte growth as well as guiding cartilage tissue regeneration.156 Although it is a field in its infancy, many investigators are currently seeking to fabricate biomimetic nanostructured tissue engineering scaffolds encapsulating cells (such as progenitor cells and chondrocytes) for repairing and regenerating cartilage tissues.
Nanofibrous or nanoporous polymer matrices can be fabricated via electrospinning, particulate leaching, chemical etching, 3D printing techniques, and phase separation. For cartilage applications, there has been great interest in incorporating chondrocytes or stem cells into the 3D polymer or composite nanofibrous scaffolds through electrospinning.111,157,158 For example, in vitro chondrogenesis of bone marrow-derived MSCs was evaluated in an electrospun PCL nanofibrous scaffold and compared with an established cell pellet culture.157 The electrospun nanofibrous PCL scaffold effectively induced chondrogenic differentiation of MSCs and finally formed a tissue engineered construct with plentiful cartilaginous matrices. In addition, the easily fabricated and modified nanofibers possessed much better mechanical properties compared to the cell pellets, and thus the electrospun nanofibrous PCL scaffold presented itself as an ideal candidate for stem cell transplantation during clinical cartilage repair. Because the small pore sizes of nanofibers may inhibit cell infiltration, uneven cell distributions may occur throughout the electrospun nanofibrous scaffolds. Therefore, a recent study improved chondrocyte seeding technology and created a more homogeneous cell–PLLA nanofiber composite.158 It was observed that chondrocytes were uniformly present throughout the entire cell-nanofiber composite, and the scaffold developed into a smooth, cartilage-like tissue with more total collagen and improved mechanical properties in a dynamic bioreactor relative to one obtained in static culture. Moreover, another study observed significantly increased chondrocyte functions (adhesion, proliferation and matrix synthesis) on 3D nanostructured PLGA created via chemical etching.159
Besides research effort of pursuing optimum cytocompatibility properties of the above mentioned biomaterial scaffolds, mechanical characteristics of biomaterials are another critical consideration for designing cartilage tissue engineering scaffolds. Due to the different tissue loading environments, different natural or synthetic scaffolds should be chosen to provide appropriate mechanical properties for cell adhesion and tissue regeneration. For example, metal, ceramics or ceramic reinforced polymer composites with robust mechanical properties have been used for bone repair, which requires more rigorous mechanical loading.156 Since articular cartilage is under continuously excessive loading environments, the mechanical mismatch between implanted scaffolds and surrounding tissues may frequently deteriorate cartilage regeneration at defect sites and then lead to implant failure. Thus, biomaterial scaffolds with both superior biocompatibility and suitable mechanical properties similar to cartilage are desirable for articular cartilage tissue engineering.
IV.D. Scaffold-free Cartilage Tissue Constructs
1. Scaffold-free Methods
Aside from using scaffolds, several scaffold-free techniques for generating neocartilage have been investigated including organ, pellet, aggregate cultures, and, more recently, a self-assembling process.160–167 These techniques do not employ exogenous materials at all and were initially used to study chondrocyte phenotype, metabolism, development, and disease. Within these methods, the state of the art in chondrocyte culture by the late ‘80s was severely limited by diffusion, and, with few exceptions,165 resulted in tissues less than 500 µm in size.165,168–170 Replacement cartilage would need to be of native articular cartilage thickness (1–3mm or thicker),17,171 and, as described below, recent techniques are capable of delivering constructs of similar size.
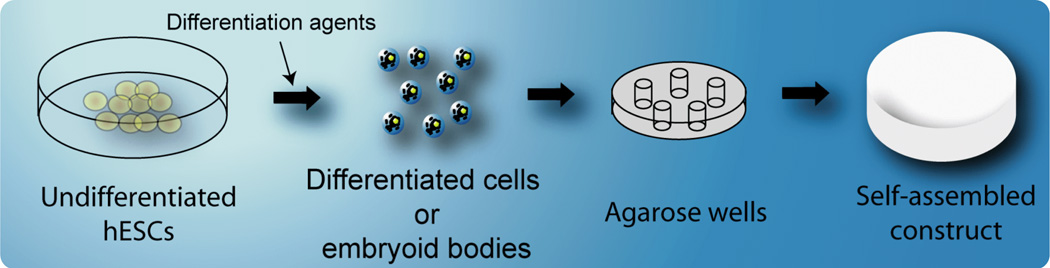
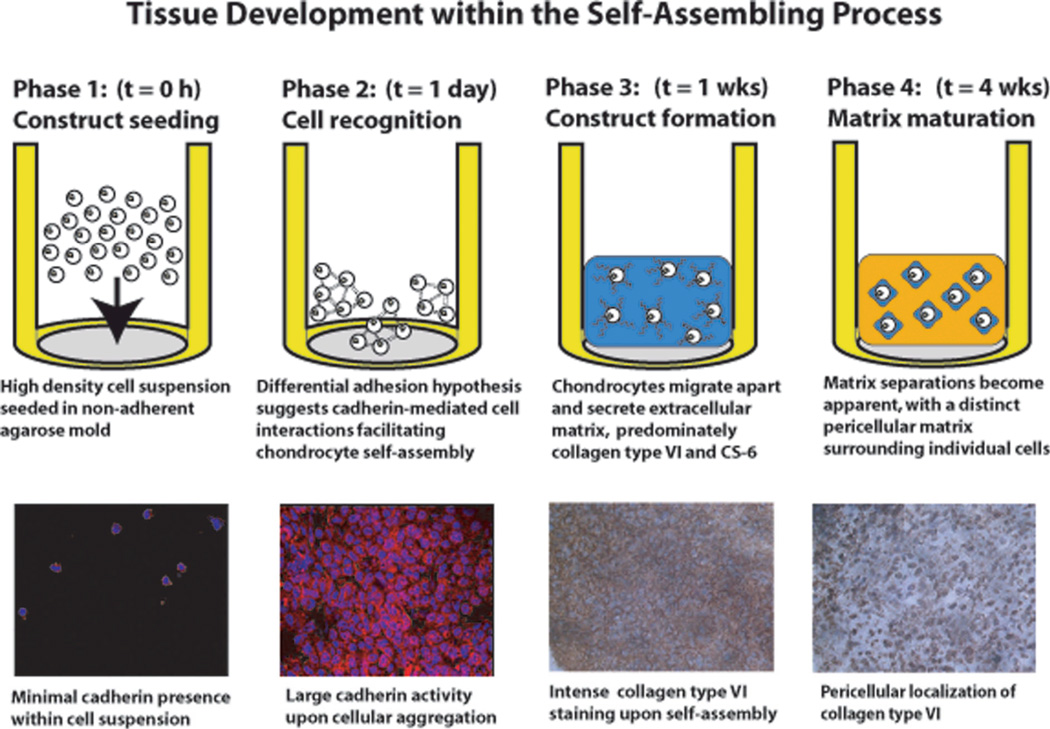
In the past few years, resurgence in scaffold-free culture has benefited from the knowledge developed using scaffold systems (e.g., growth factor and mechanical stimuli) to culminate in the production of thicker, more clinically relevant sized cartilage constructs that demonstrate functional characteristics.172–174 As an example, chondrocytes were seeded on (instead of into) a non-adhesive hydrogel mold in a process termed self-assembly.167 As the system minimized its free energy, the cells associate and coalesce to form neocartilage free of exogenous biomaterials and unaffected by adhesion to any surface other than each other (Figure 6).175 Several other forms of scaffoldless culture techniques exist. For example, high density culture of chondrocytes on tissue culture plastic, culture insert membranes, and silicon molds have been used to create constructs ranging from 0.5 mm to 2.9 mm in thickness.176 Some scaffoldless constructs have also been examined in vivo.177,178
Fig 6.
Chondrocyte self-assembly and ECM development in a scaffold-free process. Sequences of cadherin and collagen VI expression and distribution parallel those seen in native cartilage development. (Used under the Creative Commons Attribution License, from Ofek et al., PloS ONE, 2008175)
For scaffold-free constructs formed on an adhesive surface, the aggregate modulus values of engineered constructs have been reported at 41.6 kPa at 8 wks.176 In contrast, when chondrocytes self-assemble over an agarose gel (of 98% water), cell-biomaterial interactions are greatly reduced; engineered cartilage formed thusly has been shown to reach aggregate modulus values of over 150 kPa.179 The differences in the properties observed may be due to whether seeding occurs onto an adhesive or non-adhesive biomaterial. Surfaces where proteins may adsorb to allow for cell attachment or spreading can alter cartilage tissue formation, which has been shown to be mediated by cadherin175,180,181 and integrin binding.182–184 These recent observations serve to explain why, in a comparison between chondrocytes self-assembled over an adhesive TCP surface versus over agarose, the spreading and attachment onto TCP resulted in constructs with a corrugated appearance and significantly lower mechanical properties.167
Other scaffold-free methods include aggregate165,168–170 and pellet culture.185–189 In these cases, mechanical forces are generally present during construct formation. Aggregates are often formed by orbital shaker culture, and pellet cultures are formed under centrifugal forces. Shear forces present in orbital shaker culture can be detrimental to chondrocyte culture.190,191 Driven to quickly form constructs via centrifugation, pellet culture not only applies forces during construct formation, but may also alter cell-cell interactions that characterize other methods of tissue formation more akin to cartilage development.175,180–184,192 Rather than using centrifugation or rotational culture to form aggregates, chondrocyte self-assembly allows for minimization of free energy by only allowing cell-cell interactions, e.g., N-cadherin binding; in morphogenesis this is described as the Differential Adhesion Hypothesis.192–194
2. Advantages of Scaffold-free Culture
Scaffold-free cultures offer certain advantages over scaffold use. Morphological change, brought on by spreading, has been strongly linked to chondrocyte dedifferentiation via cytoskeletal changes.195,196 In retrospect, it is now known that diminished levels of collagen II and superficial zone protein (SZP) expression seen immediately upon monolayer culture due to chondrocyte dedifferentiation are unrecoverable.72 Similar to monolayer culture, cells seeded onto polymer biomaterials spread,197–200 and thus may dedifferentiate.
Though chondrocytes are allowed to retain their spherical morphology, gel encapsulation can limit cell-cell communication188,201 to inhibit ECM synthesis. This is in contrast to the high cadherin and integrin activity, shown to be active during cartilage development, observed during scaffold-free culture.175,182
Additionally, as chondrocytes are highly mechanosensitive, scaffold materials may result in stress shielding that limits beneficial mechanotransduction.202 Finally, as with any implanted biomaterial, there are concerns regarding potential toxicity of degradation byproducts and immune responses.203
IV.E. Growth Factors and Mechanical Stimuli for Improving Cartilage Tissue Repair and Regeneration
1. Growth Factors
The hormonal and growth factor regulation of chondrocyte aggregation,204 adhesion,205 growth,206 and metabolism207 have been investigated since the late ‘60s and ‘70s. Cartilage tissue engineering using growth factors coincided with the development of scaffold materials in the ‘90s. From this point, the literature can be separated into two categories: techniques to incorporate growth factors into biomaterials and the effects of growth factors on chondrocytes. For the former, specific techniques on retaining activity, controlled release, and other parameters particular to different biomaterials constitute a major area in materials research. As the technologies applicable to different scaffold systems are unique in themselves, it is impossible to present a comprehensive overview (the reader is instead directed to a recently published textbook that elaborates in depth on this subject).71 Nonetheless, the development of techniques that combine growth factors with scaffolds remains an active and populous area of research. For the latter, research in cartilage tissue engineering has reached a consensus via progress from the ‘90s and beyond that growth factors are beneficial in improving functional properties. IGF-I, basic fibroblast growth factor (bFGF), hepatocyte growth factor (HGF), and platelet-derived growth factor (PDGF) and the TGF-β superfamily are some of the most actively researched growth factors today.
Various growth factors that have shown to have effects on proliferation, differentiation, and synthesis include IGF-I, bFGF, HGF, and PDGF.208 IGF-I has been shown to mitigate injurious response of impacted cartilage by limiting the loss of matrix components.209 This may be due to IGF-I reducing apoptosis caused by a disruption of the collagen network.210 Applied to chondrocytes, IGF-I has been shown to increase collagen and proteoglycan deposition,211 though their effects are different across cartilage zones.212 Research on HGF has been limited as it is now considered to be ineffective toward chondrogenesis.213 PDGF enhances chondrocyte migration214 and increases SZP expression.215 However, it also changes chondrocyte morphology to a spindle-like shape,216 which, as shown in other studies, detracts from the chondrogenic phenotype.196 Because of this, PDGF has been used in fibrocartilage tissue engineering, though its effects have been limited.217 Aside from these growth factors, the most dramatic results have been seen with members of the TGF-β superfamily. Involved in repair and inflammation,218 the TGF-β superfamily contains several isoforms, such as TGF-β1 and TGF-β3. These have been shown to promote collagen formation and increase construct wet weight.219,220 Scaffold-free constructs stimulated using TGF-β1 showed approximately 1-fold increases in both aggregate modulus and tensile modulus over controls.221 Both TGF-β1 and TGF-β3 have been employed in combination with mechanical stimuli, to be discussed later.86,173,222,223 BMPs are also part of the TGF-β superfamily. These growth factors influence endochondral bone formation, proliferation, matrix synthesis, and defect healing in vivo.218,224 Increased matrix (e.g., proteoglycans and collagen) synthesis, and cell proliferation have been demonstrated using BMP-2,221,224–226 BMP-4,224,227 BMP-7,224,228 BMP-12, and BMP-13.225 Of these, BMP-1 and -2 have been shown to be particularly beneficial over other growth factors (e.g., other BMPs225 or TGF-β1229). Like TGF-β, BMPs have also been investigated in conjunction with mechanical forces.173,230
2. Mechanical Stimuli
In vivo, the synovial fluid reduces friction of articulating surfaces, and tissue shear is minimal. Shear has nonetheless been examined as a tool to induce matrix production. Oftentimes, the application of shear requires that a small amount of compressive strain be applied to maintain contact between the two surfaces.231–233 Dynamic shear of 1–3% at 0.01–1 Hz has been shown to increase ECM synthesis.233 Within this range (dynamic shear of 2% at 1 Hz) it has been shown that a 6-fold higher equilibrium modulus in constructs compared to unstimulated controls can be achieved with just a short (6 minutes every other day) application of this stimulus.234 Shear has also been shown to increase cartilage oligomeric matrix protein expression.235 Shear, applied onto chondrocyte monolayers using a cone viscometer at 1.6 Pa, resulted in a 10- to 20-fold increase in prostaglandin E2 release and 9-fold increase in tissue inhibitor of metalloproteinase mRNA,236 and shear also increases interleukin-6 and nitric oxide levels.191,237 These proinflammatory mediators and signs that are observed in osteoarthritis have indicated to some researchers that a shear force may not be the best stimulus for cartilage tissue engineering. Since a method to increase diffusion is via fluid flow, efforts have thus been directed toward reducing shear in systems that attempt to employ fluid flow in increasing nutrient and waste transfer.238–242 Rotating wall bioreactors are capable of applying shear on the order of ~0.15 Pa,238 as compared to the 1.6 Pa shown to increase proinflammatory mediators.236 Rotating wall bioreactors have been shown to increase GAG content within engineered constructs beyond physiological levels, while maintaining collagen levels.240
Direct compression, as applied to tissue engineered constructs, has been mostly dynamic, as native cartilage has been shown to respond negatively to static loading.142,243–247 As previously described, compressive loading has been suspected to increase solute transport248 in addition to mechanically stimulating the cells.245 For this reason, it may not be the static force that is causing decreased synthesis, but the lack of diffusion under static compression conditions. It has also been shown that static compression causes a decrease in the pH of the local environment,245 which may also inhibit synthesis. As with other forms of mechanical stimuli, the main dynamic compression parameters that have been optimized over the past decade are frequency, the duty cycle, the strain or force used, and the duration of the experiment. Over the past 15 years, frequencies ranging from 0.0001 to 3 Hz, strains from 0.1 to 25%, loads from 0.1 to 24 MPa, and durations lasting hours to weeks have been examined at various duty cycles and waveforms.87,222,243,246,249–257 Many of these experiments were performed on mature native tissue, though the developing environment in engineered constructs can be vastly different. For instance, it is well known that the pericellular environment serves a unique role in mechanotransduction,25,37 and this environment is rapidly changing during culture. The effects of ECM or scaffold stress-shielding thus become an apparent obstacle in comparing direct compression studies for tissue engineering. Nonetheless, certain results have been shown across several systems. Dynamic compression at 1 Hz or lower and 10% or lower have typically shown beneficial effects in agarose,243,250 poly(L-lactide-co-epsilon-caprolactone),256 and other scaffolds.257 Particularly, since the local microenvironment is so important in mechanotransduction, it would be interesting to examine the effects of nanomaterials on mechanotransduction. This has been investigated, preliminarily, with shear systems (though the effects of shear itself were not quantified in conjunction with the nanomaterial).158 However, the combination of nanomaterials with direct compression remains an open field with respect to direct compression.
