Abstract
Two-component systems (TCS) are principal mechanisms by which bacteria adapt to their surroundings. Borrelia burgdorferi encodes only two TCS. One is comprised of a histidine kinase, Hk2, and the response regulator Rrp2. While the contribution of Hk2 remains unclear, Rrp2 is part of a regulatory pathway involving the spirochete's alternate sigma factors, RpoN and RpoS. Genes within the Rrp2/RpoN/RpoS regulon function to promote tick transmission and early infection. The other TCS consists of a hybrid histidine kinase, Hk1, and the response regulator Rrp1. Hk1 is composed of two periplasmic sensor domains (D1 and D2), followed by conserved cytoplasmic histidine kinase core, REC, and Hpt domains. In addition to its REC domain, Rrp1 contains a GGDEF motif characteristic of diguanylate cyclases. To investigate the role of Hk1 during the enzootic cycle, we inactivated this gene in two virulent backgrounds. Extensive characterization of the resulting mutants revealed a dramatic phenotype whereby Hk1-deficient spirochetes are virulent in mice and able to migrate out of the bite site during feeding but are killed within the midgut following acquisition. We hypothesize that the phosphorelay between Hk1 and Rrp1 is initiated by the binding of feeding-specific ligand(s) to Hk1 sensor domain D1 and/or D2. Once activated, Rrp1 directs the synthesis of cyclic dimeric GMP (c-di-GMP), which, in turn, modulates the expression and/or activity of gene products required for survival within feeding ticks. In contrast to the Rrp2/RpoN/RpoS pathway, which is active only within feeding nymphs, the Hk1/Rrp1 TCS is essential for survival during both larval and nymphal blood meals.
INTRODUCTION
Two-component signal transduction systems (TCSs) are principal mechanisms by which bacteria survey and adapt to perturbations in their surroundings (29, 44). Typically, TCSs are composed of sensor histidine kinase (HK) and response regulator (RR) components, with the genes encoding a particular TCS frequently being cotranscribed (25, 29). The majority of TCS HKs consist of a variable extracytoplasmic sensor domain and conserved cytoplasmic kinase core containing catalytic ATP-binding (CA) and dimerization/histidine phosphotransfer (DHp) domains (29). RR proteins typically are comprised of a conserved receiver (REC) domain and an effector domain (26, 29). In its simplest form, regulation via TCSs begins with the binding of a specific ligand by the HK sensor domain, which in turn induces a conformation change promoting autophosphorylation of a His residue within the kinase core (29). The cognate RR then catalyzes the transfer of the phosphoryl group from the phosphorylated His (His∼P) to an Asp residue within its own REC domain (29). Once activated, the RR effector domain elicits an appropriate response, typically by altering transcription of specific genes or allosteric regulation of target proteins (26). Although examples of cross talk have been reported, bacteria have evolved multiple mechanisms to prevent inadvertent signaling between unrelated HK and RR components (69).
The genome of Borrelia burgdorferi, the Lyme disease spirochete, encodes two TCSs in addition to the CheA and CheY orthologs associated with chemotaxis (24, 31). One consists of a sensor histidine kinase (Hk2/BB0764) and response regulator (Rrp2/BB0763), both of which are predicted to localize to the cytoplasm. Although Hk2 was widely presumed to be the cognate HK for Rrp2, Xu et al. (86) recently demonstrated that the high-energy, phosphoryl donor acetyl phosphate (acetyl∼P) is capable of phosphorylating Rrp2 in vitro and, more importantly, that Hk2 is not required for activation of Rrp2 in vivo. Once activated, Rrp2∼P acts as a transcriptional activator for the alternate sigma factor RpoN (6, 87), which in turn controls expression of the spirochete's other alternate sigma factor, RpoS (7, 20, 36, 52, 70). Genes within the Rrp2/RpoN/RpoS regulon promote tick-to-mammal transmission (30) and early murine infection (10, 20, 32, 68, 80, 83).
The other borrelial TCS is composed of a sensor histidine kinase (Hk1/BB0420) and response regulator (Rrp1/BB0419) (24). Hk1 consists of a periplasmic sensor domain flanked by two transmembrane helices followed by a histidine kinase core, REC, and previously unrecognized histidine-containing phosphotransfer (Hpt; described below) domains while Rrp1 contains a REC domain as well as a GGDEF domain characteristic of diguanylate cyclases (27), the enzyme responsible for synthesis of the small nucleotide messenger bis-(3′-5′)-cyclic dimeric GMP (c-di-GMP) (59). Activation of Rrp1's diguanylate cyclase activity requires phosphorylation of its REC domain (59), presumably mediated by Hk1-dependent phosphorelay. Once produced, c-di-GMP induces a conformational change in one or more target proteins, altering their enzymatic activity or their ability to interact with DNA or other proteins (65). More recently, c-di-GMP also has been shown to directly alter gene expression via its interaction with the 5′ untranslated region of target mRNAs (45, 71). Evidence for a complete c-di-GMP signaling pathway in B. burgdorferi recently was confirmed by studies demonstrating that BB0363, an EAL domain-containing phosphodiesterase, specifically degrades c-di-GMP (76). In bacteria, c-di-GMP-mediated signaling has been associated with a wide range of adaptive processes, most notably the transition between planktonic and sessile lifestyles and biofilm formation (13, 34, 85).
Hk1 and Rrp1 are predicted to function cooperatively, and, as such, inactivation of either gene would result in a similar phenotype. Microarray analyses of a Δrrp1 mutant indentified 140 genes whose expression was influenced by this RR in vitro (58), several of which encode proteins whose annotated functions suggest a role in carbon metabolism, maintenance of the spirochete's cell envelope, and adaptation to the arthropod vector and/or mammalian host. However, because the background used to generate this Δrrp1 mutant was avirulent (58), the contribution of Rrp1 to virulence could not be determined. Therefore, to determine the role of the Hk1/Rrp1 TCS during the enzootic cycle, we sought to inactivate both genes within a virulent strain 297 background. While our attempts to isolate a strain 297 rrp1 mutant were unsuccessful, we obtained multiple hk1 mutants in strains 297 and B31 5A4 NP1. Extensive characterization of these mutants revealed a dramatic phenotype whereby Hk1-deficient spirochetes are fully virulent in mice and able to migrate into ticks during feeding but are killed within the midgut following acquisition. trans-Complementation restored the ability of the B31 hk1 mutant to survive within both larvae and nymphs. Recently, studies by two independent laboratories demonstrated that B. burgdorferi lacking Rrp1 displays an identical survival defect (33a, 41a), establishing overwhelmingly that the protective function of Hk1 is mediated via phosphorelay. In contrast to Rrp2, which is active only during the nymphal blood meal (11, 49), the Hk1/Rrp1 TCS is required during both larval and nymphal life stages. Signaling via Hk1 appears to be induced by host- and/or tick-derived stimuli generated as part of the feeding process. The sensing of feeding-specific signals, encountered within the bite site and/or tick midgut, is presumably mediated by Hk1's D1 and D2 periplasmic sensor domains, both of which share structural similarities to bacterial extracellular solute-binding proteins (78).
MATERIALS AND METHODS
Bacterial strains and culture conditions.
B. burgdorferi isolates used in these studies (Table 1) were cultivated in modified Barbour-Stoenner-Kelly medium (57) supplemented with 6% rabbit serum (Pel-Freeze Biologicals, Rogers, AK) (BSK-II). Strain 297 hk1 mutants Bb508 and Bb807 were maintained under selection using streptomycin (50 μg/ml), while the strain B31 5A4 NP1 hk1 mutant Bb1197 was maintained under selection using streptomycin (50 μg/ml) and kanamycin (400 μg/ml). B. burgdorferi isolates expressing a PflaB-gfp reporter inserted into the 26-kb circular plasmid (cp26) were maintained under selection using gentamicin (50 μg/ml). The plasmid content of all isolates was monitored as previously described (18). Standard temperature shift experiments and growth curves were performed as previously described (11). To obtain organisms in a host-adapted state, spirochetes were cultivated in dialysis membrane chambers (DMCs) implanted into the peritoneal cavities of rats as previously described (1). Escherichia coli strains were maintained in Luria-Bertani broth (LB) (1% tryptone, 0.5% yeast extract, 1% NaCl) with the appropriate antibiotic. Selection was performed on LB agar plates (LB with 1.5% agar) supplemented with the appropriate antibiotic.
Table 1.
Bacterial strains used in these studies
| Strain | Description | Reference or source |
|---|---|---|
| CE162 | Wild-type virulent strain 297 parent | 11 |
| 5A4 NP1 | Wild-type virulent strain B31 parent | 40 |
| Bb508 | CE162 transformed with pMC1389; strain 297 hk1 mutant | This study |
| Bb807 | CE162 transformed with pMC1389; strain 297 hk1 mutant | This study |
| Bb1197 | B31 5A4 NP transformed with pMC1389; strain B31 hk1 mutant | This study |
| Bb914 | CE162 cells containing PflaB-gfp reporter inserted into cp26 | 16 |
| Bb1152 | Bb914 transformed with pMC1389; hk1 mutant constitutively expressing GFP | This study |
| Bb1155 | Bb914 transformed with pMC1389; hk1 mutant constitutively expressing GFP | This study |
| Bb1363 | Bb1197 complemented in trans with hk1 contained a cp9-based shuttle vector | This study |
| Bb1367 | Bb1197 complemented in trans with hk1 contained a cp9-based shuttle vector | This study |
| 5A13-Δrrp1 | Strain B31 5A13 rrp1 mutant | 58 |
DNA manipulations and routine cloning.
Routine molecular cloning and plasmid propagation were performed using E. coli Top10 cells (Invitrogen, Carlsbad, CA). Routine and high-fidelity PCR amplification reactions were performed using Choice Taq (Denville Scientific, Metuchen, NJ) and Takara ExTaq (Fisher Scientific, Pittsburgh, PA), respectively. Plasmid DNAs were purified from E. coli using Qiagen Midi and Spin Prep Kits (Valencia, CA). Nucleotide sequencing was performed by Agencourt Bioscience Corp. (Beverly, MA).
Bioinformatics.
Routine and comparative sequence analyses were performed using MacVector (version 10.1; MacVector, Inc., Cary, NC). Conserved domain searches were performed using a conserved domains database (CDD) search either alone (http://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml) or within the NCBI Basic Local Alignment Search Tool (BLAST). Pairwise and multiple sequence alignments (PA and MSA, respectively) were performed using the ClustalW (version 1.83) (79) option within MacVector. Structural similarities and modeling were performed using Swiss-Model (http://swissmodel.expasy.org/) (2). The molecular viewer program PyMOL (www.pymol.org) (14) was used to generate the structural representations and calculate root mean square deviation (RMSD) values.
