Abstract
γ-crystallins constitute the major protein component in the nucleus of the vertebrate eye lens. Present at very high concentrations, they exhibit extreme solubility and thermodynamic stability to prevent scattering of light and the formation of cataracts. However, functions beyond this structural role have remained mostly unclear. Here, we calculate molecular refractive index increments of crystallins. We show that all lens γ-crystallins have evolved a significantly elevated molecular refractive index increment, which is far above those of most proteins, including non-lens members of the βγ-crystallin family from different species. The same trait has evolved in parallel in crystallins of different phyla, including in the S-crystallins of cephalopods. A high refractive index increment can lower the crystallin concentration required to achieve a suitable refractive power of the lens, and thereby reduce their propensity to aggregate and form cataract. To produce a significant increase of the refractive index increment, a substantial global shift in the amino acid composition is required, which can naturally explain the highly unusual amino acid composition of γ-crystallins and their functional homologues. This function provides a new perspective for interpreting their molecular structure.
Keywords: crystallin, protein structure function, protein refractive index, excluded volume
Introduction
Crystallins are the major protein component in the cytoplasm of fiber cells in the lens, a tissue where biochemical, thermodynamic, and optical properties of its constituent proteins closely intertwine 1–3. In order to refract and focus light onto the retina, crystallins need to be soluble and present in extremely high concentrations for the lens to have a high tissue refractive index above those of the aqueous and vitreous humor. The high protein concentration also serves to provide transparency of the lens due to the suppression of long-range concentration fluctuations that would be a source of light scattering 4,5. In order to diminish light scattering further, fiber cells also lose their nucleus and organelles 6, and consequently there is no protein turn-over and the crystallin molecules have to last the life-time of the individual organism 1. Light scattering arising from crystallin aggregation, crystallization, as well as liquid-liquid phase transitions, can cause cataracts 4,7, which are seemingly unavoidable in human lenses at higher age, and are the leading cause of blindness worldwide 8.
This poses extraordinary stability and solubility requirements for crystallins, which have been well studied 1,3,9–14. A common structural feature within the βγ-crystallin family is a pair of domains containing two tightly packed Greek key beta sheet motifs. This fold, with additional contributions from domain interactions, provides for an unusually high thermodynamic stability 15. High solubility is thought to be governed by weak inter-particle interactions conferred by surface properties of the proteins 11,16,17. However, crystallin functions beyond these structural aspects, as well as the purpose of their unusual amino acid compositions, have remained largely unclear.
Information about possible crystallin functions has been gathered from their molecular evolution, which has been of high interest also in the context of the evolution of the eye 18–21. Most crystallins appear to have been co-opted from proteins originally serving functions unrelated to eyes 1,21,22. The major crystallin families in vertebrates, the ubiquitous α-, β-, and γ-crystallins, have been linked to stress-related proteins 12. α-crystallin is a member of the small heat-shock protein superfamily and can function as a chaperone 23–25. β-, and γ-crystallins belong to a superfamily characterized structurally by the Greek key motif 26, which is also found, for example, in a spore coat protein S from Myxococcus xanthus 27, and in an encystment-specific protein spherulin 3A from Physarum polycephalum 28.
In addition to the ubiquitous crystallins, many vertebrate species have developed taxon-specific crystallins 1. These show a remarkable similarity or are even identical to metabolic enzymes, and often continue to be expressed to lesser extent in other tissues 29,30. In some cases, these enzyme crystallins are derived by gene sharing, with continued expression of the same protein in other tissues without loss of the enzymatic function, while in other cases gene duplication took place 1. Examples include δ-crystallin in duck and chicken, derived from argininosuccinate lyase 31, ε-crystallin in birds and crocodiles derived from lactate dehydrogenase B 32, υ-crystallin in platypus derived from lactate dehydrogenase A 33, and λ-crystallin in rabbit derived from L-gulonate 3-dehydrogenase 34.
Through parallel evolution, many invertebrates have developed camera-like eyes with structures superficially very similar to those of vertebrates, which also exhibit different classes of crystallins 35. For example, the main protein component in the lenses of squid and octopus is S-crystallin. Cephalopod S-crystallins form a large and diverse family of proteins, and their evolution has also been studied in detail 36. Remarkably, the same strategy of recruitment of metabolic enzymes as lens proteins has been used in cephalopods: S-crystallins have a high similarity to squid glutathione-S-transferase (GST), from which they emerged by gene duplication and now have little or no enzymatic activity 37.
This naturally poses again the question which property qualifies certain proteins to be recruited to serve as lens crystallins, and – if gene sharing is followed by gene duplication – which further adaptation to a lens specific function takes place that conflicts with the original function 33,38,39. For example, it was recently shown how a chromophore binding protein CRBP was recruited to serve as a taxon-specific crystallin (ι-crystallin) in the gecko, and adapted to bind a suitable chromophore to help cope with intense UV exposure of this species 40. A similar function has been proposed for some other taxon-specific crystallins 41. Unfortunately, such specific crystallin functions, beyond their purely structural properties governing thermodynamic stability and solubility, are largely unclear for most crystallins, notably including the ubiquitous vertebrate βγ-crystallins 2,3,42.
Motivated by the consideration that the refraction of light is the most fundamental function of the lens tissue, we have recently embarked on a study of a possible molecular refractive function of crystallins. As a first stage providing biophysical methodology, we have recently developed a computational tool to predict the molecular refractive index increments of proteins, abbreviated dn/dc, from their amino acid composition 43, based on the method by McMeekin 44,45. Previously, we have applied this to the entire set of predicted proteins of different species, and found that they are normally distributed and fall in a remarkably narrow range 43, but with exception of a few protein classes including crystallins. Second, with a biophysical model of macromolecular crowding in the lens tissue, we have explored the possible relevance of variations of molecular refractive indices 46. Specifically, we have analyzed the thermodynamic consequences of small reductions in protein concentration that become possible from elevated crystallin refractive index increments. We found that, in the most dense lens tissues, even only 5 – 10% higher dn/dc values can, in a non-linear fashion, translate into very significant reductions in the osmotic pressure and in the propensity for aggregation 46. We hypothesized that this can provide a driving force for the evolution of high refractive index increment crystallins.
The present communication is focused on the detailed structure and function of γ crystallins and their homologues from the perspective of their refractive index increment dn/dc. To this end, we examined the known sequences and evolutionary relationships between members of the different crystallin families. Our results show that the molecular refractive index is consistently and significantly elevated in all those crystallins prevalent in the highest density lens tissues, but not for non-lens crystallins, suggesting the molecular refractive index increment to be a tissue-specific crystallin function. To test this idea, we examined the refractive index properties of unrelated crystallins from other phyla. The results from the available sequence data on S-crystallins indicate that they have continuously evolved towards a high dn/dc. Studying this protein function in more detail, we found that no single amino acids and no single class of amino acids can be held responsible for the elevated dn/dc, which instead is generated by a global shift in amino acid composition. We show in the present paper how these findings can naturally explain the previously noted highly unusual amino acid compositions of crystallins, as well as newly identified anomalies in their composition. Finally, we discuss the implications of this dn/dc function for our understanding of the protein structure of γ crystallins, and the role of the Greek key motif to accommodate a variety of different amino acids.
Results
Refractive index increments of vertebrate crystallins
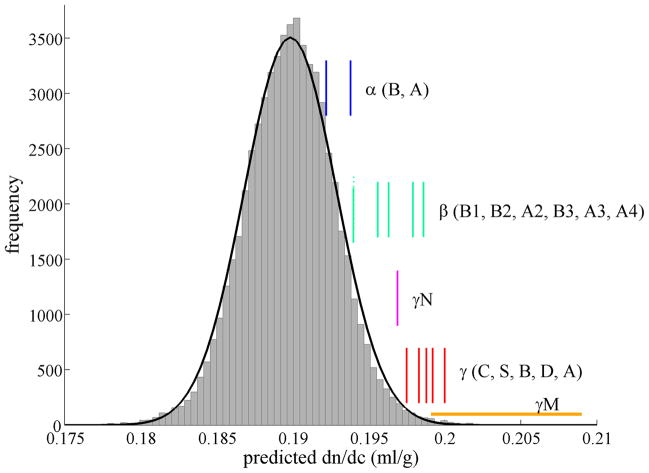
For light in the visible frequency range, the protein refractive index depends essentially only on its atomic composition and local chemical groups, and it is well-known to be independent of long-range structural details 47,48. Accordingly, it is possible to estimate the protein refractive index increment dn/dc from the amino acid composition, using known amino acid refractivities 43–45. We applied this method to the protein sequences of the ubiquitous vertebrate crystallins, as shown in Table 1. Figure 1 shows as vertical bars the dn/dc values computed for the human α-crystallin (blue), β-crystallin (green), and γcrystallin genes (red), superimposed to the dn/dc distribution previously calculated for all predicted human proteins (UCSC genome browser Feb. 2009 (GRCh37/hg19) assembly) 43. (Not shown are the dn/dc values determined for different bovine γ-crystallin genes, which range from 0.199 to 0.202 ml/g, in good agreement with the human values and the experimental value of 0.203 measured by Pierscionek et al. 49.)
Table 1.
Refractive index increment of members of the βγ-crystallin family
| protein | accession # | dn/dc (ml/g) | P& |
|---|---|---|---|
| human alpha-A-crystallin | CAG28619 | 0.1938 | 0.097 |
| human alpha-B-crystallin | NP_001876 | 0.1922 | 0.22 |
|
| |||
| human beta-A1/A3 crystallin | NP_005199 | 0.1979 | 0.0038 |
| human beta-A2-crystallin | NP_476435 | 0.1956 | 0.029 |
| human beta-A4-crystallin | NP_001877 | 0.1986 | 0.0019 |
| human beta-B1-crystallin | NP_001878 | 0.1940 | 0.086 |
| human beta-B2-crystallin | AAB39700 | 0.1940 | 0.086 |
| human beta-B3-crystallin | NP_004067 | 0.1963 | 0.016 |
|
| |||
| human gamma-A-crystallin | NP_055432 | 0.2000 | 3.8E-4 |
| human gamma-B-crystallin | NP_005201 | 0.1988 | 0.0015 |
| human gamma-C-crystallin | NP_066269 | 0.1975 | 0.0056 |
| human gamma-D-crystallin | NP_008822 | 0.1992 | 9.7E-4 |
| human gamma-S-crystallin | NP_060011 | 0.1983 | 0.0026 |
| human gamma-N-crystallin | NP_653328 | 0.1969 | 0.0098 |
|
| |||
| gamma-crystallin M1 (carp) | P10043 | 0.2037 | 2.1E-6 |
| gamma-crystallin M1-2 (teleostean fish) | AAA50960 | 0.2017 | 4.2E-5 |
| gamma-crystallin M2-2 (teleostean fish) | AAA50961 | 0.2041 | 1.1E-6 |
| gamma-crystallin M2-3 (teleostean fish) | AAA50962 | 0.1995 | 6.9E-4 |
| gamma-crystallin M1 (lip shark) | P48648 | 0.2030 | 7.3E-6 |
| gamma-crystallin M2 (lip shark) | P48649 | 0.2050 | 2.4E-7 |
| gamma-crystallin 1 (bamboo shark) | CAA55810 | 0.2031 | 6.3E-6 |
| gamma-crystallin M (Mexican tetra) | AAF07205 | 0.2091 | 1.2E-9 |
| gamma-crystallin MX (zebra fish) | NP_001013280 | 0.2003 | 2.6E-4 |
| gamma-crystallin S1 (zebra fish) | NP_001013294 | 0.1991 | 0.0011 |
| gamma-crystallin N1 (zebra fish) | Q5XTN8 | 0.2001 | 3.4E-4 |
| gamma-crystallin N2 (zebra fish) | Q6DGY7 | 0.2014 | 6.3E-5 |
|
| |||
| EDSP of the amphibious newt 119 | BAA01991 | 0.1901 | 0.47 |
| βγ-component of the protein complex βγ-CAT in toad skin 120 | ABV56830 | 0.1882 | 0.71 |
| AIM1 121 | AAB53792 | 0.1822 | 0.995 |
| protein S of Myxococcus xanthus 27 | AAA25407 | 0.1874 | 0.80 |
| spherulin 3A of Physarum polycephalum 28 | 1AG4_A | 0.1911 | 0.34 |
| Yersina crystallin Yersina pestis CO92 122 | YP_002347819 | 0.1917 | 0.27 |
| βγ-crystallin Geodia cydonium 123 | CAA70024 | 0.1923 | 0.21 |
| archaeal M-crystallin 50 | AAM05909 | 0.1961 | 0.020 |
| the urochordate βγ-crystallin 51 | 2BV2_B | 0.1918 | 0.26 |
P is the probability for obtaining just by chance a dn/dc value as large or larger, if it was a normally distributed random variable as shown in the solid line of Figure 1.
Figure 1.
