Abstract
Amelogenesis imperfecta (AI) describes a clinically and genetically heterogeneous group of disorders of biomineralization resulting from failure of normal enamel formation. AI is found as an isolated entity or as part of a syndrome, and an autosomal-recessive syndrome associating AI and gingival hyperplasia was recently reported. Using whole-exome sequencing, we identified a homozygous nonsense mutation in exon 2 of FAM20A that was not present in the Single Nucleotide Polymorphism database (dbSNP), the 1000 Genomes database, or the Centre d'Etude du Polymorphisme Humain (CEPH) Diversity Panel. Expression analyses indicated that Fam20a is expressed in ameloblasts and gingivae, providing biological plausibility for mutations in FAM20A underlying the pathogenesis of this syndrome.
Main Text
Dental enamel is the most extreme example of mammalian biomineralization, with almost 97% of the mature tissue occupied by unusually large hydroxyapatite crystals that are organized into prisms; consequently, enamel is the hardest mineralized tissue in humans.1 A number of inherited congenital defects of enamel formation have been described, together termed amelogenesis imperfecta (AI; MIM 104530), that provide the opportunity to delineate the essential events underlying biomineralization and to dissect the molecular pathogenesis of skeletal disease.2 Collectively, the various forms of AI are common, with incidence rates as high as 1 in 700 live births reported.3 In addition, AI results in considerable morbidity, because affected individuals experience difficulty maintaining oral hygiene, have lower self-esteem owing to poor dental aesthetics, and report inferior quality of life.4
AI is characterized by marked clinical and genetic heterogeneity, with at least 14 different subtypes recognized and with autosomal-dominant, autosomal-recessive, and X-linked recessive inheritance described.3 To date, six genes (AMELX, MIM 300391; ENAM, MIM 606585; MMP20, MIM 604629; KLK4, MIM 603767; FAM83H, MIM 611927; WDR72, MIM 613214) have been implicated in nonsyndromic forms of AI.5–7 Mutations in two additional genes have been shown to underlie syndromic AI, with DLX3 mutations causing tricho-dento-osseous syndrome (MIM 190320), an autosomal-dominant disorder characterized by curly hair at birth, enamel hypoplasia, taurodontism, and thick cortical bone,5 and CNNM4 mutations resulting in Jalili syndrome, which comprises autosomal-recessive cone rod dystrophy and AI (MIM 217080).8,9 Despite these findings, known loci have been excluded in many AI families. These observations, together with the lack of a consistent genotype-phenotype correlation, complicate genetic analysis of AI. Recently, whole-exome sequencing has been demonstrated to be an effective method for the identification of rare Mendelian disorders;10–13 nevertheless, considerable bioinformatics challenges remain, and linkage information together with functional data provide useful filters for reducing this complexity.14–16
Recently, we reported a large, consanguineous family affected by a syndrome characterized by dental anomalies and gingival hyperplasia (Figures 1A and 1B).17 Although thin generalized hypoplastic AI was the main dental feature observed in affected individuals (Figure 1A), intrapulpal calcifications, delayed tooth eruption, and failure of tooth development were also observed.17 Although mental retardation was superimposed on the AI and gingival hyperplasia in one individual, the observation that this feature occurred as an isolated entity in six relatives suggested the segregation of mutations in two independent loci in this family. DNA samples were obtained from nine members of the family via standard procedures and with informed consent under the relevant Institutional Review Boards and the UK National Research Ethics Service.
Figure 1.
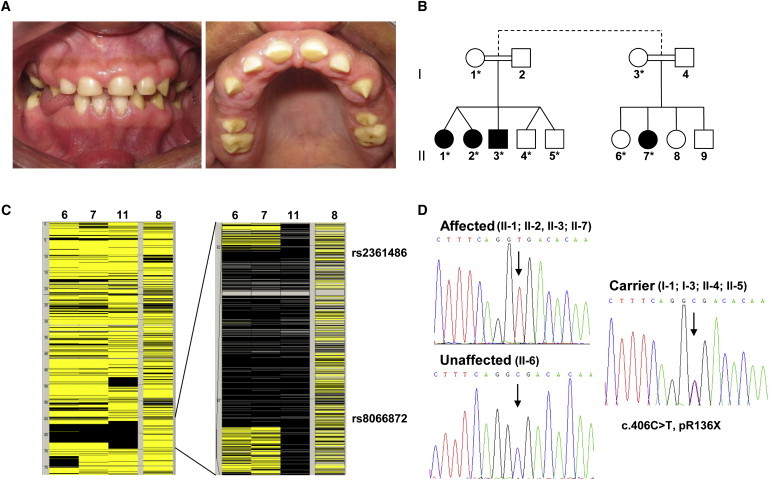
A Mutation in FAM20A Underlies AI with Gingival Hyperplasia
(A) Clinical pictures of the teeth and gingivae of individuals II-2 and II-3. Affected persons exhibit thin, hypoplastic AI, resulting in a dentition with yellow discoloration and loss of structure together with enlargement of the gingivae.
