Abstract
Borrelia burgdorferi, the agent of Lyme disease, undergoes rapid adaptive gene expression in response to signals unique to its arthropod vector or vertebrate hosts. Among the upregulated genes under vertebrate host conditions is one of the five annotated homologs of oligopeptide permease A (OppA5, BBA34). A mutant lacking oppA5 was constructed in an lp25-deficient isolate of B. burgdorferi strain B31, and the minimal regions of infectivity were restored via a shuttle vector pBBE22 with or without an intact copy of bba34. Immunoblot analysis of the bba34 mutant revealed a reduction in the levels of RpoS, BosR, and CsrABb with a concomitant reduction in the levels of OspC, DbpA, BBK32, and BBA64. There were no changes in the levels of OspA, NapA, P66, and three other OppA orthologs. Quantitative transcriptional analysis correlated with the changes in the protein levels. However, the bba34 mutant displayed comparable infectivities in the C3H/HeN mice and the wild-type strain, despite the reduction in several pathogenesis-related proteins. Supplementation of the growth medium with increased levels of select components, notably sodium acetate and sodium bicarbonate, restored the levels of several proteins in the bba34 mutant to wild-type levels. We speculate that the transport of acetate appears to contribute to the accumulation of key metabolites, like acetyl phosphate, that facilitate the adaptation of B. burgdorferi to the vertebrate host by the activation of the Rrp2-RpoN-RpoS pathway. These studies underscore the importance of solute transport to host-specific adaptation of B. burgdorferi.
INTRODUCTION
Borrelia burgdorferi, the etiological agent of Lyme disease, is transmitted to a variety of vertebrate hosts via the bite of infected Ixodes sp. ticks (13, 76). This spirochetal pathogen causes a pathognomonic, localized inflammatory skin lesion termed erythema migrans at the site of the tick bite (54). If left untreated, the spirochetes disseminate to various organs and organ systems, leading to a multiphasic disorder involving the cutaneous, musculoskeletal, cardiovascular, and nervous systems (75). Lyme disease is endemic in certain geographic areas of the United States where there are close ecological interactions between the transmitting vector, infected reservoir hosts, and human populations, resulting in a significant impact on public health.
The ability of B. burgdorferi to be transmitted between an arthropod vector and a variety of vertebrate hosts is reliant on the rapid adaptation of its gene expression profiles that facilitates survival under highly disparate environmental conditions present in these hosts (4, 23, 24, 71, 77). A broad array of studies using global gene expression arrays has provided clues to contributions of genes individually or at a global level that facilitate host-specific adaptation of B. burgdorferi (4, 6, 12, 17, 55, 65, 83). Environmental conditions such as temperature (71), cell density (40), pH (19, 20), levels of dissolved gases (39, 72), and other components that are significantly different between the tick vector and vertebrate hosts (7, 8, 23) have been used to decipher both the transcriptional levels of genes and the regulatory networks involved in modulating this response (5, 14–16, 32, 47, 49, 91).
The compact and segmented nature of the borrelial genome comprising one linear chromosome and several circular and linear plasmids also adds to the complexity of regulatory mechanisms operative in B. burgdorferi (21, 31). For example, B. burgdorferi does not possess metabolic pathways for the synthesis of amino acids, fatty acids, and nucleotides. In addition, it has a limited set of transport systems to acquire these select nutrients from the external environment (31). There is a single peptide transport system that belongs to a superfamily of ABC transporters present in B. burgdorferi with extensive similarity to oligopeptide permease (Opp) and dipeptide permease (Dpp) systems described in other bacteria (45, 48, 86). The Opp transport system is characterized by the presence of a peptide binding protein (OppA), two transmembrane proteins (OppB and OppC), and two ATP binding proteins (OppD and OppF) (2, 59). However, unlike other bacteria, B. burgdorferi has 5 open reading frames (ORFs) annotated as homologs of OppA, suggesting that these proteins could play a significant role in acquiring select nutrients/solutes in specific microenvironments (11). Three of these OppA homologs (OppA1, -2, and -3) encoded on the chromosome bound heptapeptides with high affinities in a pH-dependent manner, while the other two homologs present on plasmids (OppA4 on cp26 and OppA5 or BBA34 on lp54) did not exhibit such binding characteristics (84, 85). Additional studies also indicated that bba34 was upregulated when the culture conditions of B. burgdorferi were shifted from 25°C to 37°C and in spirochetes present in infected mouse tissues (52). Moreover, the expression level of bba34 was determined to be under the control of alternative σ factors RpoN and RpoS (52), suggesting a role for bba34 in the adaptation of B. burgdorferi to vertebrate host-specific conditions.
Several recent studies have brought into focus the importance of key metabolites contributing to adaptive gene expression in B. burgdorferi. The phosphorylation of the two-component response regulatory protein, Rrp2, which leads to the activation of the RpoN-RpoS pathway, was shown to be mediated by acetyl phosphate, a metabolite of the acetate utilization pathway (88). The accumulation of this metabolite is partly dependent on the repression of levels of an enzyme, phosphate acetyltransferase (Pta), which converts acetyl phosphate to acetyl-coenzyme A (CoA), leading to conditions that could potentially dampen the activation of the Rrp2-RpoN-RpoS pathway. The repression of Pta has been shown to be mediated by CsrABb (carbon storage regulator A of B. burgdorferi), which we and others recently demonstrated plays a crucial role in affecting differential gene expression in B. burgdorferi that facilitates adaptation to vertebrate host-specific conditions (41, 70, 82). Notably, deletion of csrABb leads to a reduction in the levels of expression of several members of the rpoS regulon. Consistent with this in vitro phenotype, the csrABb mutant was attenuated for infection in the murine model of Lyme disease (41, 82, 88). Molecular determinants that connect the external environment to the metabolic status of B. burgdorferi that eventually lead to host-specific adaptation are yet to be characterized in detail. Therefore, we focused on bba34 as a probable mediator of transport of key substances that modulate adaptation to vertebrate hosts since it was (i) upregulated under vertebrate host-specific conditions (52); (ii) downregulated in the csrABb mutant (41); (iii) similar to other bacterial solute-binding proteins (31); and (iv) incapable of binding peptides, unlike other OppA homologs of B. burgdorferi (84).