As described previously, fluid flow out of cartilage is inhibited by the dense matrix and Donnan osmotic pressure. During compression, this inhibition results in elevated hydrostatic pressures. Physiological levels of hydrostatic pressure have been determined to be 7–10 MPa,258,259 and tissue engineering studies have employed magnitudes within this range as well as hypo- and hyper-physiological forces. Constant hydrostatic pressure applied for long periods has been shown to have a negative impact on matrix secretions and cell viability.260–262 Above the physiological range, static pressure at 30 MPa in chondrocyte monolayers inhibited proteoglycan synthesis.261 Low frequencies of hydrostatic pressure (akin to a static application) have also been shown to similarly deter synthesis in isolated cells and explants in physiological and hypo-physiological magnitudes, but the same study also showed that the ECM may alter cellular perception of hydrostatic pressure.260 From these studies, it was initially presumed that static hydrostatic pressure would not be useful in engineering cartilage, but recent studies have proven otherwise. Application of hydrostatic pressure has thus far resulted in tissue engineered constructs with aggregate modulus values approaching 300 kPa,172 and its combination with growth factors has shown both additive and synergistic effects in improving construct properties.173 In this case, the developing, scaffoldless construct contained rounded cells (contrasted with monolayers261) and immature ECM that is distinctly different from the previously examined cartilage explants.260 Aside from static pressure, a window of pressures and frequencies between 0.1 and 15 MPa and 0.05 and 1 Hz have been shown to yield positive results toward cartilage tissue engineering.263–269
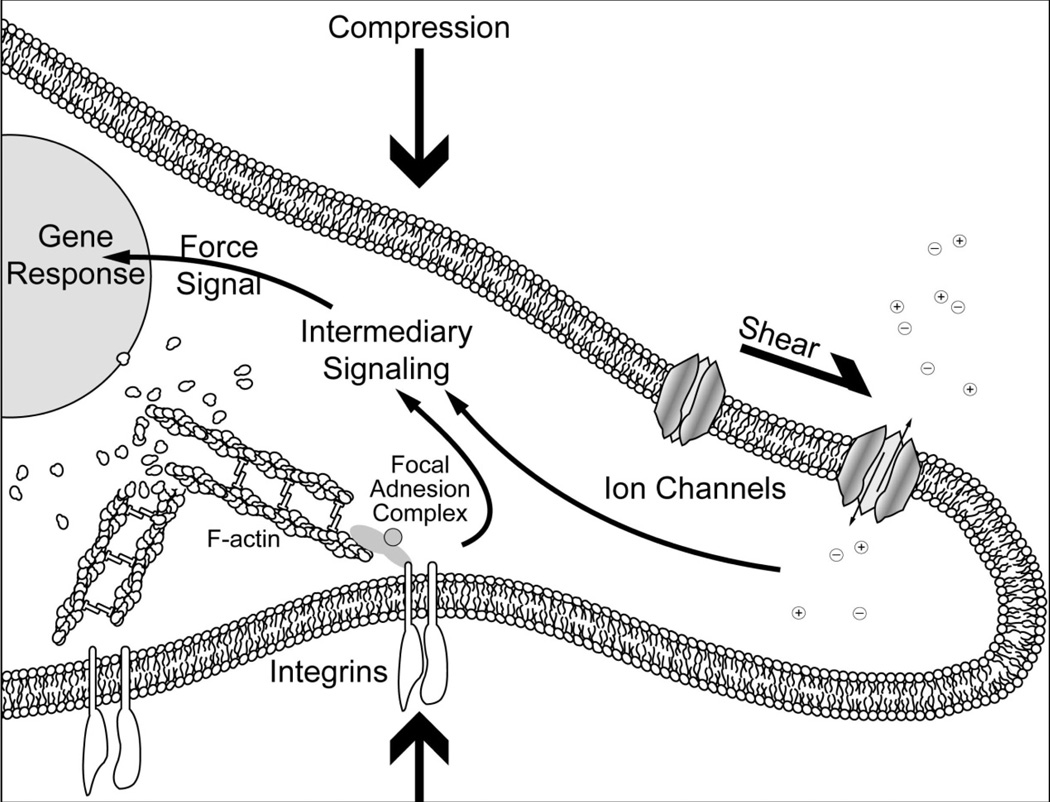
In addition, mechanotransduction has been well examined as a tool in tissue engineering, as metabolic responses to mechanical forces can precipitate via several coupling mechanisms (Figure 7). For instance, membrane deformation due to direct compression or shear can result in the activation of mechanosensitive ion channels.270,271 Hydrostatic pressure-sensitive changes to intracellular ion concentration have also been observed in chondrocytes.272 These changes in intracellular ionic concentrations can activate or suppress various genetic responses. Mechanical forces can also be coupled via integrins and the cytoskeleton. Tethering to the mechanical environment, activated integrins initiate the formation of a focal adhesion complex (FAC). The FAC is formed by recruiting not only other integrins, but also adaptor proteins and several kinases, and these proceed to activate or suppress genes and transcription factors.273
Fig 7.
Cells can respond to mechanical forces via several coupling mechanisms, such as stretch activated ion channels and focal adhesion complexes.
3. Combinations of Stimuli
BMP-2 application with IGF-I have been shown to increase the functional properties of engineered constructs.174 In this case, continuous versus intermittent growth factor treatments were compared, along with the combination of two and three growth factors (BMP-2, TGF-β1, and IGF-1). Neither synergistic nor additive response was observed, and the authors pointed to prior results showing that BMP-2 signal transduction can be inhibited by TGF-β1.274 TGF-β alone is known to cross-talk with the mitogen-activated protein kinase (MAPK), P13K/Akt, Wnt, and various other pathways.275 Application of multiple growth factors continue to be an intense area of research for cartilage tissue engineering.
Growth factors have been examined with shear, compression, and hydrostatic pressure. For instance, shear and increased diffusion combined with BMP-2 induced chondrogenic gene and protein expression when dedifferentiated chondrocytes were cultured.276 It was also deduced in a separate study that shear, when combined with IGF-I, produce a synergistic effect on chondrogenic synthesis.232 IGF-I also combined with direct compression to increase proteoglycan and collagen synthesis by 180% and 290%, respectively.246 TGF-β3 combined with direct compression223 and TGF-β1 with hydrostatic pressure173 both increased construct functional properties to native tissue ranges. In the latter case, the growth factor and mechanical stimulus were found to have additive effects on functional properties but synergistic effects on collagen content.173
V. CHALLENGES AND FUTURE DIRECTIONS
1. Technical
a. Design Specifications
Cartilage regeneration aided by tissue engineering faces varied challenges, and among them are differences in anatomical geometries and loads for different joints. Large, weight bearing joints require the replacement cartilage to have great compressive stiffness. The patellofemoral problem requires that a high shear-bearing construct be fitted. Clinical intervention in small, non-weight bearing joints suffer from the challenges of tight joint spaces and small radii of curvature. Added to these considerations is the fact that differences in integration have yet to be evaluated across different joints.
Actually, while cartilage must be engineered for functionality, a resultant engineered construct dense in collagen fibers will prove difficult to integrate. An optimal maturity for engineered constructs that balances construct stiffness with ease of integration has yet to be determined. In addition, integration techniques must be developed to ensure that the interface between engineered and native cartilages will not have stress concentrations. Along these lines, new surgical techniques may need to be developed to implant engineered cartilage.
It is difficult to design scaffolds while the design specifications for cartilage therapy are still evolving. Due to the integration issue, it is unclear whether a cartilage product should be designed to be mature (stiff but difficult to integrate) or immature (possibly easier to integrate, but with lower weight bearing capacity). Without clear design standards, the design process for scaffolds may be unclear. For instance, a parameter in scaffold design, degradation rate, will greatly influence the maturation and integrative properties of a resulting construct. It is recommended that, as researchers develop new biomaterials for cartilage engineering, they should also consider how an engineered product using such technology will be put to clinical use.
b. Stimuli interactions
Currently, the pressing questions that drive the area of multiple growth factor use are how best to employ them in sequence and in combination. The interplay of growth factors presented to chondrocytes (or progenitor cells) during cartilage formation is varied and delicate. If sequenced stimuli were to be necessary, controlled release of multiple growth factors at different sequences can be challenging in scaffold development.
Likewise, current challenges in the application of mechanical stimuli are 1) to systematically combine several mechanical stimuli at once, and 2) to investigate the coupled effects of different classes of stimuli (e.g., growth factor with mechanical stimuli). The roles that growth factors play in altering mechanotransduction constitute an area of intense interest and challenge. Past studies that have combined the two are exciting in their abilities to increase construct functional properties. Some studies have even observed synergism for these different classes of stimuli.173,232 How such synergism came about, why synergism is not seen for all properties, and, finally, what one should do in order to obtain additional synergistic effects are both interesting and perplexing questions. These must first be addressed before exogenous stimuli can be efficiently applied for cartilage tissue engineering.
Lastly, cartilage tissue engineering studies thus far have focused on anabolic processes, i.e., with creating more cartilage matrix. Recent studies using chondroitinase-ABC (C-ABC) have shown that increased functional properties can be obtained with the selective and timed application of a catabolic enzyme.277,278 This counter-intuitive method is proof that matrix turnover in engineered constructs should be examined. Thus, catabolic processes can be elucidated and harnessed in producing functional constructs.
2. Regulatory
The progress in cartilage tissue engineering during the past two decades is rapid and humbling. As with other rapidly maturing technologies, standards and regulation have not caught up with the advancements seen in this field. Clearance by the Food and Drug Administration (FDA) now poses as a significant hurdle for companies seeking to translate cartilage engineering technologies to clinical use. The different Centers of the FDA that are relevant to cartilage tissue engineering and potential pathways to market are described below.
a. Structure of the FDA
Of the seven product-oriented centers within the FDA, two are particularly relevant to articular cartilage tissue engineering: the Center for Biologics Evaluation and Research (CBER) and the Center for Devices and Radiological Health (CDRH). For cartilage tissue engineering, a product that is a combination of both biological product and a device will be assigned by The Office of Combination Products to one of the two centers, where primary jurisdiction over the product will reside.
The CBER regulates products whose primary mode of action is metabolic. Past products regulated by this center include blood, allergenics, tissues, and other cellular products derived from living sources. Manufacturers of biological products must follow current good manufacturing practices (cGMP), as described by 21 Code of Federal Regulations (CFR) Part 211279 and report adverse events to the Adverse Event Reporting System (AERS). Engineered cartilages derived from autologous, allogeneic, and xenogeneic products are produced by cells and will be regulated by CBER if their primary mode of the effects is metabolic.
For implants, the FDA has had a history of classifying most orthopaedic implants as medical devices, which are regulated by the CDRH. The CDRH regulates firms that manufacture, repackage, relabel, and/or import medical devices. A medical device is defined as “an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including any component, part, or accessory, which is intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease.”280 In these cases, the primary mode of effect for devices is mechanical or electrical.
b. Center Assignment
Potential cartilage therapies can be assigned to CBER or CDRH, depending on the therapy’s primary mode of action. Assignment to different centers translates to different filing requirements, which result in different financial burdens and time to market.
CBER oversaw the approval of ACI, whose primary mode of action is the metabolic production of cartilage matrix after implantation. The metabolic agents are manipulated, expanded autologous cells. In this case, a Biologics License Application (BLA) was filed for the permission to introduce a biologic product into the market, and the BLA is regulated under 21 CFR 600 to 680. Other requirements include complying with requirements set forth by Form 356h, which includes the applicant information, product and manufacturing information, pre-clinical studies, clinical studies, and labeling. For clearance by the CBER, clinical studies, which can be slow and costly, are required, and these are quire burdensome for companies that seek to introduce an engineered cartilage product whose primary mode of effect is metabolic.