(B) Partial pedigree of the family. Shaded symbols represent individuals affected with AI and gingival hyperplasia, with the exception of individual II-7, who is affected with AI, gingival hyperplasia, and mental retardation. DNA samples were available from those individuals indicated with an asterisk.
(C) Homozygosity mapping showing the homozygous region on chromosome 17 common to affected individuals. Black and yellow bars indicate homozygous and heterozygous SNPs, respectively.
(D) Sequence chromatograms showing segregation of the c.406C>T mutation with the affected phenotype.
Targeted enrichment and sequencing were performed on 3 μg of DNA extracted from peripheral blood of a single individual with AI and gingival hyperplasia. Enrichment was performed with SureSelect Human All Exon Kit version one (Agilent) for the AB SOLiD system, following the manufacturer's protocols. Electronic PCR was conducted on the resultant sample library, which was then sequenced across two quads of a flow cell on a SOLiD 3+ sequencer (Life Technologies), following the manufacturer's protocols.
Sequence data were mapped with SOLiD Bioscope software (Life Technologies) and with hg18 human genome as a reference. SNPs were called with the diBayes tool in the Bioscope software suite with the Med-Coverage setting and then filtered for those SNPs with less than 10× coverage. SNPs were annotated with the SeattleSeq annotation tool. A total of 5.4 gigabases of sequence mapped uniquely to the genome reference hg18, with 90.8% of the targeted exome covered at 10-fold or higher. On average, a total of 53-fold coverage was achieved across the exome (see Table S1 available online).
To reduce the complexity of the bioinformatics analysis, we performed homozygosity mapping on five individuals (two affected with AI and gingival hyperplasia, one affected with AI, gingival hyperplasia, and mental retardation, one affected with mental retardation only, and one unaffected). DNA was hybridized to Affymetrix Genome-Wide Human SNP 6.0 arrays in accordance with the manufacturer's protocol. Briefly, 500 ng total genomic DNA was digested with NspI and StyI. Primers that recognize the adaptor sequences were used to amplify adaptor-ligated DNA fragments. PCR conditions were optimized to preferentially amplify fragments in the 200–1100 bp size range. The amplified DNA was fragmented with the Affymetrix fragmentation reagent to a size of 50–200 bp and was then labeled and hybridized to a GeneChip Human Mapping SNP 6.0 array. Genotyping and copy number analysis were performed with the Genotyping Console software and Chromosome Analysis Suite (Affymetrix), respectively. Homozygous regions were identified with AutoSNPa. The results of this analysis identified a single region of homozygosity greater than 1 Mb: a 6 Mb interval flanked by nucleotides 61,966,341–67,925,728 (as defined by rs2361486 and rs8066872) on human chromosome 17q in the individuals affected with AI and gingival hyperplasia (Figure 1C). The individual affected with mental retardation alone was not homozygous for this region, indicating that this phenotype results from mutation of a different locus. Fifty-five genes were contained within this critical interval, and analysis of the captured exons indicated an average of 49-fold coverage (Figure S1; Table S1). The next-largest region of homozygosity, an 800 kb interval on human chromosome 20q, did not encompass any SNPs that were not present in dbSNP.