Refractive index increment of human crystallin genes (vertical bars): human α-crystallin (blue), β-crystallin (green), γN-crystallin (magenta), and γ-crystallin (red). They are drawn superimposed to the dn/dc distribution of all human proteins (histogram for the predicted proteins from the UCSC human genome browser for the human Feb. 2009 (GRCh37/hg19) assembly) 43. The best-fit Gaussian to the distribution of all proteins is indicated as black solid line, with mean of 0.1899 ml/g and standard deviation of 0.0031 ml/g. Also shown is the range of values computed for different fish γM-crystallins (orange horizontal bar), as listed in Table 1.
While values of α-crystallin are close to average, β-crystallins have elevated dn/dc, and those of γcrystallin are very high. Since the distribution of dn/dc for all predicted proteins – most of which will not have a refractive index function – is close to a Gaussian with an average value of 0.1899 ml/g and a small standard deviation of ±0.0030 ml/g (with nearly identical distributions obtained from several other species) 43, we can calculate a probability that a given dn/dc value will be achieved or exceeded if this were a just random variable. For γ-crystallin, these probabilities all are within the range from P = 0.01 to P = 0.0004. The dn/dc values computed for various γM-crystallins from different aquatic vertebrates are still higher (horizontal orange bar), ranging from 0.199 to 0.209 ml/g, corresponding to probabilities P < 10−9 for them to be achieved by chance. An extended list including known γ-crystallins from frog, newt, lizard, and several fish species can be found in Supplementary Table 1, consistently showing dn/dc values three standard deviations above the average.
This significantly elevated dn/dc values of all lens β– and γ–crystallins poses the question to what extent the high refractive index increment is an attribute that already existed in the ancient precursor to the βγ–crystallin family and predisposed it to serve as major lens protein, and/or to what extent this trait has evolved as specialization in the lens. In order to address this, next, we have examined sequences of current non-lens members of the βγ–crystallin family (Table 1). We found that all non-lens βγ–crystallins had low dn/dc with an average of 0.1901, nearly exactly identical to the average of all proteins. For example, Myxococcus xanthus protein S has a value of 0.187 ml/g. An exception is the recently discovered archaeal M-crystallin 50, which exhibited a moderately high value of 0.1961 ml/g (P = 0.02). Of special interest is the protein likely most closely related to vertebrate βγ–crystallin, the urochordate Ci-βγ-crystallin 51, for which we computed a value of 0.1918 ml/g that is well within the spread of dn/dc of average proteins (P = 0.26) (see below). This suggests that the high refractive index increment is a trait that likely did not pre-exist but was specifically acquired in conjunction with expression in the lens, to fulfill a specific function.
Among the different crystallins, the ranking of the crystallin dn/dc mimics conspicuously the ranking of the tissue refractive indices where these crystallin molecules are found: A common feature of eye lenses is a radial refractive index gradient that minimizes spherical aberration 52. A higher refractive index is found in the lens nucleus near the optical axis and lower refractive index in the cortex. This gradient is achieved by a variation of protein concentration and crystallin species 53–55. In human and bovine lenses, α and β crystallins are located mostly in the cortex with lower refractive index and lower protein concentration (200 – 300 mg/ml 56,57), and α crystallin was found to be excluded from the lens nucleus 58. As shown in Figure 1 and Table 1, α and β crystallins also have the lowest dn/dc values in the group of ubiquitous crystallins. In contrast, γ crystallins, which exhibit the highest dn/dc values, are most abundant in the center with highest lens refractive index and highest protein concentration (440 – 500 mg/ml and higher 56,59,60). In the innermost regions of bovine and toad lenses, it was even found to provide nearly all of the soluble protein 61,62. Generally, γ crystallins are also particularly prevalent in hard, high refractive index-lenses which are more closely packed, and relatively dehydrated 63,64. In particular, this is true for fish eyes, which lack the refractive power of the cornea provided by the interface with air, and have hard lenses with the highest known protein concentration of any tissue (estimated in excess of 1,000 mg/ml 12). Correspondingly, as shown in Figure 1 and Table 1, we found that γM crystallins of aquatic vertebrates exhibit the highest dn/dc of all.
We also computed the refractive index increments for taxon-specific crystallins (Table 2). In contrast to the lens γ crystallins, their refractive index increment, as well as those of their related enzymes, are only average (Table 2). Thus, enzymes seem not to have been recruited for an amino acid composition generating unusually high dn/dc. One explanation for this could be that enzyme crystallins continue to be expressed to a lesser extent in other tissue 29,30, and that, therefore, constraints from non-lens functions may have prevented an adaptation to develop a high molecular refractive index increment. On the other hand, many taxon-specific crystallins do not constitute major fractions of the total protein content comparable to those of the highly conserved ubiquitous crystallins 65, and therefore the impact of their refractive index increment on the local tissue refractive index would be minimal. A notable exception is δ-crystallin which replaces γ-crystallin in the soft lenses of birds 41. Interestingly, the comparatively low protein content in these lenses, which might be only between 200 – 300 mg/ml 66, in conjunction with the low refractive index increment of these δ-crystallins fits well to the observed correlation between molecular and tissue refractive index.
Table 2.
Refractive index increment of taxon-specific crystallins
| species | related protein/enzyme | dn/dc (ml/g) | crystallin | dn/dc (ml/g) |
|---|---|---|---|---|
| lampreys, fish, reptiles, birds 124 | α-enolasea | 0.189 | τ | 0.189 |
| duck 32 | lactate dehydrogenase Bb | 0.187 | ε | 0.187 |
| duck/chicken 31 | argininosuccinate lyasec | 0.185 | δ | 0.185–0.187 |
| rabbit 34 | L-gulonate 3-dehydrogenased | 0.188 | λ | 0.188 |
| elephant shrew 125 | aldehyde dehydrogenasee | 0.190 | η | 0.189 |
| gecko 40 | retinol binding protein Ih | 0.195 | ι | 0.195 |
| platypus 33 | lactate dehydrogynase Aj | 0.189 | υ | 0.188 |
| octopus 70 | aldehyde dehydrogenasef | 0.190 | Ω | 0.193 |
| scallop 30 | aldehyde dehydrogenasef | 0.190 | Ω | 0.192 |
| octopus 73 | Lipid-binding protein PEBPg | 0.191 | Ο | 0.194 |
Refractive index increments of invertebrate crystallins
In lieu of an experimental test of the hypothesis that the crystallin molecular refractive index increment has specifically evolved towards higher than average values in high density lens tissue, we next studied crystallins from other phyla, which have evolved independently. Through parallel evolution, some invertebrates have developed camera-like eyes with structures superficially very similar to those of vertebrates 52 but with very different crystallins. Amino acid compositions of invertebrate crystallins are known for squid, octopus, jellyfish, and scallop species.
S-crystallins represent the major component of the hard lenses of cephalopods. From the refractive index in the core of the cephalopod eye lens of 1.485, we can estimate the protein concentration to be ~760 mg/ml 67, which is comparable to the total protein concentration in lenses of fish 1. S-crystallins are also thought to have lens (and cornea) specific expression, where they provide 70–90% of all soluble protein 41. Like γM -crystallins, S-crystallins have acquired extremely high methionine and tyrosine content (see below), and similar to γ-crystallins the specific functions of S-crystallin other than their stability at high concentrations are unknown. S-crystallins form a large and diverse family of proteins, and their evolution has been studied in detail 36. Thus, they provide an excellent model system for the present purpose of testing our refractive index hypothesis. The calculated refractive index increments of different S-crystallins and their relatives are shown in Table 3.
Table 3.
Cephalopod S-crystallins and glutathione-S-transferases
| protein | accession number | dn/dc (ml/g) | P |
|---|---|---|---|
| S-crystallin (SL4) | AAA91343 | 0.1954 | 0.033 |
| S-crystallin (SL11) | AAA29403 | 0.1940 | 0.086 |
| S-crystallin (SL18) | AAA29404 | 0.1965 | 0.014 |
| S-crystallin (SL20) | AAA29402 | 0.1987 | 0.0017 |
|
| |||
| Lops1 | AAA97541 | 0.1900 | 0.49 |
| Lops4 | AAA97540 | 0.1951 | 0.042 |
| Lops5 | AAA97542 | 0.2015 | 5.5E-05 |
| Lops6 | AAA97543 | 0.1995 | 6.9E-04 |
| Lops7 | AAA97544 | 0.2011 | 9.4E-05 |
| Lops8 | AAA97547 | 0.2010 | 1.1E-04 |
| Lops9 | AAA97545 | 0.1988 | 0.0015 |
| Lops10 | AAA97546 | 0.2003 | 0.00030 |
| Lops12 | AAA97548 | 0.1985 | 0.0021 |
| Lops13 | AAA97549 | 0.1984 | 0.0023 |
| Lops16 | AAA97550 | 0.1991 | 1.1E-03 |
| Lops18 | AAB01053 | 0.2019 | 3.2E-05 |
| Lops19 | AAB01054 | 0.1984 | 0.0023 |
| Lops20 | AAB01055 | 0.1984 | 0.0023 |
| Lops21 | AAB01056 | 0.2021 | 2.4E-05 |
| Lops22 | AAB01057 | 0.2029 | 7.3E-06 |
| Lops23 | AAB01058 | 0.2015 | 5.5E-05 |
| Lops25 | AAB01059 | 0.1998 | 4.8E-04 |
| Lops102 | AAA97551 | 0.2002 | 3.0E-04 |
|
| |||
| OctS1 | P27013 | 0.1969 | 0.0098 |
| OctS2 | P27014 | 0.1975 | 0.0056 |
| OctS3 | Q25626 | 0.1975 | 0.0056 |
|
| |||
| OL1 | P27009 | 0.1967 | 0.012 |
| OL2 | P27010 | 0.1963 | 0.016 |
| OL3 | P27011 | 0.1977 | 0.0047 |
| OL4 | P27012 | 0.1968 | 0.011 |
|
| |||
| S I (squid Nototodarus gouldi) | N/A* | 0.1964 | 0.015 |
| S II (squid Nototodarus gouldi) | N/A* | 0.1982 | 0.0028 |
| S III (squid Nototodarus gouldi) | N/A* | 0.1994 | 7.7E-04 |
|
| |||
| glutathione-S-transferases | |||
| 1GSQ [Ommastrephes sloani pacificus (squid)] | 1GSQ_A | 0.1906 | 0.41 |
| 1GNE [Schistosoma Japonicum] | 1GNE | 0.1942 | 0.076 |
| 1GUH [human] | 1GUH_A | 0.1899 | 0.50 |
| 6GST [Rattus rattus (black rat)] | 6GST_A | 0.1955 | 0.031 |
| 2GSR [Sus scrofa (pig)] | 2GSR_A | 0.1911 | 0.34 |
In the squid Loligo opalescens, there are 24 isoforms of S-crystallins with known sequences (termed Lops) that are 46 –99 % identical to each other, and exhibit 36 – 44% sequence identity to squid glutathione-S-transferase (GST), from which they emerged by gene duplication, but now lack enzymatic activity 36. We found that the ancestral enzyme, squid GST, has a predicted dn/dc of 0.191 ml/g, similar to those of GST proteins from other organisms (0.192 ± 0.002) ml/g. In contrast, the known sequences of S-crystallins from Loligo opalescens have an average value of (0.199 ± 0.002) ml/g. Lops12 has been identified a species of major abundance, does not exhibit GST activity 36, and has a dn/dc value of 0.198 ml/g. The S-crystallin species thought to be closest to GST is termed Lops4, exhibits similar enzymatic activity as GST, and has the lowest dn/dc value of 0.195 ml/g.
In order to investigate the relationships between the different Lops molecules and their refractive properties, a phylogenetic tree was constructed with the neighbor-joining method (Figure 2 Top). (The resulting tree is very similar to the Bayesian tree reported by Sweeney et al. 68.) It can be discerned that both Lops4 and Lops20 are closest to 1GSQ, with relatively low but elevated dn/dc. Then, the tree branches off into an upper cluster with relatively high dn/dc values that contains the most abundant Lops12 (Lops102 – Lops13), and a lower cluster (Lops16 and below) that exhibits the highest dn/dc values. In order to more directly visualize the relationship between dn/dc and the sequence distance from 1GSQ (which we assume is closet of all to the ancestral precursor enzyme), we plotted dn/dc versus the sequence distance as extracted from the distance matrix in the software PHYLIP (Figure 2 Bottom). A strong correlation between sequence distance and dn/dc can be discerned. Indicated in black are the isoforms in the lower cluster of the tree (Lops16 and below), which have the largest sequence distance. This is consistent with the notion that the advantages from optimizing the refractive index were at least one of the driving forces in the evolution of Lops.
Figure 2.