Generation and complementation of B. burgdorferi hk1 mutants.
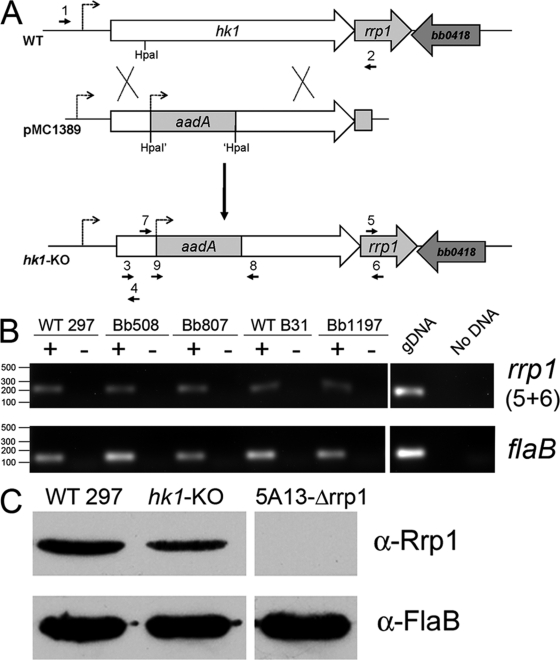
A 4.9-kb region containing hk1 and flanking sequences was amplified from strain 297 using primers ups.hk1-5′ and dwns.hk1-3′ (Table 2) and cloned into the pCR2.1-TOPO vector (Invitrogen). The resulting plasmid was digested with HpaI and ligated with a PflgB-aadA cassette conferring resistance to spectinomycin (E. coli; 100 μg/ml) and streptomycin (B. burgdorferi; 50 μg/ml) (23), to yield pMC1389. The orientation of the hk1 and aadA genes within pMC1389 was assessed by PCR using primers ups.hk1-KO-5′ (where KO is knockout) and aadA-5′ and subsequently confirmed by sequencing. Competent CE162 cells were prepared as previously described (60) and electrotransformed with 15 to 20 μg of purified pMC1389. Streptomycin-resistant transformants were assessed for an insertion within hk1 using primers hk1-KO-5′ and hk1-KO-3′. Two transformants (Bb508 and Bb807) derived from independent batches of CE162, each retaining a full plasmid complement, were selected for further analyses. A strain B31 hk1 mutant (Bb1197) was generated by transforming competent 5A4 NP1 (40) with pMC1389 as described above.
Table 2.
Oligonucleotide primers used in these studies
| Primera | Sequence (5′–3′)b | Purpose | Reference or source |
|---|---|---|---|
| hk1-F | CGTCAATTTATTTTCTAAGGATATTTTC | qRT-PCR | This work |
| hk1-R | TGCTTCGTCTTCAATTTCACT | qRT-PCR | This work |
| rrp1-F | AAGGTGCTTACGAGATTGAG | qRT-PCR | This work |
| rrp1-R | TCTGTGGAACTTCTTGAACTAA | qRT-PCR | This work |
| ups.hk1-5′ | GGGTCCTGGAAGAATACCAGGTTG | Cloning and mutagenesis | This work |
| dwns.hk1-3′ | GTGGGGAGAATCATCCACAATTAA | Cloning and mutagenesis | This work |
| hk1-KO-5′ | CCCATTCAACATTTTTATCCAATTTT | Confirm insertion within hk1 | This work |
| hk1-KO-3′ | TGGACCAGCATCATCATTGCTTAGGTCTTTTG | Confirm insertion within hk1 | This work |
| hk1-KO junc-5′ | AGGTTAAAAAACGTTAACACCAT | Confirm complementation | This work |
| flgB-5′ | GCGCCATGGTACCCGAGCTTCAAGGAAGA | Construction pSP1G | This work |
| gent-3′ | GCGCCATGGTTAGGTGGCGGTACTTGGG | Construction pSP1G | This work |
| Hk1 compl-5′ | GCGGGATCCGGGTCCTGGAAGAATACCAG | Complementation | This work |
| Hk1 compl-3′ | GCGCTGCAGTTCCACTGCTAATATCTCTTATT | Complementation | This work |
| flaB-F | CTTTTCTCTGGTGAGGGAGCTC | qRT-PCR, qPCR | 53 |
| flaB-R | GCTCCTTCCTGTTGAACACCC | qRT-PCR, qPCR | 53 |
| flaB-Probe | CTTGAACCGGTGCAGCCTGAGCA | qRT-PCR, qPCR | 53 |
| nidogen-F | CCCCAGCCACAGAATACCAT | qPCR | 81 |
| nidogen-R | AAAGGCGCTACTGAGCCGA | qPCR | 81 |
| nidogen-probe | CCGGAACCTTCCCACCCAGC | qPCR | 81 |
F, forward; R, reverse.
Restriction sites are in boldface.
Bb1197 was complemented with a wild-type copy of hk1 inserted into pSP1G, a gentamicin-resistant derivative of pBSV2 (75). To modify pBSV2, the gentamicin resistance cassette was amplified from pSPCG (41) using primer flgB-5′ and primer gent-3′ and inserted into the NcoI site of pBSV2. The pBSV2 kanamycin resistance cassette was inactivated by digesting the vector with PvuI, which removed the flgB promoter and the first 425 bp of the cassette. After the gentamicin-resistant shuttle vector was generated, a full-length copy of hk1 plus 325 bp of upstream sequence was amplified with primers Hk1 compl-5′ and Hk1 compl-3′ and cloned into the BamHI and PstI sites of pSP1G. The resulting vector, Hk1-pSP1G, was electrotransformed into Bb1197, and two gentamicin-resistant transformants (Bb1363 and Bb1367) were selected. The presence of the complementing copy of hk1 was confirmed using primers hk1-KO junc-5′ and hk1-KO-3′; expression of hk1 in the complemented mutants was confirmed by reverse transcription-PCR (RT-PCR) using cDNAs derived from in vitro grown organisms using these same primers.
SDS-PAGE and Western blot analyses.
Whole-cell lysates were prepared from spirochetes cultivated either in vitro at 23°C and following a temperature shift to 37°C or within DMCs as previously described (11). Equivalent amounts of lysate (∼2 × 107 spirochetes) were separated through 12.5% separating polyacrylamide mini-gels and visualized by silver staining. For immunoblotting, proteins were transferred to nylon-supported nitrocellulose and incubated with rat polyclonal antiserum directed against FlaB (9), BBA62/Lp6.6 (42), BBA24/DbpA (33), OspE (1), or Rrp1 (58), followed by goat anti-rat secondary antibody (Southern Biotechnology Associates, Birmingham, AL). Blots were developed using the SuperSignal West Pico chemiluminescence substrate (Pierce, Rockford, IL).
Animal infectivity and tick-related studies.
To assess infectivity of wild-type and hk1 mutant strains, 5-to 8-week-old female C3H/HeJ mice (five per group, per isolate) were inoculated intradermally with either 104 or 103 spirochetes. Infection was assessed at 2 and 4 weeks postinfection by serology and cultivation of tissues in BSK-II medium containing an antibiotic cocktail (0.05 mg/ml sulfamethoxazole, 0.02 mg/ml phosphomycin, 0.05 mg/ml rifampin, 0.01 mg/ml trimethoprim, and 0.0025 mg/ml amphotericin B) to minimize contamination. Cultures were monitored weekly by dark-field microscopy.
To generate naturally infected ticks, approximately 300 to 400 pathogen-free I. scapularis larvae (Oklahoma State University, Stillwater, OK) were placed on infected C3H/HeJ mice 2 to 3 weeks after syringe inoculation; the ticks were allowed to feed to repletion and then held in an environmental incubator until they had molted to the nymphal stage. To obtain fed nymphs, 10 to 12 infected flat I. scapularis nymphs were confined to a capsule affixed to the backs of naïve C3H/HeJ mice as previously described (49). Unless otherwise indicated, nymphs were allowed to feed until fully engorged. Immersion-fed larvae were generated according to the method described by Policastro and Schwan (56).
All animal experimentation was conducted following the NIH guidelines for housing and care of laboratory animals and was performed in accordance with the University of Connecticut Health Center and University of Maryland institutional regulations after review and approval by Institutional Animal Care and Use Committees at each respective institution.
Quantitative real-time RT-PCR.
Total RNA was isolated from infected ticks using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. Contaminating genomic DNA was removed using Turbo DNAfree (Ambion, Inc., Austin, TX). DNase-treated RNAs (1 to 4 μg of total RNA per sample) were converted to cDNA using SuperScript III (Invitrogen) in the presence and absence of reverse transcriptase (RT) according to the manufacturer's instructions. cDNAs (with RT) were assayed in quadruplicate using iQ Supermix (Bio-Rad). Transcript copy numbers were calculated using the iCycler postrun analysis software based on internal standard curves and then normalized against copies of flaB. Normalized copy number values were compared within Prism, version 5.00 (GraphPad Software, San Diego, CA) using an unpaired t test with two-tailed P values and a 95% confidence interval.
Assessment of B. burgdorferi burdens within infected murine tissues.
Spirochete burdens within infected murine tissues were assessed at 4 weeks postinfection. Twenty to 80 mg of each tissue was digested with 20× (vol/wt) 0.1% type I collagenase A (Sigma-Aldrich) at 37°C for 4 h and then mixed with an equal volume of 0.2 mg/ml proteinase K in 200 mM NaCl, 20 mM Tris-HCl (pH 8.0), 50 mM EDTA, and 1% sodium dodecyl sulfate. After overnight incubation at 55°C, 200 μl of each digested tissue was mixed with an equal volume of ATL buffer (Qiagen). Subsequent steps were performed using a Qiagen DNeasy blood and tissue kit according to the manufacturer's instructions. TaqMan-based flaB (88) and nidogen (81) assays were performed using iQ Supermix (Bio-Rad). Each DNA sample was assayed in quadruplicate, and genome copy numbers were calculated using the CFX Manager (Bio-Rad) postrun analysis software based on internal standard curves. Normalized values were compared within Prism, version 5.00, using an unpaired t test with two-tailed P values and a 95% confidence interval.
Assessment of B. burgdorferi burdens within I. scapularis ticks.