In this study, we generated a deletion mutant in bba34 and confirmed that BBA34 was indeed synthesized at higher levels under conditions mimicking the fed ticks. Most interestingly, deletion of bba34 resulted in reduction of the levels of key regulators of gene expression in B. burgdorferi, such as RpoS, BosR, and CsrABb, along with levels of lipoproteins associated with pathogenic mechanisms, such as OspC, DbpA (34), BBK32 (60), and BBA64 (33, 50, 51). In spite of the reduction in the levels of these determinants of infection, there was no deficit in the colonization capabilities of the bba34 mutant, suggesting that there was a compensatory mechanism that restored the ability of the mutant to colonize C3H/HeN mice. Further in vitro phenotypic analysis provided evidence that BBA34 contributes to the transport of select solutes, such as acetate and bicarbonate, which in turn modulate levels of vertebrate host-specific determinants. Based on these observations, we discuss how this protein could facilitate the adaptation of B. burgdorferi to host-specific conditions based on levels of external signals.
MATERIALS AND METHODS
Bacterial strains and media.
A noninfectious, clonal derivative of B. burgdorferi strain B31 lacking lp25 (ML23) was used in transformation experiments (44). An infectious, clonal isolate of B. burgdorferi strain B31 containing all the plasmids (MSK5) was used as a source of total genomic DNA. All borrelial strains were grown in Barbour-Stoenner-Kelly II (BSK-II) liquid medium supplemented with 6% normal rabbit serum (NRS; Pel-Freeze Biological, Rogers, AR) and 1% CO2 at 32°C (9). The borrelial strains were also initially propagated in BSK-II medium with 6% NRS at conditions mimicking the midgut of unfed ticks (pH 7.6/23°C) and shifted to those mimicking fed ticks (pH 6.8/37°C).
Expression and purification of recombinant proteins for generation of monospecific sera against OppA homologs.
The purification of BBA34/OppA5 was done using procedures described previously (26). Total genomic DNA obtained from B. burgdorferi clonal isolate MSK5 (Table 1) was used as the template to PCR amplify OppA1, OppA2, and OppA4 by using forward and reverse primers containing appropriate engineered restriction enzyme sites (Table 2). The leader sequences of lipoproteins were not included to generate the expression constructs in order to facilitate the induction of increased levels of recombinant proteins in Escherichia coli. The amplicons were cloned into pCR2.1-TOPO vector (Invitrogen) and transformed into E. coli TOP 10 cells and subjected to blue/white colony screening in the presence of ampicillin (100 μg/ml) and kanamycin (50 μg/ml). The inserts were excised with NdeI/XhoI and ligated into the pET23a expression vector. The ligated products were electrotransformed into E. coli TOP 10 cells and screened by restriction enzyme digestion for the presence of insert of appropriate size. The junctions of plasmids containing inserts of expected size were sequenced, and the plasmids were used to transform the E. coli expression host (Rosetta, Novagen). Recombinant OppA1, OppA2, and OppA4 with a C-terminal 6-histidine tag were overexpressed by inducing E. coli strains containing appropriate plasmids with 1 mM IPTG (isopropyl-β-d-1-thiogalactopyranoside) for 2 h and subjected to affinity purification using Ni-nitrilotriacetic acid (NTA) beads (Qiagen, Valencia, CA) as per the manufacturer's instructions. The bound 6-histidine proteins were eluted as 0.5-ml fractions with elution buffer (8 M urea; pH 4.5) and analyzed by sodium dodecyl sulfate (SDS)-12.5% polyacrylamide gel electrophoresis (PAGE). Select fractions with the largest concentrations of eluted proteins were further purified using Amicon centrifugal filters (Millipore, Bedford, MA), and in some cases the proteins were purified to homogeneity using the S&S Elutrap electroseparation system as described previously (42). Recombinant proteins purified to homogeneity (data not shown) were quantified by bicinchoninic acid (BCA) (Pierce, Thermo Fisher Scientific, Rockford, IL) assay and stored at −80°C until further use. The purified recombinant proteins were emulsified in equal volumes of Titermax (Sigma, St. Louis MO) and used to immunize 6- to 8-week-old female BALB/c mice. Booster immunizations were given at days 14 and 21. Immunoblot analysis was carried out to determine the specificity of the antibodies against the recombinant proteins and total borrelial lysates with the serum obtained on day 28 postimmunization (data not shown). All animal procedures were done in accordance with the approved animal use protocol from the Institutional Animal Care and Use Committee of The University of Texas at San Antonio.
Table 1.
Plasmids and strains used in this study
| Plasmid or strain | Descriptiona | Source or reference |
|---|---|---|
| Plasmids | ||
| pCR2.1-TOPO | PCR cloning vector | Invitrogen |
| pML102 | Customized donor plasmid for in vitro mutagenesis | 74 |
| pBVS2 | Borrelial shuttle confers Kanr | 78 |
| pBBE22 | Borrelial shuttle vector confers Kanr derived from pBSV2 with BBE22 region of lp25 | 61 |
| pSVR1 | 1.9 kb of lp54 spanning BBA34 in pCR2.1-TOPO | This study |
| pSVR2 | SVR1 with 1.6-kb insertion of PflgB-aadA at the nucleotide 154 bp from the 5′ end of BBA34 | This study |
| pSVR3 | Borrelial shuttle vector pBBE22 with functional copy of bba34 | This study |
| B. burgdorferi strains | ||
| MSK5 | B31 isolate with all infection-associated plasmids | 44 |
| ML23 | B31, lp25-negative, noninfectious clonal isolate | 44 |
| ML23/pBBE22 (wt) | ML23 with pBBE22 (BBE22+), Kanr | 74 |
| SVR2 | bba34 mutant in ML23; lp25−, Strr | This study |
| SVR2/pBBE22 (mt) | SVR2 with pBBE22; Kanr; Strr | This study |
| SVR3 (ct) | SVR2 with pSVR3 (complementation plasmid, pBBE22/bba34+) | This study |
Strr, streptomycin resistance; Kanr, kanamycin resistance.