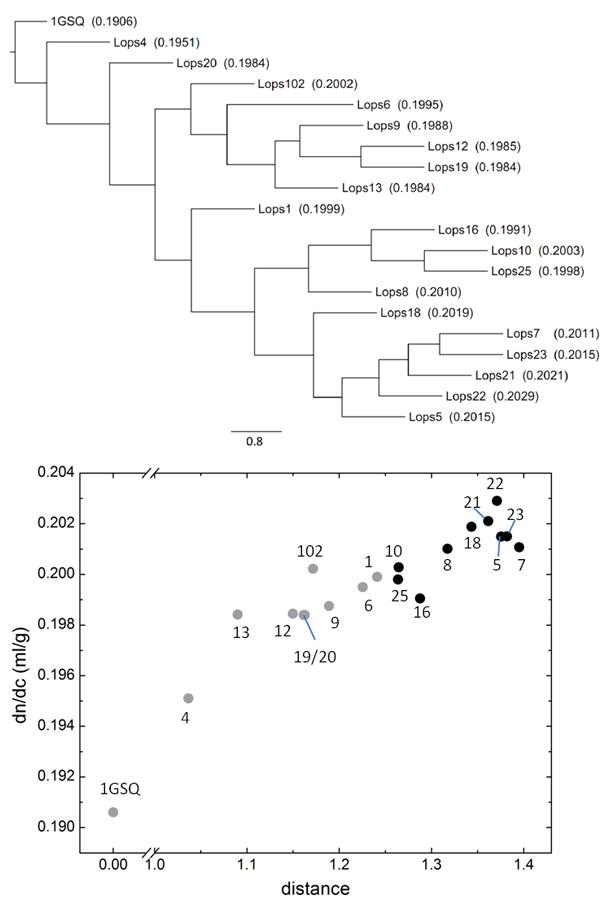
Relationship between the 24 known S-crystallins from Loligo opalescens and glutathione-S-transferase, in conjunction with their refractive properties. Top: Consensus phylogenetic tree of S-crystallins and squid glutathione-S-transferase, using 1GSQ from Ommastrephes sloani as a reference, from a 500-replicate bootstrap analysis of the neighbor-joining tree. The edge lengths represent the value resolved from bootstrap analysis. The scale bar represents a bootstrap value of 80%. The values in parentheses are the calculated refractive index increment (dn/dc) values. Bottom: Correlation of dn/dc with the distance between each protein to 1GSQ. The distances were calculated by the program protdist in PHYLIP. The identity of the molecules corresponding to each data point is indicated by the associated number. The black circles represent the cluster Lops16 and below in the tree above, all other isoforms are in grey.
A consistent pattern was found in other squid and octopus species, for which less detail is known (Table 3): For S-crystallins of the squid Nototodarus gouldi 69, high refractive increments (0.198 ml/g) were obtained, although no sequences from intermediates are known. In the squid Ommastrephes sloani, there are four known members of S-crystallins that exhibit 42–44% sequence identity to squid glutathione-S-transferase (GST) 37. Their average dn/dc is (0.196 ± 0.002) ml/g. Mirroring the findings from Loligo opalescens, in Ommastrephes sloani the most abundant and putatively most recent member, SL20 (exhibits 80% sequence identity to Lops12) has no enzymatic activity and exhibits a value of 0.199 ml/g. One difference between GST and most S-crystallins are repeats of an insert of up to 193 residues with several repeats of RGDGGYXVQG/S 37, a sequence with relatively high value of 0.196 ml/g. The S-crystallin species thought to be closest to GST and orthologous to Lops4 is termed SL11, and has similarly high enzymatic activity. It does not have an insert, and is thought to be an evolutionary intermediate between GST and the fully developed SL20 36. We found it to have the lowest dn/dc value at 0.195 ml/g, close to the value of squid GST. The correlation between decrease of enzyme activity and increase in dn/dc implies that the lens-specific S-crystallins in the two types of cephalopods have undergone the adaption into structural proteins from their ancestor to provide high refractivity in eye.
S-crystallin sequences have also been determined for two octopus species 70,71. Like in squid, they are related to GST (20–25% sequence similarity 70), and exhibit a 48–58% sequence similarity with squid SL20 and 42–80% similarity between the octopus species 71. We calculated a high dn/dc, with values of ~0.197 ml/g (Table 3). In addition, octopus has taxon-specific crystallins in low abundance that show only slightly elevated dn/dc values (Table S3), including Ω-crystallin related to aldehyde dehydrogenase (~10% of lens proteins) 70,72, as well as an O-crystallin (3%-5% of lens proteins) 73. An orthologue of Ω-crystallin is also found in the soft lens of scallop 74, but with an only average dn/dc of 0.190 ml/g. Although it is the sole crystallin in the scallop, it is also found in significant amounts in non-ocular tissue 74.
In a different phylum, box jellyfish also have sophisticated eyes with relatively high refractive index lenses 75. Their crystallins are J-crystallins, which may also serve some non-lens function 30. They have a 43–50% sequence similarity to saposins 76 (which have a predicted dn/dc of 0.188 ml/g), and 36% identity to the ADP-ribosylation enzyme SelJ 77 (with 0.192 ml/g). The average dn/dc of the known J-crystallins was calculated to be 0.195 ml/g.
In summary, our results show that the development of a high dn/dc trait of the vertebrate γ-crystallins from low dn/dc non-lens precursors seems to be repeated independently in different evolutionary lineages. This signifies a functional role of the molecular refractive index in lens crystallins.
Amino acid composition
It is interesting to study the protein amino acid composition and structure from this perspective. As mentioned above, the overall distribution of protein dn/dc values is very narrow with a standard deviation of 0.003 ml/g. This is due to the fact that the amino acid refractivities are little correlated with their chemical properties 43. Amino acids with high polarizability and refractive index increment are those containing aromatic rings, sulfur, or double-bonds in the R-group, the highest ones being tryptophan, phenylalanine, tyrosine, histidine, cysteine, arginine, and methionine. However, for achieving a high refractive index increment through amino acid substitution, the most drastic single amino acid change possible (a substitution of a proline to a tryptophan) would change the γ-crystallin dn/dc only by 0.0009 ml/g, and most single substitutions would change it much less (for example, a single lysine to arginine substitution would change dn/dc by only 0.0002 ml/g). This is small compared to the difference in refractive index of γ-crystallins to average proteins, which can be achieved only by a major overall change towards highly unusual composition.
The amino acid composition from the perspective of its impact on dn/dc is illustrated in Figure 3 Top Panel for several vertebrate γ-crystallins in comparison with the putative ancient non-lens relative M. Xanthus protein S. In the abscissa of Figure 3 are shown all amino acids, ranked by their refractivity. The ordinate shows the relative abundance of each amino acid (in %) as a difference to the normally expected percentage of each amino acid, the latter determined from the set of all predicted human proteins (UCSC Feb. 2009 (GRCh37/hg19) assembly). In order to visualize how unusual a certain deviation from the average abundance is, their full frequency distribution of % content for each amino acid is shown as a color contour plot in the background. It highlights in red to white the extremely rare percentages for each amino acid (frequencies of lower than 0.05 and higher than 0.95 of the cumulative frequency distribution, as shown in the color bar). Examples of the full frequency distribution of % abundance and the observed percentages for individual amino acids are shown in the insets.
Figure 3.
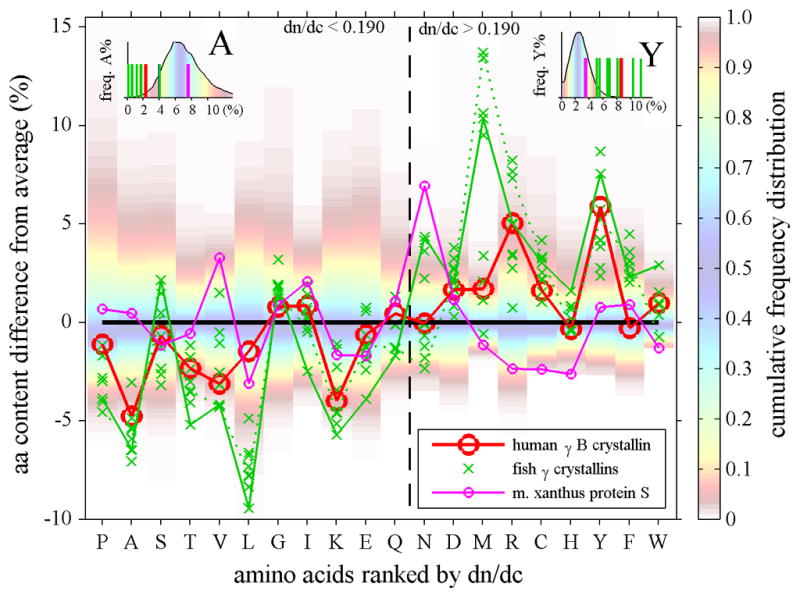
Top Panel: Amino acid composition of γ-crystallins (crosses and triangles) and M. xanthus protein S (magenta line with small circles), plotted as difference of their percent % content from the average content observed in human proteins. Each amino acid is represented in one vertical column. In the background of each column, the color patches indicate the spread of the distribution of fractional amino acid content in all human proteins (Feb. 2009 (GRCh37/hg19) assembly), using the color temperature scale on the right. For example, the range between the red shaded areas (from yellow to blue to yellow) encompasses the content of the amino acid found in approximately the central 80 percent of all proteins, whereas dark red shade fading to white indicates unusually high and low amino acid content among the lowest 5 or highest 95 percentile of all proteins. The insets for A and Y highlight the relationship between the observed amino acid content in crystallins (bars) and the histogram of the frequency distribution of that amino acid content in all proteins (in absolute %). The abscissa shows all amino acids ranked by increasing dn/dc. We can discern that in γ-crystallins the content of low dn/dc residues is mostly lower than average, whereas their content of high dn/dc residues is mostly higher than average. For example, human γB-crystallin (red circles and solid red line) shows values predominantly in the lower left and upper right quadrant. This behavior is particularly evident for A, V, L, T and M, R, Y residues in γ-crystallins of fish (green crosses, showing data from carp, teleostean fish, lip shark, Mexican tetra, and zebra fish), which have contents far outside the normal distribution. Highlighted by the green solid line are the data for (green solid line) and lip shark γ-M2 (green dotted line), which have the highest and second highest values, respectively. In contrast, while Protein S (magenta line and small circles) exhibits unusual content of N and H, it shows values scattered more evenly for both high and low dn/dc residues. Bottom Panel: In the same comparison with the average abundances of the amino acids, shown are the data for different human γ-crystallins (blue symbols, γS is highlighted by a blue line and γD highlighted by a green line) and its closest known ancestor, the urochordate Ci-βγ-crystallin (magenta).
In this representation, we can discern several aspects of the origin of the high dn/dc of vertebrate γ-crystallins. First, the fact that the predominant population of the symbols are in the lower left and upper right quadrant of this plot shows that there is not just one or two specific amino acids that are in high abundance and serve to alter the dn/dc, but rather there is an overall shift in amino acid composition. Second, the elevation of the relative abundance of highest dn/dc residues, such as aromatic amino acids, cysteine, arginine, and methionine, is accompanied by the elimination of amino acids with low refractivity, such as alanine, valine, threonine, and leucine. Third, the comparison with the natural distribution of these amino acids’ abundance in human proteins indicates that several of the deviations are very significant. For example, for human γB-crystallin highlighted by the red line, this can be discerned from the data points being located in the red to white bands. In contrast, the putative ancient relative M. Xanthus protein S (magenta line), even though it does not have a perfectly average content of all amino acids, exhibits examples for both high contents of some low dn/dc amino acids as well as low content of high dn/dc amino acids.
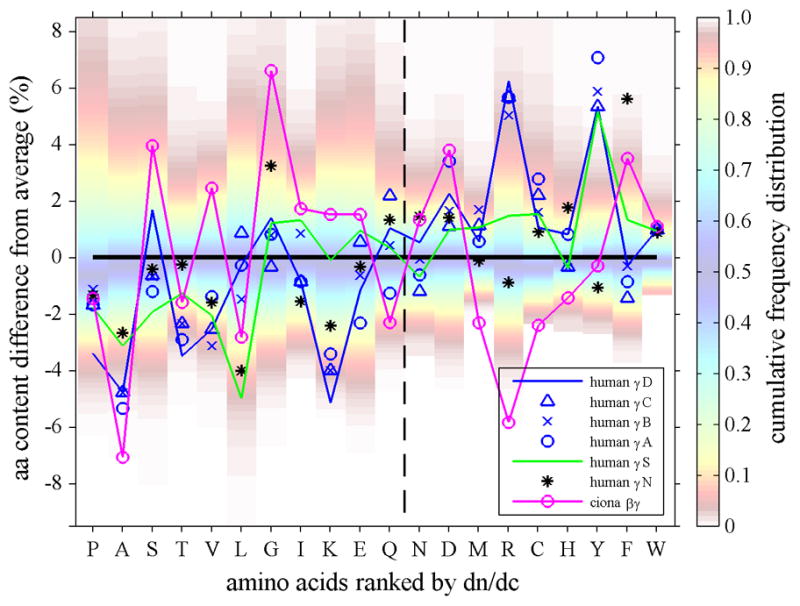
This pattern is exacerbated in fish, which has the highest density lenses and the highest dn/dc crystallins. For example, highlighted in Figure 3 Top Panel by the green line are the data from lip shark γM2. In fact, several fish γ-crystallins are very extreme in that they contain no alanine, lysine, and/or threonine at all, but unusually high fractions of phenylalanine, tyrosine, arginine, and methionine. The most extreme example is the Mexican tetra, which has very few low dn/dc residues – a single alanine, a single leucine, no lysine and no threonine, but extremely high numbers of dn/dc ones with 4.2 % tryptophan, 10.2% tyrosine, 10.8% arginine, and 12.6% methionine. In a similar presentation, Figure 3 Bottom Panel shows the deviation from average amino acid composition for the different human γ-crystallins (blue symbols) in comparison with their closest known ancient relative, the urochordate Ci-βγ-crystallin (magenta) which has a dn/dc value of 0.1918 ml/g. In this plot, γS is highlighted (blue line), which was found to be expressed in the cortex 78 and has a dn/dc value of 0.1983 ml/g, as well as γD (green line), which is a major component of the lens nucleus with a dn/dc value of 0.1992 ml/g.