Spirochete burdens were assessed by quantitative PCR (qPCR) using individual pools of 15 larvae fed to repletion on syringe-inoculated mouse (3 mice per group, per isolate) or triplicate pools of 15 larvae infected by immersion and fed to repletion on a naïve mouse. Total genomic DNA was isolated from surface-sterilized larvae using a Gentra Puregene Yeast and Bacteria kit (Qiagen) according to the manufacturer's instructions. DNAs were diluted 1:10 in water prior to being assayed for flaB as described above. Spirochete viability was assessed by plating on semisolid medium (pBSK) as previously described (60). Plates were monitored for up to 4 weeks for the appearance of colonies. For immunofluorescence, pools of 15 larvae were crushed into 500 μl of ice-cold 1× CMRL medium, centrifuged for 10 min at 4,000 × g, washed twice with ice-cold 1× CMRL medium, and resuspended in 40 μl. Aliquots of each suspension (four per pool) were smeared on polylysine-treated slides, and spirochetes were detected using fluorescein isothiocyanate (FITC)-conjugated anti-Borrelia antibody (Kirkegaard and Perry Laboratories, Gaithersburg, MD) as previously described (49).
Microinjection of B. burgdorferi into naïve I. scapularis nymphs and confocal immunofluorescence microscopy.
Spirochetes were microinjected into the rectal openings of naïve nymphal ticks as described previously (54, 88). Forty-eight hours after injection, nymphs (15 per mouse) were allowed to feed on naïve C3H/HeN mice. Spirochetes within dissected midguts were detected using FITC-conjugated anti-Borrelia antibody, counterstained with propidium iodide, and mounted in antifade reagent for examination. Spirochetes within unfed nymphs were similarly analyzed at 10 days postinjection. Specimens were viewed using an LSM 510 scanning laser confocal microscope equipped with an argon/krypton laser. Images were acquired using a 40× (1.2 numerical aperture [NA]) water immersion objective with 512- by 512-pixel resolution at 1-μm intervals through the full thickness of the sample. Image acquisition and analyses were performed using LSM 5 AIM, version 4.2, software.
Time-lapse epifluorescence imaging of spirochetes within gelatin matrices.
The time-lapse imaging studies described here and below require the use spirochetes that constitutively express green fluorescent protein (GFP). We therefore used pMC1389 to inactivate hk1 within Bb914, a virulent CE162 isolate containing a highly stable PflaB-gfp reporter (16); two GFP-positive (GFP+) hk1 mutants (Bb1152 and Bb1155) were selected. Gelatin matrices (∼1 mm thick) were prepared as previously described (16). Approximately 1 × 108 in vitro cultivated spirochetes were added to each chamber well, and slides were incubated for 1 h at room temperature. Each chamber was rinsed twice with sterile phosphate-buffered saline (PBS) before being viewed by epifluorescent microscopy on an Olympus BX41 microscope (Center Valley, PA) using a 40× (1.3 NA) oil immersion objective. Motility was recorded using Streampix high-speed imaging software (Norpix, Canada) at 40 frames per s over 10-s intervals using a Retiga EXi charge-coupled device (CCD) camera (Q Imaging, Canada). A minimum of 200 organisms for each isolate were categorized per experiment. Each isolate was assayed in at least two independent experiments.
RESULTS
The Hk1/Rrp1 phosphorelay scheme involves a previously unrecognized Hpt domain within Hk1.
Autophosphorylation and subsequent phosphorelay by HKs typically are mediated by kinase core (DHp and CA domains) (17), REC (28), and histidine-containing phosphotransfer (Hpt) domains (39). Using the NCBI Conserved Domain Database (CDD), we were able to localize kinase core and REC domains within Hk1, but we initially were unable to identify the Hpt domain required for phosphorelay between Hk1 and Rrp1. We confirmed the presence of this requisite domain within the C terminus of Hk1 by multiple sequence alignment with seven prototypical hybrid HKs (see Fig. S1A in the supplemental material). The predicted Hk1 Hpt domain contains three residues, including the active-site histidine (H1252), that are highly conserved across a broad range of Hpt domains (see Fig. S1A) (39, 48). The Hk1 Hpt domain also closely modeled the corresponding domain from a newly described hybrid HK (Protein Data Bank [PDB] code 3MYF; RMSD of 0.1 Å) from Shewanella sp. W3-18-1 (see Fig. S1B) and displayed an overall fold that was highly similar to the folds of the well-characterized Hpt domains from BarA (PDB code 3IQT; RMSD of 1.3 Å) and ArcB (PDB code 1FR0; RMSD of 3.2 Å). Identification of this phosphotransfer domain enables us to propose a complete phosphorelay scheme for the Hk1/Rrp1 TCS based on established models for other HKs (39, 84) (see Fig. S1C).
hk1 and rrp1 are expressed by B. burgdorferi throughout the enzootic cycle.
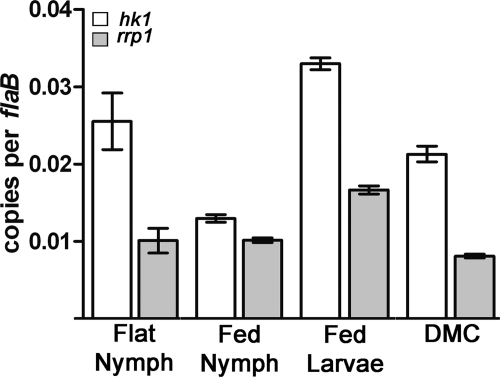
Expression profiling of hk1 and rrp1 was performed to gain insight into the function of this TCS during the enzootic cycle. In preliminary studies, we used semiquantitative RT-PCR across the hk1-rrp1 intergenic region to confirm that these genes are cotranscribed in vitro and within with dialysis membrane chambers (DMCs) (data not shown). We then performed quantitative RT-PCR (qRT-PCR) on RNAs isolated from I. scapularis ticks infected with wild-type strain 297 while the mammalian host phase was represented by DMC-cultivated organisms. Overall, both genes were expressed at low levels (∼1 to 3 copies per 100 copies of flaB). We observed higher levels of hk1, the upstream gene, than of rrp1 under all conditions (Fig. 1), which is not surprising given that the gene length of hk1 is ∼4.5 kb. While both genes were expressed within all tick stages and DMCs, transcript levels were highest in larvae fed to repletion and flat nymphs (Fig. 1). Given prior data demonstrating that the alternate sigma factor RpoS is not expressed within either fed larvae or flat nymphs (11, 49), we postulate that transcription of hk1 and rrp1 is controlled by the housekeeping sigma factor, RpoD. Indeed, we identified a putative σ70 promoter (TTGCCA-18-TTTAAA) located 77 nucleotides upstream of the Hk1 ATG start codon. Constitutive expression of hk1 and rrp1 implies that the corresponding TCS functions at multiple points within the tick-mouse cycle.
Fig. 1.
Expression profiling of hk1 and rrp1. Values represent the average flaB-normalized transcript copy number ± standard error of the mean for each gene. Values for hk1 were significantly different (P < 0.05) for the following comparisons: flat nymph versus fed larvae and fed nymph, fed larvae versus fed nymph and DMC, and fed nymph versus DMC. Values for rrp1 were significantly different (P < 0.05) for the following comparisons: fed larvae versus flat nymph, fed nymph and DMC, and DMC versus flat nymph and fed larvae. hk1 was expressed at significantly (P < 0.05) higher levels than rrp1 in the same sample under all four conditions examined. The sequences of the forward and reverse primers used to detect hk1 and rrp1 are provided in Table 2, and their locations are shown in Fig. 2A (hk1-F and hk1-R are designated by arrows 3 and 4, respectively; rrpl-F and rrpl-R are designated by arrows 5 and 6, respectively).
Hk1 is not required for mammalian host adaptation.
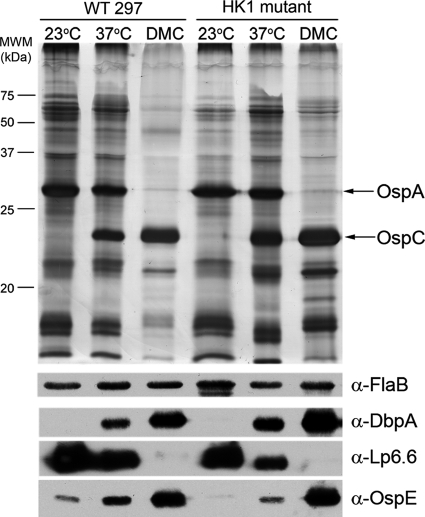
To functionally characterize Hk1, we inactivated hk1 by insertion of a PflgB-aadA cassette conferring resistance to streptomycin in B. burgdorferi (Fig. 2 A). The point of insertion within Hk1 (Val153) is located within the predicted periplasmic sensor domain, and, as such, the mutant polypeptide would lack both sensing and signal transduction capabilities. Two independently derived hk1 mutants (Bb508 and Bb807) containing full plasmid complements were obtained using our virulent strain 297 isolate CE162 (Table 1). Insertion and orientation of the PflgB-aadA cassette were confirmed by PCR (see Fig. S2A in the supplemental material; also data not shown). Both mutants grew identically to their parent at 23°C and 37°C in vitro and within DMCs (data not shown). The PflgB-aadA cassette used to inactivate hk1 does not contain a transcriptional terminator and therefore allows for transcriptional read-through and continued expression of rrp1. Using RT-PCR and immunoblotting, we first confirmed that our hk1 mutant isolates continue to express Rrp1 at or near wild-type levels (Fig. 2B and C). We observed few differences between the polypeptide profiles of Bb508 and its parent CE162 following temperature shift in vitro and cultivation within DMCs (Fig. 3). The high degree of similarity between the wild-type and mutant DMC proteomes was further substantiated by comparative two-dimensional (2-D) isoelectric focusing (IEF)-SDS-PAGE (data not shown). We also compared the expression profiles of prototypical σ70- and RpoS-dependent lipoproteins associated with mammalian host adaptation and/or virulence (1, 9, 11). Like its parent, Bb508 induced expression of OspC, DbpA, and OspE in response to temperature shift and further enhanced their expression within DMCs (Fig. 3). Moreover, RpoS-mediated repression of OspA and Lp6.6 within DMCs was unaffected by loss of Hk1 (Fig. 3).
Fig. 2.
(A) Strategy for inactivation of hk1. The hk1 coding sequence plus upstream and downstream flanking regions was amplified from strain 297 using primers ups.hk1-5′ and dwns.hk1-3′ (1 and 2). The hk1 coding sequence was disrupted by insertion of a PflgB-aadA antibiotic resistance cassette into an HpaI restriction site present within the endogenous hk1 gene, yielding pMC1389. Only the relevant portion of pMC1389 is shown. Insertion of the hk1 KO allele was confirmed in strain 297 and B31 mutant isolates using primers hk1-KO-5′ and hk1-KO-3′ (7 and 8) (see Fig. S2 in the supplemental material). Primer sequences are provided in Table 2. (B) Hk1-deficient spirochetes continue to express Rrp1. Semiquantitative RT-PCR was performed on RNAs isolated from a wild-type (WT) (297 and B31) and hk1 KO strains (Bb508, Bb807, and Bb1197) using primers specific for rrp1 (5 and 6) and flaB. RT indicates the absence (−) or presence (+) of reverse transcriptase in the reaction mixture. Purified genomic DNA (gDNA) was used as positive controls. (C) Detection of Rrp1 by immunoblotting. Whole-cell lysates of CE162 (WT 297), Bb508 (hk1-KO), and a previously characterized B31 5A13 Δrrp1 isolate (58) were immunoblotted with rat polyclonal antisera directed against Rrp1 (58) with FlaB used as a loading control. α, anti.