Implants, whose primary function is to bear mechanical load (as tissue engineered cartilage is designed to function), may have CDRH as its primary regulator. Contrast this with the case of ACI: while ACI may eventually result in tissue that bears load, the initial implant does not act through a mechanical effect; instead, a metabolic process takes place where tissues are formed in situ. Another example is Medtronic’s InFuse Bone Graft/LT-Cage, which is regulated as a medical device, despite containing recombinant human BMP (rhBMP). In this case, the primary mode of action for the product is mechanical. Other products regulated as devices include bone void fillers and demineralized bone matrix, both of which can be biologically derived, but serve mechanical functions during implantation.
Researchers and companies should take into consideration the Center assignment as they develop cartilage therapies, because the pathways to clinical usage are substantially different for each Center. Furthermore, if a company would like to be regulated under CDRH, it should have a plan for which class a product falls into and project the time and financial burdens from there. It is expected that tissue engineered cartilage implants would be examined by either CBER or CDRH. Center assignment is both an industrial and a scientific question. While the industrial aspect is obvious, scientists should likewise be cognizant of how safety and efficacy are demonstrated in designing their studies.
c. Device Classes
Orthopaedic device manufacturing companies, where tissue engineered cartilage therapies are likely to arise, are typically familiar with the CDRH. Devices regulated within the CDRH fall into three classes, each with different requirements that the manufacturer must fulfill prior to introducing a product to market. Again, the relevance to tissue engineering is that the time and money spent under each class differs and will affect whether and how long a therapy takes to reach the clinic.
Class I devices are low risk and pose minimal potential harm. For a Class I device to be approved, a company must demonstrate that it has implemented “general controls,” which include quality system regulation (QSR), as described by 21 CFR 820,281 to ensure adherence to predefined design controls and good manufacturing practices (GMP), label requirements to prevent product mislabeling, and the use of Medical Device Reporting (MDR) (in contrast with the AERS for biological products) to maintain records for the reporting of adverse events. Medical devices must use forms FDA-2891 and FDA-2892 for establishment registration and medical device listing.
Tissue engineered cartilage is more likely to be regulated as Class II or Class III devices. Class II devices are of moderate risk and often require a Premarket Notification 510(k) pathway to market. A manufacturer must notify the FDA 90 days before marketing a Class II device to show that it is substantially equivalent to a predicate device legally in commercial distribution in the US before May 28, 1976. Either a traditional, special, or abbreviated 510(k) may be filed. A traditional 510(k) takes about 90 days to review. When a device is modified without changes to the intended use, a special 510(k) can be filed, which generally takes 30 days. An abbreviated 510(k) relies on use of guidance documents or special controls to provide a summary report that describes adherence to the relevant guidance document, and can also take less than 90 days. Class II devices may require special controls, such as postmarket surveillance, patient registries, guidances, and standards, after it has been marketed. Most joint arthroplasty components are cleared as Class II devices.
Devices that support or sustain human life, are of substantial importance in preventing impairment of human health, or present a potential, unreasonable risk of illness or injury are classified as Class III. Particularly relevant to tissue engineering, devices for which substantially equivalent predicates are non-existent also fall into this class. New device that are deemed to be substantially equivalent to a predicate Class III will also be classified as Class III. Finally, new devices determined to be substantially equivalent to Class I or II devices that were developed after 1976 will also be classified as Class III. Before legal distribution can occur, a company must submit a premarket approval application (PMA) or Product Development Protocol (PDP). Both preclinical and clinical data are needed to demonstrate safety and efficacy. A new device must first have an investigational device exemption (IDE) (see 21 CFR 812282) before it can be used in humans to collect clinical data. A company may petition to have a new device reclassified to Class I or Class II.
Additional pathways to market include the Humanitarian Device Exemption (HDE), which is intended for the development of devices to treat rare (<4,000 patients per year) conditions, and the PDP (for Class III devices). An alternative to the PMA, companies seeking to purse the PDP pathway, will work with the FDA in designing preclinical and clinical studies, protocols, assessment methods, and acceptance criteria.
VI. CONCLUSIONS
In summary, there is a great promise to advance current cartilage therapies toward achieving a consistently successful approach for addressing cartilage afflictions. Tissue engineering may be the best way to reach this objective via the use of promising cell sources such as stem cells, novel biologically inspired scaffolds or scaffoldless approaches, emerging nanotechnology, chondrogenic factors, and physical stimuli. Undoubtedly, there are challenges and a significant number of unanswered questions about cartilage pathophysiology that may hinder the progress. Furthermore, the regulatory pathways that future cartilage therapies may need to follow are still unfolding. Nevertheless, significant evidence exists now supporting the idea that tissue engineered articular cartilage represents a potentially cogent approach to effectively treat cartilage injury or trauma.
VII. ACKNOWLEDGEMENTS
The authors acknowledge support from the National Institutes of Health grants NIH R01 DE015038, NIH R01 AR053286, and NIH R01 AR047839, and from the Arthritis Foundation. The authors would also like to thank Justin Chen for his assistance during manuscript preparation.
Contributor Information
Lijie Zhang, Email: lgzhang@ucdavis.edu.
Jerry Hu, Email: jcyhu@ucdavis.edu.
IX. References
- 1.CDC.org. Atlanta: Centers for Disease Control and Prevention; 2009. [cited 2009 August 15th]. [homepage on the Internet] Available from http://www.cdc.gov/ [Google Scholar]
- 2.Romanelli D, Watanabe DS, Mand BR. Articular cartilage injuries. Rosemont: American Orthopaedic Society for Sports Medicine; 2008. [updated 2008; cited 2009 August 15th]. Available from http://www.sportsmed.org/tabs/patienteducation/SportsTipDetails.aspx?DID=310. [Google Scholar]
- 3.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008 Jan;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.CDC.org. Atlanta: National Center for Chronic Disease Prevention and Health Promotion; 2009. [cited 2009 September 1st]. [homepage on the Internet] Available from http://www.cdc.gov/arthritis/arthritis/osteoarthritis.html. [Google Scholar]
- 5.AAOS.org. The Burden of Musculoskeletal Diseases in the United States. Rosemont: American Academy of Orthopaedic Surgeons; 2009. [monograph on the Internet] Available from http://www.boneandjointburden.org. [Google Scholar]
- 6.Cartilage.org. Zürich: International Cartilage Repair Society; 2009. [cited 2009 September 1st]. [homepage on the Internet] Available from http://www.cartilage.org/index.php?pid=22. [Google Scholar]
- 7.Responte DJ, Natoli RM, Athanasiou KA. Collagens of articular cartilage: structure, function, and importance in tissue engineering. Crit Rev Biomed Eng. 2007;35(5):363–411. doi: 10.1615/critrevbiomedeng.v35.i5.20. [DOI] [PubMed] [Google Scholar]
- 8.Ateshian GA, Warden WH, Kim JJ, Grelsamer RP, Mow VC. Finite deformation biphasic material properties of bovine articular cartilage from confined compression experiments. J Biomech. 1997 Nov–Dec;30(11–12):1157–1164. doi: 10.1016/s0021-9290(97)85606-0. [DOI] [PubMed] [Google Scholar]
- 9.Vasita R, Katti DS. Nanofibers and their applications in tissue engineering. Int J Nanomedicine. 2006;1(1):15–30. doi: 10.2147/nano.2006.1.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reddi AH. Role of morphogenetic proteins in skeletal tissue engineering and regeneration. Nat Biotechnol. 1998 Mar;16(3):247–252. doi: 10.1038/nbt0398-247. [DOI] [PubMed] [Google Scholar]
- 11.Temenoff JS, Mikos AG. Review: tissue engineering for regeneration of articular cartilage. Biomaterials. 2000 Mar;21(5):431–440. doi: 10.1016/s0142-9612(99)00213-6. [DOI] [PubMed] [Google Scholar]
- 12.Clair BL, Johnson AR, Howard T. Cartilage Repair: Current and Emerging Options in Treatment Foot & Ankle Specialist. 2009;2(4):179–188. doi: 10.1177/1938640009342272. [DOI] [PubMed] [Google Scholar]
- 13.O'Driscoll SW. The healing and regeneration of articular cartilage. J Bone Joint Surg Am. 1998 Dec;80(12):1795–1812. [PubMed] [Google Scholar]
- 14.Zhang L, Sirivisoot S, Balasundaram G, Webster TJ. In: Nanoengineering for Bone Tissue Engineering. Khademhosseini A, Borenstein J, Toner M, Takayama S, editors. Micro and Nanoengineering of the Cell Microenvironment: Technologies and Applications: Artech House; 2008. pp. 431–460. [Google Scholar]
- 15.Rubash HE, Berry J. Revisions of hip and knee replacements in Canada. Canadian Joint Replacement Registry Analytic Bulletin: Canadian Institute for Health Information. 2004;603:1–20. [Google Scholar]
- 16.Eckstein F, Reiser M, Englmeier KH, Putz R. In vivo morphometry and functional analysis of human articular cartilage with quantitative magnetic resonance imaging--from image to data, from data to theory. Anat Embryol (Berl) 2001 Mar;203(3):147–173. doi: 10.1007/s004290000154. [DOI] [PubMed] [Google Scholar]
- 17.Athanasiou KA, Rosenwasser MP, Buckwalter JA, Malinin TI, Mow VC. Interspecies comparisons of in situ intrinsic mechanical properties of distal femoral cartilage. J Orthop Res. 1991 May;9(3):330–340. doi: 10.1002/jor.1100090304. [DOI] [PubMed] [Google Scholar]
- 18.Setton LA, Elliott DM, Mow VC. Altered mechanics of cartilage with osteoarthritis: human osteoarthritis and an experimental model of joint degeneration. Osteoarthritis Cartilage. 1999 Jan;7(1):2–14. doi: 10.1053/joca.1998.0170. [DOI] [PubMed] [Google Scholar]
- 19.Buckwalter JA, Hunziker EB, Rosenberg LC, Coutts R, Adams M, Eyre D. Articular cartilage: composition and structure. In: Woo SL-Y, Buckwalter JA, editors. Injury and Repair of the Musculoskeletal Soft Tissues. Park Ridge: American Academy of Orthopaedic Surgeons; 1991. pp. 405–425. [Google Scholar]
- 20.Parsons J. Cartilage. In: Black J, Hastings G, editors. Handbook of Biomaterial Properties. Springer - Verlag; 1998. pp. 40–47. [Google Scholar]
- 21.Ethier CR, Simmons CA. Introductory Biomechanics - From Cells to Organisms. Cambridge University Press; 2007. [Google Scholar]
- 22.Ofek G, Athanasiou KA. Micromechanical properties of chondrocytes and chondrons: relevance to articular cartilage tissue engineering. Journal of Mechanics of Materials and Structures. 2007;2(6):1059–86. [Google Scholar]
- 23.Eyre DR, Weis MA, Wu JJ. Articular cartilage collagen: an irreplaceable framework? Eur Cell Mater. 2006;12:57–63. doi: 10.22203/ecm.v012a07. [DOI] [PubMed] [Google Scholar]
- 24.Poole CA, Ayad S, Schofield JR. Chondrons from articular cartilage: I.Immunolocalization of type VI collagen in the pericellular capsule of isolated canine tibial chondrons. J Cell Sci. 1988;90(Pt 4):635–643. doi: 10.1242/jcs.90.4.635. [DOI] [PubMed] [Google Scholar]
- 25.Guilak F, Alexopoulos LG, Upton ML, Youn I, Choi JB, Cao L, et al. The pericellular matrix as a transducer of biomechanical and biochemical signals in articular cartilage. Annals of the New York Academy of Sciences. 2006;1068:498–512. doi: 10.1196/annals.1346.011. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz MH, Leo PH, Lewis JL. A microstructural model for the elastic response of articular cartilage. J Biomech. 1994 Jul;27(7):865–873. doi: 10.1016/0021-9290(94)90259-3. [DOI] [PubMed] [Google Scholar]
- 27.Kovach IS, Athanasiou KA. Small-angle HeNe laser light scatter and the compressive modulus of articular cartilage. J Orthop Res. 1997 May;15(3):437–441. doi: 10.1002/jor.1100150317. [DOI] [PubMed] [Google Scholar]