For Lops proteins, a pattern emerges similar to that of vertebrate γ-crystallins (Figure 4). While squid GST shows relatively evenly distributed values, Lops crystallins have systematically lower fractions of low dn/dc residues, especially serine and leucine, accompanied by high fractions of high dn/dc residues, such as asparagines, methionine, tyrosine, and phenylalanine. Further, one can discern that the evolution from the most GST-like Lops4 to the currently Lops12 has caused, with few exceptions, the systematic exacerbation of this pattern.
Figure 4.
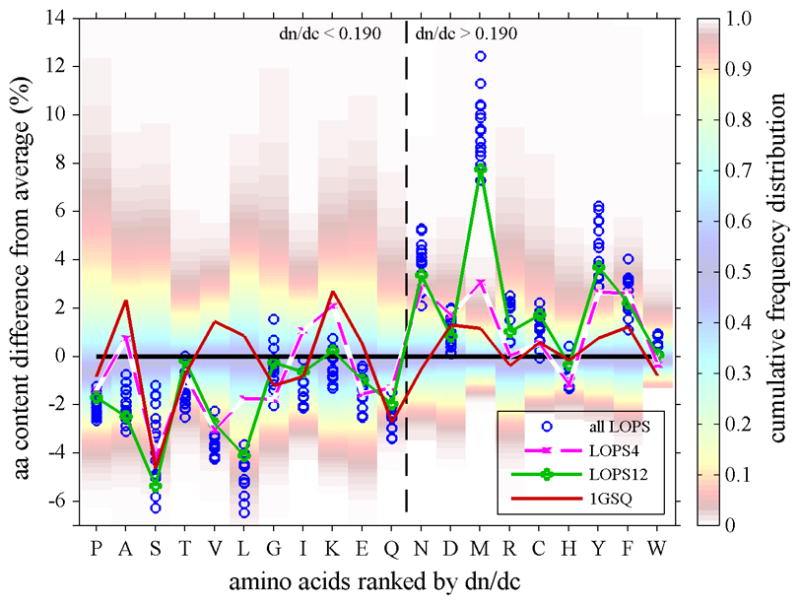
Amino acid composition of different S-crystallins isoforms from Loligo opalescens (symbols) and of 1GSQ (red solid line), plotted as difference of their percent % content from the average content observed in human proteins, in the same presentation as in Figure 4. Highlighted are the most ancient S-crystallin (Lops4, magenta dashed line), and the most abundant and more recent S-crystallin (Lops12, green solid line). With the exception of F and D, all high dn/dc residues in the more recent molecule Lops12 are higher, and with the exception of G and E all low dn/dc residues in the more recent molecule are lower than in the most ancient Lops4. This results in the increase of refractive index in the order from GSQ to Lops4 to Lops12.
It is interesting to look more quantitatively at the contributions of the abundances of each amino acid in the crystallin sequence towards the cumulative elevation of the crystallin dn/dc above that of average proteins. As outlined in the Methods section Eqs. 1 – 3, dn/dc is not simply additive. Nevertheless, we can calculate the change in dn/dc for a hypothetical sequence where, separately for each amino acid, their relative abundance in crystallin is adjusted to match that of average proteins. This will quantify how important the contribution of that amino acid is towards maintaining the elevated crystallin dn/dc. The results are listed in Table 4, normalized by the total difference in dn/dc between the crystallin and the average protein. The values in parenthesis quantify how, from a background of an average protein, the average dn/dc will increase if a particular amino acid abundance is adjusted to the level occurring in the crystallin. Both viewpoints provide slightly different numbers, especially for those amino acids with dn/dc in between those of the average protein and crystallin (which could potentially play a role an evolutionary path).
Table 4.
Contribution of different amino acids to the elevation of crystallin refractive indices
| amino acid | (dn/dc)a (ml/g)(1) | normalized dn/dc contribution of each amino acid (%)(2) | ||
|---|---|---|---|---|
| γ B(3) | γM (4) | Lops22 (5) | ||
| Pro | 0.166 | 3.6 (2.9) | 6.5 (2.0) | 5.3 (2.2) |
| Ala | 0.167 | 9.5 (7.7) | 8.2 (2.9) | 4.3 (1.3) |
| Ser | 0.170 | 1.3 (1.0) | −3.6 (−4.2) | 5.6 (2.2) |
| Thr | 0.172 | 5.8 (4.3) | 8.3 (2.5) | 5.2 (1.7) |
| Val | 0.172 | 8.5 (6.5) | 6.6 (1.4) | 7.0 (2.9) |
| Leu | 0.173 | 4.8 (3.5) | 15.9 (7.4) | 14.0 (7.9) |
| Gly | 0.175 | −0.7 (−0.5) | −1.5 (−3.0) | 0.5 (−1.5) |
| Ile | 0.179 | −1.2 (−0.8) | 3.5 (−0.8) | 3.9 (0.3) |
| Lys | 0.181 | 8.5 (5.0) | 8.1 (0.8) | 1.6 (−1.0) |
| Glu | 0.183 | 1.1 (0.6) | 5.8 (−0.5) | 4.3 (0.0) |
| Gln | 0.186 | −0.9 (−0.2) | 2.1 (−2.0) | 4.6 (−0.7) |
|
| ||||
| Asn | 0.192 | −0.4 (0.1) | −3.6 (−1.8) | −3.7 (−1.1) |
| Asp | 0.197 | − 0.4 (1.2)
|
−1.1 (−1.7) | −0.8 (−0.9)
|
| Met | 0.204 | 1.1 (3.1) | −3.2 (6.0) | 1.5 (12.6) |
| Arg | 0.206 | 5.5 (11.7) | −1.2 (3.0) | 0.6 (1.9) |
| Cys | 0.206 | 1.1 (2.7) | −0.5 (−0.1)
|
0.3 (0.2) |
| His | 0.219 | −1.5 (−2.2) | 1.0 (0.9) | −1.8 (−5.4) |
| Tyr | 0.240 | 38.2 (42.4) | 18.2 (2.2) | 25.7 (30.1) |
| Phe | 0.244 | −3.5 (−4.5) | 5.3 (6.4) | 8.7 (10.1) |
| Trp | 0.278 | 15.7 (18.1) | 16.7 (19.3) | 7.9 (8.0) |
Calculated for a hypothetical poly-peptide of that amino acid.
Difference between the dn/dc calculated from the protein sequence (dn/dc)P and a sequence where the relative abundance of amino acid X is changed to that of average proteins (dn/dc)PXavg, normalized by the total difference (dn/dc)P – 0.190 ml/g. The values in parenthesis are the difference between (dn/dc)avgXP of an average protein with relative abundance of X set to that in crystallin minus 0.190, normalized by the total difference (dn/dc)P – 0.190 ml/g.
human γB crystalline (NP_005201).
Mexican tetra γM-crystallin (AAF07205).
squid S-crystallin (AAB01057). The solid horizontal line separates amino acids with lower than average from those with higher than the dn/dc of average proteins. The horizontal lines in the columns of the individual proteins similarly separates the amino acids lower and higher relative to the dn/dc of that crystallin.
From either perspective, the data highlight that a global shift in amino acid composition is required for achieving the high molecular refractive index increment of lens crystallins. Although the tyrosine residues make the biggest contribution, they provide little more than a third of the refractive index elevation of human γB, and substantially less in the other species examined. In contrast, the cumulative contribution from the relative lack of low dn/dc residues is larger than the effect from tyrosine or any other high dn/dc residues. For example, in the γM from Mexican tetra the extremely low leucine content alone has almost the same effect on dn/dc as the elevated tyrosine content.
Refractive index increments and protein structures
Even though the refractive properties are independent of the three-dimensional structure, it is of interest to inspect the location of the amino acids that are particularly enriched in lens crystallins. To this end, Figure 5 shows a structural comparison of human γB-crystallin and the homologous spore coat protein from M. Xanthus protein S 79, highlighting the high dn/dc amino acids Met, Arg, Cys, Tyr, and Trp, which are significantly more abundant in the lens protein than in its ancient relative. It is well possible that the increased fraction of at least some of these high dn/dc residues could additionally support other essential functions. In particular, their surface accessibility is significant, as the surface characteristics of γcrystallin will govern inter-particle interactions and solubility. (For simplicity, in Figure 5, we do not show the low dn/dc residues that are more abundant in protein S, the lack of which would be difficult to attribute an alternative function in γB-crystallin; see below).
Figure 5.
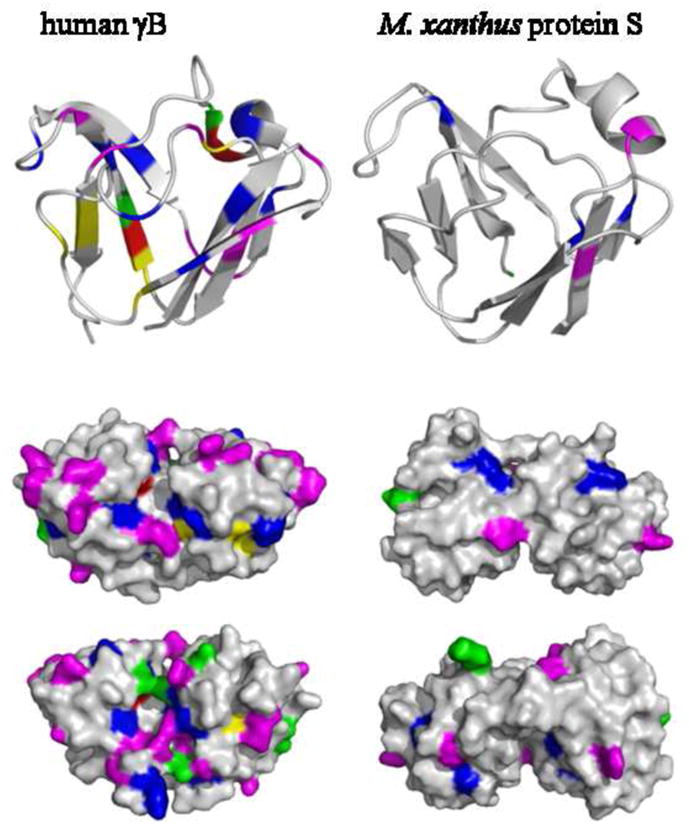
Top: Ribbon representation of the N-terminal domain of human γB-crystallin (2JDF) and M. xanthus protein S (1PRR). Highlighted residues are in high relative abundance in γB-crystallin with high refractive index increment: methionine (green), arginine (magenta), cysteine (yellow), tryptophan (red), and tyrosine (blue). Middle and Bottom: Surface representation of the whole molecules containing two domains, viewed from two opposite angles.
In human γB-crystallin, there are 19 arginines compared to 6 in Protein S (Figure 5, magenta). They are indeed preferentially located at the surface (which may be discerned from the fact that they are ~11% of total amino acids providing ~21% of the total protein surface area), and therefore could fulfill dual roles in contributing both to surface characteristics and dn/dc. However, it appears that the high dn/dc residues overall are rather evenly distributed between surface and interior, contributing to the solvent accessible area approximately in proportion of their relative abundance, representing 35% of the total amino acids and providing 38% of solvent accessible area in human γB-crystallin. In particular, the four tryptophan residues of human γB-crystallin are nearly entirely buried 10, and most of the 15 tyrosine residues are largely solvent inaccessible, and are therefore difficult to imagine contributing significantly to solubility and interaction properties.
A high abundance of methionine in γ-crystallin of aquatic vertebrates has been proposed to fulfill a role in mediating intermolecular interactions that aid in dense packing 80. Similarly, there are many surface accessible arginine and methionine residues in the S-crystallins of cephalopods. However, a homology model of OctS3 81 shows methionine contributing approximately 10% of the total amino acids while also providing ~10% of the surface area (see Supplemental Figure 1), the even distribution suggesting that they may at least not have an exclusive role in mediating surface properties, consistent with their role in aiding the molecular refractive index, a function for which pragmatically the location of the residues within the three-dimensional protein structure is not relevant.