Fig. 3.
Hk1 is not required for mammalian host adaptation. Whole-cell lysates from CE162 (WT 297) and Bb508 (Hk1 mutant) were separated by SDS-PAGE, stained with silver, and immunoblotted using antisera directed against DbpA (33), Lp6.6/BBA62 (42), and OspE (1) with FlaB used as a loading control. MWM, molecular weight marker in thousands; α, anti.
hk1 mutant B. burgdorferi organisms are fully virulent in mice but were detected at low levels in larvae following acquisition.
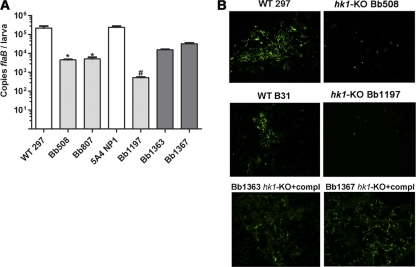
While not required for mammalian host adaptation within DMCs, Hk1 may contribute to other virulence-related aspects of the tick-mouse cycle, such as surface adhesion, dissemination, and/or immune evasion. To test this, we syringe inoculated C3H/HeJ mice (5 per group) with 1 × 104 spirochetes of either hk1 mutant (Bb508 and Bb807) or of their parent, CE162. At 2 and 4 weeks postinoculation, all mice showed evidence of seroconversion and were culture positive for spirochetes (data not shown). Bb508 also was highly infectious at a dose of 103 spirochetes (9/10 mice were culture positive for Bb508 at 4 weeks postinoculation compared to 4/10 mice infected with CE162 at this same dose). We next assessed spirochete burdens within infected tissues at 4 weeks postinoculation by qPCR. With the exception of somewhat lower burdens of Bb807 in ear tissue (P = 0.012), we detected comparable numbers of wild-type and hk1 mutant spirochetes in all tissues examined (Fig. 4). Prior to being sacrificed, these same mice were used to assess whether Hk1 is required for larval acquisition. By qPCR, we observed a >1.5 log10-fold (P < 0.01) decrease in the flaB copy numbers in larvae fed on mice infected with the hk1 mutants compared to larvae fed on mice infected with their wild-type counterpart (Fig. 5 A). The difference between the wild-type and mutant burdens was even more pronounced, as determined by plating in pBSK (Table 3) and immunofluorescence assay (IFA) (Fig. 5B); in contrast to the large numbers of viable wild-type spirochetes detected by both methods, few, if any, intact organisms were recovered from or visualized within larvae fed to repletion on mice infected with the hk1 mutants.
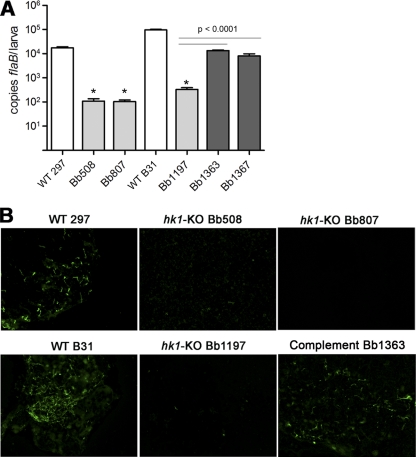
Fig. 4.
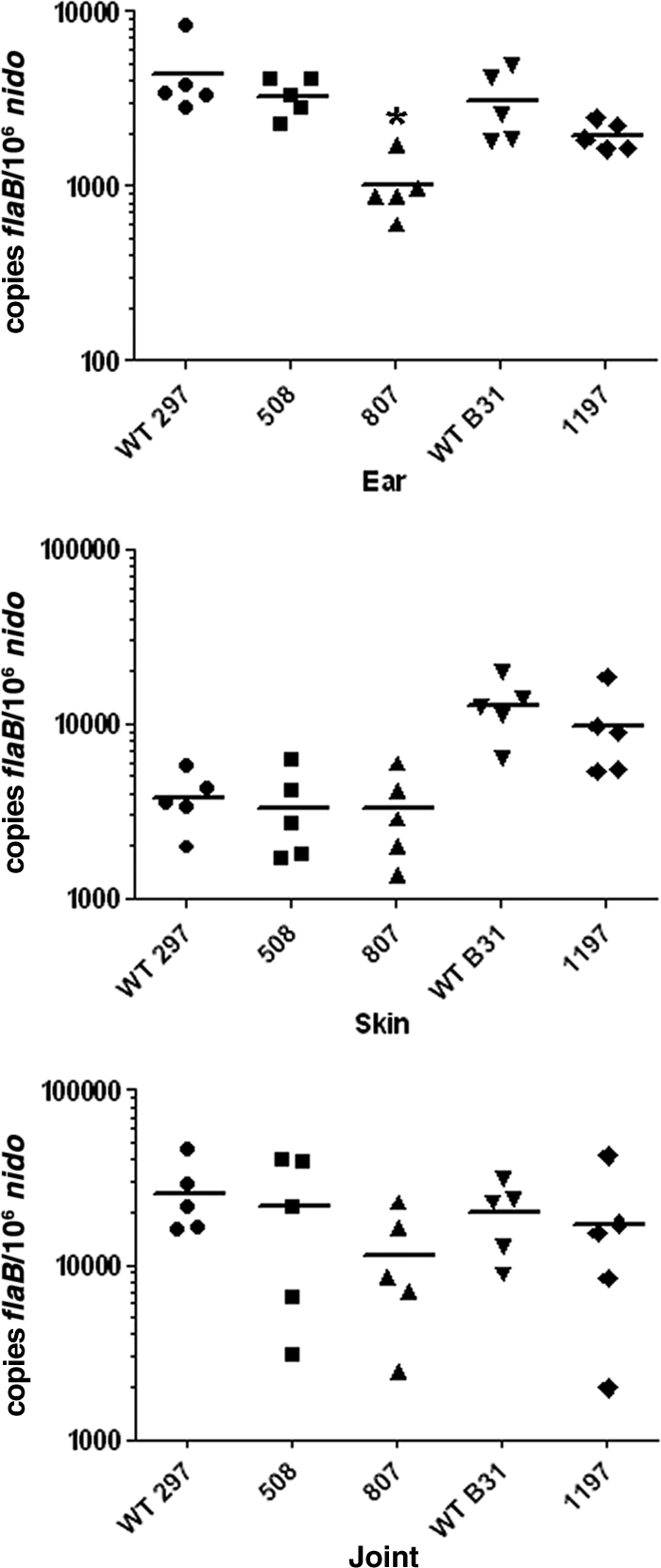
Spirochetes lacking Hk1 are infectious in mice by syringe inoculation. Tissues were collected from infected mice (5 mice per group) at 4 weeks postinoculation with 104 spirochetes of CE162 (WT 297), Bb508, Bb807, B31 5A4 NP1 (WT B31), or Bb1197. Spirochete genome copies, here and elsewhere, were determined using TaqMan assays for spirochetal flaB and murine nidogen (nido). The mean nido-normalized flaB value for all mice within a group is indicated by a horizontal line. The asterisk indicates a statistical difference (P < 0.05) between CE162 and Bb807 in ear tissue.
Fig. 5.
hk1 mutants are killed within larvae fed to repletion on syringe-inoculated mice. Data represent spirochete burdens within larvae fed to repletion on C3H/HeJ mice 2 to 3 weeks following syringe inoculation with wild-type parents (CE162 and B31 5A4 NP1), hk1 mutants (Bb508, Bb807, and Bb1197), or complemented hk1 mutants (Bb1363 and Bb1367). (A) Spirochete genome copies within pools of 15 larvae collected from individual mice (3 mice per isolate) were determined using a TaqMan assay for flaB. Bars represent the mean ± standard error of the mean for each isolate. The normalized flaB values for larvae fed on mice infected with hk1 mutants (hk1 KO strains) was significantly lower (*, P = 0.0021; #, P < 0.0001) than that for larvae fed on mice infected with the corresponding parent or complements (compl). (B) Representative micrographs of larvae fed to repletion on syringe-inoculated mice. Pools of 15 larvae from each mouse (3 mice per group) were assessed by IFA using FITC-conjugated anti-Borrelia antibody.
Table 3.
Semisolid plating of larvae fed to repletion on syringe-inoculated mice
| Isolate and groupa | No. of CFU per larva (avg ± SD)b |
|---|---|
| WT 297 | |
| Larval pool 1 | 1,395.7 ± 513.4 |
| Larval pool 2 | 1,629.1 ± 377.3 |
| Larval pool 3 | 4,650 ± 2,192 |
| Bb508 | |
| Larval pool 1 | ND |
| Larval pool 2 | 6 ± 6.8 |
| Larval pool 3 | ND |
| Bb807 | |
| Larval pool 1 | 4.4 ± 3.9 |
| Larval pool 2 | 0.2 ± 0.4 |
| Larval pool 3 | ND |
| Bb1197 | |
| Larval pool 1 | ND |
| Larval pool 2 | ND |
| Larval pool 3 | ND |
Strain used to syringe inoculate the mice used for larval infestation.
The number of CFU per larva is based on larvae (15 per pool) fed to repletion on individual infected mice, with 3 mice per isolate (animals M1 to M3). Values represent the average number of CFU (± standard deviation) from three serial dilutions (undiluted, 10−1, and 10−2), plated in duplicate, for each larval pool. ND, no CFU detected.
B. burgdorferi spirochetes lacking Hk1 are acquired normally during feeding but are killed within the larval midgut during the blood meal.