- 28.Kovach IS. A molecular theory of cartilage viscoelasticity. Biophys Chem. 1996 Mar 7;59(1–2):61–73. doi: 10.1016/0301-4622(95)00115-8. [DOI] [PubMed] [Google Scholar]
- 29.Hu J. Chondrocyte self-assembly and culture in bioreactors [Ph.D. Thesis] Houston: Rice University; 2005. [Google Scholar]
- 30.Sun DDN, Leong KW. Functional engineering of load-supporting soft tissues. In: Milne I, Ritchie RO, Karihaloo B, editors. Comprehensive Structural Integrity. Elsevier; 2003. pp. 97–113. [Google Scholar]
- 31.Lai WM, Mow VC, Zhu W. Constitutive modeling of articular cartilage and biomacromolecular solutions. J Biomech Eng. 1993 Nov;115(4B):474–480. doi: 10.1115/1.2895527. [DOI] [PubMed] [Google Scholar]
- 32.Buckwalter JA, Mankin HJ. Articular cartilage: tissue design and chondrocyte}matrix interactions. AAOS Inst Course Lect. 1998;47:477–486. [PubMed] [Google Scholar]
- 33.Fassbender HG. Articular cartilage and osteoarthrosis. Vienna: Hans Huber; 1983. [Google Scholar]
- 34.Wong M, Wuethrich P, Eggli P, Hunziker E. Zone-specific cell biosynthetic activity in mature bovine articular cartilage: a new method using confocal microscopic stereology and quantitative autoradiography. J Orthop Res. 1996 May;14(3):424–432. doi: 10.1002/jor.1100140313. [DOI] [PubMed] [Google Scholar]
- 35.Siczkowski M, Watt FM. Subpopulations of chondrocytes from different zones of pig articular cartilage. Isolation, growth and proteoglycan synthesis in culture. J Cell Sci. 1990 Oct;97(Pt 2):349–360. doi: 10.1242/jcs.97.2.349. [DOI] [PubMed] [Google Scholar]
- 36.Bhosale AM, Richardson JB. Articular cartilage: structure, injuries and review of management. Br Med Bull. 2008;87:77–95. doi: 10.1093/bmb/ldn025. [DOI] [PubMed] [Google Scholar]
- 37.Alexopoulos LG, Setton LA, Guilak F. The biomechanical role of the chondrocyte pericellular matrix in articular cartilage. Acta Biomater. 2005 May;1(3):317–325. doi: 10.1016/j.actbio.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 38.Moutos FT. Biomimetic Composite Scaffolds for the Functional Tissue Engineering of Articular Cartilage. Duke University; 2009. [Google Scholar]
- 39.Mow VC, Kuei SC, Lai WM, Armstrong CG. Biphasic creep and stress relaxation of articular cartilage in compression? Theory and experiments. J Biomech Eng. 1980 Feb;102(1):73–84. doi: 10.1115/1.3138202. [DOI] [PubMed] [Google Scholar]
- 40.Zhu W, Mow VC, Koob TJ, Eyre DR. Viscoelastic shear properties of articular cartilage and the effects of glycosidase treatments. J Orthop Res. 1993 Nov;11(6):771–781. doi: 10.1002/jor.1100110602. [DOI] [PubMed] [Google Scholar]
- 41.McCutchen CW. Joint lubrication. Bull Hosp Jt Dis Orthop Inst. 1983 Fall;43(2):118–129. [PubMed] [Google Scholar]
- 42.Akizuki S, Mow VC, Muller F, Pita JC, Howell DS, Manicourt DH. Tensile properties of human knee joint cartilage: I. Influence of ionic conditions, weight bearing, and fibrillation on the tensile modulus. J Orthop Res. 1986;4(4):379–392. doi: 10.1002/jor.1100040401. [DOI] [PubMed] [Google Scholar]
- 43.Shin D, Lin JH, Agrawal CM, Athanasiou K, editors. Zonal variations in microindentation properties of articular cartilage. Orthopaedic research society 44th annual meeting; 1998. [Google Scholar]
- 44.Schinagl RM, Gurskis D, Chen AC, Sah RL. Depth-dependent confined compression modulus of full-thickness bovine articular cartilage. J Orthop Res. 1997 Jul;15(4):499–506. doi: 10.1002/jor.1100150404. [DOI] [PubMed] [Google Scholar]
- 45.Athanasiou KA, Agarwal A, Dzida FJ. Comparative study of the intrinsic mechanical properties of the human acetabular and femoral head cartilage. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 1994 May;12(3):340–349. doi: 10.1002/jor.1100120306. [DOI] [PubMed] [Google Scholar]
- 46.Athanasiou KA, Liu GT, Lavery LA, Lanctot DR, Schenck RC., Jr Biomechanical topography of human articular cartilage in the first metatarsophalangeal joint. Clinical orthopaedics and related research. 1998;348:269–281. [PubMed] [Google Scholar]
- 47.Athanasiou KA, Niederauer GG, Schenck RC., Jr Biomechanical topography of human ankle cartilage. Annals of biomedical engineering. 1995 Sep–Oct;23(5):697–704. doi: 10.1007/BF02584467. [DOI] [PubMed] [Google Scholar]
- 48.Laurencin CT, Ambrosio AM, Borden MD, Cooper JA., Jr Tissue engineering: orthopedic applications. Annu Rev Biomed Eng. 1999;1:19–46. doi: 10.1146/annurev.bioeng.1.1.19. [DOI] [PubMed] [Google Scholar]
- 49.Hunziker EB. Articular cartilage repair: are the intrinsic biological constraints undermining this process insuperable? Osteoarthritis Cartilage. 1999 Jan;7(1):15–28. doi: 10.1053/joca.1998.0159. [DOI] [PubMed] [Google Scholar]
- 50.Shapiro F, Koide S, Glimcher MJ. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1993 Apr;75(4):532–553. doi: 10.2106/00004623-199304000-00009. [DOI] [PubMed] [Google Scholar]
- 51.Caplan AI, Elyaderani M, Mochizuki Y, Wakitani S, Goldberg VM. Principles of cartilage repair and regeneration. Clin Orthop Relat Res. 1997 Sep;(342):254–269. [PubMed] [Google Scholar]
- 52.Campbell CJ. The healing of cartilage defects. Clin Orthop Relat Res. 1969 May–Jun;64:45–63. [PubMed] [Google Scholar]
- 53.Buckwalter JA. Articular cartilage: injuries and potential for healing. J Orthop Sports Phys Ther. 1998 Oct;28(4):192–202. doi: 10.2519/jospt.1998.28.4.192. [DOI] [PubMed] [Google Scholar]
- 54.Bergman RJ, Gazit D, Kahn AJ, Gruber H, McDougall S, Hahn TJ. Age-related changes in osteogenic stem cells in mice. J Bone Miner Res. 1996 May;11(5):568–577. doi: 10.1002/jbmr.5650110504. [DOI] [PubMed] [Google Scholar]
- 55.Stolzing A, Scutt A. Age-related impairment of mesenchymal progenitor cell function. Aging Cell. 2006 Jun;5(3):213–224. doi: 10.1111/j.1474-9726.2006.00213.x. [DOI] [PubMed] [Google Scholar]
- 56.Goldberg VM, Caplan AI. Biological resurfacing: an alternative to total joint arthroplasty. Orthopedics. 1994 Sep;17(9):819–821. doi: 10.3928/0147-7447-19940901-25. [DOI] [PubMed] [Google Scholar]
- 57.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994 Oct 6;331(14):889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 58.Peterson L, Menche D, Grande D, Klein M, Burmester G, Pugh J, et al. Chondrocyte transplantation-an experimental model in the rabbit. Trans Orthop Res Soc. 1984;9:218. [Google Scholar]
- 59.Gikas PD, Bayliss L, Bentley G, Briggs TW. An overview of autologous chondrocyte implantation. J Bone Joint Surg Br. 2009 Aug;91(8):997–1006. doi: 10.1302/0301-620X.91B8.21824. [DOI] [PubMed] [Google Scholar]
- 60.Massachusetts: Genzyme Corporation; [cited 2009 October 14th]. Carticel.com. [homepage on the Internet] Available from http://www.carticel.com/patients/treatment.aspx. [Google Scholar]
- 61.Chiang H, Jiang CC. Repair of articular cartilage defects: review and perspectives. J Formos Med Assoc. 2009 Feb;108(2):87–101. doi: 10.1016/S0929-6646(09)60039-5. [DOI] [PubMed] [Google Scholar]
- 62.Bobacz K, Erlacher L, Smolen J, Soleiman A, Graninger WB. Chondrocyte number and proteoglycan synthesis in the aging and osteoarthritic human articular cartilage. Ann Rheum Dis. 2004 Dec;63(12):1618–1622. doi: 10.1136/ard.2002.002162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stockwell RA. The cell density of human articular and costal cartilage. J Anat. 1967 Sep;101(Pt 4):753–763. [PMC free article] [PubMed] [Google Scholar]
- 64.Revell CM, Athanasiou KA. Success rates and immunologic responses of autogenic, allogenic, and xenogenic treatments to repair articular cartilage defects. Tissue Eng Part B Rev. 2009 Mar;15(1):1–15. doi: 10.1089/ten.teb.2008.0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hangody L, Kish G, Karpati Z, Udvarhelyi I, Szigeti I, Bely M. Mosaicplasty for the treatment of articular cartilage defects: application in clinical practice. Orthopedics. 1998 Jul;21(7):751–756. doi: 10.3928/0147-7447-19980701-04. [DOI] [PubMed] [Google Scholar]
- 66.Hangody L, Vasarhelyi G, Hangody LR, Sukosd Z, Tibay G, Bartha L, et al. Autologous osteochondral grafting--technique and long-term results. Injury. 2008 Apr;39 Suppl 1:S32–S39. doi: 10.1016/j.injury.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 67.Solheim E, Hegna J, Oyen J, Austgulen OK, Harlem T, Strand T. Osteochondral autografting (mosaicplasty) in articular cartilage defects in the knee: Results at 5 to 9 years. Knee. 2009 Aug 8; doi: 10.1016/j.knee.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 68.Bell E. Tissue engineering: a perspective. Journal of cellular biochemistry. 1991 Mar;45(3):239–241. doi: 10.1002/jcb.240450302. [DOI] [PubMed] [Google Scholar]
- 69.Cima LG, Vacanti JP, Vacanti C, Ingber D, Mooney D, Langer R. Tissue engineering by cell transplantation using degradable polymer substrates. J Biomech Eng. 1991 May;113(2):143–151. doi: 10.1115/1.2891228. [DOI] [PubMed] [Google Scholar]
- 70.Langer R, Vacanti JP. Tissue engineering. Science. 1993 May 14;260(5110):920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 71.Athanasiou KA, Darling EM, Hu JC. In: Articular Cartilage Tissue Engineering. Athanasiou KA, editor. San Rafael: Morgan & Claypool; 2009. [Google Scholar]
- 72.Darling EM, Athanasiou KA. Rapid phenotypic changes in passaged articular chondrocyte subpopulations. J Orthop Res. 2005 Mar;23(2):425–432. doi: 10.1016/j.orthres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 73.Yu H, Grynpas M, Kandel RA. Composition of cartilagenous tissue with mineralized and non-mineralized zones formed in vitro. Biomaterials. 1997 Nov;18(21):1425–1431. doi: 10.1016/s0142-9612(97)00071-9. [DOI] [PubMed] [Google Scholar]
- 74.Aydelotte MB, Greenhill RR, Kuettner KE. Differences between sub-populations of cultured bovine articular chondrocytes. II. Proteoglycan metabolism. Connect Tissue Res. 1988;18(3):223–234. doi: 10.3109/03008208809016809. [DOI] [PubMed] [Google Scholar]
- 75.Aydelotte MB, Kuettner KE. Differences between sub-populations of cultured bovine articular chondrocytes. I. Morphology and cartilage matrix production. Connect Tissue Res. 1988;18(3):205–222. doi: 10.3109/03008208809016808. [DOI] [PubMed] [Google Scholar]
- 76.Richardson SM, Hoyland JA, Mobasheri R, Csaki C, Shakibaei M, Mobasheri A. Mesenchymal stem cells in regenerative medicine: Opportunities and challenges for articular cartilage and intervertebral disc tissue engineering. J Cell Physiol. 2009 Sep 1; doi: 10.1002/jcp.21915. [DOI] [PubMed] [Google Scholar]
- 77.Koga H, Engebretsen L, Brinchmann JE, Muneta T, Sekiya I. Mesenchymal stem cell-based therapy for cartilage repair: a review. Knee Surg Sports Traumatol Arthrosc. 2009 Mar 31; doi: 10.1007/s00167-009-0782-4. [DOI] [PubMed] [Google Scholar]
- 78.Johnstone B, Yoo J. Mesenchymal cell transfer for articular cartilage repair. Expert Opin Biol Ther. 2001 Nov;1(6):915–921. doi: 10.1517/14712598.1.6.915. [DOI] [PubMed] [Google Scholar]
- 79.van Osch GJ, Brittberg M, Dennis JE, Bastiaansen-Jenniskens YM, Erben RG, Konttinen YT, et al. Cartilage repair: past and future--lessons for regenerative medicine. J Cell Mol Med. 2009 May;13(5):792–810. doi: 10.1111/j.1582-4934.2009.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ashton BA, Allen TD, Howlett CR, Eaglesom CC, Hattori A, Owen M. Formation of bone and cartilage by marrow stromal cells in diffusion chambers in vivo. Clin Orthop Relat Res. 1980 Sep;151:294–307. [PubMed] [Google Scholar]
- 81.Csaki C, Schneider PR, Shakibaei M. Mesenchymal stem cells as a potential pool for cartilage tissue engineering. Ann Anat. 2008 Nov 20;190(5):395–412. doi: 10.1016/j.aanat.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 82.Magne D, Vinatier C, Julien M, Weiss P, Guicheux J. Mesenchymal stem cell therapy to rebuild cartilage. Trends Mol Med. 2005 Nov;11(11):519–526. doi: 10.1016/j.molmed.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 83.Noel D, Gazit D, Bouquet C, Apparailly F, Bony C, Plence P, et al. Short-term BMP-2 expression is sufficient for in vivo osteochondral differentiation of mesenchymal stem cells. Stem Cells. 2004;22(1):74–85. doi: 10.1634/stemcells.22-1-74. [DOI] [PubMed] [Google Scholar]
- 84.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999 Apr 2;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 85.Sekiya I, Colter DC, Prockop DJ. BMP-6 enhances chondrogenesis in a subpopulation of human marrow stromal cells. Biochem Biophys Res Commun. 2001 Jun 8;284(2):411–418. doi: 10.1006/bbrc.2001.4898. [DOI] [PubMed] [Google Scholar]
- 86.Miyanishi K, Trindade MC, Lindsey DP, Beaupre GS, Carter DR, Goodman SB, et al. Effects of hydrostatic pressure and transforming growth factor-beta 3 on adult human mesenchymal stem cell chondrogenesis in vitro. Tissue Eng. 2006 Jun;12(6):1419–1428. doi: 10.1089/ten.2006.12.1419. [DOI] [PubMed] [Google Scholar]