A more detailed structural comparison of the amino acid replacements from lens and non-lens crystallin is possible from a structure-based sequence alignment of human γB-crystallin (2JDF) and its closest known ancient relative, the βγ-crystallin from C. intestinalis (2BV2) 51. The high degree of sequence similarity (49%) and structural similarity 51 makes it possible to ask where individual amino acid substitutions occurred that led to the increase from low dn/dc of 0.1918 ml/g in C. intestinalis to the high value of 0.1988 ml/g. To this end, Figure 6 shows a structural alignment and a structure-based sequence alignment (γB-crystallin in yellow, C. intestinalis βγ-crystallin in grey). It is interesting here that the set of identical residues alone (marked blue in the γB-crystallin sequence and structure) are of a composition with predicted dn/dc value of 0.201 ml/g. Elevation of the dn/dc of the complete protein for the lens crystallin is accomplished by substitution of the majority of remaining residues replacing low with high dn/dc residues (marked in red). In our view, these substitutions are not systematically coincident with structural features.
Figure 6.
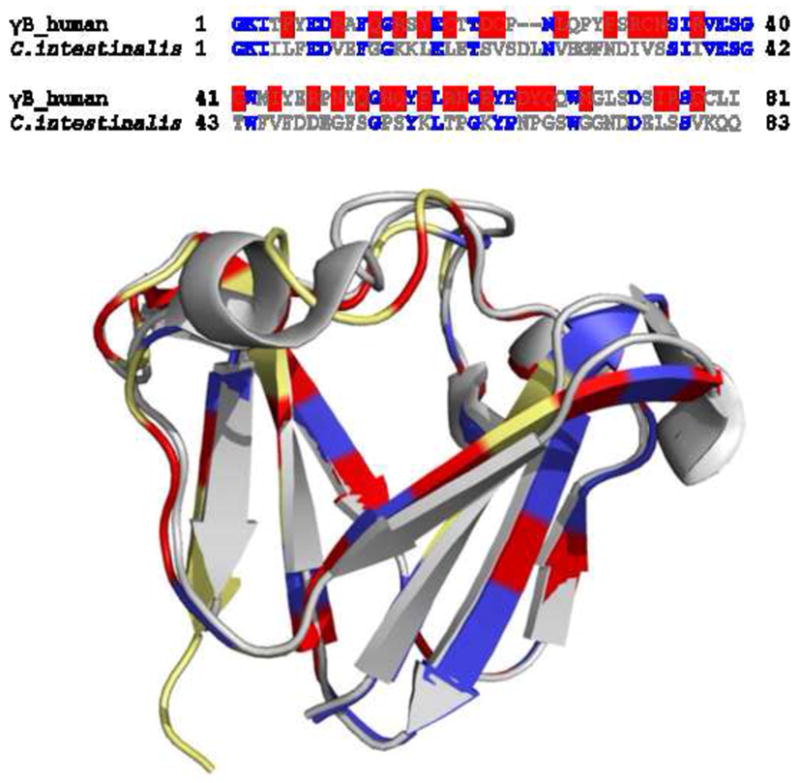
Sequence and structure comparison of N-terminal domain of human γB crystallin (2JDF) and βγ-crystallin from C. intestinalis (2BV2). (A) Sequence alignment of the two polypeptides. The identical residues are in blue, and the residues with higher dn/dc in human γB crystallin are highlighted in red. (B) Structural overlay of the two constructs. The N-terminal domain of human γB crystalline is in yellow and βγ-crystallin from C. intestinalis is in gray. The residues highlighted in blue are the identical ones. The residues in human γB crystalline which have higher dn/dc values compared to those at the same positions in βγ-crystallin C. intestinalis are highlighted in red.
Discussion
Our data show that, independently in different phyla, lens crystallins from the highest density tissues have specifically evolved a significantly elevated molecular refractive index. The observation of an elevated protein refractive index may be unexpected considering the wide-spread assumption that dn/dc of all proteins can be considered in good approximation to be identical, with values expected to be between 0.18 – 0.19 ml/g 82–84. However, this conventional wisdom has been questioned previously by Pierscionek et al., who have measured an unusually high value for bovine γ-crystallin of 0.203 ml/g 49. Consistent with this, a detailed bioinformatics analysis of the distribution of refractive index increments of predicted proteins from different species generally confirmed the narrow spread of values for most proteins, but with distinct exceptions, including the βγ-crystallin family 43. Another class of proteins with highly elevated dn/dc are reflectins 43, which were identified in reflector platelets of squid light organ reflector tissues that serve to provide thin film interference 85,86.
Since the increased crystallin refractive index could only be achieved by virtue of a substantial, global shift in amino acid composition, and yet, the relative increase over average protein generally amounts to 10% or less, one can suspect that a high dn/dc must confer a substantial evolutionary advantage. The question arises in which way such a molecular refractive index function would be helpful. We believe the answer to this can be found in the correlation of high dn/dc with the intracellular protein concentration in the lens tissue, and in the observation that with crystallin molecules of higher refractive index increment, the same lens refractive index can be achieved with a proportionally lower concentration of crystallins.
In a related work we have explored this question from a theoretical perspective 46. We have shown with a model of macromolecular crowding of non-interacting hard spheres, that in the extreme environment of fiber cells (which in the center of high density lenses may have total protein concentrations 500 mg/ml and above) even a small reduction of protein concentration afforded by the higher macromolecular dn/dc can have profound thermodynamic consequences. With average free distances between crystallins only being approximately half their radius, the cytosol is highly thermodynamically non-ideal. Further increasing the protein concentration is associated with a steeply increasing osmotic pressure and steeply increasing chemical activity and energetic cost to keep the proteins soluble. Using scaled particle theory to capture the effect of volume exclusion in this solution, we have calculated the saving in osmotic pressure from lower protein concentrations that become possible with a 6.5% higher dn/dc crystallin (at the same tissue refractive index). This relative reduction in osmotic pressure is shown in Figure 7 Top as a function of total protein lenticular protein concentration. In the highest density lenses, it can amount to 20 – 25%. Similarly, we have calculated the reduction in the chemical activity (Figure 7 Bottom Inset), and – in a thermodynamic model originally introduced in the context of hemoglobin sickling 87,88 – the increase in the stability of crystallin to be soluble relative to it being in a polymer or crystallin phase (Figure 7, Bottom). In lenses of greater than 500 mg/ml total protein, this can amount to more than 1 kcal/mol. Although the thermodynamic consequences of volume exclusion are obligatory and will qualitatively affect all species, interactions between different protein species (which were not accounted for in our model46) can further modulate their stability in the crowded environment 17.
Figure 7.
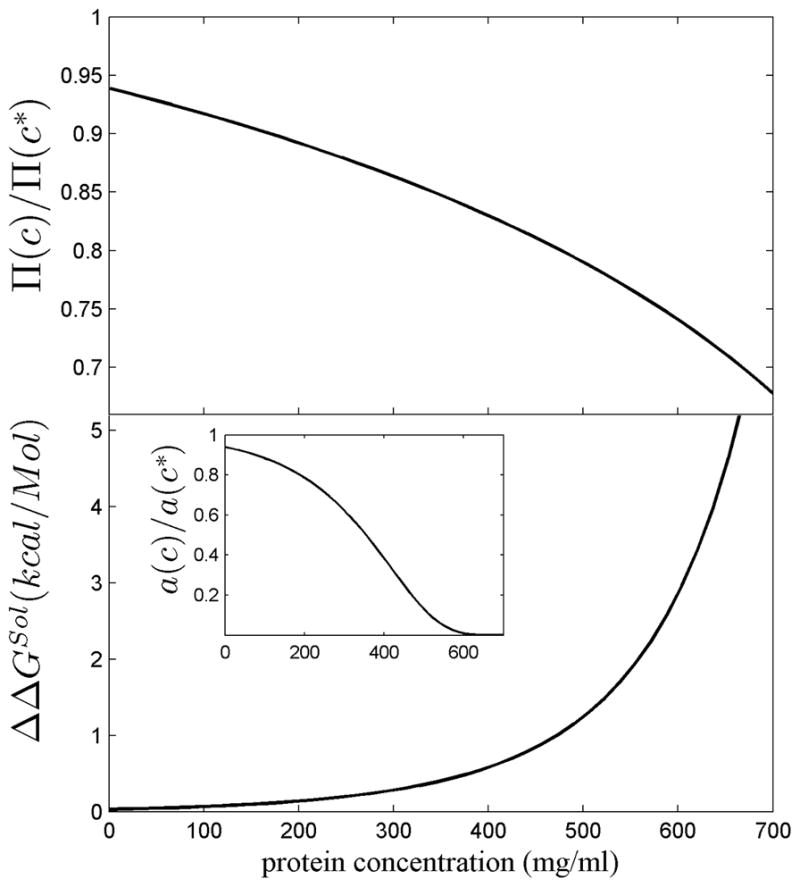
Thermodynamic effect of a crystallin refractive index 6.5% above that of average proteins, corresponding to the value of bovine γ-crystallin. Results are based on a non-interacting hard sphere model of crystallin calculating the excluded volume by scaled particle theory 126, as described in detail in 46, and presented as a function of total protein concentration c. In order to reach the same tissue refractive index as high dn/dc molecules at concentration c, molecules with average dn/dc would have to be present at a higher concentration c*, in the present case c* = 1.065 c. Top Panel: The ratio of the osmotic pressure Π (c)/Π(c*), corresponding to the reduction in osmotic pressure arising from the high dn/dc. Bottom: The difference in the free energy of transfer of a crystallin molecule from the soluble phase into a polymer phase, ΔGSol(c), to the same free energy at a higher crystallin concentration, ΔGSol(c*), with ΔΔGSol(c) = ΔGS(c) - ΔGS(c*). The calculations are based on a ‘crystal approximation’ 87,88. The positive sign of ΔΔGSol means that at the lower concentration afforded by the increase in dn/dc makes the transition into the polymer phase less favorable. The insert shows the ratio of the chemical activity (i.e. ratio of ‘effective’ concentrations) arising from a 6.5% lower actual protein concentration.
The salient aspect of these calculations is that, because at higher concentrations it becomes increasingly more difficult to find sufficient space for accommodating more proteins, small changes in the actual protein concentration can be strongly non-linearly amplified into very significant changes in thermodynamic parameters governing reactivity and aggregation. Since the formation of crystalline or polymer phases leads to light scattering and cataract, as shown by many forms of cataract caused by point mutants of γ crystallin that cause reduced solubility and/or crystallization 89–95, the improved thermodynamic stability of the soluble state afforded by molecules with higher dn/dc (and at correspondingly lower concentration) could well explain the evolutionary driving force for the adaptation to high dn/dc of crystallins prevalent in the highest density lenses. This hypothesis can explain the correlation between molecular refractive index increment and the tissue density of the ubiquitous vertebrate crystallins, as well as the distribution of different crystallins between lens cortex and lens nucleus observed in several vertebrate species. Further, it is consistent with the absence of adaptation to high dn/dc of taxon-specific crystallins in low density lens tissues (as in the lenses of birds as well as in the cortex of human lenses) 46Footnote 1
From our data it is evident that substitution of a single or a few amino acid residues is insufficient to generate significant increase in dn/dc, but requires an overall shift, replacing many low dn/dc residues with many residues of above-average dn/dc. (This is evident, similarly, in the amino acid composition of reflectins, which are highly enriched in sulfur-containing and aromatic amino acids and contain no alanine, isoleucine, leucine, and lysine 85.) This provides a natural explanation for the puzzle of an unusual amino acid composition in γ-crystallins and cephalopod S-crystallins that have been noted previously by several groups: Vertebrate γ-crystallins exhibit an unusually high tyrosine content, conserved in most vertebrate γ-crystallins 41, and an unusually high number of cysteine residues 80. Similarly, an unusually high fraction of arginine relative to lysine in human γ-crystallins 41 would be naturally explained by the higher refractive index increment of the former (0.206 ml/g) compared to that of the latter (0.181 ml/g). The present work led to the identification of another strong anomaly in crystallins, which is the relative lack of alanine, threonine, valine, and leucine.
A prominently noted anomaly in the amino acid composition of γ-crystallin is an extremely high content of methionine residues in γM -crystallins of fish (e.g., 15% in catfish) 96,97, mirrored in S-crystallins of cephalopods 69,70, which also have very high fraction of arginine and methionine residues (11.6% and 10.7%, respectively). The purpose of the high methionine content in fish γ-crystallin is unknown, and was speculated to contribute to protein stability 97, aid solubility and cold adaptation 98, and to lead to an increased refractive index increment 65. From the present work, it seems that high methionine content would be suitable to contribute to an increase dn/dc for most proteins.
On the other hand, it is interesting to consider ‘extreme’ crystallins such as γM-crystallin from Mexican tetra or Lops22. They have overall refractive index increments similar or in excess of that of a hypothetical polypeptide of methionine (0.204 ml/g). Therefore, the methionine residues do actually not appear to be very helpful anymore, or might even be detrimental, for achieving dn/dc-values in excess of 0.204 ml/g. This points to the fact that more crucial for extremely high dn/dc proteins is the balance between the aromatic amino acids and the elimination of low dn/dc residues (compare Table 4). However, at some point it seems certain that other requirements for γ-crystallins, such as high thermal stability and solubility and low hydration, would be conflicting with such optimization. In this regard, reflectins are noteworthy in that they have a dn/dc substantially higher than crystallins, but exhibit strong self-assembly 86 that would be most detrimental for a crystallin. The fact that even single amino acid replacements have been identified as the cause of altered phase transition leading to the formation of cataracts 91,93,99 highlights the delicate balance of forces for maintaining solubility.