The most straightforward interpretation of the above data is that Hk1-deficient spirochetes are being destroyed within the midguts of feeding larvae following acquisition. c-di-GMP has emerged as an important regulator of bacterial virulence (38, 85). Indeed, Sultan et al. (76) recently demonstrated that B. burgdorferi lacking BB0363, a c-di-GMP-specific phosphodiesterase, is unable to translate laterally (i.e., reverse their direction of swimming) in vitro. The predicted role for Hk1 in activating Rrp1 raised the possibility that the larval acquisition defect displayed by our hk1 mutants could stem, in part, from their inability to migrate out of the bite site. As a first step toward understanding the contribution of Hk1 to acquisition, we used a gelatin matrix-based assay (16) to compare the motility patterns of wild-type and hk1 mutant isolates in vitro. Although we observed fewer nonmotile hk1 mutant spirochetes than wild-type organisms (Table 4), these differences were not statistically significant. Moreover, comparable percentages of wild-type and hk1 mutant organisms could be observed translating laterally within the matrix (Table 4). We next assessed whether Hk1-deficient organisms are acquired normally during feeding. Technical limitations related to the small size and fragile nature of larval midguts required that these timed-feeding studies be performed using naïve nymphs. Previously, Schwan and Piesman (66) demonstrated that spirochetes could be detected within naïve nymphs within 24 h of attachment to an infected mouse. At 24 to 36 h postattachment, we detected comparable numbers of spirochetes in nymphs fed on C3H/HeJ mice infected with either wild-type or hk1 mutant isolates (see Fig. S3 in the supplemental material; also data not shown). Lastly, we artificially infected naïve larvae with CE162, Bb508, and Bb807 by immersion (56), thereby eliminating the migratory aspect of acquisition entirely. Results obtained using immersion-fed larvae were identical to those obtained using larvae fed on syringe-inoculated mice; we observed a marked decrease in the numbers of hk1 mutant compared to wild-type organisms in fed larvae, as determined by qPCR (Fig. 6 A), semisolid plating (Table 5), and IFA (Fig. 6B).
Table 4.
hk1 mutant B. burgdorferi display normal motility in vitro
| Strain | Motility profile (% [mean ± SD])a |
Total no. of organisms | ||
|---|---|---|---|---|
| Nonmotile | Motile | Translating | ||
| WT 297 (Bb914) | 12.67 ± 6.75 | 87.33 ± 6.74 | 35.54 ± 8.93 | 601 |
| hk1 mutant (Bb1152) | 4.10 ± 4.55 | 95.90 ± 4.55 | 24.42 ± 2.27 | 465 |
| hk1 mutant (Bb1155) | 3.13 ± 2.22 | 96.88 ± 2.23 | 30.60 ± 8.10 | 427 |
Motility categories are defined as the following: nonmotile, organisms that displayed no discernible signs of motility throughout 10-s imaging interval; motile, organisms that displayed obvious signs of motility at any point during imaging; and translating, organisms within the motile category that also displayed lateral translation in the x or y axis. Values represent results from at least two independent experiments. A minimum of 200 organisms were scored in each experiment.
Fig. 6.
hk1 mutants are killed within the midguts of larvae infected by immersion. Larvae infected by immersion with wild-type 297 and B31 (CE162 and 5A4 NP1), hk1-KO mutants (Bb508, Bb807 and Bb1197), or complement (Bb1363) were fed to repletion on naïve mice. (A) Spirochete genome copies within triplicate pools of 15 fed larvae. Bars represent the mean flaB values per larva ± standard error of the mean for each isolate. Asterisks indicate significantly (P < 0.0001) lower values for hk1 mutants than for the corresponding parent. (B) Representative immunofluorescence micrographs of fed larvae infected by immersion. Pools of 15 fed larvae infected with each strain were assessed by IFA using FITC-conjugated anti-Borrelia antibody.
Table 5.
Semisolid plating of replete larvae infected by immersion
| Isolatea | No. of CFU per larva (avg ± SD)b |
|---|---|
| WT 297 | 227.3 ± 94.4 |
| Bb508 | 0.1 ± 1.3 |
| Bb807 | 0.3 ± 0.5 |
| WT B31 | 1,226 ± 855 |
| Bb1197 | 9.0 ± 9.0 |
Strain used to immerse naïve larvae. Viability of spirochetes within immersion-fed larvae was assessed after ticks had fed to repletion on naïve mice.
The number of CFU is based on pools of 15 larvae per isolate. Values represent results from three serial dilutions (undiluted, 10−1, and 10−2), plated in triplicate, for each larval pool.
Generation and characterization of a B. burgdorferi strain B31 hk1 mutant and complementation.
The dramatic tick phase phenotype displayed by both of our independently derived strain 297 hk1 mutants is unlikely to be due to a secondary mutation within an unrelated gene(s). Nevertheless, to prove definitively that hk1 alone is responsible for this phenotype, we attempted to complement both hk1 mutants with a wild-type copy of hk1 contained on a shuttle vector. Despite exhaustive efforts, we were unable to transform either Bb508 or Bb807 with this construct. To garner more definitive evidence that the observed phenotype is due to loss of hk1, we inactivated hk1 in the highly transformable, virulent B31 isolate 5A4 NP1 (40) using the same strategy as used for strain 297, yielding Bb1197 (Table 1). Like its strain 297 mutant counterparts, Bb1197 expressed normal levels of rrp1 (Fig. 2B), was fully virulent in mice by syringe inoculation (Fig. 4), and was acquired by nymphs during the first 24 h of feeding (see Fig. S3 in the supplemental material). Equally important, Bb1197 was highly sensitive to killing by the larval blood meal (Fig. 5 and 6; Tables 3 and 5). Unlike our strain 297 hk1 mutants, we obtained multiple complemented isolates using Bb1197 (Table 1; see also Fig. S2C). Using two independent isolates (Bb1363 and Bb1367), we confirmed that survival of Hk1-deficient spirochetes within fed larvae could be restored by trans-complementation with a wild-type copy of hk1 contained on a cp9-based shuttle vector (Fig. 5 to 7 and data not shown).
Fig. 7.
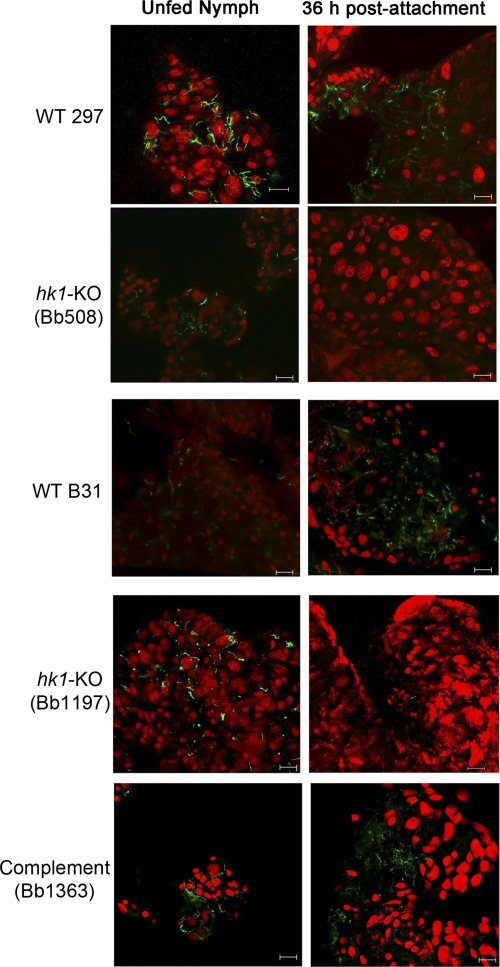
Hk1 is required for survival within fed but not flat nymphal midguts. Composite confocal image showing the distribution of spirochetes within nymphs infected by rectal microinjection with CE162 (WT 297), Bb807 (hk1-KO mutant), Bb1197 (hk1-KO mutant), 5A4 NP1 (WT B31), or complement (Bb1363). Spirochetes were detected within midguts carefully dissected from unfed and fed nymphs, forcibly removed at 36 h postattachment, using FITC-conjugated anti-Borrelia antibody. The tick midgut was counterstained with propidium iodide (red). Scale bar, 20 μm.
hk1 mutant B. burgdorferi organisms are killed within nymphal as well as larval midguts.
Our studies thus far have focused on the contribution of Hk1 to survival within feeding larvae. We reasoned, however, that the protection afforded by Hk1 during acquisition also would be required during the nymphal blood meal. But because hk1 mutants are eliminated from the midguts of fed larvae, we could not assess the role of this gene product within nymphs following the molt. To circumvent this limitation, we used the microinjection technique developed by Pal et al. (53) to administer spirochetes directly into the midguts of naïve nymphs via the rectal opening. Up to 10 days postinjection, we detected similar numbers of wild-type (CE162 and 5A4 NP1) and hk1 mutant (Bb508, Bb807 and Bb1197) spirochetes within unfed nymphal midguts (Fig. 7 and data not shown), indicating that Hk1 is not required when the midgut epithelium is quiescent. As early as 36 h postattachment, on the other hand, we saw a dramatic decrease in the burdens of all three hk1 mutants in fed versus unfed midguts (Fig. 7 and data not shown). The numbers of wild-type organisms, in contrast, remained relatively unchanged in response to early feeding (Fig. 7). Survival of spirochetes lacking Hk1 was restored in feeding nymphs by trans-complementation (Fig. 7 and data not shown).
The Hk1 sensor domain contains two distinct regions with homology to different amino acid substrate-binding proteins.
The molecule(s) responsible for activating the Hk1 sensor domain must be able to traverse the spirochete's outer membrane. We performed detailed in silico analyses of this region as a first step toward identifying potential ligands. CDD searches revealed that Hk1's periplasmic sensor consists of two discrete solute-binding domains, designated D1 and D2, both belonging to the Pfam PF00497 family of bacterial extracellular solute-binding proteins. This highly diverse family is typically associated with substrate-binding proteins (SBPs) of ABC-type transporters (47, 78) but recently has been expanded to include a number of sensor histidine kinases (5). Based on homology searches using the Swiss-Model server (2), D1 modeled most closely with ArtJ (PDB 2Q2A; E value of 8.10e−34), an arginine-, lysine-, histidine-binding protein from Geobacillus stearothermophilus (82) (see Fig. S4A in the supplemental material), while D2 best matched GlnBP (PDB 1WDN; E value of 1.00e−22), a glutamine-binding protein from Escherichia coli (77) (see Fig. S4B). The structural models for D1 and D2 each display features common to ABC transporter- and sensor-type SBPs, namely, two mixed α/β-fold globular lobes connected by a flexible hinge region with a predicted binding pocket located at the interface between the two lobes in each domain (5). Nonsynonymous amino acid substitutions within the predicted binding pockets for D1 and D2 compared to ArtJ and GlnBP (see Fig. S4A and B), respectively, imply that the ligands recognized by D1 and D2 differ from those of their respective structural homolog.