- 87.Pelaez D, Huang CY, Cheung HS. Cyclic Compression Maintains Viability and Induces Chondrogenesis of Human Mesenchymal Stem Cells in Fibrin Gel Scaffolds. Stem Cells Dev. 2008 Apr 9; doi: 10.1089/scd.2008.0030. [DOI] [PubMed] [Google Scholar]
- 88.Koga H, Muneta T, Nagase T, Nimura A, Ju YJ, Mochizuki T, et al. Comparison of mesenchymal tissues-derived stem cells for in vivo chondrogenesis: suitable conditions for cell therapy of cartilage defects in rabbit. Cell Tissue Res. 2008 Aug;333(2):207–215. doi: 10.1007/s00441-008-0633-5. [DOI] [PubMed] [Google Scholar]
- 89.Winter A, Breit S, Parsch D, Benz K, Steck E, Hauner H, et al. Cartilage-like gene expression in differentiated human stem cell spheroids: a comparison of bone marrow-derived and adipose tissue-derived stromal cells. Arthritis Rheum. 2003 Feb;48(2):418–429. doi: 10.1002/art.10767. [DOI] [PubMed] [Google Scholar]
- 90.Liu TM, Martina M, Hutmacher DW, Hui JH, Lee EH, Lim B. Identification of common pathways mediating differentiation of bone marrow- and adipose tissue-derived human mesenchymal stem cells into three mesenchymal lineages. Stem Cells. 2007 Mar;25(3):750–760. doi: 10.1634/stemcells.2006-0394. [DOI] [PubMed] [Google Scholar]
- 91.Im GI, Shin YW, Lee KB. Do adipose tissue-derived mesenchymal stem cells have the same osteogenic and chondrogenic potential as bone marrow-derived cells? Osteoarthritis Cartilage. 2005 Oct;13(10):845–853. doi: 10.1016/j.joca.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 92.Yoo JU, Barthel TS, Nishimura K, Solchaga L, Caplan AI, Goldberg VM, et al. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone Joint Surg Am. 1998 Dec;80(12):1745–1757. doi: 10.2106/00004623-199812000-00004. [DOI] [PubMed] [Google Scholar]
- 93.Wakitani S, Goto T, Pineda SJ, Young RG, Mansour JM, Caplan AI, et al. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1994 Apr;76(4):579–592. doi: 10.2106/00004623-199404000-00013. [DOI] [PubMed] [Google Scholar]
- 94.Solchaga LA, Gao J, Dennis JE, Awadallah A, Lundberg M, Caplan AI, et al. Treatment of osteochondral defects with autologous bone marrow in a hyaluronan-based delivery vehicle. Tissue Eng. 2002 Apr;8(2):333–347. doi: 10.1089/107632702753725085. [DOI] [PubMed] [Google Scholar]
- 95.Zhou XZ, Leung VY, Dong QR, Cheung KM, Chan D, Lu WW. Mesenchymal stem cell-based repair of articular cartilage with polyglycolic acid-hydroxyapatite biphasic scaffold. Int J Artif Organs. 2008 Jun;31(6):480–489. doi: 10.1177/039139880803100603. [DOI] [PubMed] [Google Scholar]
- 96.Guo X, Zheng Q, Yang S, Shao Z, Yuan Q, Pan Z, et al. Repair of full-thickness articular cartilage defects by cultured mesenchymal stem cells transfected with the transforming growth factor beta1 gene. Biomed Mater. 2006 Dec;1(4):206–215. doi: 10.1088/1748-6041/1/4/006. [DOI] [PubMed] [Google Scholar]
- 97.Nimura A, Muneta T, Koga H, Mochizuki T, Suzuki K, Makino H, et al. Increased proliferation of human synovial mesenchymal stem cells with autologous human serum: comparisons with bone marrow mesenchymal stem cells and with fetal bovine serum. Arthritis Rheum. 2008 Feb;58(2):501–510. doi: 10.1002/art.23219. [DOI] [PubMed] [Google Scholar]
- 98.Guilak F, Awad HA, Fermor B, Leddy HA, Gimble JM. Adipose-derived adult stem cells for cartilage tissue engineering. Biorheology. 2004;41(3–4):389–399. [PubMed] [Google Scholar]
- 99.Awad HA, Wickham MQ, Leddy HA, Gimble JM, Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials. 2004 Jul;25(16):3211–3222. doi: 10.1016/j.biomaterials.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 100.Wakitani S, Imoto K, Yamamoto T, Saito M, Murata N, Yoneda M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage. 2002 Mar;10(3):199–206. doi: 10.1053/joca.2001.0504. [DOI] [PubMed] [Google Scholar]
- 101.Wakitani S, Mitsuoka T, Nakamura N, Toritsuka Y, Nakamura Y, Horibe S. Autologous bone marrow stromal cell transplantation for repair of full-thickness articular cartilage defects in human patellae: two case reports. Cell Transplant. 2004;13(5):595–600. doi: 10.3727/000000004783983747. [DOI] [PubMed] [Google Scholar]
- 102.Wakitani S, Nawata M, Tensho K, Okabe T, Machida H, Ohgushi H. Repair of articular cartilage defects in the patello-femoral joint with autologous bone marrow mesenchymal cell transplantation: three case reports involving nine defects in five knees. J Tissue Eng Regen Med. 2007 Jan–Feb;1(1):74–79. doi: 10.1002/term.8. [DOI] [PubMed] [Google Scholar]
- 103.Kuroda R, Ishida K, Matsumoto T, Akisue T, Fujioka H, Mizuno K, et al. Treatment of a full-thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone-marrow stromal cells. Osteoarthritis Cartilage. 2007 Feb;15(2):226–231. doi: 10.1016/j.joca.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 104.Kramer J, Hegert C, Guan K, Wobus AM, Muller PK, Rohwedel J. Embryonic stem cell-derived chondrogenic differentiation in vitro: activation by BMP-2 and BMP-4. Mech Dev. 2000 Apr;92(2):193–205. doi: 10.1016/s0925-4773(99)00339-1. [DOI] [PubMed] [Google Scholar]
- 105.Nakayama N, Duryea D, Manoukian R, Chow G, Han CY. Macroscopic cartilage formation with embryonic stem-cell-derived mesodermal progenitor cells. J Cell Sci. 2003 May 15;116(Pt 10):2015–2028. doi: 10.1242/jcs.00417. [DOI] [PubMed] [Google Scholar]
- 106.Vats A, Bielby RC, Tolley N, Dickinson SC, Boccaccini AR, Hollander AP, et al. Chondrogenic differentiation of human embryonic stem cells: the effect of the micro-environment. Tissue Eng. 2006 Jun;12(6):1687–1697. doi: 10.1089/ten.2006.12.1687. [DOI] [PubMed] [Google Scholar]
- 107.Sui Y, Clarke T, Khillan JS. Limb bud progenitor cells induce differentiation of pluripotent embryonic stem cells into chondrogenic lineage. Differentiation. 2003 Dec;71(9–10):578–585. doi: 10.1111/j.1432-0436.2003.07109001.x. [DOI] [PubMed] [Google Scholar]
- 108.Koay EJ, Hoben GM, Athanasiou KA. Tissue engineering with chondrogenically differentiated human embryonic stem cells. Stem Cells. 2007 Sep;25(9):2183–2190. doi: 10.1634/stemcells.2007-0105. [DOI] [PubMed] [Google Scholar]
- 109.Koay EJ, Athanasiou KA. Development of serum-free, chemically defined conditions for human embryonic stem cell-derived fibrochondrogenesis. Tissue Eng Part A. 2009 Aug;15(8):2249–2257. doi: 10.1089/ten.tea.2008.0320. [DOI] [PubMed] [Google Scholar]
- 110.Hwang NS, Varghese S, Lee HJ, Zhang Z, Ye Z, Bae J, et al. In vivo commitment and functional tissue regeneration using human embryonic stem cell-derived mesenchymal cells. Proc Natl Acad Sci U S A. 2008 Dec 30;105(52):20641–20646. doi: 10.1073/pnas.0809680106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fecek C, Yao D, Kacorri A, Vasquez A, Iqbal S, Sheikh H, et al. Chondrogenic derivatives of embryonic stem cells seeded into 3D polycaprolactone scaffolds generated cartilage tissue in vivo. Tissue Eng Part A. 2008 Aug;14(8):1403–1413. doi: 10.1089/ten.tea.2007.0293. [DOI] [PubMed] [Google Scholar]
- 112.Jukes JM, Moroni L, van Blitterswijk CA, de Boer J. Critical Steps toward a tissue-engineered cartilage implant using embryonic stem cells. Tissue Eng Part A. 2008 Jan;14(1):135–147. doi: 10.1089/ten.a.2006.0397. [DOI] [PubMed] [Google Scholar]
- 113.Hwang NS, Kim MS, Sampattavanich S, Baek JH, Zhang Z, Elisseeff J. Effects of three-dimensional culture and growth factors on the chondrogenic differentiation of murine embryonic stem cells. Stem Cells. 2006 Feb;24(2):284–291. doi: 10.1634/stemcells.2005-0024. [DOI] [PubMed] [Google Scholar]
- 114.Lee EH, Hui JH. The potential of stem cells in orthopaedic surgery. J Bone Joint Surg Br. 2006 Jul;88(7):841–851. doi: 10.1302/0301-620X.88B7.17305. [DOI] [PubMed] [Google Scholar]
- 115.Bajada S, Mazakova I, Richardson JB, Ashammakhi N. Updates on stem cells and their applications in regenerative medicine. J Tissue Eng Regen Med. 2008 Jun;2(4):169–183. doi: 10.1002/term.83. [DOI] [PubMed] [Google Scholar]
- 116.Richards M, Fong CY, Chan WK, Wong PC, Bongso A. Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nat Biotechnol. 2002 Sep;20(9):933–936. doi: 10.1038/nbt726. [DOI] [PubMed] [Google Scholar]
- 117.Mizuno S, Glowacki J. Low oxygen tension enhances chondroinduction by demineralized bone matrix in human dermal fibroblasts in vitro. Cells Tissues Organs. 2005;180(3):151–158. doi: 10.1159/000088243. [DOI] [PubMed] [Google Scholar]
- 118.Glowacki J, Yates KE, Maclean R, Mizuno S. In vitro engineering of cartilage: effects of serum substitutes, TGF-beta, and IL-1alpha. Orthod Craniofac Res. 2005 Aug;8(3):200–208. doi: 10.1111/j.1601-6343.2005.00333.x. [DOI] [PubMed] [Google Scholar]
- 119.Yates KE, Forbes RL, Glowacki J. New chondrocyte genes discovered by representational difference analysis of chondroinduced human fibroblasts. Cells Tissues Organs. 2004;176(1–3):41–53. doi: 10.1159/000075026. [DOI] [PubMed] [Google Scholar]
- 120.Mizuno S, Glowacki J. Chondroinduction of human dermal fibroblasts by demineralized bone in three-dimensional culture. Exp Cell Res. 1996 Aug 25;227(1):89–97. doi: 10.1006/excr.1996.0253. [DOI] [PubMed] [Google Scholar]
- 121.Yates KE, Glowacki J. Gene expression changes in an in vitro model of chondroinduction: a comparison of two methods. Wound Repair Regen. 2003 Sep–Oct;11(5):386–392. doi: 10.1046/j.1524-475x.2003.11512.x. [DOI] [PubMed] [Google Scholar]
- 122.Deng Y, Hu JC, Athanasiou KA. Isolation and chondroinduction of a dermis-isolated, aggrecan-sensitive subpopulation with high chondrogenic potential. Arthritis Rheum. 2007 Jan;56(1):168–176. doi: 10.1002/art.22300. [DOI] [PubMed] [Google Scholar]
- 123.Chunmeng S, Tianmin C, Yongping S, Xinze R, Yue M, Jifu Q, et al. Effects of dermal multipotent cell transplantation on skin wound healing. J Surg Res. 2004 Sep;121(1):13–19. doi: 10.1016/j.jss.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 124.Shi CM, Cheng TM. Differentiation of dermis-derived multipotent cells into insulin-producing pancreatic cells in vitro. World J Gastroenterol. 2004 Sep 1;10(17):2550–2552. doi: 10.3748/wjg.v10.i17.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gharzi A, Reynolds AJ, Jahoda CA. Plasticity of hair follicle dermal cells in wound healing and induction. Exp Dermatol. 2003 Apr;12(2):126–136. doi: 10.1034/j.1600-0625.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- 126.Glowacki J. Cellular reactions to bone-derived material. Clin Orthop Relat Res. 1996 Mar;(324):47–54. doi: 10.1097/00003086-199603000-00007. [DOI] [PubMed] [Google Scholar]
- 127.Glowacki J, Trepman E, Folkman J. Cell shape and phenotypic expression in chondrocytes. Proc Soc Exp Biol Med. 1983 Jan;172(1):93–98. doi: 10.3181/00379727-172-41533. [DOI] [PubMed] [Google Scholar]
- 128.Kozma EM, Olczyk K, Wisowski G, Glowacki A, Bobinski R. Alterations in the extracellular matrix proteoglycan profile in Dupuytren's contracture affect the palmar fascia. J Biochem (Tokyo) 2005 Apr;137(4):463–476. doi: 10.1093/jb/mvi054. [DOI] [PubMed] [Google Scholar]
- 129.Mizuno S, Glowacki J. Three-dimensional composite of demineralized bone powder and collagen for in vitro analysis of chondroinduction of human dermal fibroblasts. Biomaterials. 1996 Sep;17(18):1819–1825. doi: 10.1016/0142-9612(96)00041-5. [DOI] [PubMed] [Google Scholar]
- 130.Yates KE, Glowacki J. Altered expression of connective tissue genes in postnatal chondroinduced human dermal fibroblasts. Connect Tissue Res. 2003;44(3–4):121–127. doi: 10.1080/03008200390223891. [DOI] [PubMed] [Google Scholar]
- 131.Yates KE, Mizuno S, Glowacki J. Early shifts in gene expression during chondroinduction of human dermal fibroblasts. Exp Cell Res. 2001 May 1;265(2):203–211. doi: 10.1006/excr.2001.5192. [DOI] [PubMed] [Google Scholar]
- 132.Zhou S, Glowacki J, Yates KE. Comparison of TGF-beta/BMP pathways signaled by demineralized bone powder and BMP-2 in human dermal fibroblasts. J Bone Miner Res. 2004 Oct;19(10):1732–1741. doi: 10.1359/JBMR.040702. [DOI] [PubMed] [Google Scholar]
- 133.French MM, Rose S, Canseco J, Athanasiou KA. Chondrogenic differentiation of adult dermal fibroblasts. Ann Biomed Eng. 2004 Jan;32(1):50–56. doi: 10.1023/b:abme.0000007790.65773.e0. [DOI] [PubMed] [Google Scholar]
- 134.Fries KM, Blieden T, Looney RJ, Sempowski GD, Silvera MR, Willis RA, et al. Evidence of fibroblast heterogeneity and the role of fibroblast subpopulations in fibrosis. Clin Immunol Immunopathol. 1994 Sep;72(3):283–292. doi: 10.1006/clin.1994.1144. [DOI] [PubMed] [Google Scholar]