Table 2.
Oligonucleotides used in this study
| Name | Sequence (5′→3′)a |
|---|---|
| P1 | ACGCCATATGTCTAAACCAAAAGATA |
| P2 | ACGCCTCGAGTTCTTCTATAGGTTT |
| P3 | AATGTATTTCTGGGGGAGTG |
| P4 | GGTCATAAGGGTCGACCAGAAAGATTAGCCCTATCAG |
| P5 | AATCTTTCTGGTCGACCCTTATGACCTTCCTTTAGC |
| P6 | ACAGGTTTTTGAACACTCTCATC |
| P7 (34S1F) | GCACGGGTTAGTAGAAAAAAG |
| P8 (34S2R) | CTTTTTATCCCATTTTATCCC |
| P9 (BBA34-compF) | ACGCGGATCCGAGCTTTCTTTAGCTGAAAG |
| P10 (BBA34-compR) | ACGCGTCGACCAATTTCTGTAAGCGATTTAAC |
| PflgB-aadAF | ACGCGTCGACGGAAGATTTCCTATTAAGG |
| PflgB-aadAR | ACGCGTCGACTTATTTGCCGACTACCTTGGT |
| BosRFRq | GCATTGGAAAAAGTCGGCATT |
| BosRRRq | GATGCTTTCATTCCTAATTCTGATGTT |
| RpoSFRq | AGATATGCGGGTAAAGGGTTAAAA |
| RpoSRRq | CAGCAGCTCTTATTAATCCCAAGTT |
| E22F | ACGCGGTACCTTTTTATATTGTGAGCCGGT |
| E22R | ACGCGGTACCTCTATGTATCCCCTTGTTCA |
Restriction sites are underlined.
Construction of bba34 mutant.
A two-step overlap PCR strategy was employed to generate the plasmid used for the deletion of bba34 as described previously (51). In the first step, ∼1-kb regions of lp54 flanking the bba34 gene were PCR amplified from the total genomic DNA from an infectious, clonal isolate of B. burgdorferi B31 (MSK5). This was done using primer sets P3/P4 and P5/P6 for generating the upstream and downstream regions of bba34, respectively (Table 2). Primers P4 and P5 had engineered SalI sites as well as a 10-nucleotide overlap to facilitate the deletion of bba34 by using overlap PCR. In the second step, a 1.9-kb amplicon was PCR amplified by LATaq polymerase (Takara) using primers P3 and P6 using the PCR products from the first step as DNA templates. The amplicon was cloned into the pCR2.1 cloning vector (Invitrogen) and transformed into TOP 10 E. coli. A plasmid designated pSVR1 with an appropriate insert was identified following blue/white screening, and the PflgB-aadA with flanking SalI sites was cloned into pSVR1 digested with the same enzyme. A plasmid designated pSVR2 with PflgB-aadA inserted between flanking regions of bba34 (between coordinates 21663 and 23212 of lp54) was used for the generation of the mutant in the noninfectious lp25− strain of B. burgdorferi B31 (ML23).
Transformation of B. burgdorferi.
The transformation of ML23 with pSVR2 was done as described previously (68, 73, 78). After electroporation, the transformants were plated (after a 24-h incubation in BSK-II medium without antibiotics) on BSK-II agar overlays containing 50 μg/ml of streptomycin. Plates were incubated at 32°C in 1% CO2 for 14 to 18 days until individual colonies were visible (78) in the overlays. Colonies were isolated aseptically into BSK-II liquid medium containing 50 μg/ml of streptomycin and incubated at 32°C until the spirochetes reached a density of 5 × 107/ml. One milliliter of this culture was used to extract total genomic DNA, and the presence of PflgB-aadA cassette within oppA5 was determined using primers specific to the upstream and downstream areas of OppA5 (Table 2). Several positive clones were identified, and one clone, designated SVR2, was further analyzed for the confirmation of the loss of bba34 by Southern blot and immunoblot analysis.
Southern blot analysis.
To further confirm the deletion of bba34, total genomic DNA was isolated from the parental control strain (ML23) and the bba34 mutant (SVR2) (73) and digested with either HindIII or HindIII/NdeI, separated on a 1% agarose gel, and transferred onto a nylon membrane. The membranes were probed either with the aadA gene (Strr marker) or with the PCR-amplified, full-length bba34 gene by using the ECL labeling and detection system (GE Healthcare). The membranes were hybridized with labeled probes overnight at 42°C and developed per the manufacturer's instructions (GE Healthcare).
Complementation and restoration of minimal region of lp25 required for infectivity of bba34 mutant.