Among the sequence variants identified in the interval defined by rs2361486 and rs8066872, a single homozygous C>T transition was noted in exon 2 of FAM20A (MIM 611062) (genomic DNA: Chr17[NCBI 36]:g.64063478C>T; cDNA: NM_017565.2:c.406C>T), which resulted in the homozygous nonsense mutation p.Arg136X (Figures S1 and S2). Sanger sequencing confirmed that the mutation cosegregated with the dental phenotype, but not with the mental retardation (Figure 1D; Table S2). Reanalysis of those individuals who carried one mutated allele confirmed that they did not manifest any clinical signs of AI, confirming autosomal-recessive transmission. Bioinformatics analysis confirmed that this SNP was not present in either dbSNP or the 1000 Genomes database (Table S1). Allelic discrimination PCR analysis (Table S2) further indicated that the mutation was not present in 952 DNA samples from the CEPH Diversity Panel, including 43 Brazilian individuals, nor in DNA samples from an additional 159 individuals of Brazilian ancestry. The mutation underlying the mental retardation observed in this family has not, to date, been elucidated.
To provide direct biological plausibility for Fam20a playing a role in amelogenesis, we performed in situ hybridization of Fam20a mRNA and immunolocalization of the three FAM20 family members in the mouse incisor tooth, which, in adult mice, forms and erupts continuously throughout life, offering access to all stages of enamel development along the axial length of a single tooth (Figure 2; Figure S3). Mandibles from 2- to 3-month-old wild-type mice (n = 4) were dissected following cervical dislocation and were fixed in neutral buffered formalin at room temperature for 48 hr. Following fixation, the mandibles were decalcified in 0.5 M EDTA, dehydrated through a graded ethanol series, cleared in chloroform, embedded as hemi-mandibles in paraffin wax, and sectioned.
Figure 2.

In Situ Hybridization of Fam20a and Immunolocalization of Fam20a in the Mouse Mandibular Incisor
(A) Fam20a mRNA expression is observed in the secretory ameloblasts, stratum intermedium, and stellate reticulum analog.
(B) Fam20a mRNA is also expressed in the maturation stage ameloblasts, papillary layer, and the cells of the underlying connective tissue bed.
(C and D) Fam20a is also expressed in the suprabasal layers of the gingivae (C) and in the odontoblasts (D).
(E–H) The specificity of the in situ hybridization is demonstrated by use of a Fam20a sense riboprobe, which produces only background levels of alkaline phosphatase activity.
(I–L) Consistent with the results of the in situ hybridization analysis, Fam20a immunoreactivity was observed in the secretory (I) and maturation (J) stage ameloblasts, the suprabasal layers of the gingival epithelium (K), and the odontoblasts (L).
(M–P) The specificity of the immunolabeling is demonstrated by use of nonimmune serum, which produces only autofluorescence in erythrocytes (asterisks). For immunofluorescence, sections were counterstained with DAPI. The following abbreviations are used: a, ameloblasts; b, basal cell layer; c, cornified layer; ct, connective tissue bed; dp, dental pulp; o, odontoblasts; p, papillary layer; sb, suprabasal layer; si, stratum intermedium; sr, stellate reticulum analog. Scale bars represent 50 μm (C, G, I–P) and 25 μm (A, B, D, E, F, H).
For in situ hybridization analysis, a 370 bp fragment of Fam20a exon 1 was PCR amplified (Table S2) and TA cloned into the pGEM-T Easy vector (Promega), and the resulting construct was used to synthesize digoxygenin-labeled Fam20a sense and antisense riboprobes with T7 and SP6 polymerases (Promega) and digoxygenin-conjugated dUTP (Roche). In situ hybridization was performed as described previously;18 however, riboprobe binding was detected immunohistochemically with an alkaline phosphatase-conjugated, anti-digoxygenin antibody (Roche) followed by histochemical demonstration of alkaline phosphatase activity that used BM purple (Roche) as the chromogenic substrate. In situ hybridization for Fam20a transcripts with the antisense riboprobe showed expression within the secretory ameloblasts, stratum intermedium cells, and the underlying analog of the stratum intermedium (Figure 2A). Fam20a mRNA expression was also observed within maturation stage ameloblasts, the associated papillary layer, and connective tissue bed (Figure 2B). In addition, Fam20a mRNA was detected in the suprabasal cells of the gingivae (Figure 2C), odontoblasts, and dental pulp cells (Figure 2D). In parallel experiments with the Fam20a sense riboprobe, only background levels of alkaline phosphatase activity were detected (Figures 2E–2H).