- 135.Mathisen B, Loennechen T, Gedde-Dahl T, Winberg JO. Fibroblast heterogeneity in collagenolytic response to colchicine. Biochem Pharmacol. 2006 Feb 28;71(5):574–583. doi: 10.1016/j.bcp.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 136.Maxwell DB, Grotendorst CA, Grotendorst GR, LeRoy EC. Fibroblast heterogeneity in scleroderma: Clq studies. J Rheumatol. 1987 Aug;14(4):756–759. [PubMed] [Google Scholar]
- 137.Getgood A, Brooks R, Fortier L, Rushton N. Articular cartilage tissue engineering: today's research, tomorrow's practice? J Bone Joint Surg Br. 2009 May;91(5):565–576. doi: 10.1302/0301-620X.91B5.21832. [DOI] [PubMed] [Google Scholar]
- 138.Grigolo B, Roseti L, Fiorini M, Fini M, Giavaresi G, Aldini NN, et al. Transplantation of chondrocytes seeded on a hyaluronan derivative (hyaff-11) into cartilage defects in rabbits. Biomaterials. 2001 Sep;22(17):2417–2424. doi: 10.1016/s0142-9612(00)00429-4. [DOI] [PubMed] [Google Scholar]
- 139.Kujawa MJ, Caplan AI. Hyaluronic acid bonded to cell-culture surfaces stimulates chondrogenesis in stage 24 limb mesenchyme cell cultures. Dev Biol. 1986 Apr;114(2):504–518. doi: 10.1016/0012-1606(86)90214-9. [DOI] [PubMed] [Google Scholar]
- 140.Murphy CL, Sambanis A. Effect of oxygen tension and alginate encapsulation on restoration of the differentiated phenotype of passaged chondrocytes. Tissue Eng. 2001 Dec;7(6):791–803. doi: 10.1089/107632701753337735. [DOI] [PubMed] [Google Scholar]
- 141.Diduch DR, Jordan LC, Mierisch CM, Balian G. Marrow stromal cells embedded in alginate for repair of osteochondral defects. Arthroscopy. 2000 Sep;16(6):571–577. doi: 10.1053/jars.2000.4827. [DOI] [PubMed] [Google Scholar]
- 142.Elder SH, Goldstein SA, Kimura JH, Soslowsky LJ, Spengler DM. Chondrocyte differentiation is modulated by frequency and duration of cyclic compressive loading. Ann Biomed Eng. 2001 Jun;29(6):476–482. doi: 10.1114/1.1376696. [DOI] [PubMed] [Google Scholar]
- 143.Kleinman HK, Klebe RJ, Martin GR. Role of collagenous matrices in the adhesion and growth of cells. J Cell Biol. 1981 Mar;88(3):473–485. doi: 10.1083/jcb.88.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nehrer S, Breinan HA, Ramappa A, Shortkroff S, Young G, Minas T, et al. Canine chondrocytes seeded in type I and type II collagen implants investigated in vitro. J Biomed Mater Res. 1997 Summer;38(2):95–104. doi: 10.1002/(sici)1097-4636(199722)38:2<95::aid-jbm3>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 145.Dorotka R, Bindreiter U, Macfelda K, Windberger U, Nehrer S. Marrow stimulation and chondrocyte transplantation using a collagen matrix for cartilage repair. Osteoarthritis Cartilage. 2005 Aug;13(8):655–664. doi: 10.1016/j.joca.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 146.Lee CR, Breinan HA, Nehrer S, Spector M. Articular cartilage chondrocytes in type I and type II collagen-GAG matrices exhibit contractile behavior in vitro. Tissue Eng. 2000 Oct;6(5):555–565. doi: 10.1089/107632700750022198. [DOI] [PubMed] [Google Scholar]
- 147.Coombes AG, Heckman JD. Gel casting of resorbable polymers. 2. In-vitro degradation of bone graft substitutes. Biomaterials. 1992;13(5):297–307. doi: 10.1016/0142-9612(92)90053-q. [DOI] [PubMed] [Google Scholar]
- 148.Grande DA, Halberstadt C, Naughton G, Schwartz R, Manji R. Evaluation of matrix scaffolds for tissue engineering of articular cartilage grafts. J Biomed Mater Res. 1997 Feb;34(2):211–220. doi: 10.1002/(sici)1097-4636(199702)34:2<211::aid-jbm10>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 149.Freed LE, Hollander AP, Martin I, Barry JR, Langer R, Vunjak-Novakovic G. Chondrogenesis in a cell-polymer-bioreactor system. Exp Cell Res. 1998 Apr 10;240(1):58–65. doi: 10.1006/excr.1998.4010. [DOI] [PubMed] [Google Scholar]
- 150.Mehlhorn AT, Zwingmann J, Finkenzeller G, Niemeyer P, Dauner M, Stark B, et al. Chondrogenesis of adipose-derived adult stem cells in a poly-lactide-co-glycolide scaffold. Tissue Eng Part A. 2009 May;15(5):1159–1167. doi: 10.1089/ten.tea.2008.0069. [DOI] [PubMed] [Google Scholar]
- 151.Park K, Cho KJ, Kim JJ, Kim IH, Han DK. Functional PLGA scaffolds for chondrogenesis of bone-marrow-derived mesenchymal stem cells. Macromol Biosci. 2009 Mar 10;9(3):221–229. doi: 10.1002/mabi.200800187. [DOI] [PubMed] [Google Scholar]
- 152.Wu SC, Chang JK, Wang CK, Wang GJ, Ho ML. Enhancement of chondrogenesis of human adipose derived stem cells in a hyaluronan-enriched microenvironment. Biomaterials. 2009 Oct 9; doi: 10.1016/j.biomaterials.2009.09.089. [DOI] [PubMed] [Google Scholar]
- 153.Ameer GA, Mahmood TA, Langer R. A biodegradable composite scaffold for cell transplantation. J Orthop Res. 2002 Jan;20(1):16–19. doi: 10.1016/S0736-0266(01)00074-2. [DOI] [PubMed] [Google Scholar]
- 154.Huang Q, Goh JC, Hutmacher DW, Lee EH. In vivo mesenchymal cell recruitment by a scaffold loaded with transforming growth factor beta1 and the potential for in situ chondrogenesis. Tissue Eng. 2002 Jul;8(3):469–482. doi: 10.1089/107632702760184727. [DOI] [PubMed] [Google Scholar]
- 155.Marijnissen WJ, van Osch GJ, Aigner J, Verwoerd-Verhoef HL, Verhaar JA. Tissue-engineered cartilage using serially passaged articular chondrocytes. Chondrocytes in alginate, combined in vivo with a synthetic (E210) or biologic biodegradable carrier (DBM) Biomaterials. 2000 Mar;21(6):571–580. doi: 10.1016/s0142-9612(99)00218-5. [DOI] [PubMed] [Google Scholar]
- 156.Zhang L, Webster TJ. Nanotechnology and Nanomaterials: Promises for Improved Tissue Regeneration. Nanotoday. 2009;4(1):66–80. [Google Scholar]
- 157.Li WJ, Tuli R, Okafor C, Derfoul A, Danielson KG, Hall DJ, et al. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials. 2005 Feb;26(6):599–609. doi: 10.1016/j.biomaterials.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 158.Li WJ, Jiang YJ, Tuan RS. Cell-nanofiber-based cartilage tissue engineering using improved cell seeding, growth factor, and bioreactor technologies. Tissue Eng Part A. 2008 May;14(5):639–648. doi: 10.1089/tea.2007.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Park GE, Pattison MA, Park K, Webster TJ. Accelerated chondrocyte functions on NaOH-treated PLGA scaffolds. Biomaterials. 2005 Jun;26(16):3075–3082. doi: 10.1016/j.biomaterials.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 160.Yasumoto S, Kondo S, Kato Y. Growth and differentiation of primary chick embryonic chondrocytes on agar plates. Jpn J Exp Med. 1980 Jun;50(3):221–224. [PubMed] [Google Scholar]
- 161.Castagnola P, Moro G, Descalzi-Cancedda F, Cancedda R. Type X collagen synthesis during in vitro development of chick embryo tibial chondrocytes. J Cell Biol. 1986 Jun;102(6):2310–2317. doi: 10.1083/jcb.102.6.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lindahl A, Isgaard J, Nilsson A, Isaksson OG. Growth hormone potentiates colony formation of epiphyseal chondrocytes in suspension culture. Endocrinology. 1986 May;118(5):1843–1848. doi: 10.1210/endo-118-5-1843. [DOI] [PubMed] [Google Scholar]
- 163.Lindahl A, Isgaard J, Carlsson L, Isaksson OG. Differential effects of growth hormone and insulin-like growth factor I on colony formation of epiphyseal chondrocytes in suspension culture in rats of different ages. Endocrinology. 1987 Sep;121(3):1061–1069. doi: 10.1210/endo-121-3-1061. [DOI] [PubMed] [Google Scholar]
- 164.Lindahl A, Nilsson A, Isaksson OG. Effects of growth hormone and insulin-like growth factor-I on colony formation of rabbit epiphyseal chondrocytes at different stages of maturation. J Endocrinol. 1987 Nov;115(2):263–271. doi: 10.1677/joe.0.1150263. [DOI] [PubMed] [Google Scholar]
- 165.Tacchetti C, Quarto R, Nitsch L, Hartmann DJ, Cancedda R. In vitro morphogenesis of chick embryo hypertrophic cartilage. J Cell Biol. 1987 Aug;105(2):999–1006. doi: 10.1083/jcb.105.2.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bassleer C, Gysen P, Foidart JM, Bassleer R, Franchimont P. Human chondrocytes in tridimensional culture. In Vitro Cell Dev Biol. 1986 Mar;22(3 Pt 1):113–119. doi: 10.1007/BF02623497. [DOI] [PubMed] [Google Scholar]
- 167.Hu JC, Athanasiou KA. A self-assembling process in articular cartilage tissue engineering. Tissue engineering. 2006 Apr;12(4):969–979. doi: 10.1089/ten.2006.12.969. [DOI] [PubMed] [Google Scholar]
- 168.Furukawa KS, Suenaga H, Toita K, Numata A, Tanaka J, Ushida T, et al. Rapid and large-scale formation of chondrocyte aggregates by rotational culture. Cell Transplant. 2003;12(5):475–479. doi: 10.3727/000000003108747037. [DOI] [PubMed] [Google Scholar]
- 169.Vunjak-Novakovic G, Obradovic B, Martin I, Bursac PM, Langer R, Freed LE. Dynamic cell seeding of polymer scaffolds for cartilage tissue engineering. Biotechnol Prog. 1998 Mar–Apr;14(2):193–202. doi: 10.1021/bp970120j. [DOI] [PubMed] [Google Scholar]
- 170.Bueno EM, Bilgen B, Carrier RL, Barabino GA. Increased rate of chondrocyte aggregation in a wavy-walled bioreactor. Biotechnol Bioeng. 2004 Dec 20;88(6):767–777. doi: 10.1002/bit.20261. [DOI] [PubMed] [Google Scholar]
- 171.Shepherd DE, Seedhom BB. Thickness of human articular cartilage in joints of the lower limb. Ann Rheum Dis. 1999 Jan;58(1):27–34. doi: 10.1136/ard.58.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Elder BD, Athanasiou KA. Effects of Temporal Hydrostatic Pressure on Tissue-Engineered Bovine Articular Cartilage Constructs. Tissue Eng Part A. 2008 Oct 2; doi: 10.1089/ten.tea.2008.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Elder BD, Athanasiou KA. Synergistic and additive effects of hydrostatic pressure and growth factors on tissue formation. PLoS ONE. 2008;3(6):e2341. doi: 10.1371/journal.pone.0002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Elder BD, Athanasiou KA. Systematic assessment of growth factor treatment on biochemical and biomechanical properties of engineered articular cartilage constructs. Osteoarthritis Cartilage. 2009 Jan;17(1):114–123. doi: 10.1016/j.joca.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ofek G, Revell CM, Hu JC, Allison DD, Grande-Allen KJ, Athanasiou KA. Matrix development in self-assembly of articular cartilage. PLoS ONE. 2008;3(7):e2795. doi: 10.1371/journal.pone.0002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Novotny JE, Turka CM, Jeong C, Wheaton AJ, Li C, Presedo A, et al. Biomechanical and magnetic resonance characteristics of a cartilage-like equivalent generated in a suspension culture. Tissue Eng. 2006 Oct;12(10):2755–2764. doi: 10.1089/ten.2006.12.2755. [DOI] [PubMed] [Google Scholar]
- 177.Brehm W, Aklin B, Yamashita T, Rieser F, Trub T, Jakob RP, et al. Repair of superficial osteochondral defects with an autologous scaffold-free cartilage construct in a caprine model: implantation method and short-term results. Osteoarthritis Cartilage. 2006 Jun 30; doi: 10.1016/j.joca.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 178.Park K, Huang J, Azar F, Jin RL, Min BH, Han DK, et al. Scaffold-free, engineered porcine cartilage construct for cartilage defect repair--in vitro and in vivo study. Artif Organs. 2006 Aug;30(8):586–596. doi: 10.1111/j.1525-1594.2006.00267.x. [DOI] [PubMed] [Google Scholar]
- 179.Revell CM, Reynolds CE, Athanasiou KA. Effects of initial cell seeding in self assembly of articular cartilage. Ann Biomed Eng. 2008 Sep;36(9):1441–1448. doi: 10.1007/s10439-008-9524-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Morrison EH, Ferguson MW, Bayliss MT, Archer CW. The development of articular cartilage: I. The spatial and temporal patterns of collagen types. J Anat. 1996 Aug;189(Pt 1):9–22. [PMC free article] [PubMed] [Google Scholar]
- 181.Oberlender SA, Tuan RS. Expression and functional involvement of N-cadherin in embryonic limb chondrogenesis. Development. 1994 Jan;120(1):177–187. doi: 10.1242/dev.120.1.177. [DOI] [PubMed] [Google Scholar]
- 182.Gigout A, Jolicoeur M, Nelea M, Raynal N, Farndale R, Buschmann MD. Chondrocyte aggregation in suspension culture is GFOGER-GPP- and beta1 integrin-dependent. J Biol Chem. 2008 Nov 14;283(46):31522–31530. doi: 10.1074/jbc.M804234200. [DOI] [PubMed] [Google Scholar]
- 183.Camper L, Hellman U, Lundgren-Akerlund E. Isolation, cloning, and sequence analysis of the integrin subunit alpha10, a beta1-associated collagen binding integrin expressed on chondrocytes. J Biol Chem. 1998 Aug 7;273(32):20383–20389. doi: 10.1074/jbc.273.32.20383. [DOI] [PubMed] [Google Scholar]
- 184.Camper L, Holmvall K, Wangnerud C, Aszodi A, Lundgren-Akerlund E. Distribution of the collagen-binding integrin alpha10beta1 during mouse development. Cell Tissue Res. 2001 Oct;306(1):107–116. doi: 10.1007/s004410100385. [DOI] [PubMed] [Google Scholar]