In order to complement the bba34 mutant, a region of lp54 corresponding to the nucleotide coordinates 21137 and 23797 was amplified using primers P9 and P10 with engineered SalI sites. The amplicon was cloned into the borrelial shuttle vector pBBE22 (61, 78) at the SalI site to generate a plasmid designated pSVR3, which also has the minimal region of lp25 (BBE22 region) needed to restore the infectivity of ML23 in the C3H/HeN mouse model of Lyme disease. The bba34 mutant (SVR2) was transformed by electroporation either with pSVR3 or with pBBE22 alone so as to generate a complemented strain or a bba34-deficient strain, respectively, with all the genetic elements needed for infectivity analysis in the mouse model of Lyme disease. The transformants were selected on BSK-II agarose overlays supplemented with 200 μg/ml of kanamycin and 50 μg/ml of streptomycin, and the presence of pBBE22 was verified using primers described previously (26, 50). The plasmid profiles of wild-type (wt; ML23/pBBE22), mutant (mt; SVR2/pBBE22), and complemented (ct; SVR2/pBBE22-bba34+) strains were verified using primer sets described previously (44) and observed to be the same in all three strains (data not shown).
SDS-PAGE and immunoblot analysis.
B. burgdorferi parental, mutant, and complemented strains were cultivated under conditions that mimic the midgut of the tick before (23°C/pH 7.6) or after (37°C/pH 6.8) a blood meal to a density of 1 × 108 spirochetes/ml in BSK-II growth medium as described above. Whole-cell lysates were prepared and separated on SDS-12.5% PAGE gels as described previously (72). The separated proteins were either visualized by Coomassie brilliant blue staining or transferred to polyvinylidene difluoride (PVDF) membranes and subjected to immunoblot analysis as described previously (26, 41, 50, 70). The membranes were probed with α-OspA monoclonal antibodies, α-BBA34, α-BBK32, α-DbpA, α-SodA, α-P66, α-BBA64, α-BosR, α-CsrABb, α-RpoS, and α-NapA antibodies. The blots were incubated with appropriate concentrations of horseradish peroxidase (HRP)-conjugated anti-mouse, anti-rat, or anti-rabbit secondary antibodies and developed using ECL Western blotting detection reagents (GE Healthcare).
Protease accessibility.
Protease accessibility studies were conducted as described previously (42). Briefly, the parental, mutant, and complemented strains (∼2 × 108 cells) were centrifuged at 4,300 × g for 15 min at 4°C, and the pellet was resuspended in equal volumes of phosphate-buffered saline (PBS; pH 7.4), 5 mM MgCl2, and 50 mM sucrose (42). After pelleting, the samples were split into equal volumes and incubated in either 50 μl of sterile water (proteinase K-negative control) or with proteinase K (final concentration of 200 μg/ml) at 20°C for 40 min. After 40 min, phenylmethylsulfonyl fluoride (PMSF) was added to a concentration of 1 mM, and samples were examined by dark-field microscopy to assess their motility as a measure for viability. The samples were pelleted and washed again as described above in the presence of 1 mM PMSF and resuspended in SDS-PAGE sample buffer. The proteins were separated by SDS-PAGE and subjected to immunoblot analysis as described above with α-BBA34, α-FlaB, and α-P66 antibodies.
PCR analysis of plasmid profiles of strains used in infectivity.
Plasmid profile analysis was done as described previously (44, 50). Primer sets used to amplify specific target regions in different linear and circular plasmids have been described previously (44). The amplicons were separated on a 1.5% agarose gel and visualized by staining with ethidium bromide (data not shown). A primer set (Table 2, E22F/E22R) was also used to amplify the BBE22 region of lp25 present in the borrelial shuttle vector pBBE22.
In vitro phenotypic analysis.
The parental, mutant, and complemented strains were propagated in BSK-II medium supplemented with 6% NRS and appropriate antibiotics at 32°C with 1% CO2. The spirochetes were enumerated every 12 h using dark-field microscopy, and all experiments were conducted in triplicate. The protein profiles of these strains were also monitored following growth in media with different levels of Bacto TC Yeastolate (Becton, Dickinson and Company, Sparks, MD), dialyzed yeastolate (Gibco; Invitrogen; 50× solution), and other solutes as indicated in the respective figure legends. All the strains were tested in triplicate, and the experiments were repeated at least three times.
Infectivity studies.
Groups of 6-week-old female C3H/HeN mice (n = 3) were intradermally inoculated at doses of 102, 103, 104, and 105 spirochetes per mouse with wild-type (ML23/pBBE22), bba34 mutant, and complemented strains. Twenty-one days after inoculation, the spleens, tibiotarsal joints, inguinal lymph nodes, hearts, bladders, and pieces of abdominal skin were collected and cultured in BSK-II growth medium to facilitate the isolation of spirochetes as previously described (50, 51). The cultures were scored for growth of B. burgdorferi after 2 to 3 weeks by using dark-field microscopy. All animal procedures were performed in accordance with the approved animal use protocol from the Institutional Animal Care and Use Committee at The University of Texas at San Antonio.
RESULTS
Generation of a deletion mutant of oppA5.