DISCUSSION
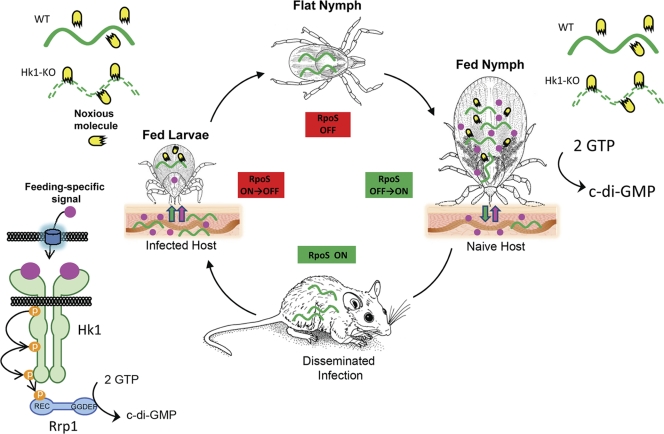
Maintenance of B. burgdorferi within its enzootic cycle depends upon the spirochete's ability to sense and respond to environmental stimuli encountered within the arthropod vector and mammalian host. Signals contained within the nymphal blood meal trigger a complex series of transcriptional, antigenic, and physiological changes that enable spirochetes colonizing the midgut to disseminate through tick tissues while preparing for growth within the mammalian host (11, 15, 16, 67); the Rrp2/RpoN/RpoS regulatory pathway is central to this transmission-associated program (6, 7, 11, 15, 20, 52, 86, 87). The signaling pathways underlying the converse process, whereby spirochetes transit from the mammalian host into naïve I. scapularis ticks, have received comparatively little attention. Here, we demonstrate that the hybrid histidine kinase Hk1 is required for B. burgdorferi to survive within the fed midgut environment. Recently, studies by two independent laboratories demonstrated that spirochetes lacking Rrp1 display an identical survival defect (33a, 41a), thereby confirming that the protective function of Hk1 is mediated via phosphorelay. Importantly, spirochetes lacking Hk1 survive for prolonged periods within the midguts of unfed nymphs but are destroyed at the onset of the nymphal blood meal, indicating that the adaptive response mediated by Hk1 is not tick stage specific. Thus, in contrast to the Rrp2/RpoN/RpoS pathway, which functions exclusively during nymphal transmission and subsequent mammalian infection (7, 10, 11, 49, 86), the Hk1/Rrp1 TCS functions during both acquisition and transmission.
Inactivation of hk1 had no effect on the ability of spirochetes to adapt to the host, disseminate within mice following syringe inoculation, or establish murine infection. Indeed, despite being present at wild-type levels within skin, hk1 mutant spirochetes were not recovered from larvae fed on syringe-inoculated mice. One can envision two non-mutually exclusive explanations for this defect: (i) hk1 mutants are unable to exit the feeding site, or (ii) spirochetes lacking Hk1 are being killed following acquisition. Multiple lines of evidence argue in favor of the latter. First, using a gelatin matrix-based assay (16), we determined that loss of Hk1 had no discernible effect on motility; this finding is particularly noteworthy in light of the established link between c-di-GMP and motility in other bacteria (13, 85) and a report by Sultan et al. (76), demonstrating that the manipulation of c-di-GMP levels in B. burgdorferi engenders a motility defect in vitro. Second, hk1 mutant organisms could be visualized within the midguts of naïve nymphs at ≤24 h postattachment during the so-called preparatory phase that precedes the ingress of blood and differentiation of midgut epithelial cells (3). Third, we detected appreciable amounts of borrelial genomic DNA by qPCR but scant viable or intact organisms by semisolid plating and IFA, respectively, in larvae fed on hk1 mutant-infected mice. Lastly, using immersion feeding to circumvent infected mice as a means of introducing hk1 mutants into naïve larvae, we observed a similar stark difference between spirochete burdens detected by qPCR and the numbers of live and intact organisms detected by plating and IFA.
While further experimentation will be required to establish a definitive biochemical link between Hk1 and Rrp1, the similar phenotypes displayed by our hk1 mutants and those lacking Rrp1 (33a, 41a) provide compelling evidence that these proteins work cooperatively to promote the synthesis of c-di-GMP. Typically, c-di-GMP exerts its regulatory effects by binding to a wide range of effector molecules, altering either transcription or enzymatic activity (34, 65). In the case of B. burgdorferi, the time frame for activation of the Hk1/Rrp1 TCS is more than sufficient to encompass both transcription and de novo synthesis of borrelial gene products. We envision two types of adaptive responses that could be initiated within ticks. First, activation of Hk1 may be required for spirochetes to evade killing by noxious substances generated during digestion of the blood meal and/or elaborated by the midgut epithelium (21, 50, 74). Based on the near-complete destruction of Hk1-deficient organisms within fed ticks, we hypothesize that the initial lesion is likely a breach in the spirochete's fragile outer membrane that exposes the underlying cell envelope (4, 8, 43). Our observation that prolonged incubation of hk1 mutants with 72-h-fed nymphal midguts ex vivo did not replicate the killing observed within feeding nymphs (data not shown) implies that killing requires proximity to the midgut epithelium. Spirochetes maintain extensive and prolonged contact with the midgut epithelium within feeding ticks (16), suggesting that the protection afforded by Hk1 extends beyond the early feeding time point in which we observed destruction of our hk1 mutants. Alternatively, c-di-GMP may regulate borrelial gene products involved in metabolic adaptation to the fed midgut. Along these lines, two independent microarray studies have shown that Rrp1 promotes the transcription of glp genes (bb0240-bb0243) involved in glycerol uptake and utilization (58; also He et al., submitted); in addition to its role in membrane biogenesis, glycerol is thought to be the principal carbon/energy source for spirochetes within feeding ticks (55; also He et al., submitted). However, constitutive expression of the glp operon in spirochetes lacking Rrp1 only partially alleviates the survival defect within engorged ticks (He et al., submitted), implicating additional gene products as part of a larger Hk1/Rrp1-mediated response. In both of the above scenarios, one would predict that Hk1 would be required during the nymphal as well as larval blood meal. We confirmed this supposition by demonstrating that hk1 mutants survive for prolonged periods in unfed nymphal midguts infected by microinjection but are destroyed as early as 36 h postattachment to a naïve host.
Our working model, presented in Fig. 8, proposes that the periplasmic portion of Hk1 senses host- and/or tick-derived molecules generated during feeding. Tick saliva contains a plethora of bioactive molecules (22, 63, 72). Destruction of host tissues during creation of the feeding lesion and the ensuing inflammatory response would also give rise to numerous small molecules (e.g., histamine and serotonin) with signaling potential (46, 61). Thus, the bite site represents an extraordinarily rich milieu for generating ligands able to rapidly traverse the spirochete's outer membrane and engage one or both of the D1 and D2 sensor domains of Hk1. Based on in silico structural modeling, we predict that D1 and D2 recognize amino acids (or their derivatives). Interestingly, Scheckelhoff et al. (64) demonstrated that the administration of the β-adrenergic antagonist propranolol to infected mice significantly reduced spirochete burdens within fed larvae; their findings implicate catecholamines, which are derivatives of phenylalanine and tyrosine and plentiful in tick saliva (62), as attractive candidate signaling molecules for Hk1. The requirement for Hk1 at the onset of the nymphal blood meal, on the other hand, implies that signaling molecule(s) involved in activating this sensor histidine kinase also are present within the midgut early during feeding, either imbibed from the bite site as part of the blood meal or elaborated by the differentiating midgut epithelium.
Fig. 8.
Working model for Hk1/Rrp1 and Rrp2/RpoN/RpoS TCS during the enzootic cycle. At the onset of feeding, Hk1 senses unique host- and/or tick-derived molecules generated within the feeding site. These feeding-specific molecules must be small enough to rapidly traverse the spirochete's outer membrane and engage Hk1's D1 and D2 periplasmic sensor domains. During acquisition, spirochetes first encounter these molecules as they migrate into the feeding site, while spirochetes within flat nymphs (or larvae infected by immersion) would encounter these ligands solely within the midgut (3). The Hk1/Rrp1-directed synthesis of c-di-GMP initiates an adaptive response that enables spirochetes either to evade killing by noxious substances within midgut epithelium as feeding progresses (21, 37, 50, 73, 74) or to adjust metabolically to growth within the arthropod vector. In contrast to the Rrp2/RpoN/RpoS pathway, which is active (ON) only within feeding nymphs, the Hk1/Rrp1 TCS is essential for survival during both the larval and nymphal blood meals.
Sensor-type SBPs, such D1 and D2, have emerged as a new structural class of HKs that are thought to function by analogy to the classic “Venus flytrap” model for ABC transporters (12, 35). With transporter SBPs, occupancy of the binding pocket favors a closed conformation, allowing the protein to interact specifically with its cognate permease (19). Ligand binding by sensor SBPs, in contrast, is thought to stabilize an open conformation (12, 35), with the resulting piston-like conformational change stimulating the autophosphorylation of a conserved histidine (predicted to be H773 in Hk1) within the cytoplasmic kinase core (see Fig. S1 in the supplemental material) (29). In a recent study, Herrou et al. (35) proposed that BvgS, a prototype for this new class of HKs, is constitutively active in the unbound (i.e., closed) state and is deactivated by ligand binding. This scenario seems unlikely for Hk1 because phosphorylation of Rrp1 is a prerequisite for diguanylate cyclase activity (59). While the presence of tandem SBPs is common among periplasmic histidine kinase sensor domains (Microbial Signal Transduction Database; Agile Genomics, Mount Pleasant, SC), little is known regarding whether these domains function cooperatively. One intriguing possibility is that D1 and D2 recognize signaling molecules that are unique to the acquisition and transmission phases of the enzootic cycle and function independently to promote activation of Hk1 within feeding larvae and nymphs.