Immunofluorescence analysis was performed with antibodies raised against Fam20a, Fam20b, and Fam20C (Sigma-Aldrich). Primary antibodies were detected with an Alexa Fluor 488-conjugated secondary antibody (Invitrogen) and mounted in DAPI-containing mountant (Vector Laboratories). Sections were examined with a DMRB microscope (Leica) with Spot digital camera and associated software (RTKE/SE; Diagnostic Instruments). Consistent with the clinical phenotype of AI and gingival hyperplasia and with results of the in situ hybridization analysis, Fam20a was observed throughout the tissues of the mandibular incisor, including the secretory and maturation stage ameloblasts (Figures 2I and 2J), the suprabasal layers of the gingival epithelium (Figure 2K), and the odontoblasts (Figure 2L). Despite the presence of a signal sequence within the primary peptide,19 there was only weak immunolabeling of Fam20a in the enamel matrix, suggesting limited secretion with localization to nuclei in the maturation stage enamel organ and gingivae (Figures 2I and 2J).
Little or no immunoreactivity for Fam20b was detected in the secretory stage enamel organ (Figure S3). In contrast, weak to moderate Fam20b localization was observed in the gingivae and odontoblasts (Figure S3), with strong immunolocalization in the cytoplasm of maturation stage ameloblasts (Figure S3). Fam20c was the only Fam20 member that showed evidence of secretion, being detected in the matrix of the secretory stage enamel organ and within the cytoplasm of the ameloblasts (Figure S3). Strong immunolabeling for Fam20c at this stage of amelogenesis was observed within the cells adjoining the stratum intermedium (a tissue layer analogous to the stellate reticulum seen in the developing molar; Figure S3). Strong Fam20c immunoreactivity was seen in the cytoplasm of maturation stage ameloblasts and throughout the noncornified layers of the gingival epithelium (Figure S3). As with the other Fam20 proteins, Fam20c was observed in the cytoplasm of the odontoblasts (Figure S3).
Although the function of FAM20A is unknown, the genetic results and tissue localization analyses presented here indicate that FAM20A plays a fundamental role in enamel development and gingival homeostasis. Although further functional domains have not been identified, comparative sequence analysis of the FAM20 protein family has indicated that the highest homology lies in the C-terminal two-thirds of the protein.19 Although the p.Arg136X change identified in FAM20A in the current study would spare the signal sequence, the entire conserved C-terminal domain would be lost (Figure S2); nevertheless, the position of the termination codon in exon 2 is most likely to result in nonsense-mediated degradation of the mutant transcript and a consequent loss of FAM20A protein.20 Notably, the closely related FAM20C has been shown to be a secreted, calcium-binding protein that modulates differentiation of the dentin-producing odontoblasts,21 and loss-of-function mutations in this protein result in the autosomal-recessive lethal osteosclerotic bone dysplasia Raine syndrome, indicating that this protein plays a crucial role in mineralization.22 The nonsynonymous mutations found in this condition fall exclusively in the conserved C-terminal domain.22 Recently, a transgenic mouse line with a 58 kb deletion that encompasses part of Fam20a and its upstream region was shown to exhibit cessation of growth 5 weeks after birth;23 analysis of the dentition of these mice may provide further insights into the role of Fam20a during amelogenesis.
In summary, we report the use of whole-exome sequencing to elucidate the molecular basis of a syndrome that encompasses AI. Importantly, this study illustrates the utility of this approach in finding rare mutations in single families affected by conditions that are characterized by extreme phenotypic variability and genetic heterogeneity, particularly when combined with methods for reducing the complexity of data analysis such as homozygosity mapping.
Acknowledgments
We express our gratitude to the family members for participating in the study and to Aline Petrin, University of Iowa, for collection of the control DNA samples from Brazilian individuals. We gratefully acknowledge financial support from the Wellcome Trust (075945, 093113), the National Institute for Health Research Manchester Biomedical Research Centre, the State Minas Gerais Research Foundation (Fundação de Amparo à Pesquisa do Estado de Minas Gerais), and the Brazil National Council for Scientific and Technological Development (CNPq). C.C.B. was supported by grants from Fundação Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), Brazil.
Contributor Information
Graeme C.M. Black, Email: graeme.black@manchester.ac.uk.
Michael J. Dixon, Email: mike.dixon@manchester.ac.uk.