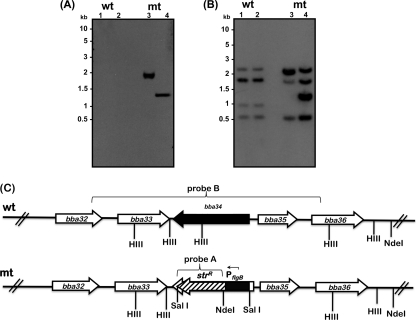
We generated a plasmid, designated pSVR2, by a two-step PCR process as described previously, where the bba34 gene was replaced with PflgB-aadA (30, 51). In order to inactivate the bla gene present on the vector used to generate pSVR2, we linearized this plasmid at the ScaI site prior to electrotransformation of the ML23 strain of B. burgdorferi (43, 44, 68, 74). The mutant borrelial colonies were selected in BSK-II agar overlays supplemented with 50 μg/ml of streptomycin and initially screened by PCR using primers specific to bba34. One mutant, designated SVR2, was further analyzed by Southern hybridization. There was hybridization of 1,836-bp and 1,276-bp fragments when the total genomic DNA from bba34 mutant was digested with HindIII or HindIII/NdeI, while there was no such hybridization with the DNA from the parental strain when the blots were probed with the aadA (Strr) marker (Fig. 1A). Moreover, when bba34 was used as a probe (Fig. 1B), there was hybridization to fragments (Fig. 1B, lane 1, wt, 2,386 bp, 1,770 bp, 816 bp, 519 bp) in the total genomic DNA of the parental strain (wt), consistent with the presence of HindIII sites, while the hybridization pattern was significantly different in the mutant (mt) strain digested with HindIII due to the presence of the streptomycin resistance cassette (Fig. 1B, lane 1, mt, 2,218 bp, 1,869 bp, 1,530 bp, 439 bp) or due to the presence of the NdeI site within the PflgB-aadA marker (Fig. 1B, lane 2, mt, 2,218 bp, 1,530 bp, 1,230 bp, 439 bp). Based on the schematic representation of the region of lp54 shown (Fig. 1C), we should have also observed hybridization to a 187-bp fragment in both the parental and mutant strains digested with either HindIII or HindIII/NdeI. Due to the relatively small size of the fragment and partly due to the hybridization conditions employed, we did not observe hybridization to this fragment. However, this does not alter the interpretation that only bba34 was deleted and replaced with PflgB-aadA. Although extensive similarity exists in the sequences of oppA homologs, Southern hybridization analysis confirmed the inactivation and clonality of the bba34 mutant.
Fig. 1.
Southern blotting confirms the mutation in the bba34 gene in ML23 (noninfectious, lp25−, B. burgdorferi strain B31). (A and B) Total genomic DNA from the ML23 parental strain (wt, lanes 1 and 2) and the bba34 mutant (mt, lanes 3 and 4) was digested with HindIII (lanes 1 and 3) and HindIII/NdeI (lanes 2 and 4) and probed with aadA (Strr marker) (A) or bba34 (PCR-amplified using P3, P6) (Table 2) (B). The numbers on the left of each panel indicate the size of the markers in kilobases. (C) Schematic of the bba34 region in the lp54 plasmid of B. burgdorferi. The gene designations based on TIGR annotations are indicated on each corresponding locus. Probe B was PCR amplified using P3/P6 primers sets with total genomic DNA from B. burgdorferi as the template, and probe A was amplified using aadAF/aadAR primers using pML102 as the template.
Complementation of the bba34 mutant.
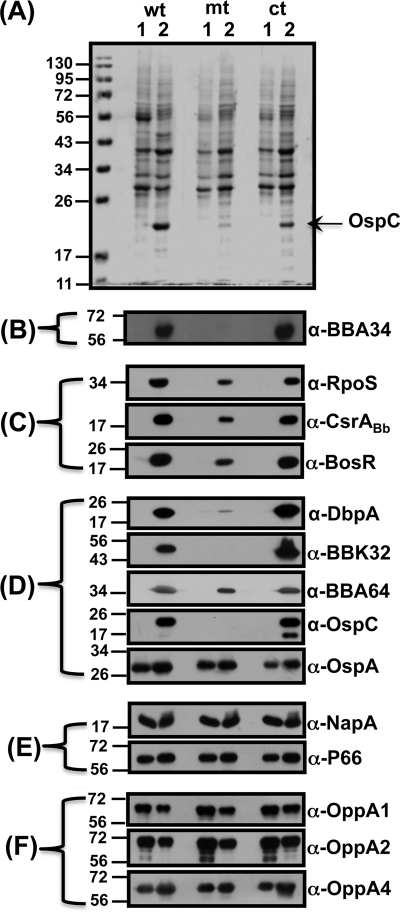
We complemented the bba34 mutant with the borrelial shuttle vector pBBE22 alone or with an intact copy of bba34 (including its upstream region). This was done not only to restore the minimal region of lp25 required for infectivity in the mutant but also to functionally complement the mutant with an intact copy of bba34 to test the molecular Koch's postulates (61, 62, 74). The transformants were selected in the presence of 200 μg/ml kanamycin and 50 μg/ml of streptomycin and were screened using primers specific for pBBE22 (50). Immunoblot analysis of the complemented strain confirmed the synthesis of BBA34 only upon propagation under fed-tick conditions (pH 6.8/37°C) consistent with the expression of this homolog in the parental strain (Fig. 2B; wt; ct; lane 2). Taken together, we have isolated a bba34-deficient strain and genetically complemented the mutant such that the levels of bba34 are similar to those of the parental strain and have the requisite genetic background to evaluate the in vitro and in vivo phenotypic effects of targeted deletion of bba34.
Fig. 2.
Inactivation of bba34 results in reduced levels of regulators of gene expression and key pathogenesis-related proteins of B. burgdorferi. Immunoblot analysis of total protein lysates of wild-type (wt), mutant (mt), and complemented (ct) strains propagated in either unfed (lane 1, pH 7.6/23°C)- or fed (lane 2, pH 6.8/37°C)-tick conditions was performed with monospecific serum against the antigen indicated to the right of each blot. (A) Coomassie blue-stained 12.5% SDS-PAGE of whole-cell lysates of borrelial strains. (B) Immunoblot analysis of whole-cell lysates with mouse anti-A34 antibodies indicates lack of synthesis of BBA34 in the bba34 mutant. (C) Reduced levels of regulators of gene expression in the bba34 mutant. Immunoblot analysis of total lysates was done with mouse or rabbit anti-RpoS, anti-CsrABb, and anti-BosR antibodies. (D) Reduced levels of key lipoproteins associated with pathogenesis in the bba34 mutant. Immunoblot analysis was done with mouse or rat anti-DbpA, anti-BBK32, anti-BBA64, anti-OspC, and anti-OspA monoclonal antibodies. (E) Lack of difference in the levels of select proteins in the wt, mt, and ct strains. Immunoblot analysis was done with rabbit or mouse anti-NapA and anti-P66 antibodies. (F) Lack of compensatory change in the levels of other OppA homologs. Immunoblot analysis of wt, mt, or ct strains with mouse anti-OppA1, anti-OppA2, and anti-OppA4 antibodies. The molecular size standards in kilodaltons are indicated to the left. The blots were developed using the Enhanced Chemiluminescence System.