The data in this paper, together with other studies (9, 11, 15, 20, 49, 52, 70, 86), allow us to contrast the Hk1/Rrp1 and Hk2/Rrp2 regulatory pathways while, at the same time, envisioning how these two TCSs may collaborate to promote the maintenance of B. burgdorferi in nature (Fig. 8). The Rrp2/RpoN/RpoS pathway is induced during the nymphal blood meal and presumably stays ON throughout infection, transitioning from an ON to OFF state during larval acquisition (11, 49). The apparent lack of cross talk between the spirochete's two TCSs is consistent with the intrinsic ability of HKs to recognize their cognate response regulator to the exclusion of all others (69). The role of Hk2 as the principle means of activating Rrp2 was unexpectedly called into question by Xu et al. (86), who demonstrated that phosphorylation of Rrp2 also can be mediated via the high-energy phosphate donor acetyl∼P. The dramatic phenotype associated with loss of Hk1 within feeding nymphs indicates that acetyl∼P is unable to promote phosphorylation of Rrp1, thereby creating a definitive barrier between these two signal transduction pathways. That the Hk1/Rrp1 is ON during the larval and nymphal blood meals is strong evidence not only that the Hk1/Rrp1 and Rrp2/RpoN/RpoS pathways are activated by disparate environmental stimuli but also that the physiological cues that promote activation of Rrp2 are specific to the nymphal blood meal. Despite their strict segregation, the Hk1/Rrp1 and Rrp2/RpoN/RpoS regulatory pathways are, nevertheless, clearly interdependent. The most obvious example is the protective function of Hk1/Rrp1, without which spirochetes could not be transmitted by feeding nymphs. While destruction of spirochetes lacking either Hk1 or Rrp1 (33a, 41a) precludes a direct examination of the transcriptional changes elicited by this TCS during tick feeding, microarray analyses performed using in vitro cultivated organisms indicate that Rrp1 and, by extension, c-di-GMP, move the spirochete's transcriptional set point toward the expression of tick phase genes (58). Thus, it is tempting to speculate that “pressure” from the Hk1/Rrp1 TCS drives the transition from an RpoS-ON to RpoS-OFF state during acquisition and delays the downregulation of tick phase genes until spirochetes have been successfully transmitted to the mammalian host. Regarding the latter, we have shown that downregulation of tick phase genes, such as ospA, is RpoS dependent but occurs slowly over the course of the nymphal blood meal (9, 11, 49), while Ohnishi et al. (51) elegantly documented that many spirochetes continue to express OspA within the feeding site. We hypothesize that mammalian host adaption is not complete until spirochetes have migrated away from the bite site and are no longer subject to the regulatory effects of c-di-GMP.
Supplementary Material
ACKNOWLEDGMENTS
We thank Anna Allard for her technical assistance and Meghan Lybecker for her efforts regarding the transformation of strain 297 hk1 mutant isolates. We are indebted to Daniel Sonenshine for this many helpful suggestions and advice on the tick physiology.
This work was supported in part by NIH/NIAID grants AI-29735 and 3R01AI029735-20S1 (J.D.R. and M.J.C.), AI085248 (M.J.C.), A1080615 (U.P.), and AI059373 and AI085310 (D.R.A.), along with grants from the Oklahoma Center for the Advancement of Science and Technology (HR09-002 to D.R.A.), the National Research Fund for Tick-Borne Diseases (M.J.C.), and a New England Regional Center of Excellence Fellowship (U54 AI-057159 to S.D.-E.).
Footnotes
Supplemental material for this article may be found at http://iai.asm.org/.
Published ahead of print on 23 May 2011.
REFERENCES
- 1. Akins D. R., Bourell K. W., Caimano M. J., Norgard M. V., Radolf J. D. 1998. A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. J. Clin. Invest. 101:2240–2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arnold K., Bordoli L., Kopp J., Schwede T. 2006. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22:195–201 [DOI] [PubMed] [Google Scholar]
- 3. Balashov Y. S. 1972. Blood-sucking ticks (Ixodidae)-vectors of disease of man and animals. Misc. Pub. Entomol. Soc. Am. 8:161–376 [Google Scholar]
- 4. Bergstrom S., Zuckert W. R. 2010. Structure, function and biogenesis of the Borrelia cell envelope, p. 139–166 In Samuels D. S., Radolf J. D. (ed.), Borrelia: molecular biology, host interaction and pathogenesis. Calister Academic Press, Norfolk, United Kingdom [Google Scholar]
- 5. Berntsson R. P. A., Smits S. H. J., Schmitt L., Slotboom D.-J., Poolman B. 2010. A structural classification of substrate-binding proteins. FEBS Lett. 584:2606–2617 [DOI] [PubMed] [Google Scholar]
- 6. Blevins J. S., et al. 2009. Rrp2, a σ54-dependent transcriptional activator of Borrelia burgdorferi, activates rpoS in an enhancer-independent manner. J. Bacteriol. 191:2902–2905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boardman B. K., et al. 2008. Essential role of the response regulator Rrp2 in the infectious cycle of Borrelia burgdorferi. Infect. Immun. 76:3844–3853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boylan J. A., Lawrence K. A., Downey J. S., Gherardini F. C. 2008. Borrelia burgdorferi membranes are the primary targets of reactive oxygen species. Mol. Microbiol. 68:786–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Caimano M. J., Eggers C. H., Gonzalez C. A., Radolf J. D. 2005. Alternate sigma factor RpoS is required for the in vivo-specific repression of Borrelia burgdorferi plasmid lp54-borne ospA and lp6.6 genes. J. Bacteriol. 187:7845–7852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Caimano M. J., Eggers C. H., Hazlett K. R., Radolf J. D. 2004. RpoS is not central to the general stress response in Borrelia burgdorferi but does control expression of one or more essential virulence determinants. Infect. Immun. 72:6433–6445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Caimano M. J., et al. 2007. Analysis of the RpoS regulon in Borrelia burgdorferi in response to mammalian host signals provides insight into RpoS function during the enzootic cycle. Mol. Microbiol. 65:1193–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cheung J., Le-Khac M., Hendrickson W. A. 2009. Crystal structure of a histidine kinase sensor domain with similarity to periplasmic binding proteins. Proteins 77:235–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cotter P. A., Stibitz S. 2007. c-di-GMP-mediated regulation of virulence and biofilm formation. Curr. Opin. Microbiol. 10:17–23 [DOI] [PubMed] [Google Scholar]
- 14. DeLano W. L. 2002. The PyMOL molecular graphics system. DeLano Scientific, San Carlos, CA [Google Scholar]
- 15. de Silva A. M., Tyson K. R., Pal U. 2009. Molecular characterization of the tick-Borrelia interface. Front. Biosci. 14:3051–3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dunham-Ems S. M., et al. 2009. Live imaging reveals a novel, biphasic mode of dissemination of Borrelia burgdorferi within ticks. J. Clin. Invest. 119:3652–3665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dutta R., Qin L., Inouye M. 1999. Histidine kinases: diversity of domain organization. Mol. Microbiol. 34:633–640 [DOI] [PubMed] [Google Scholar]
- 18. Eggers C. H., et al. 2002. Identification of loci critical for replication and compatibility of a Borrelia burgdorferi cp32 plasmid and use of a cp32-based shuttle vector for the expression of fluorescent reporters in the Lyme disease spirochaete. Mol. Microbiol. 43:281–295 [DOI] [PubMed] [Google Scholar]
- 19. Felder C. B., Graul R. C., Lee A. Y., Merkle H.-P., Sadee W. 1999. The Venus flytrap of periplasmic binding proteins: an ancient protein module present in multiple drug receptors. AAPS Pharm. Sci. 1:1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fisher M. A., et al. 2005. Borrelia burgdorferi σ54 is required for mammalian infection and vector transmission but not for tick colonization. Proc. Natl. Acad. Sci. U. S. A. 102:5162–5167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fogaça A. C., et al. 1999. Antimicrobial activity of a bovine hemoglobin fragment in the tick Boophilus microplus. J. Biol. Chem. 274:25330–25334 [DOI] [PubMed] [Google Scholar]
- 22. Francischetti I. M., Sa-Nunes A., Mans B. J., Santos I. M., Ribeiro J. M. 2009. The role of saliva in tick feeding. Front. Biosci. 14:2051–2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Frank K. L., Bundle S. F., Kresge M. E., Eggers C. H., Samuels D. S. 2003. aadA confers streptomycin resistance in Borrelia burgdorferi. J. Bacteriol. 185:6723–6727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fraser C. M., et al. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580–586 [DOI] [PubMed] [Google Scholar]
- 25. Galperin M. 2005. A census of membrane-bound and intracellular signal transduction proteins in bacteria: bacterial IQ, extroverts and introverts. BMC Microbiol. 5:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Galperin M. Y. 2010. Diversity of structure and function of response regulator output domains. Curr. Opin. Microbiol. 13:150–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Galperin M. Y., Nikolskaya A. N., Koonin E. V. 2001. Novel domains of the prokaryotic two-component signal transduction systems. FEMS Microbiol. Lett. 203:11–21 [DOI] [PubMed] [Google Scholar]
- 28. Gao R., Mack T. R., Stock A. M. 2007. Bacterial response regulators: versatile regulatory strategies from common domains. Trends Biochem. Sci. 32:225–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gao R., Stock A. M. 2009. Biological insights from structures of two-component proteins. Annu. Rev. Microbiol. 63:133–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gilmore R. D., et al. 2010. The bba64 gene of Borrelia burgdorferi, the Lyme disease agent, is critical for mammalian infection via tick bite transmission. Proc. Natl. Acad. Sci. U. S. A. 107:7515–7520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goldstein S. F., et al. 2010. The chic motility and chemotaxis of Borrelia burgdorferi, p. 167–188 In Samuels D. S., Radolf J. D., (ed.), Borrelia: molecular biology, host interaction, and pathogenesis. Caister Academic Press, Norfolk, United Kingdom [Google Scholar]
- 32. Grimm D., et al. 2004. Outer-surface protein C of the Lyme disease spirochete: A protein induced in ticks for infection of mammals. Proc. Natl. Acad. Sci. U. S. A. 101:3142–3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hagman K. E., et al. 1998. Decorin-binding protein of Borrelia burgdorferi is encoded within a two-gene operon and is protective in the murine model of Lyme borreliosis. Infect. Immun. 66:2674–2683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33a. He M., et al. 2011. Cyclic di-GMP is essential for the survival of the Lyme disease spirochete in ticks. PLoS Pathog. 7:e1002133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hengge R. 2009. Principles of c-di-GMP signalling in bacteria. Nat. Rev. Microbiol. 7:263–273 [DOI] [PubMed] [Google Scholar]
- 35. Herrou J., et al. 2010. Periplasmic domain of the sensor-kinase BvgS reveals a new paradigm for the Venus flytrap mechanism. Proc. Natl. Acad. Sci. U. S. A. 107:17351–17355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hubner A., et al. 2001. Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN-RpoS regulatory pathway. Proc. Natl. Acad. Sci. U. S. A. 98:12724–12729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hynes W. L., Ceraul S. M., Todd S. M., Seguin K. C., Sonenshine D. E. 2005. A defensin-like gene expressed in the black-legged tick, Ixodes scapularis. Med. Vet. Entomol. 19:339–344 [DOI] [PubMed] [Google Scholar]