The most effective residues for increasing dn/dc appear to be tryptophan and tyrosine. The highly elevated tyrosine content does currently not seem well justified by their UV absorption property, considering that most of the UV-B radiation at wavelengths < 300 nm is filtered out already in the cornea and anterior chamber 100, and that at wavelengths > 295 nm the tyrosine residues do not absorb much. Tryptophan absorption extends to higher wavelengths, and it has been shown that the γ-crystallin structure allows for a very efficient quenching by charge transfer, likely as a protection from photodamage 101,102. One could speculate that this would make the acquisition of additional tryptophan residues for the purpose of elevating dn/dc structurally complicated. Generally, photodamage might represent less of a constraint for most aquatic species.
Structurally, the shift in amino acid composition from an ancient precursor protein necessary to produce high dn/dc γ-crystallins poses the problem of accommodating many replacements, the location of which is irrelevant for the refractive index property, but without compromising the extraordinarily high thermal stability of the Greek key domains 12. This is highlighted, for example, in Figure 6. Already Blundell and colleagues 9 have remarked on the surprisingly high degree of variability how side chains in the hydrophobic core of these domains can pack while maintaining close three-dimensional homology. Such sequence variability is mirrored also by non-lens members of the βγ-crystallin family 103. Thus, one could speculate that this flexibility of the Greek key motif to accommodate different residues lends itself well as a stable scaffold for a lens protein, providing the opportunity of replacing residues of low dn/dc with high ones. In fact, this property has recently been exploited to engineer new proteins 104,105, such as affilins from combinatorial libraries randomizing eight solvent-exposed amino acid residues of γB-crystallins.
In summary, the most compelling explanation for the unusual amino acid composition of crystallin molecules, specifically from the highest refractive index tissue, is a molecular refractive index function. The same trait has evolved in parallel across different phyla, consistent with the notion of universal physical constraints arising in protein solutions at extremely high concentrations, which make solubility easier to maintain at slightly lower concentrations afforded by higher refractive index molecules. Such molecules have evolved by directed amino acid substitutions without altering the three dimensional structure. This shows for the first time that a protein refractive index may be more than a coincidental property, and that the refractive function of the eye lens is manifest on a molecular level.
Methods
Sequence-based analysis of the protein refractive index increment
The experimental measurement of a protein dn/dc requires tens of mg of highly pure and soluble material 43, which would pose formidable difficulties for a systematic study of many crystallins using either bacterial over-expression or purification from biological samples. Therefore, the computational prediction of the protein refractive index increment from the amino acid sequence was adopted in the present work, following the method of McMeekin 44,45, as recently described 43. In brief, the refractivity per gram of protein, Rp, is calculated from its amino acid composition as the weight average
| (Eq. 1) |
of the contributions of the amino acid refractivities Ra, experimentally measured and tabulated for 589 nm and 25 ºC in 45 and reproduced in 43. The data are supported by ab initio calculations of mean residue polarizabilities 106–108. Ma is the residue molecular weight. Analogously, the protein partial specific volume vp is predicted as a weight-average from composition, using the tables from Cohn & Edsall 109. The Lorentz-Lorenz formula then predicts the protein refractive index as
| (Eq. 2) |
, and the refractive index increment was obtained with the Wiener equation for dilute solutions as
| (Eq. 3) |
110 (with solvent refractive index n0 taken as 1.3340, corresponding to water at 25 ºC with 150 mM sodium chloride).
This calculation assumes unconjugated polypeptides, without any preferential hydration. Appropriate corrections could in principle be applied for contributions from preferential solvation, post-translational modifications, temperature effects, and solution refractive index 111–114. The validity of the computational method was verified experimentally by refractometry and dry weight measurement of common proteins 44,45 and peptides specifically constructed to span a large refractive index range 43.
Sequence alignment and phylogenetic analysis of Lops proteins
An initial global sequence alignment was performed with CLUSTAL W2 115 on all known sequences of GST from O. pacificus as well as the S-crystallins and GST from L. opalescens found in the NCBI protein database. The BLOSUM model was chosen for the sequence alignment option. Next, a distance matrix was calculated using the protdist program of the PHYLIP package version 3.57c (kindly provided by Dr. J. Felsenstein), using the Jones-Taylor-Thornton substitution matrix 116. The NEIGHBOR program of PHYLIP was then used to construct a neighbor-joining tree 117. A bootstrap analysis with 500 replicates was performed to determine the levels of support for the interior nodes, and a consensus tree was constructed.
Protein structure analysis
For the crystal structure analysis, the solvent accessibility was calculated with the program AREAIMOL in the CCP4 Suite 118. Graphics of protein structures were created with the software PyMOL.
Supplementary Material
Acknowledgments
We thank Dr. Gu-Gang Chang for providing the coordinates from the homology model of octopus S-crystallin, Dr. Andor Kiss for providing some protein sequences, and Dr. Graeme Wistow for critically reading an initial draft of the manuscript. This work was supported by the Intramural Research Program of the National Institute of Biomedical Imaging and Bioengineering, National Institutes of Health.
Footnotes
Only for squid proteins, at first sight our hypothesis appears to be in conflict with the report by Sweeney et al. 68, who have measured the radial concentration distribution of ‘long loop S-crystallins’ (which are those molecules in lower branch in our tree Figure 2 Top, indicated by black dots in Figure 2 Bottom), and reported them to be located preferentially in the periphery with lower concentrations and to be ‘nearly absent in the centre’. According to our data, these are the isoforms with highest molecular refractive index increment (Figure 2 Bottom) (not to be confused with the tissue refractive index discussed by 68). We cannot resolve this problem, since, unfortunately, no absolute values for the protein concentration measured by Sweeney et al. were reported in the paper 68. On the other hand, no real conflict may exist: In our reading, the MALDI-TOF data shown in the Supplement of 68 do not allow a clear localization of the molecules; contrary to the conclusions proposed by Sweeney and co-workers, they seem to show tryptic digest peptides stemming from many of the high dn/dc isoforms being present also in the innermost lens regions (including Lops5, Lops7, Lops23, Lops18, Lops10, Lops25, Lops16, and Lops8), even with most of these being present in the SDS gel band increasing in density towards the center of the lens (including Lops5, Lops7, Lops23, Lops18, and Lops16).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Wistow GJ, Piatigorsky J. Lens crystallins: the evolution and expression of proteins for a highly specialized tissue. Annu Rev Biochem. 1988;57:479–504. doi: 10.1146/annurev.bi.57.070188.002403. [DOI] [PubMed] [Google Scholar]
- 2.Zigler JS. Lens proteins. In: Albert DM, Jakobiec FA, editors. The Principles and Practice of Ophthalmology: Basic Sciences. W.B. Saunders Co; Philadelphia, PA: 1994. pp. 97–112. [Google Scholar]
- 3.Bloemendal de Jong W, Jaenicke R, Lubsen NH, Slingsby C, Tardieu A. Ageing and vision: structure, stability and function of lens crystallins. Progr Biophys Mol Biol. 2004;86:407–485. doi: 10.1016/j.pbiomolbio.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Benedek GB. Theory of transparency of the eye. Appl Optics. 1971;10:459–473. doi: 10.1364/AO.10.000459. [DOI] [PubMed] [Google Scholar]
- 5.Delaye M, Tardieu A. Short-range order of crystallin proteins accounts for eye lens transparency. Nature. 1983;302:415–7. doi: 10.1038/302415a0. [DOI] [PubMed] [Google Scholar]
- 6.Bassnett S. On the mechanism of organelle degradation in the vertebrate lens. Exp Eye Res. 2009;88:133–9. doi: 10.1016/j.exer.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graw J. Genetics of crystallins: cataract and beyond. Exp Eye Res. 2009;88:173–89. doi: 10.1016/j.exer.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 8.WHO. Fact Sheet No 282. Vol. 2011. WHO; 2009. Visual impairment and blindness. [Google Scholar]
- 9.Blundell T, Lindley P, Miller L, Moss D, Slingsby C, Tickle I, Turnell B, Wistow G. The molecular structure and stability of the eye lens: x-ray analysis of gamma-crystallin II. Nature. 1981;289:771–7. doi: 10.1038/289771a0. [DOI] [PubMed] [Google Scholar]
- 10.Wistow G, Turnell B, Summers L, Slingsby C, Moss D, Miller L, Lindley P, Blundell T. X-ray analysis of the eye lens protein gamma-II crystallin at 1.9 A resolution. J Mol Biol. 1983;170:175–202. doi: 10.1016/s0022-2836(83)80232-0. [DOI] [PubMed] [Google Scholar]
- 11.Tardieu A, Veretout F, Krop B, Slingsby C. Protein interactions in the calf eye lens: interactions between beta-crystallins are repulsive whereas in gamma-crystallins they are attractive. Eur Biophys J. 1992;21:1–12. doi: 10.1007/BF00195438. [DOI] [PubMed] [Google Scholar]
- 12.Jaenicke R, Slingsby C. Lens crystallins and their microbial homologs: structure, stability, and function. Crit Rev Biochem Mol Biol. 2001;36:435–99. doi: 10.1080/20014091074237. [DOI] [PubMed] [Google Scholar]
- 13.Pande A, Annunziata O, Asherie N, Ogun O, Benedek GB, Pande J. Decrease in protein solubility and cataract formation caused by the Pro23 to Thr mutation in human gamma D-crystallin. Biochemistry. 2005;44:2491–500. doi: 10.1021/bi0479611. [DOI] [PubMed] [Google Scholar]
- 14.Mills IA, Flaugh SL, Kosinski-Collins MS, King JA. Folding and stability of the isolated Greek key domains of the long-lived human lens proteins gammaD-crystallin and gammaS-crystallin. Protein Sci. 2007;16:2427–44. doi: 10.1110/ps.072970207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaenicke R. Stability and folding of domain proteins. Progr Biophys Mol Biol. 1999;71:155–241. doi: 10.1016/s0079-6107(98)00032-7. [DOI] [PubMed] [Google Scholar]
- 16.Purkiss AG, Bateman OA, Wyatt K, Wilmarth PA, David LL, Wistow GJ, Slingsby C. Biophysical properties of gammaC-crystallin in human and mouse eye lens: the role of molecular dipoles. J Mol Biol. 2007;372:205–22. doi: 10.1016/j.jmb.2007.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dorsaz N, Thurston GM, Stradner A, Schurtenberger P, Foffi G. Phase separation in binary eye lens protein mixtures. Soft Matter. 2010;7:1763–1776. doi: 10.1021/jp807103f. [DOI] [PubMed] [Google Scholar]
- 18.Darwin C. On the origin of species. Harvard University Press; Cambridge, MA: 1859. [Google Scholar]
- 19.De Jong W. Evolution of lens and crystallins. In: Bloemendal H, editor. Molecular and cellular biology of the eye lens. Wiley; New York: 1981. pp. 221–278. [Google Scholar]
- 20.Fernald RD. Casting a genetic light on the evolution of eyes. Science. 2006;313:1914–8. doi: 10.1126/science.1127889. [DOI] [PubMed] [Google Scholar]
- 21.Weadick CJ, Chang BS. Molecular evolution of the betagamma lens crystallin superfamily: evidence for a retained ancestral function in gamma N crystallins? Mol Biol Evol. 2009;26:1127–42. doi: 10.1093/molbev/msp028. [DOI] [PubMed] [Google Scholar]