Supplemental Data
Web Resources
The URLs for data presented herein are as follows:
AutoSNPa, http://www.autozygosity.org
Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim
SeattleSeq annotation tool, http://gvs.gs.washington.edu/SeattleSeqAnnotation/HelpAbout.jsp
References
- 1.Smith C.E. Cellular and chemical events during enamel maturation. Crit. Rev. Oral Biol. Med. 1998;9:128–161. doi: 10.1177/10454411980090020101. [DOI] [PubMed] [Google Scholar]
- 2.Hu J.C., Chun Y.H., Al Hazzazzi T., Simmer J.P. Enamel formation and amelogenesis imperfecta. Cells Tissues Organs (Print) 2007;186:78–85. doi: 10.1159/000102683. [DOI] [PubMed] [Google Scholar]
- 3.Bäckman B., Holm A.K. Amelogenesis imperfecta: Prevalence and incidence in a northern Swedish county. Community Dent. Oral Epidemiol. 1986;14:43–47. doi: 10.1111/j.1600-0528.1986.tb01493.x. [DOI] [PubMed] [Google Scholar]
- 4.Coffield K.D., Phillips C., Brady M., Roberts M.W., Strauss R.P., Wright J.T. The psychosocial impact of developmental dental defects in people with hereditary amelogenesis imperfecta. J. Am. Dent. Assoc. 2005;136:620–630. doi: 10.14219/jada.archive.2005.0233. [DOI] [PubMed] [Google Scholar]
- 5.Stephanopoulos G., Garefalaki M.E., Lyroudia K. Genes and related proteins involved in amelogenesis imperfecta. J. Dent. Res. 2005;84:1117–1126. doi: 10.1177/154405910508401206. [DOI] [PubMed] [Google Scholar]
- 6.Kim J.W., Lee S.K., Lee Z.H., Park J.C., Lee K.E., Lee M.H., Park J.T., Seo B.M., Hu J.C., Simmer J.P. FAM83H mutations in families with autosomal-dominant hypocalcified amelogenesis imperfecta. Am. J. Hum. Genet. 2008;82:489–494. doi: 10.1016/j.ajhg.2007.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Sayed W., Parry D.A., Shore R.C., Ahmed M., Jafri H., Rashid Y., Al-Bahlani S., Al Harasi S., Kirkham J., Inglehearn C.F., Mighell A.J. Mutations in the beta propeller WDR72 cause autosomal-recessive hypomaturation amelogenesis imperfecta. Am. J. Hum. Genet. 2009;85:699–705. doi: 10.1016/j.ajhg.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parry D.A., Mighell A.J., El-Sayed W., Shore R.C., Jalili I.K., Dollfus H., Bloch-Zupan A., Carlos R., Carr I.M., Downey L.M. Mutations in CNNM4 cause Jalili syndrome, consisting of autosomal-recessive cone-rod dystrophy and amelogenesis imperfecta. Am. J. Hum. Genet. 2009;84:266–273. doi: 10.1016/j.ajhg.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polok B., Escher P., Ambresin A., Chouery E., Bolay S., Meunier I., Nan F., Hamel C., Munier F.L., Thilo B. Mutations in CNNM4 cause recessive cone-rod dystrophy with amelogenesis imperfecta. Am. J. Hum. Genet. 2009;84:259–265. doi: 10.1016/j.ajhg.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walsh T., Shahin H., Elkan-Miller T., Lee M.K., Thornton A.M., Roeb W., Abu Rayyan A., Loulus S., Avraham K.B., King M.C., Kanaan M. Whole exome sequencing and homozygosity mapping identify mutation in the cell polarity protein GPSM2 as the cause of nonsyndromic hearing loss DFNB82. Am. J. Hum. Genet. 2010;87:90–94. doi: 10.1016/j.ajhg.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng S.B., Buckingham K.J., Lee C., Bigham A.W., Tabor H.K., Dent K.M., Huff C.D., Shannon P.T., Jabs E.W., Nickerson D.A. Exome sequencing identifies the cause of a mendelian disorder. Nat. Genet. 2010;42:30–35. doi: 10.1038/ng.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoischen A., van Bon B.W., Gilissen C., Arts P., van Lier B., Steehouwer M., de Vries P., de Reuver R., Wieskamp N., Mortier G. De novo mutations of SETBP1 cause Schinzel-Giedion syndrome. Nat. Genet. 2010;42:483–485. doi: 10.1038/ng.581. [DOI] [PubMed] [Google Scholar]