In vitro growth phenotype of bba34 mutant.
We first evaluated the in vitro growth phenotype of the parental, mutant, and complemented strains in BSK-II growth medium supplemented with 6% NRS under conditions where we expected the levels of BBA34 to be maximal (pH 6.8/37°C) or minimal (pH 7.6/23°C) at 1% CO2 (26). We found no difference in the growth rates of the bba34 mutant or the control strains under the conditions examined, suggesting that the lack of BBA34 does not alter the in vitro survival and duplication time of B. burgdorferi (data not shown).
Deletion of bba34 alters levels of key regulators of gene expression.
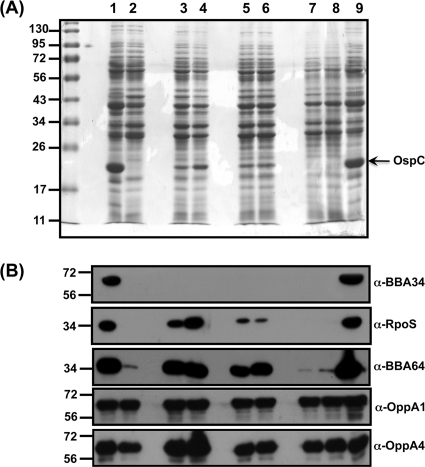
We first established the growth conditions at which the parental and complemented strains synthesize increased amounts of BBA34 by using the bba34 mutant as a negative control. Immunoblot analysis of total proteins from wild-type and complemented strains propagated under conditions that mimicked the midgut of fed ticks (pH 6.8/37°C) showed increased synthesis of BBA34 (Fig. 2B, wt, ct, lane 2), while there was no expression of BBA34 (Fig. 2B, wt, ct, lane 1) at unfed-tick conditions (pH 7.6/23°C). As expected, there was no synthesis of BBA34 at either condition in the mutant strain (Fig. 2B, mt, lanes 1 and 2).
To our surprise, the Coomassie blue-stained gel (Fig. 2A) of the above-described samples indicated that the levels of OspC were conspicuously lower in the bba34 mutant strain grown at fed-tick conditions (Fig. 2A, mt, lane 2). As expected, the control strains had increased levels of OspC only under the above-described conditions (Fig. 2A, wt, ct, lane 2). We then examined if the bba34 mutant had the requisite gene regulatory network intact to establish infection in the murine model of Lyme disease. We therefore determined both the levels of key regulators of gene expression and the members of their respective regulons, which are directly or indirectly controlled by these proteins. The levels of RpoS, CsrABb, and BosR that have been previously shown to be increased at the fed-tick conditions were consistently lower in the bba34 mutant (Fig. 2C, mt, lane 2) than in the parental or complemented strains (Fig. 2C, wt, ct, lane 2) (41). The levels of these regulators, as expected, were barely detectable in all three strains when propagated at the unfed-tick midgut conditions (Fig. 2C, wt, mt, ct, lane 1).
Reduced levels of select pathogenesis-related proteins in bba34 mutant.
We further examined the levels of several pathogenesis-related proteins in the bba34 mutant that are modulated by the above regulators. Immunoblot analysis of total proteins from the mutant grown at conditions mimicking the fed ticks (pH 6.8/37°C) showed reduction in the levels of DbpA, BBK32, BBA64, and OspC, while the wild-type and complemented strains grown under these conditions had increased levels of these lipoproteins (Fig. 2D, wt, mt, ct, lane 2). The levels of these proteins, as expected, were lower when each of the strains was propagated at unfed-tick conditions (Fig. 2D, wt, mt, ct, lane 1). These observations indicated that the inactivation of bba34 appears to have an effect both on the levels of key regulators of gene expression in B. burgdorferi and on the levels of lipoproteins that are under the control of these regulators. Furthermore, the reduction in the levels of these regulators and the lipoproteins is due to the lack of bba34 because the levels of these proteins were restored in the genetically complemented strain similar to the parental control strain (Fig. 2A to D, wt, ct, lane 2).
We also observed that the inactivation of bba34 did not result in a reduction in several other proteins (Fig. 2D and E, wt, mt, ct, lane 2). We found that the levels of OspA, P66 (a surface-exposed integrin binding porin), and NapA were similar in all three strains and did not exhibit any difference upon propagation of these strains under our experimental conditions (53, 70). Contrary to a recent observation (38), we did not observe a change in the levels of NapA under growth conditions that resulted in reduced levels of BosR either in the mutant or in the control strains (Fig. 2E, wt, mt, ct, lanes 1 and 2). This could presumably be due to the growth conditions employed in this study, where the levels of CO2 were maintained consistently at 1% in all experiments.
Lack of upregulation of other OppA homologs in the bba34 mutant.
Since bba34 is annotated as one of the five OppA homologs present in B. burgdorferi, we decided to determine if there are any alterations in the levels of other OppA homologs that could functionally compensate for the lack of BBA34. Immunoblot analysis using sera specific to OppA1, OppA2, and OppA4 demonstrated that there were no dramatic differences in the levels of these OppA homologs between the mutant and the control strains. All three strains showed a slight increase in the levels of OppA1 and OppA2 when propagated at unfed-tick conditions (Fig. 2F, wt, mt, ct, lane 1; α-OppA1, α-OppA2) compared to fed-tick conditions (Fig. 2F, wt, mt, ct, lane 2; α-OppA1, α-OppA2). The opposite was true with OppA4, where all the strains exhibited a slight increase in the levels of OppA4 at fed-tick condition compared to the unfed-tick conditions (Fig. 2F, wt, mt, ct, lanes 1 and 2; α-OppA4). Taken together, these observations indicate that there were minimal direct compensatory changes in the levels of other OppA homologs tested in the bba34 mutant.