- 185.Graff RD, Kelley SS, Lee GM. Role of pericellular matrix in development of a mechanically functional neocartilage. Biotechnol Bioeng. 2003 May 20;82(4):457–464. doi: 10.1002/bit.10593. [DOI] [PubMed] [Google Scholar]
- 186.Zhang Z, McCaffery JM, Spencer RG, Francomano CA. Hyaline cartilage engineered by chondrocytes in pellet culture: histological, immunohistochemical and ultrastructural analysis in comparison with cartilage explants. J Anat. 2004 Sep;205(3):229–237. doi: 10.1111/j.0021-8782.2004.00327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lubke C, Ringe J, Krenn V, Fernahl G, Pelz S, Kreusch-Brinker R, et al. Growth characterization of neo porcine cartilage pellets and their use in an interactive culture model. Osteoarthritis Cartilage. 2005 Jun;13(6):478–487. doi: 10.1016/j.joca.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 188.Elder SH, Sanders SW, McCulley WR, Marr ML, Shim JW, Hasty KA. Chondrocyte response to cyclic hydrostatic pressure in alginate versus pellet culture. J Orthop Res. 2006 Apr;24(4):740–747. doi: 10.1002/jor.20086. [DOI] [PubMed] [Google Scholar]
- 189.Masuda K, Sah RL, Hejna MJ, Thonar EJ. A novel two-step method for the formation of tissue-engineered cartilage by mature bovine chondrocytes: the alginate-recovered-chondrocyte (ARC) method. J Orthop Res. 2003 Jan;21(1):139–148. doi: 10.1016/S0736-0266(02)00109-2. [DOI] [PubMed] [Google Scholar]
- 190.Hu JC, Athanasiou KA. The effects of intermittent hydrostatic pressure on self-assembled articular cartilage constructs. Tissue Eng. 2006 May;12(5):1337–1344. doi: 10.1089/ten.2006.12.1337. [DOI] [PubMed] [Google Scholar]
- 191.Smith RL, Trindade MC, Ikenoue T, Mohtai M, Das P, Carter DR, et al. Effects of shear stress on articular chondrocyte metabolism. Biorheology. 2000;37(1–2):95–107. [PubMed] [Google Scholar]
- 192.Foty RA, Steinberg MS. The differential adhesion hypothesis: a direct evaluation. Dev Biol. 2005 Feb 1;278(1):255–263. doi: 10.1016/j.ydbio.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 193.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005 Nov 18;310(5751):1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 194.Steinberg MS. Differential adhesion in morphogenesis: a modern view. Curr Opin Genet Dev. 2007 Aug;17(4):281–286. doi: 10.1016/j.gde.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 195.Brown PD, Benya PD. Alterations in chondrocyte cytoskeletal architecture during phenotypic modulation by retinoic acid and dihydrocytochalasin B-induced reexpression. J Cell Biol. 1988 Jan;106(1):171–179. doi: 10.1083/jcb.106.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Benya PD. Modulation and reexpression of the chondrocyte phenotype; mediation by cell shape and microfilament modification. Pathol Immunopathol Res. 1988;7(1–2):51–54. doi: 10.1159/000157093. [DOI] [PubMed] [Google Scholar]
- 197.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997 May 30;276(5317):1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 198.Meredith JC, Sormana JL, Keselowsky BG, Garcia AJ, Tona A, Karim A, et al. Combinatorial characterization of cell interactions with polymer surfaces. J Biomed Mater Res A. 2003 Sep 1;66(3):483–490. doi: 10.1002/jbm.a.10004. [DOI] [PubMed] [Google Scholar]
- 199.Yoon DM, Fisher JP. Chondrocyte signaling and artificial matrices for articular cartilage engineering. Adv Exp Med Biol. 2006;585:67–86. doi: 10.1007/978-0-387-34133-0_5. [DOI] [PubMed] [Google Scholar]
- 200.Barbero A, Grogan SP, Mainil-Varlet P, Martin I. Expansion on specific substrates regulates the phenotype and differentiation capacity of human articular chondrocytes. J Cell Biochem. 2006 Aug 1;98(5):1140–1149. doi: 10.1002/jcb.20754. [DOI] [PubMed] [Google Scholar]
- 201.Bryant SJ, Chowdhury TT, Lee DA, Bader DL, Anseth KS. Crosslinking density influences chondrocyte metabolism in dynamically loaded photocrosslinked poly(ethylene glycol) hydrogels. Ann Biomed Eng. 2004 Mar;32(3):407–417. doi: 10.1023/b:abme.0000017535.00602.ca. [DOI] [PubMed] [Google Scholar]
- 202.Bryant SJ, Anseth KS, Lee DA, Bader DL. Crosslinking density influences the morphology of chondrocytes photoencapsulated in PEG hydrogels during the application of compressive strain. J Orthop Res. 2004 Sep;22(5):1143–1149. doi: 10.1016/j.orthres.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 203.Anderson JM. Biological responses to materials. Annual Reviews in Materials Research. 2001;31:81–110. [Google Scholar]
- 204.Gaillard PJ, Moskalewski S, Verhoog MJ, Wassenaar AM. The influence of parathyroid hormone on the aggregation of isolated chondrocytes. Calcif Tissue Res. 1968 Suppl doi: 10.1007/BF02065231. 49-a. [DOI] [PubMed] [Google Scholar]
- 205.Takeichi M. The factor affecting the spreading of chondrocytes upon inorganic substrate. J Cell Sci. 1973 Jul;13(1):193–204. doi: 10.1242/jcs.13.1.193. [DOI] [PubMed] [Google Scholar]
- 206.Jones KL, Addison J. Pituitary fibroblast growth factor as a stimulator of growth in cultured rabbit articular chondrocytes. Endocrinology. 1975 Aug;97(2):359–365. doi: 10.1210/endo-97-2-359. [DOI] [PubMed] [Google Scholar]
- 207.Corvol MT, Malemud CJ, Sokoloff L. A pituitary growth-promoting factor for articular chondrocytes in monolayer culture. Endocrinology. 1972 Jan;90(1):262–271. doi: 10.1210/endo-90-1-262. [DOI] [PubMed] [Google Scholar]
- 208.Darling EM, Athanasiou KA. Bioactive scaffold design for articular cartilage engineering. In: Moore J, Zouridakis G, editors. Biomedical Technology and Devices Handbook. Chapter 21. Boca Raton, FL: CRC Press LLC; 2004. [Google Scholar]
- 209.Natoli RM, Athanasiou KA. P188 Reduces Cell Death and IGF-I Reduces GAG Release Following Single-Impact Loading of Articular Cartilage. J Biomech Eng. 2008 Aug;130(4) doi: 10.1115/1.2939368. 041012. [DOI] [PubMed] [Google Scholar]
- 210.Lo MY, Kim HT. Chondrocyte apoptosis induced by collagen degradation: inhibition by caspase inhibitors and IGF-1. J Orthop Res. 2004 Jan;22(1):140–144. doi: 10.1016/S0736-0266(03)00117-7. [DOI] [PubMed] [Google Scholar]
- 211.Jenniskens YM, Koevoet W, de Bart AC, Weinans H, Jahr H, Verhaar JA, et al. Biochemical and functional modulation of the cartilage collagen network by IGF1, TGFbeta2 and FGF2. Osteoarthritis Cartilage. 2006 Nov;14(11):1136–1146. doi: 10.1016/j.joca.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 212.Darling EM, Athanasiou KA. Growth factor impact on articular cartilage subpopulations. Cell Tissue Res. 2005 Jul;27:1–11. doi: 10.1007/s00441-005-0020-4. [DOI] [PubMed] [Google Scholar]
- 213.Bau B, McKenna LA, Soeder S, Fan Z, Pecht A, Aigner T. Hepatocyte growth factor/scatter factor is not a potent regulator of anabolic and catabolic gene expression in adult human articular chondrocytes. Biochem Biophys Res Commun. 2004 Apr 16;316(4):984–990. doi: 10.1016/j.bbrc.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 214.Mishima Y, Lotz M. Chemotaxis of human articular chondrocytes and mesenchymal stem cells. J Orthop Res. 2008 Oct;26(10):1407–1412. doi: 10.1002/jor.20668. [DOI] [PubMed] [Google Scholar]
- 215.Khalafi A, Schmid TM, Neu C, Reddi AH. Increased accumulation of superficial zone protein (SZP) in articular cartilage in response to bone morphogenetic protein-7 and growth factors. J Orthop Res. 2007 Mar;25(3):293–303. doi: 10.1002/jor.20329. [DOI] [PubMed] [Google Scholar]
- 216.Weiser L, Bhargava M, Attia E, Torzilli PA. Effect of serum and platelet-derived growth factor on chondrocytes grown in collagen gels. Tissue Eng. 1999 Dec;5(6):533–544. doi: 10.1089/ten.1999.5.533. [DOI] [PubMed] [Google Scholar]
- 217.Pangborn CA, Athanasiou K. Effects of growth factors on meniscal fibrochondrocytes. Tissue Engineering. 2005;11(7–8):1141–1148. doi: 10.1089/ten.2005.11.1141. [DOI] [PubMed] [Google Scholar]
- 218.Frenkel SR, Saadeh PB, Mehrara BJ, Chin GS, Steinbrech DS, Brent B, et al. Transforming growth factor beta superfamily members: role in cartilage modeling. Plast Reconstr Surg. 2000 Mar;105(3):980–990. doi: 10.1097/00006534-200003000-00022. [DOI] [PubMed] [Google Scholar]
- 219.Guerne PA, Sublet A, Lotz M. Growth factor responsiveness of human articular chondrocytes: distinct profiles in primary chondrocytes, subcultured chondrocytes, and fibroblasts. J Cell Physiol. 1994;158(3):476–484. doi: 10.1002/jcp.1041580312. [DOI] [PubMed] [Google Scholar]
- 220.Blunk T, Sieminski AL, Gooch KJ, Courter DL, Hollander AP, Nahir AM, et al. Differential effects of growth factors on tissue-engineered cartilage. Tissue Eng. 2002;8(1):73–84. doi: 10.1089/107632702753503072. [DOI] [PubMed] [Google Scholar]
- 221.Elder BD, Athanasiou KA. Systematic assessment of growth factor treatment on biochemical and biomechanical properties of engineered articular cartilage constructs. Osteoarthritis Cartilage. 2008 Jun 18; doi: 10.1016/j.joca.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Campbell JJ, Lee DA, Bader DL. Dynamic compressive strain influences chondrogenic gene expression in human mesenchymal stem cells. Biorheology. 2006;43(3–4):455–470. [PubMed] [Google Scholar]
- 223.Lima EG, Bian L, Ng KW, Mauck RL, Byers BA, Tuan RS, et al. The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-beta3. Osteoarthritis Cartilage. 2007 Sep;15(9):1025–1033. doi: 10.1016/j.joca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Pecina M, Jelic M, Martinovic S, Haspl M, Vukicevic S. Articular cartilage repair: the role of bone morphogenetic proteins. Int Orthop. 2002;26(3):131–136. doi: 10.1007/s00264-002-0338-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Gooch KJ, Blunk T, Courter DL, Sieminski AL, Vunjak-Novakovic G, Freed LE. Bone morphogenetic proteins-2, -12, and -13 modulate in vitro development of engineered cartilage. Tissue Eng. 2002 Aug;8(4):591–601. doi: 10.1089/107632702760240517. [DOI] [PubMed] [Google Scholar]
- 226.Valcourt U, Gouttenoire J, Moustakas A, Herbage D, Mallein-Gerin F. Functions of transforming growth factor-beta family type I receptors and Smad proteins in the hypertrophic maturation and osteoblastic differentiation of chondrocytes. J Biol Chem. 2002 Sep 13;277(37):33545–33558. doi: 10.1074/jbc.M202086200. [DOI] [PubMed] [Google Scholar]
- 227.Luyten FP, Yu YM, Yanagishita M, Vukicevic S, Hammonds RG, Reddi AH. Natural bovine osteogenin and recombinant human bone morphogenetic protein-2B are equipotent in the maintenance of proteoglycans in bovine articular cartilage explant cultures. J Biol Chem. 1992 Feb 25;267(6):3691–3695. [PubMed] [Google Scholar]
- 228.Mattioli-Belmonte M, Gigante A, Muzzarelli RA, Politano R, De Benedittis A, Specchia N, et al. N,N-dicarboxymethyl chitosan as delivery agent for bone morphogenetic protein in the repair of articular cartilage. Med Biol Eng Comput. 1999;37(1):130–134. doi: 10.1007/BF02513279. [DOI] [PubMed] [Google Scholar]
- 229.O'Connor WJ, Botti T, Khan SN, Lane JM. The use of growth factors in cartilage repair. Orthop Clin North Am. 2000 Jul;31(3):399–410. doi: 10.1016/s0030-5898(05)70159-0. [DOI] [PubMed] [Google Scholar]
- 230.Ando K, Imai S, Isoya E, Kubo M, Mimura T, Shioji S, et al. Effect of dynamic compressive loading and its combination with a growth factor on the chondrocytic phenotype of 3-dimensional scaffold-embedded chondrocytes. Acta Orthop. 2009 Dec;80(6):724–733. doi: 10.3109/17453670903413111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Frank EH, Jin M, Loening AM, Levenston ME, Grodzinsky AJ. A versatile shear and compression apparatus for mechanical stimulation of tissue culture explants. J Biomech. 2000 Nov;33(11):1523–1527. doi: 10.1016/s0021-9290(00)00100-7. [DOI] [PubMed] [Google Scholar]
- 232.Jin M, Emkey GR, Siparsky P, Trippel SB, Grodzinsky AJ. Combined effects of dynamic tissue shear deformation and insulin-like growth factor I on chondrocyte biosynthesis in cartilage explants. Arch Biochem Biophys. 2003 Jun 15;414(2):223–231. doi: 10.1016/s0003-9861(03)00195-4. [DOI] [PubMed] [Google Scholar]
- 233.Jin M, Frank EH, Quinn TM, Hunziker EB, Grodzinsky AJ. Tissue shear deformation stimulates proteoglycan and protein biosynthesis in bovine cartilage explants. Arch Biochem Biophys. 2001 Nov 1;395(1):41–48. doi: 10.1006/abbi.2001.2543. [DOI] [PubMed] [Google Scholar]
- 234.Waldman SD, Spiteri CG, Grynpas MD, Pilliar RM, Kandel RA. Long-term intermittent shear deformation improves the quality of cartilaginous tissue formed in vitro. J Orthop Res. 2003 Jul;21(4):590–596. doi: 10.1016/S0736-0266(03)00009-3. [DOI] [PubMed] [Google Scholar]