- 22.Riyahi K, Shimeld SM. Chordate betagamma-crystallins and the evolutionary developmental biology of the vertebrate lens. Comp Biochem Physiol B Biochem Mol Biol. 2007;147:347–57. doi: 10.1016/j.cbpb.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 23.Ingolia TD, Craig EA. Four small Drosophila heat shock proteins are related to each other and to mammalian alpha-crystallin. Proc Natl Acad Sci U S A. 1982;79:2360–4. doi: 10.1073/pnas.79.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci U S A. 1992;89:10449–53. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Acosta-Sampson L, King J. Partially folded aggregation intermediates of human gammaD-, gammaC-, and gammaS-crystallin are recognized and bound by human alphaB-crystallin chaperone. J Mol Biol. 401:134–52. doi: 10.1016/j.jmb.2010.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lubsen NH, Aarts HJ, Schoenmakers JG. The evolution of lenticular proteins: the beta- and gamma-crystallin super gene family. Prog Biophys Mol Biol. 1988;51:47–76. doi: 10.1016/0079-6107(88)90010-7. [DOI] [PubMed] [Google Scholar]
- 27.Wistow G, Summers L, Blundell T. Myxococcus xanthus spore coat protein S may have a similar structure to vertebrate lens beta gamma-crystallins. Nature. 1985;315:771–3. doi: 10.1038/315771a0. [DOI] [PubMed] [Google Scholar]
- 28.Wistow G. Evolution of a protein superfamily: relationships between vertebrate lens crystallins and microorganism dormancy proteins. J Mol Evol. 1990;30:140–5. doi: 10.1007/BF02099940. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Wistow GJ, Piatigorsky J. Linkage and expression of the argininosuccinate lyase/delta-crystallin genes of the duck: insertion of a CR1 element in the intergenic spacer. Biochim Biophys Acta. 1995;1261:25–34. doi: 10.1016/0167-4781(94)00211-k. [DOI] [PubMed] [Google Scholar]
- 30.Piatigorsky J. Gene sharing, lens crystallins and speculations on an eye/ear evolutionary relationship. Integr Comp Biol. 2003;43:492–499. doi: 10.1093/icb/43.4.492. [DOI] [PubMed] [Google Scholar]
- 31.Wistow GJ, Piatigorsky J. Gene conversion and splice-site slippage in the argininosuccinate lyases/delta-crystallins of the duck lens: members of an enzyme superfamily. Gene. 1990;96:263–70. doi: 10.1016/0378-1119(90)90262-p. [DOI] [PubMed] [Google Scholar]
- 32.Wistow GJ, Mulders JW, de Jong WW. The enzyme lactate dehydrogenase as a structural protein in avian and crocodilian lenses. Nature. 1987;326:622–4. doi: 10.1038/326622a0. [DOI] [PubMed] [Google Scholar]
- 33.van Rheede T, Amons R, Stewart N, de Jong WW. Lactate dehydrogenase A as a highly abundant eye lens protein in platypus (Ornithorhynchus anatinus): upsilon (upsilon)-crystallin. Mol Biol Evol. 2003;20:994–8. doi: 10.1093/molbev/msg116. [DOI] [PubMed] [Google Scholar]
- 34.Mulders JW, Hendriks W, Blankesteijn WM, Bloemendal H, de Jong WW. Lambda-crystallin, a major rabbit lens protein, is related to hydroxyacyl-coenzyme A dehydrogenases. J Biol Chem. 1988;263:15462–6. [PubMed] [Google Scholar]
- 35.Piatigorsky J. Evolution of mollusc lens crystallins: Glutathione S-transferase/S-crystallins and aldehyde dehydrogenase/Omega-crystallins. Amer Malac Bull. 2008;26:73–81. [Google Scholar]
- 36.Tomarev SI, Chung S, Piatigorsky J. Glutathione S-transferase and S-crystallins of cephalopods: evolution from active enzyme to lens-refractive proteins. J Mol Evol. 1995;41:1048–56. doi: 10.1007/BF00173186. [DOI] [PubMed] [Google Scholar]
- 37.Tomarev SI, Zinovieva RD, Piatigorsky J. Characterization of squid crystallin genes. Comparison with mammalian glutathione S-transferase genes. J Biol Chem. 1992;267:8604–12. [PubMed] [Google Scholar]
- 38.Piatigorsky J, Wistow G. The recruitment of crystallins: new functions precede gene duplication. Science. 1991;252:1078–9. doi: 10.1126/science.252.5009.1078. [DOI] [PubMed] [Google Scholar]
- 39.Wistow G. Lens crystallins: gene recruitment and evolutionary dynamism. Trends Biochem Sci. 1993;18:301–6. doi: 10.1016/0968-0004(93)90041-k. [DOI] [PubMed] [Google Scholar]
- 40.Werten PJ, Roll B, van Aalten DM, de Jong WW. Gecko iota-crystallin: how cellular retinol-binding protein became an eye lens ultraviolet filter. Proc Natl Acad Sci U S A. 2000;97:3282–7. doi: 10.1073/pnas.050500597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wistow G. Molecular biology and evolution of crystallins: Gene recruitment and multifunctional proteins in the eye lens. R.G. Landes Company; Georgetown, TX: 1995. [Google Scholar]
- 42.Bhat SP. Transparency and non-refractive functions of crystallins--a proposal. Exp Eye Res. 2004;79:809–16. doi: 10.1016/j.exer.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 43.Zhao H, Brown PH, Schuck P. Biophys J. Vol. 100. 2011. On the distribution of protein refractive index increments; pp. 2309–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McMeekin TL, Groves ML, Hipp NJ. Refractive indices of amino acids, proteins, and related substances. In: Stekol J, editor. Amino Acids and Serum Proteins. American Chemical Society; Washington DC: 1964. [Google Scholar]
- 45.McMeekin TL, Wilensky M, Groves ML. Refractive indices of proteins in relation to amino acid composition and specific volume. Biochem Biophys Res Commun. 1962;7:151–156. [Google Scholar]
- 46.Zhao H, Magone MT, Schuck P. The role of macromolecular crowding in the evolution of lens crystallins with high molecular refractive index. Phys Biol. 2011;8:046004. doi: 10.1088/1478-3975/8/4/046004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanford C. Physical Chemistry of Macromolecules. Wiley; New York: 1961. [Google Scholar]
- 48.Born M, Wolf E. Principles of Optics. 7. University Press; Cambridge: 1999. [Google Scholar]
- 49.Pierscionek B, Smith G, Augusteyn RC. The refractive increments of bovine alpha-, beta-, and gamma-crystallins. Vision Res. 1987;27:1539–41. doi: 10.1016/0042-6989(87)90162-3. [DOI] [PubMed] [Google Scholar]
- 50.Barnwal RP, Jobby MK, Devi KM, Sharma Y, Chary KV. Solution structure and calcium-binding properties of M-crystallin, a primordial betagamma-crystallin from archaea. J Mol Biol. 2009;386:675–89. doi: 10.1016/j.jmb.2008.12.058. [DOI] [PubMed] [Google Scholar]
- 51.Shimeld SM, Purkiss AG, Dirks RP, Bateman OA, Slingsby C, Lubsen NH. Urochordate betagamma-crystallin and the evolutionary origin of the vertebrate eye lens. Curr Biol. 2005;15:1684–9. doi: 10.1016/j.cub.2005.08.046. [DOI] [PubMed] [Google Scholar]
- 52.Land MF, Nilsson D-E. Animal eyes. Oxford University Press; Oxford: 2002. [Google Scholar]
- 53.Campbell MC. Measurement of refractive index in an intact crystalline lens. Vision Res. 1984;24:409–15. doi: 10.1016/0042-6989(84)90039-7. [DOI] [PubMed] [Google Scholar]
- 54.Veretout F, Tardieu A. The protein concentration gradient within eye lens might originate from constant osmotic pressure coupled to differential interactive properties of crystallins. Eur Biophys J. 1989;17:61–8. doi: 10.1007/BF00257103. [DOI] [PubMed] [Google Scholar]
- 55.Keenan J, Orr DF, Pierscionek BK. Patterns of crystallin distribution in porcine eye lenses. Mol Vis. 2008;14:1245–53. [PMC free article] [PubMed] [Google Scholar]
- 56.Amoore JE, Bartley W, Van Heyningen R. Distribution of sodium and potassium within cattle lens. Biochem J. 1959;72:126–33. doi: 10.1042/bj0720126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stevens A, Wang SX, Caines GH, Schleich T. 13C-NMR off-resonance rotating frame spin-lattice relaxation studies of bovine lens gamma-crystallin self association: effect of ‘macromolecular crowding’. Biochim Biophys Acta. 1995;1246:82–90. doi: 10.1016/0167-4838(94)00172-d. [DOI] [PubMed] [Google Scholar]
- 58.Grey AC, Schey KL. Distribution of bovine and rabbit lens alpha-crystallin products by MALDI imaging mass spectrometry. Mol Vis. 2008;14:171–9. [PMC free article] [PubMed] [Google Scholar]
- 59.Huizinga A, Bot ACC, De Mul FFM, Vrensen GF, Greve J. Local variation in absolute water content of human and rabbit eye lenses measured by raman microspectroscopy. Exp Eye Res. 1989;48:487–496. doi: 10.1016/0014-4835(89)90032-8. [DOI] [PubMed] [Google Scholar]
- 60.Uhlhorn SR, Borja D, Manns F, Parel JM. Refractive index measurement of the isolated crystalline lens using optical coherence tomography. Vision Res. 2008;48:2732–8. doi: 10.1016/j.visres.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blundell T, Lindley PF, Miller LR, Moss DS, Slingsby C, Turnell WG, Wistow G. Lens Res. 1983;1:109–131. doi: 10.1016/s0022-2836(83)80232-0. [DOI] [PubMed] [Google Scholar]
- 62.Keenan J, Elia G, Dunn MJ, Orr DF, Pierscionek BK. Crystallin distribution patterns in concentric layers from toad eye lenses. Proteomics. 2009;9:5340–9. doi: 10.1002/pmic.200800986. [DOI] [PubMed] [Google Scholar]
- 63.Slingsby C. Structural variation in lens crystallins. TIBS. 1985;10:281–284. [Google Scholar]
- 64.White HE, Driessen HP, Slingsby C, Moss DS, Lindley PF. Packing interactions in the eye-lens. Structural analysis, internal symmetry and lattice interactions of bovine gamma IVa-crystallin. J Mol Biol. 1989;207:217–35. doi: 10.1016/0022-2836(89)90452-x. [DOI] [PubMed] [Google Scholar]
- 65.Kappe G, Purkiss AG, van Genesen ST, Slingsby C, Lubsen NH. Explosive expansion of betagamma-crystallin genes in the ancestral vertebrate. J Mol Evol. 2010;71:219–30. doi: 10.1007/s00239-010-9379-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Simpson A, Moss D, Slingsby C. The avian eye lens protein delta-crystallin shows a novel packing arrangement of tetramers in a supramolecular helix. Structure. 1995;3:403–12. doi: 10.1016/s0969-2126(01)00171-x. [DOI] [PubMed] [Google Scholar]
- 67.Sivak JG. Optical properties of a cephalopod eye. J Comp Physiol. 1982;147:323–327. [Google Scholar]
- 68.Sweeney AM, Des Marais DL, Ban YE, Johnsen S. Evolution of graded refractive index in squid lenses. J R Soc Interface. 2007;4:685–98. doi: 10.1098/rsif.2006.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Siezen RJ, Shaw DC. Physicochemical characterization of lens proteins of the squid Nototodarus gouldi and comparison with vertebrate crystallins. Biochim Biophys Acta. 1982;704:304–20. doi: 10.1016/0167-4838(82)90160-1. [DOI] [PubMed] [Google Scholar]
- 70.Tomarev SI, Zinovieva RD, Piatigorsky J. Crystallins of the octopus lens. Recruitment from detoxification enzymes. J Biol Chem. 1991;266:24226–31. [PubMed] [Google Scholar]
- 71.Chiou SH, Yu CW, Lin CW, Pan FM, Lu SF, Lee HJ, Chang GG. Octopus S-crystallins with endogenous glutathione S-transferase (GST) activity: sequence comparison and evolutionary relationships with authentic GST enzymes. Biochem J. 1995;309 ( Pt 3):793–800. doi: 10.1042/bj3090793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chiou SH. A novel crystallin from octopus lens. FEBS Lett. 1988;241:261–4. doi: 10.1016/0014-5793(88)81073-1. [DOI] [PubMed] [Google Scholar]
- 73.Zinovieva RD, Piatigorsky J, Tomarev SI. O-Crystallin, arginine kinase and ferritin from the octopus lens. Biochim Biophys Acta. 1999;1431:512–7. doi: 10.1016/s0167-4838(99)00066-7. [DOI] [PubMed] [Google Scholar]
- 74.Piatigorsky J, Kozmik Z, Horwitz J, Ding L, Carosa E, Robison WG, Jr, Steinbach PJ, Tamm ER. Omega -crystallin of the scallop lens. A dimeric aldehyde dehydrogenase class 1/2 enzyme-crystallin. J Biol Chem. 2000;275:41064–73. doi: 10.1074/jbc.M005625200. [DOI] [PubMed] [Google Scholar]
- 75.Nilsson DE, Gislen L, Coates MM, Skogh C, Garm A. Advanced optics in a jellyfish eye. Nature. 2005;435:201–5. doi: 10.1038/nature03484. [DOI] [PubMed] [Google Scholar]