Lack of bba34 results in transcriptional downregulation of select ORFs.
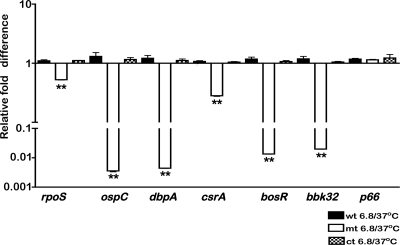
In order to determine if the reduction in the levels of various proteins in the bba34 mutant was due to transcriptional or posttranscriptional changes, we analyzed the cDNA generated from total RNA from the control and mutant strains by using quantitative real-time PCR analysis (70). As shown in Fig. 3, the transcript levels of rpoS, ospC, dbpA, csrABb, bosR, and bbk32 exhibited significant downregulation in the bba34 mutant compared to the parental strain (P < 0.01). The levels of p66, however, remained the same in both the parental and mutant strains. We also observed that the levels of transcriptional reduction of select ORFs in the mutant compared to the complemented strain were similar to the reduction observed between the mutant and wild-type strains. These analyses were carried out using cDNA from strains propagated at pH 6.8/37°C where the transcript levels of bba34 were maximal (data not shown). The quantitative real-time PCR analyses indicated that the reduction in the levels of select proteins in the bba34 mutant (Fig. 2) was due to reduction in their transcriptional levels rather than due to posttranscriptional changes.
Fig. 3.
Quantitative real-time reverse transcription-PCR (RT-PCR) analysis of select genes in the bba34 mutant. Total RNA was isolated from wild-type (wt), mutant (mt), or complemented (ct) strains, and cDNA was synthesized as described in Materials and Methods followed by quantitative real-time PCR. All samples were normalized relative to recA, and ΔCT values were obtained as an average of each sample analyzed in triplicate. The ΔΔCT for each transcript from mutant (mt; open bars) relative to wild-type (wt; filled bars) strains (A) or mutant (mt; open bars) relative to complemented (wt; filled bars) strains (B) are shown as fold differences on the y axis with error bars indicated. The ΔCT values obtained for mt and wt or mt and ct strains were subjected to unpaired Student's t testing implemented in PRISM. The asterisk indicates samples whose CT values are statistically significant between mt and wt or mt and ct strains (**, P < 0.01).
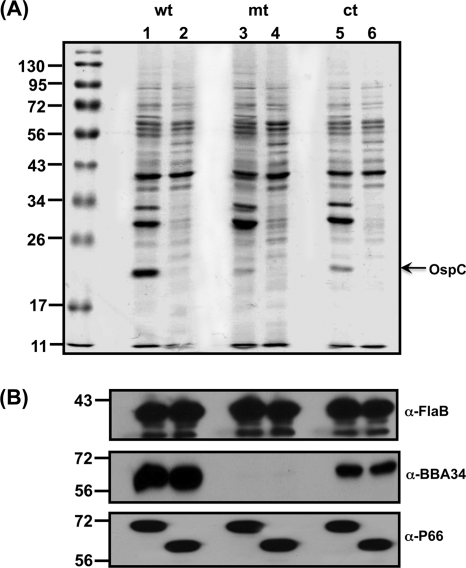
BBA34 is a periplasmic protein.
Sequence analysis indicated that BBA34 is probably localized to the periplasmic space, while other topological analyses suggested that it may have surface-exposed domains. It has been previously shown that OppA4 is a periplasmic protein, and hence the localization of these proteins could be suggestive of their physiological roles (11). Hence, we treated the parental, mutant, and complemented strains propagated at pH 6.8/37°C to ensure that there were maximal levels of BBA34 with proteinase K and determined the reactivity of these strains with the anti-BBA34 serum (42). The presence of OspC was detectable in the untreated samples compared to the proteinase K-treated samples (Fig. 4A, wt, mt, ct, lanes 1 and 2, respectively). Immunoblot analysis revealed no change in the levels of BBA34 in the parental and complemented strains, with or without proteinase K treatment, suggesting that it is localized in the periplasm (Fig. 4). The levels of another periplasmic protein, FlaB, also did not exhibit any change following proteolytic treatment (a control for proteins in the periplasm), while a surface-exposed porin, P66 (a control for a surface-exposed protein), exhibited reduction in size only in the treated samples (Fig. 4, wt, mt, ct, lane 2). This analysis demonstrated that BBA34 is not accessible on the surface and is presumably localized in the periplasm of B. burgdorferi.
Fig. 4.
BBA34 is periplasmic protein of B. burgdorferi. Intact wild-type (wt), mutant (mt), and complemented (ct) spirochetes propagated at fed (pH 6.8/37°C)-tick conditions either were left untreated (lane 1) or were treated with proteinase K (lane 2) and subjected to immunoblot analysis. (A) Coomassie blue-stained 12.5% SDS-PAGE gel of borrelial strains untreated or treated with proteinase K. (B) Immunoblot analysis of B. burgdorferi strains using monoclonal antibodies against FlaB or monospecific serum against BBA34 or P66. The numbers to the left indicate the protein markers in kilodaltons.
The bba34 mutant has no colonization deficit in the C3H/HeN mouse model of Lyme disease following intradermal needle inoculation.