- 38. Jenal U., Malone J. 2006. Mechanisms of cyclic-di-GMP signaling in bacteria. Annu. Rev. Genet. 40:385–407 [DOI] [PubMed] [Google Scholar]
- 39. Kato M., Mizuno T., Shimizu T., Hakoshima T. 1997. Insights into multistep phosphorelay from the crystal structure of the C-terminal Hpt domain of ArcB. Cell 88:717–723 [DOI] [PubMed] [Google Scholar]
- 40. Kawabata H., Norris S. J., Watanabe H. 2004. BBE02 disruption mutants of Borrelia burgdorferi B31 have a highly transformable, infectious phenotype. Infect. Immun. 72:7147–7154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kenedy M. R., Vuppala S. R., Siegel C., Kraiczy P., Akins D. R. 2009. CspA-mediated binding of human factor H inhibits complement deposition and confers serum resistance in Borrelia burgdorferi. Infect. Immun. 77:2773–2782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41a. Kostick J. L., et al. 2011. The diguanylate cyclase, Rrp1, regulates critical steps in the enzootic cycle of the Lyme disease spirochetes. Mol. Microbiol. doi:10.1111/j.1365-2958.2011.07687.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lahdenne P., et al. 1997. Molecular characterization of a 6.6-kilodalton Borrelia burgdorferi outer membrane-associated lipoprotein (lp6.6) which appears to be downregulated during mammalian infection. Infect. Immun. 65:412–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. LaRocca T. J., et al. 2010. Cholesterol lipids of Borrelia burgdorferi form lipid rafts and are required for the bactericidal activity of a complement-independent antibody. Cell Host Microbe 8:331–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Laub M. T. 2010. The role of two-component signal transduction systems in bacterial stress responses, p. 45–58 In Storz G., Hengge R. (ed.), Bacterial stress responses, 2nd ed. ASM Press, Washington, DC [Google Scholar]
- 45. Lee E. R., Baker J. L., Weinberg Z., Sudarsan N., Breaker R. R. 2010. An allosteric self-splicing ribozyme triggered by a bacterial second messenger. Science 329:845–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mans B. J., et al. 2008. Comparative sialomics between hard and soft ticks: implications for the evolution of blood-feeding behavior. Insect Biochem. Mol. Biol. 38:42–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mascher T., Helmann J. D., Unden G. 2006. Stimulus perception in bacterial signal-transducing histidine kinases. Microbiol. Mol. Biol. Rev. 70:910–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Matsushika A., Mizuno T. 1998. The structure and function of the histidine-containing phosphotransfer (Hpt) signaling domain of the Escherichia coli ArcB sensor. J. Biochem. 124:440–445 [DOI] [PubMed] [Google Scholar]
- 49. Mulay V. B., et al. 2009. Borrelia burgdorferi bba74 is expressed exclusively during tick feeding and is regulated by both arthropod- and mammalian host-specific signals. J. Bacteriol. 191:2783–2794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nakajima Y., Taylor D., Yamakawa M. 2002. Involvement of antibacterial peptide defensin in tick midgut defense. Exp. Appl. Acarol. 28:135–140 [DOI] [PubMed] [Google Scholar]
- 51. Ohnishi J., Piesman J., de Silva A. M. 2001. Antigenic and genetic heterogeneity of Borrelia burgdorferi populations transmitted by ticks. Proc. Natl. Acad. Sci. U. S. A. 98:670–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ouyang Z., Blevins J. S., Norgard M. V. 2008. Transcriptional interplay among the regulators Rrp2, RpoN and RpoS in Borrelia burgdorferi. Microbiology 154:2641–2658 [DOI] [PubMed] [Google Scholar]
- 53. Pal U., et al. 2004. TROSPA, an Ixodes scapularis receptor for Borrelia burgdorferi. Cell 119:457–468 [DOI] [PubMed] [Google Scholar]
- 54. Pal U., et al. 2004. OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J. Clin. Invest. 113:220–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pappas C. J., et al. 2011. Borrelia burgdorferi requires glycerol for maximum fitness during the tick phase of the enzootic cycle. PLoS Pathog. 7:e1002102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Policastro P. F., Schwan T. G. 2003. Experimental infection of Ixodes scapularis larvae (Acari: Ixodidae) by immersion in low passage cultures of Borrelia burgdorferi. J. Med. Entomol. 40:364–370 [DOI] [PubMed] [Google Scholar]
- 57. Pollack R. J., Telford S. R., Spielman A. 1993. Standardization of medium for culturing Lyme disease spirochetes. J. Clin. Microbiol. 31:1251–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rogers E. A., et al. 2009. Rrp1, a cyclic-di-GMP-producing response regulator, is an important regulator of Borrelia burgdorferi core cellular functions. Mol. Microbiol. 71:1551–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ryjenkov D. A., Tarutina M., Moskvin O. V., Gomelsky M. 2005. Cyclic diguanylate is a ubiquitous signaling molecule in bacteria: insights into biochemistry of the GGDEF protein domain. J. Bacteriol. 187:1792–1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Samuels D. S. 1995. Electrotransformation of the spirochete Borrelia burgdorferi. Electrotransformation protocols for microorgansims. Methods Mol. Biol. 47:253–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sangamnatdej S., Paesen G. C., Slovak M., Nuttall P. A. 2002. A high affinity serotonin- and histamine-binding lipocalin from tick saliva. Insect Mol. Biol. 11:79–86 [DOI] [PubMed] [Google Scholar]
- 62. Sauer J. R., Essenberg R. C., Bowman A. S. 2000. Salivary glands in ixodid ticks: control and mechanism of secretion. J. Insect Physiol. 46:1069–1078 [DOI] [PubMed] [Google Scholar]
- 63. Sauer J. R., McSwain J. L., Bowman A. S., Essenberg R. C. 1995. Tick salivary gland physiology. Annu. Rev. Entomol. 40:245–267 [DOI] [PubMed] [Google Scholar]
- 64. Scheckelhoff M. R., Telford S. R., Wesley M., Hu L. T. 2007. Borrelia burgdorferi intercepts host hormonal signals to regulate expression of outer surface protein A. Proc. Natl. Acad. Sci. U. S. A. 104:7247–7252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Schirmer T., Jenal U. 2009. Structural and mechanistic determinants of c-di-GMP signalling. Nat. Rev. Microbiol. 7:724–735 [DOI] [PubMed] [Google Scholar]
- 66. Schwan T. G., Piesman J. 2000. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J. Clin. Microbiol. 38:382–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Schwan T. G., Piesman J., Golde W. T., Dolan M. C., Rosa P. A. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. U. S. A. 92:2909–2913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Seshu J., et al. 2006. Inactivation of the fibronectin-binding adhesin gene bbk32 significantly attenuates the infectivity potential of Borrelia burgdorferi. Mol. Microbiol. 59:1591–1601 [DOI] [PubMed] [Google Scholar]
- 69. Skerker J. M., et al. 2008. Rewiring the specificity of two-component signal transduction systems. Cell 133:1043–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Smith A. H., Blevins J. S., Bachlani G. N., Yang X. F., Norgard M. V. 2007. Evidence that RpoS (σS) in Borrelia burgdorferi is controlled directly by RpoN (σ54/σN). J. Bacteriol. 189:2139–2144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Smith K. D., et al. 2009. Structural basis of ligand binding by a c-di-GMP riboswitch. Nat. Struct. Mol. Biol. 16:1218–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sonenshine D. E. 1993. Biology of the ticks. Oxford University Press, New York, NY [Google Scholar]
- 73. Sonenshine D. E., Ceraul S. M., Hynes W. E., Macaluso K. R., Azad A. F. 2002. Expression of defensin-like peptides in tick hemolymph and midgut in response to challenge with Borrelia burgdorferi, Escherichia coli and Bacillus subtilis. Exp. Appl. Acarol. 28:127–134 [DOI] [PubMed] [Google Scholar]
- 74. Sonenshine D. E., Hynes W. L. 2008. Molecular characterization and related aspects of the innate immune response in ticks. Front. Biosci. 13:7046–7063 [DOI] [PubMed] [Google Scholar]
- 75. Stewart P. E., Thalken R., Bono J. L., Rosa P. 2001. Isolation of a circular plasmid region sufficient for autonomous replication and transformation of infectious Borrelia burgdorferi. Mol. Microbiol. 39:714–721 [DOI] [PubMed] [Google Scholar]
- 76. Sultan S. Z., Pitzer J. E., Miller M. R., Motaleb M. A. 2010. Analysis of a Borrelia burgdorferi phosphodiesterase demonstrates a role for cyclic-di-guanosine monophoase in motility and virulence. Mol. Microbiol. 77:128–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sun Y. J., Rose J., Wang B. C., Hsiao C. D. 1998. The structure of glutamine-binding protein complexed with glutamine at 1.94 Å resolution: comparison with other amino acid binding proteins. J. Mol. Biol. 278:219–229 [DOI] [PubMed] [Google Scholar]
- 78. Tam R., Saier M. H., Jr 1993. Structural, functional, and evolutionary relationships among extracellular solute-binding receptors of bacteria. Microbiol. Rev. 57:320–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Thompson J. D., Higgins D. G., Gibson T. J. 1994. Clustal W: improving the sensitivity of progressive multiple sequence alignment through weighting positions-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tilly K., Bestor A., Jewett M. W., Rosa P. 2007. Rapid clearance of Lyme disease spirochetes lacking OspC from skin. Infect. Immun. 75:1517–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Tupin E., et al. 2008. NKT cells prevent chronic joint inflammation after infection with Borrelia burgdorferi. Proc. Natl. Acad. Sci. U. S. A. 105:19863–19868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Vahedi-Faridi A., et al. 2007. Crystal structures and mutational analysis of the arginine-, lysine, and histidine-binding protein ArtJ from Geobacillus stearothermophilus. Implications for interactions of ArtJ with its cognate ATP-binding cassette transporter, Art(MP) 2. J. Mol. Biol. 375:448–459 [DOI] [PubMed] [Google Scholar]
- 83. Weening E. H., et al. 2008. Borrelia burgdorferi lacking dbpBA exhibits an early survival defect during experimental infection. Infect. Immun. 76:5694–5705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. West A. H., Stock A. M. 2001. Histidine kinases and response regulator proteins in two-component signaling systems. Trends Biochem. Sci. 26:369–376 [DOI] [PubMed] [Google Scholar]
- 85. Wolfe A. J., Visick K. L. 2008. Get the message out: cyclic-di-GMP regulates multiple levels of flagellum-based motility. J. Bacteriol. 190:463–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Xu H., et al. 2010. Role of acetyl-phosphate in activation of the Rrp2-RpoN-RpoS pathway in Borrelia burgdorferi, p. PLoS Pathog. 6:piie1001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yang X. F., Alani S. M., Norgard M. V. 2003. The response regulator Rrp2 is essential for the expression of major membrane lipoproteins in Borrelia burgdorferi. Proc. Natl. Acad. Sci. U. S. A. 100:11001–11006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yang X. F., Pal U., Alani S. M., Fikrig E., Norgard M. V. 2004. Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J. Exp. Med. 199:641–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.