- 235.Wimmer MA, Grad S, Kaup T, Hanni M, Schneider E, Gogolewski S, et al. Tribology approach to the engineering and study of articular cartilage. Tissue Eng. 2004 Sep–Oct;10(9–10):1436–1445. doi: 10.1089/ten.2004.10.1436. [DOI] [PubMed] [Google Scholar]
- 236.Smith RL, Donlon BS, Gupta MK, Mohtai M, Das P, Carter DR, et al. Effects of fluid-induced shear on articular chondrocyte morphology and metabolism in vitro. J Orthop Res. 1995;13(6):824–831. doi: 10.1002/jor.1100130604. [DOI] [PubMed] [Google Scholar]
- 237.Lee MS, Trindade MC, Ikenoue T, Schurman DJ, Goodman SB, Smith RL. Effects of shear stress on nitric oxide and matrix protein gene expression in human osteoarthritic chondrocytes in vitro. J Orthop Res. 2002 May;20(3):556–561. doi: 10.1016/S0736-0266(01)00149-8. [DOI] [PubMed] [Google Scholar]
- 238.Freed LE, Vunjak-Novakovic G. Cultivation of cell-polymer tissue constructs in simulated microgravity. Biotechnol Bioeng. 1995;46:306. doi: 10.1002/bit.260460403. [DOI] [PubMed] [Google Scholar]
- 239.Martin I, Obradovic B, Freed LE, Vunjak-Novakovic G. Method for quantitative analysis of glycosaminoglycan distribution in cultured natural and engineered cartilage. Ann Biomed Eng. 1999;27(5):656–662. doi: 10.1114/1.205. [DOI] [PubMed] [Google Scholar]
- 240.Martin I, Obradovic B, Treppo S, Grodzinsky AJ, Langer R, Freed LE, et al. Modulation of the mechanical properties of tissue engineered cartilage. Biorheology. 2000;37(1–2):141–147. [PubMed] [Google Scholar]
- 241.Vunjak-Novakovic G, Martin I, Obradovic B, Treppo S, Grodzinsky AJ, Langer R, et al. Bioreactor cultivation conditions modulate the composition and mechanical properties of tissue-engineered cartilage. J Orthop Res. 1999;17(1):130–138. doi: 10.1002/jor.1100170119. [DOI] [PubMed] [Google Scholar]
- 242.Begley CM, Kleis SJ. The fluid dynamic and shear environment in the NASA/JSC rotating-wall perfused-vessel bioreactor. Biotechnol Bioeng. 2000;70(1):32–40. doi: 10.1002/1097-0290(20001005)70:1<32::aid-bit5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 243.Buschmann MD, Gluzband YA, Grodzinsky AJ, Hunziker EB. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J Cell Sci. 1995;108(Pt 4):1497–1508. doi: 10.1242/jcs.108.4.1497. [DOI] [PubMed] [Google Scholar]
- 244.Torzilli PA, Grigiene R, Huang C, Friedman SM, Doty SB, Boskey AL, et al. Characterization of cartilage metabolic response to static and dynamic stress using a mechanical explant test system. J Biomech. 1997;30(1):1–9. doi: 10.1016/s0021-9290(96)00117-0. [DOI] [PubMed] [Google Scholar]
- 245.Kim YJ, Sah RL, Grodzinsky AJ, Plaas AH, Sandy JD. Mechanical regulation of cartilage biosynthetic behavior: physical stimuli. Arch Biochem Biophys. 1994;311(1):1–12. doi: 10.1006/abbi.1994.1201. [DOI] [PubMed] [Google Scholar]
- 246.Bonassar LJ, Grodzinsky AJ, Frank EH, Davila SG, Bhaktav NR, Trippel SB. The effect of dynamic compression on the response of articular cartilage to insulin-like growth factor-I. J Orthop Res. 2001;19(1):11–17. doi: 10.1016/S0736-0266(00)00004-8. [DOI] [PubMed] [Google Scholar]
- 247.Davisson T, Kunig S, Chen A, Sah R, Ratcliffe A. Static and dynamic compression modulate matrix metabolism in tissue engineered cartilage. J Orthop Res. 2002 Jul;20(4):842–848. doi: 10.1016/S0736-0266(01)00160-7. [DOI] [PubMed] [Google Scholar]
- 248.Mauck RL, Hung CT, Ateshian GA. Modeling of neutral solute transport in a dynamically loaded porous permeable gel: implications for articular cartilage biosynthesis and tissue engineering. J Biomech Eng. 2003 Oct;125(5):602–614. doi: 10.1115/1.1611512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Wang QG, Magnay JL, Nguyen B, Thomas CR, Zhang Z, El Haj AJ, et al. Gene expression profiles of dynamically compressed single chondrocytes and chondrons. Biochem Biophys Res Commun. 2009 Feb 13;379(3):738–742. doi: 10.1016/j.bbrc.2008.12.111. [DOI] [PubMed] [Google Scholar]
- 250.Mauck RL, Soltz MA, Wang CC, Wong DD, Chao PH, Valhmu WB, et al. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000 Jun;122(3):252–260. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 251.Lee DA, Noguchi T, Frean SP, Lees P, Bader DL. The influence of mechanical loading on isolated chondrocytes seeded in agarose constructs. Biorheology. 2000;37(1–2):149–161. [PubMed] [Google Scholar]
- 252.Sah RL, Kim YJ, Doong JY, Grodzinsky AJ, Plaas AH, Sandy JD. Biosynthetic response of cartilage explants to dynamic compression. J Orthop Res. 1989;7(5):619–636. doi: 10.1002/jor.1100070502. [DOI] [PubMed] [Google Scholar]
- 253.Mauck RL, Yuan X, Tuan RS. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthritis Cartilage. 2006 Feb;14(2):179–189. doi: 10.1016/j.joca.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 254.Bougault C, Paumier A, Aubert-Foucher E, Mallein-Gerin F. Molecular analysis of chondrocytes cultured in agarose in response to dynamic compression. BMC Biotechnol. 2008;8:71. doi: 10.1186/1472-6750-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Wang PY, Chow HH, Tsai WB, Fang HW. Modulation of gene expression of rabbit chondrocytes by dynamic compression in polyurethane scaffolds with collagen gel encapsulation. J Biomater Appl. 2009 Jan;23(4):347–366. doi: 10.1177/0885328208093684. [DOI] [PubMed] [Google Scholar]
- 256.Jung Y, Kim SH, Kim SH, Kim YH, Xie J, Matsuda T, et al. Cartilaginous tissue formation using a mechano-active scaffold and dynamic compressive stimulation. J Biomater Sci Polym Ed. 2008;19(1):61–74. doi: 10.1163/156856208783227712. [DOI] [PubMed] [Google Scholar]
- 257.Kisiday JD, Jin M, DiMicco MA, Kurz B, Grodzinsky AJ. Effects of dynamic compressive loading on chondrocyte biosynthesis in self-assembling peptide scaffolds. J Biomech. 2004 May;37(5):595–604. doi: 10.1016/j.jbiomech.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 258.Hall AC, Horwitz ER, Wilkins RJ. The cellular physiology of articular cartilage. Exp Physiol. 1996;81(3):535–545. doi: 10.1113/expphysiol.1996.sp003956. [DOI] [PubMed] [Google Scholar]
- 259.Stockwell RA. The interrelationship of cell density and cartilage thickness in mammalian articular cartilage. J Anat. 1971;109(3):411–421. [PMC free article] [PubMed] [Google Scholar]
- 260.Parkkinen JJ, Ikonen J, Lammi MJ, Laakkonen J, Tammi M, Helminen HJ. Effects of cyclic hydrostatic pressure on proteoglycan synthesis in cultured chondrocytes and articular cartilage explants. Arch Biochem Biophys. 1993;300(1):458–465. doi: 10.1006/abbi.1993.1062. [DOI] [PubMed] [Google Scholar]
- 261.Lammi MJ, Inkinen R, Parkkinen JJ, Hakkinen T, Jortikka M, Nelimarkka LO, et al. Expression of reduced amounts of structurally altered aggrecan in articular cartilage chondrocytes exposed to high hydrostatic pressure. Biochem J. 1994;304(Pt 3):723–730. doi: 10.1042/bj3040723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Takahashi K, Kubo T, Arai Y, Kitajima I, Takigawa M, Imanishi J, et al. Hydrostatic pressure induces expression of interleukin 6 and tumour necrosis factor alpha mRNAs in a chondrocyte-like cell line. Ann Rheum Dis. 1998;57(4):231–236. doi: 10.1136/ard.57.4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Smith RL, Rusk SF, Ellison BE, Wessells P, Tsuchiya K, Carter DR, et al. In vitro stimulation of articular chondrocyte mRNA and extracellular matrix synthesis by hydrostatic pressure. J Orthop Res. 1996;14(1):53–60. doi: 10.1002/jor.1100140110. [DOI] [PubMed] [Google Scholar]
- 264.Smith RL, Lin J, Trindade MC, Shida J, Kajiyama G, Vu T, et al. Time-dependent effects of intermittent hydrostatic pressure on articular chondrocyte type II collagen and aggrecan mRNA expression. J Rehabil Res Dev. 2000;37(2):153–161. [PubMed] [Google Scholar]
- 265.Hansen U, Schünke M, Domm C, Ioannidis N, Hassenpflug J, Gehrke T, et al. Combination of reduced oxygen tension and intermittent hydrostatic pressure: a useful tool in articular cartilage tissue engineering. J Biomech. 2001;34:941–949. doi: 10.1016/s0021-9290(01)00050-1. [DOI] [PubMed] [Google Scholar]
- 266.Carver SE, Heath CA. Semi-continuous perfusion system for delivering intermittent physiological pressure to regenerating cartilage. Tissue Eng. 1999;5(1):1–11. doi: 10.1089/ten.1999.5.1. [DOI] [PubMed] [Google Scholar]
- 267.Heath CA, Magari SR. Mini-review: Mechanical factors affecting cartilage regeneration in vitro. Biotechnol Bioeng. 1996;50:430–437. doi: 10.1002/(SICI)1097-0290(19960520)50:4<430::AID-BIT10>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 268.Domm C, Fay J, Schunke M, Kurz B. Redifferentiation of dedifferentiated joint cartilage cells in alginate culture. Effect of intermittent hydrostatic pressure and low oxygen partial pressure. Orthopade. 2000;29(2):91–99. doi: 10.1007/s001320050015. [DOI] [PubMed] [Google Scholar]
- 269.Hall AC, Urban JP, Gehl KA. The effects of hydrostatic pressure on matrix synthesis in articular cartilage. J Orthop Res. 1991;9(1):1–10. doi: 10.1002/jor.1100090102. [DOI] [PubMed] [Google Scholar]
- 270.Wu QQ, Chen Q. Mechanoregulation of chondrocyte proliferation, maturation, and hypertrophy: ion-channel dependent transduction of matrix deformation signals. Exp Cell Res. 2000 May 1;256(2):383–391. doi: 10.1006/excr.2000.4847. [DOI] [PubMed] [Google Scholar]
- 271.Martina M, Mozrzymas JW, Vittur F. Membrane stretch activates a potassium channel in pig articular chondrocytes. Biochim Biophys Acta. 1997 Oct 23;1329(2):205–210. doi: 10.1016/s0005-2736(97)00154-5. [DOI] [PubMed] [Google Scholar]
- 272.Browning JA, Saunders K, Urban JP, Wilkins RJ. The influence and interactions of hydrostatic and osmotic pressures on the intracellular milieu of chondrocytes. Biorheology. 2004;41(3–4):299–308. [PubMed] [Google Scholar]
- 273.Zhu C, Bao G, Wang N. Cell mechanics: mechanical response, cell adhesion, and molecular deformation. Annu Rev Biomed Eng. 2000;2:189–226. doi: 10.1146/annurev.bioeng.2.1.189. [DOI] [PubMed] [Google Scholar]
- 274.Suzuki S, Nagano T, Yamakoshi Y, Gomi K, Arai T, Fukae M, et al. Enamel matrix derivative gel stimulates signal transduction of BMP and TGF-{beta} J Dent Res. 2005 Jun;84(6):510–514. doi: 10.1177/154405910508400605. [DOI] [PubMed] [Google Scholar]
- 275.Guo X, Wang XF. Signaling cross-talk between TGF-beta/BMP and other pathways. Cell Res. 2009 Jan;19(1):71–88. doi: 10.1038/cr.2008.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Chen HC, Sung LY, Lo WH, Chuang CK, Wang YH, Lin JL, et al. Combination of baculovirus-expressed BMP-2 and rotating-shaft bioreactor culture synergistically enhances cartilage formation. Gene Ther. 2008 Feb;15(4):309–317. doi: 10.1038/sj.gt.3303087. [DOI] [PubMed] [Google Scholar]
- 277.Natoli RM, Revell CM, Athanasiou KA, editors. Trans Orthopaedic Res. San Francisco: 2008. Chondroitinase ABC Treatment Results in Increased Tensile Properties in a Scaffold-less, Serum-free Model of Articular Cartilage Tissue Engineering. [Google Scholar]
- 278.Natoli RM, Responte DJ, Lu BY, Athanasiou KA. Effects of multiple chondroitinase ABC applications on tissue engineered articular cartilage. J Orthop Res. 2009 Jul;27(7):949–956. doi: 10.1002/jor.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Current good manufacturing practice for finished pharmaceuticals. Pt. 211. [Revised as of April 1, 2009]. [Google Scholar]
- 280.Commerce CoEa, editor. Washington: U.S. GPO; 2005. Compilation of Selected Acts within the Jurisdiction of the Committee on Energy and Commerce: Food, Drug, and Related Law, as Amended through December 31, 2004; p. 545. [Google Scholar]
- 281.Quality system regulation. Pt. 820. [Revised as of April 1, 2009]. [Google Scholar]
- 282.Investigational device exemptions. Pt. 812. [Revised as of April 1, 2009]. [Google Scholar]
- 283.Mak AF. The apparent viscoelastic behavior of articular cartilage--the contributions from the intrinsic matrix viscoelasticity and interstitial fluid flows. J Biomech Eng. 1986 May;108(2):123–130. doi: 10.1115/1.3138591. [DOI] [PubMed] [Google Scholar]
- 284.Freeman MAR. Adult articular cartilage. Tunbridge Wells: Pitman Medical; 1979. [Google Scholar]
- 285.Weiss C, Rosenberg L, Helfet AJ. An ultrastructural study of normal young adult human articular cartilage. J Bone Joint Surg Am. 1968 Jun;50(4):663–674. doi: 10.2106/00004623-196850040-00002. [DOI] [PubMed] [Google Scholar]
- 286.Langsjo TK, Hyttinen M, Pelttari A, Kiraly K, Arokoski J, Helminen HJ. Electron microscopic stereological study of collagen fibrils in bovine articular cartilage: volume and surface densities are best obtained indirectly (from length densities and diameters) using isotropic uniform random sampling. J Anat. 1999 Aug;195(Pt 2):281–293. doi: 10.1046/j.1469-7580.1999.19520281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]