- 76.Piatigorsky J, Norman B, Dishaw LJ, Kos L, Horwitz J, Steinbach PJ, Kozmik Z. J3-crystallin of the jellyfish lens: similarity to saposins. Proc Natl Acad Sci U S A. 2001;98:12362–7. doi: 10.1073/pnas.231310698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Castellano S, Lobanov AV, Chapple C, Novoselov SV, Albrecht M, Hua D, Lescure A, Lengauer T, Krol A, Gladyshev VN, Guigo R. Diversity and functional plasticity of eukaryotic selenoproteins: identification and characterization of the SelJ family. Proc Natl Acad Sci U S A. 2005;102:16188–93. doi: 10.1073/pnas.0505146102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wenk M, Herbst R, Hoeger D, Kretschmar M, Lubsen NH, Jaenicke R. Gamma S-crystallin of bovine and human eye lens: solution structure, stability and folding of the intact two-domain protein and its separate domains. Biophys Chem. 2000;86:95–108. doi: 10.1016/s0301-4622(00)00161-7. [DOI] [PubMed] [Google Scholar]
- 79.Bagby S, Harvey TS, Eagle SG, Inouye S, Ikura M. Structural similarity of a developmentally regulated bacterial spore coat protein to beta gamma-crystallins of the vertebrate eye lens. Proc Natl Acad Sci U S A. 1994;91:4308–12. doi: 10.1073/pnas.91.10.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Srikanthan D, Bateman OA, Purkiss AG, Slingsby C. Sulfur in human crystallins. Exp Eye Res. 2004;79:823–31. doi: 10.1016/j.exer.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 81.Chuang CC, Wu SH, Chiou SH, Chang GG. Homology modeling of cephalopod lens S-crystallin: a natural mutant of sigma-class glutathione transferase with diminished endogenous activity. Biophys J. 1999;76:679–90. doi: 10.1016/S0006-3495(99)77235-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Babul J, Stellwagen E. Measurement of protein concentration with interference optics. Anal Biochem. 1969;28:216–221. doi: 10.1016/0003-2697(69)90172-9. [DOI] [PubMed] [Google Scholar]
- 83.Barer R, Josephs R. Refractometry of living cells. Quart J Microcop Sci. 1954;95:399–423. [Google Scholar]
- 84.Wen J, Arakawa T, Philo JS. Size-exclusion chromatography with on-line light-scattering, absorbance, and refractive index detectors for studying proteins and their interactions. Anal Biochem. 1996;240:155–66. doi: 10.1006/abio.1996.0345. [DOI] [PubMed] [Google Scholar]
- 85.Crookes WJ, Ding LL, Huang QL, Kimbell JR, Horwitz J, McFall-Ngai MJ. Reflectins: the unusual proteins of squid reflective tissues. Science. 2004;303:235–8. doi: 10.1126/science.1091288. [DOI] [PubMed] [Google Scholar]
- 86.Kramer RM, Crookes-Goodson WJ, Naik RR. The self-organizing properties of squid reflectin protein. Nat Mater. 2007;6:533–8. doi: 10.1038/nmat1930. [DOI] [PubMed] [Google Scholar]
- 87.Eaton WA, Hofrichter J. Sickle cell hemoglobin polymerization. Adv Protein Chem. 1990;40:63–279. doi: 10.1016/s0065-3233(08)60287-9. [DOI] [PubMed] [Google Scholar]
- 88.Liu Z, Weng W, Bookchin RM, Lew VL, Ferrone FA. Free energy of sickle hemoglobin polymerization: a scaled-particle treatment for use with dextran as a crowding agent. Biophys J. 2008;94:3629–34. doi: 10.1529/biophysj.107.117465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Heon E, Priston M, Schorderet DF, Billingsley GD, Girard PO, Lubsen N, Munier FL. The gamma-crystallins and human cataracts: a puzzle made clearer. Am J Hum Genet. 1999;65:1261–7. doi: 10.1086/302619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kmoch S, Brynda J, Asfaw B, Bezouska K, Novak P, Rezacova P, Ondrova L, Filipec M, Sedlacek J, Elleder M. Link between a novel human gammaD-crystallin allele and a unique cataract phenotype explained by protein crystallography. Hum Mol Genet. 2000;9:1779–86. doi: 10.1093/hmg/9.12.1779. [DOI] [PubMed] [Google Scholar]
- 91.Pande A, Pande J, Asherie N, Lomakin A, Ogun O, King J, Benedek GB. Crystal cataracts: human genetic cataract caused by protein crystallization. Proc Natl Acad Sci USA. 2001;98:6116–6120. doi: 10.1073/pnas.101124798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Basak A, Bateman O, Slingsby C, Pande A, Asherie N, Ogun O, Benedek GB, Pande J. High-resolution X-ray crystal structures of human gammaD crystallin (1.25 A) and the R58H mutant (1.15 A) associated with aculeiform cataract. J Mol Biol. 2003;328:1137–47. doi: 10.1016/s0022-2836(03)00375-9. [DOI] [PubMed] [Google Scholar]
- 93.Evans P, Wyatt K, Wistow GJ, Bateman OA, Wallace BA, Slingsby C. The P23T cataract mutation causes loss of solubility of folded gammaD-crystallin. J Mol Biol. 2004;343:435–44. doi: 10.1016/j.jmb.2004.08.050. [DOI] [PubMed] [Google Scholar]
- 94.McManus JJ, Lomakin A, Ogun O, Pande A, Basan M, Pande J, Benedek GB. Altered phase diagram due to a single point mutation in human gamma-D crystallin. Proc Natl Acad Sci USA. 2007;104:16856–16861. doi: 10.1073/pnas.0707412104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Moreau KL, King J. Hydrophobic core mutations associated with cataract development in mice destabilize human gammaD-crystallin. J Biol Chem. 2009;284:33285–95. doi: 10.1074/jbc.M109.031344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pan FM, Chang WC, Chao YK, Chiou SH. Characterization of gamma-crystallins from a hybrid teleostean fish: multiplicity of isoforms as revealed by cDNA sequence analysis. Biochem Biophys Res Commun. 1994;202:527–34. doi: 10.1006/bbrc.1994.1960. [DOI] [PubMed] [Google Scholar]
- 97.Chang T, Jiang YJ, Chiou SH, Chang WC. Carp gamma-crystallins with high methionine content: cloning and sequencing of the complementary DNA. Biochim Biophys Acta. 1988;951:226–9. doi: 10.1016/0167-4781(88)90044-9. [DOI] [PubMed] [Google Scholar]
- 98.Kiss AJ, Cheng CH. Molecular diversity and genomic organisation of the alpha, beta and gamma eye lens crystallins from the Antarctic toothfish Dissostichus mawsoni. Comp Biochem Physiol Part D Genomics Proteomics. 2008;3:155–71. doi: 10.1016/j.cbd.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 99.Lee S, Mahler B, Toward J, Jones B, Wyatt K, Dong L, Wistow G, Wu Z. A single destabilizing mutation (F9S) promotes concerted unfolding of an entire globular domain in gammaS-crystallin. J Mol Biol. 399:320–30. doi: 10.1016/j.jmb.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sliney DH. How light reaches the eye and its components. Int J Toxicol. 2002;21:501–9. doi: 10.1080/10915810290169927. [DOI] [PubMed] [Google Scholar]
- 101.Chen J, Callis PR, King J. Mechanism of the very efficient quenching of tryptophan fluorescence in human gamma D- and gamma S-crystallins: the gamma-crystallin fold may have evolved to protect tryptophan residues from ultraviolet photodamage. Biochemistry. 2009;48:3708–16. doi: 10.1021/bi802177g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xu J, Chen J, Toptygin D, Tcherkasskaya O, Callis P, King J, Brand L, Knutson JR. Femtosecond fluorescence spectra of tryptophan in human gamma-crystallin mutants: site-dependent ultrafast quenching. J Am Chem Soc. 2009;131:16751–7. doi: 10.1021/ja904857t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Aravind P, Wistow G, Sharma Y, Sankaranarayanan R. Exploring the limits of sequence and structure in a variant betagamma-crystallin domain of the protein absent in melanoma-1 (AIM1) J Mol Biol. 2008;381:509–18. doi: 10.1016/j.jmb.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ebersbach H, Fiedler E, Scheuermann T, Fiedler M, Stubbs MT, Reimann C, Proetzel G, Rudolph R, Fiedler U. Affilin-novel binding molecules based on human gamma-B-crystallin, an all beta-sheet protein. J Mol Biol. 2007;372:172–85. doi: 10.1016/j.jmb.2007.06.045. [DOI] [PubMed] [Google Scholar]
- 105.Kapoor D, Singh B, Subramanian K, Guptasarma P. Creation of a new eye lens crystallin (Gambeta) through structure-guided mutagenic grafting of the surface of betaB2 crystallin onto the hydrophobic core of gammaB crystallin. Febs J. 2009;276:3341–53. doi: 10.1111/j.1742-4658.2009.07059.x. [DOI] [PubMed] [Google Scholar]
- 106.Hansen T, Jensen L, Åstrand PO, Mikkelson KV. Frequency-dependent polarizabilities of amino acids as calculated by an electrostatic interaction model. J Chem Theory Comput. 2005;1:626–633. doi: 10.1021/ct050053c. [DOI] [PubMed] [Google Scholar]
- 107.Millefiori S, Alparone A, Millefiori A, Vanella A. Electronic and vibrational polarizabilities of the twenty naturally occurring amino acids. Biophys Chem. 2008;132:139–47. doi: 10.1016/j.bpc.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 108.Kassimi NEB, Thakkar AJ. A simple additive model for polarizabilities: Application to amino acids. Chem Phys Lett. 2009;472:232–236. [Google Scholar]
- 109.Cohn EJ, Edsall JT. Density and apparent specific volume of proteins. In: Cohn EJ, Edsall JT, editors. Proteins, Amino Acids and Peptides. Van Nostrand-Reinhold; Princeton, NJ: 1943. pp. 370–381. [Google Scholar]
- 110.Heller W. Remarks on the refractive index mixture rules. J Phys Chem. 1965;69:1123–1129. [Google Scholar]
- 111.Perlman GE, Longsworth LG. The specific refractive increment of some purified proteins. J Am Chem Soc. 1948;70:2719–2724. doi: 10.1021/ja01188a027. [DOI] [PubMed] [Google Scholar]
- 112.Pittz EP, Lee JC, Bablouzian B, Townend R, Timasheff SN. Light scattering and differential refractometry. Methods Enzymol. 1973;27:209–256. doi: 10.1016/s0076-6879(73)27012-x. [DOI] [PubMed] [Google Scholar]
- 113.Eisenberg H. Biological macromolecules and polyelectrolytes in solution. Clarendon Press; Oxford: 1976. [Google Scholar]
- 114.Eisenberg H. Halophilic malate dehydrogenase - a case history of biophysical investigations: ultracentrifugation, light-, x-ray- and neutron scattering. Biochem Soc Symp. 1992;58:113–125. [PubMed] [Google Scholar]
- 115.Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, Higgins DG, Thompson JD. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 2003;31:3497–500. doi: 10.1093/nar/gkg500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Henikoff S, Henikoff JG. Amino acid substitution matrices from protein blocks. Proc Natl Acad Sci U S A. 1992;89:10915–9. doi: 10.1073/pnas.89.22.10915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–25. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 118.The CCP4 suite: programs for protein crystallography. Acta Crystallogr D Biol Crystallogr. 1994;50:760–3. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 119.Ogawa M, Takahashi TC, Takabatake T, Takeshima K. Isolation and characterization of a gene expressed mainly in the gastric epithelium, a novel member of the ep37 family that belongs to the betagamma-crystallin superfamily. Dev Growth Differ. 1998;40:465–73. doi: 10.1046/j.1440-169x.1998.t01-2-00001.x. [DOI] [PubMed] [Google Scholar]
- 120.Liu SB, He YY, Zhang Y, Lee WH, Qian JQ, Lai R, Jin Y. A novel non-lens betagamma-crystallin and trefoil factor complex from amphibian skin and its functional implications. PLoS One. 2008;3:e1770. doi: 10.1371/journal.pone.0001770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ray ME, Wistow G, Su YA, Meltzer PS, Trent JM. AIM1, a novel non-lens member of the betagamma-crystallin superfamily, is associated with the control of tumorigenicity in human malignant melanoma. Proc Natl Acad Sci U S A. 1997;94:3229–34. doi: 10.1073/pnas.94.7.3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jobby MK, Sharma Y. Calcium-binding crystallins from Yersinia pestis. Characterization of two single betagamma-crystallin domains of a putative exported protein. J Biol Chem. 2005;280:1209–16. doi: 10.1074/jbc.M409253200. [DOI] [PubMed] [Google Scholar]
- 123.Di Maro A, Pizzo E, Cubellis MV, D’Alessio G. An intron-less betagamma-crystallin-type gene from the sponge Geodia cydonium. Gene. 2002;299:79–82. doi: 10.1016/s0378-1119(02)01014-4. [DOI] [PubMed] [Google Scholar]
- 124.Wistow GJ, Lietman T, Williams LA, Stapel SO, de Jong WW, Horwitz J, Piatigorsky J. Tau-crystallin/alpha-enolase: one gene encodes both an enzyme and a lens structural protein. J Cell Biol. 1988;107:2729–36. doi: 10.1083/jcb.107.6.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Graham C, Hodin J, Wistow G. A retinaldehyde dehydrogenase as a structural protein in a mammalian eye lens. Gene recruitment of eta-crystallin. J Biol Chem. 1996;271:15623–8. doi: 10.1074/jbc.271.26.15623. [DOI] [PubMed] [Google Scholar]
- 126.Reiss H, Frisch HL. Statistical mechanics of rigid spheres. J Chem Phys. 1959;31:369–380. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.