- 13.Krawitz P.M., Schweiger M.R., Rödelsperger C., Marcelis C., Kölsch U., Meisel C., Stephani F., Kinoshita T., Murakami Y., Bauer S. Identity-by-descent filtering of exome sequence data identifies PIGV mutations in hyperphosphatasia mental retardation syndrome. Nat. Genet. 2010;42:827–829. doi: 10.1038/ng.653. [DOI] [PubMed] [Google Scholar]
- 14.Ng S.B., Bigham A.W., Buckingham K.J., Hannibal M.C., McMillin M.J., Gildersleeve H.I., Beck A.E., Tabor H.K., Cooper G.M., Mefford H.C. Exome sequencing identifies MLL2 mutations as a cause of Kabuki syndrome. Nat. Genet. 2010;42:790–793. doi: 10.1038/ng.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Otto E.A., Hurd T.W., Airik R., Chaki M., Zhou W., Stoetzel C., Patil S.B., Levy S., Ghosh A.K., Murga-Zamalloa C.A. Candidate exome capture identifies mutation of SDCCAG8 as the cause of a retinal-renal ciliopathy. Nat. Genet. 2010;42:840–850. doi: 10.1038/ng.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Funari V.A., Krakow D., Nevarez L., Chen Z., Funari T.L., Vatanavicharn N., Wilcox W.R., Rimoin D.L., Nelson S.F., Cohn D.H. BMPER mutation in diaphanospondylodysostosis identified by ancestral autozygosity mapping and targeted high-throughput sequencing. Am. J. Hum. Genet. 2010;87:532–537. doi: 10.1016/j.ajhg.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martelli-Júnior H., Bonan P.R., Dos Santos L.A., Santos S.M., Cavalcanti M.G., Coletta R.D. Case reports of a new syndrome associating gingival fibromatosis and dental abnormalities in a consanguineous family. J. Periodontol. 2008;79:1287–1296. doi: 10.1902/jop.2008.070520. [DOI] [PubMed] [Google Scholar]
- 18.Knight A.S., Schutte B.C., Jiang R., Dixon M.J. Developmental expression analysis of the mouse and chick orthologues of IRF6: The gene mutated in Van der Woude syndrome. Dev. Dyn. 2006;235:1441–1447. doi: 10.1002/dvdy.20598. [DOI] [PubMed] [Google Scholar]
- 19.Nalbant D., Youn H., Nalbant S.I., Sharma S., Cobos E., Beale E.G., Du Y., Williams S.C. FAM20: An evolutionarily conserved family of secreted proteins expressed in hematopoietic cells. BMC Genomics. 2005;6:11. doi: 10.1186/1471-2164-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frischmeyer P.A., Dietz H.C. Nonsense-mediated mRNA decay in health and disease. Hum. Mol. Genet. 1999;8:1893–1900. doi: 10.1093/hmg/8.10.1893. [DOI] [PubMed] [Google Scholar]
- 21.Hao J., Narayanan K., Muni T., Ramachandran A., George A. Dentin matrix protein 4, a novel secretory calcium-binding protein that modulates odontoblast differentiation. J. Biol. Chem. 2007;282:15357–15365. doi: 10.1074/jbc.M701547200. [DOI] [PubMed] [Google Scholar]
- 22.Simpson M.A., Hsu R., Keir L.S., Hao J., Sivapalan G., Ernst L.M., Zackai E.H., Al-Gazali L.I., Hulskamp G., Kingston H.M. Mutations in FAM20C are associated with lethal osteosclerotic bone dysplasia (Raine syndrome), highlighting a crucial molecule in bone development. Am. J. Hum. Genet. 2007;81:906–912. doi: 10.1086/522240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.An C., Ide Y., Nagano-Fujii M., Kitazawa S., Shoji I., Hotta H. A transgenic mouse line with a 58-kb fragment deletion in chromosome 11E1 that encompasses part of the Fam20a gene and its upstream region shows growth disorder. Kobe J. Med. Sci. 2010;55:E82–E92. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