We then analyzed the capability of the bba34 mutant strain to establish infection following intradermal needle inoculation using the C3H/HeN mouse model of Lyme disease (26, 50, 51, 74). As shown in Table 3, the mutant was able to colonize two out of three mice at 102 spirochetes per mouse, while all three mice were infected with the wild-type strain at the same dose. Even though there are a few tissues that did not support the growth of the mutant, all the mice challenged with 103, 104, or 105 mutant spirochetes were infected. It was possible to isolate viable spirochetes from all three mice that were infected with 102 spirochetes per mouse of parental strain, and every tissue except for one supported the growth of spirochetes at all other doses. This analysis clearly demonstrated that the absence of bba34 had no drastic effects on the colonization capabilities of B. burgdorferi in the C3H/HeN mouse model of Lyme disease following intradermal needle inoculation. The in vivo phenotype of the complemented strain was similar to that of the parental control strain (Table 3), indicating that there are no obvious defects in the genetically complemented strain.
Table 3.
Infectivity analysis of the bba34 mutant in C3H/HeN mice at 21 days postinfection
| Strain/dose | No. of cultures positive/no. tested |
No. of mice infected/no. tested | ||||||
|---|---|---|---|---|---|---|---|---|
| Skin | Spleen | Joint | Lymph node | Heart | Bladder | All sites | ||
| wt | ||||||||
| 102 | 3/3 | 2/3 | 3/3 | 3/3 | 3/3 | 3/3 | 17/18 | 3/3 |
| 103 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 18/18 | 3/3 |
| 104 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 18/18 | 3/3 |
| 105 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 18/18 | 3/3 |
| mt | ||||||||
| 102 | 2/3 | 0/3 | 2/3 | 2/3 | 2/3 | 2/3 | 10/18 | 2/3 |
| 103 | 3/3 | 2/3 | 2/3 | 3/3 | 3/3 | 3/3 | 16/18 | 3/3 |
| 104 | 3/3 | 2/3 | 3/3 | 2/3 | 3/3 | 3/3 | 16/18 | 3/3 |
| 105 | 3/3 | 1/3 | 3/3 | 2/3 | 3/3 | 3/3 | 15/18 | 3/3 |
| ct | ||||||||
| 102 | 2/3 | 0/3 | 2/3 | 2/3 | 2/3 | 2/3 | 10/18 | 2/3 |
| 103 | 3/3 | 2/3 | 3/3 | 3/3 | 3/3 | 3/3 | 17/18 | 3/3 |
| 104 | 3/3 | 0/3 | 3/3 | 2/3 | 3/3 | 3/3 | 14/18 | 3/3 |
| 105 | 3/3 | 0/3 | 3/3 | 3/3 | 3/3 | 3/3 | 14/18 | 3/3 |
We were surprised to note that the reduction in the levels of key regulators of gene expression, such as RpoS, BosR, and CsrABb, and of members of their respective regulons that contribute to the pathogenic processes of the bba34 mutant had no impact on the levels of infectivity in the C3H/HeN mouse model of Lyme disease. We hypothesized that while the absence of expression of bba34 translated into reduced levels of pathogenesis-related proteins in B. burgdorferi under in vitro growth conditions, undefined signals under in vivo conditions could offset the functions mediated by BBA34. This could result in the appropriate induction of RpoS, BosR, CsrABb, as well as the known and unknown members of their respective regulons leading to an in vivo phenotype that is similar to that seen for infection with the wild-type parental strain. In order to further delineate the mechanisms responsible for the differences in the in vitro and in vivo phenotypes, we decided to alter the levels of specific ingredients used in the preparation of BSK-II growth medium to obtain insights into how the levels of pathogenesis-related proteins are modulated in the bba34 mutant.
Increased levels of yeastolate and sodium bicarbonate correlate with increased levels of RpoS.
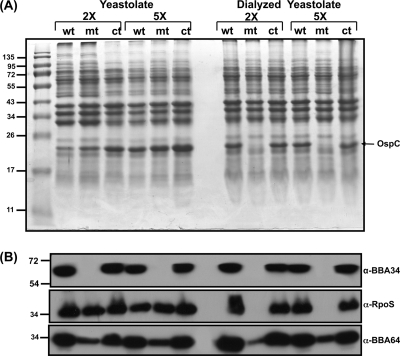
We propagated the bba34 mutant strain by shifting the spirochetes from pH 7.6/23°C to pH 6.8/37°C in BSK-II growth medium supplemented with increasing concentrations of key ingredients used in its preparation, such as bovine serum albumin (BSA), NRS, neopeptone, yeastolate, and sodium bicarbonate. As shown below (Fig. 5), there were increased levels of RpoS when the bba34 mutant was propagated with 2× and 5× concentrations of yeastolate (Fig. 5B, lanes 3 and 4, respectively; α-RpoS) compared to what was observed with the mutant propagated at the normal (1×) levels of yeastolate (Fig. 5B, lane 2; α-RpoS). An increase in the levels of RpoS was also observed in the bba34 mutant when the levels of sodium bicarbonate in the growth media was supplemented at 2× and 5× (Fig. 5, lanes 5 and 6, respectively; α-RpoS) compared to the normal levels of sodium bicarbonate (Fig. 5, lane 2; 1× sodium bicarbonate). We did not see any change in the levels of RpoS when the concentration of either normal rabbit serum or bovine serum albumin was doubled to 12% or 10% in the BSK-II medium (Fig. 5B, lanes 7 and 8, respectively; α-RpoS). The increase in the levels of RpoS in response to the increase in select nutrient signals was also reflected in the increased levels of OspC and BBA64 (Fig. 5A and B). There was no increase in the levels of BBA64 upon growth of the bba34 mutant with increased amounts of NRS and BSA (Fig. 5B, lanes 7 and 8, respectively; α-BBA64). We also determined that there was no dramatic increase in the levels of either OppA1 or OppA4 following propagation of the bba34 mutant in the presence of increased concentrations of select nutrients (Fig. 5B, lanes 2 to 8; α-OppA1 or α-OppA4). Both the parental wild-type and complemented strains propagated in conventional BSK-II growth medium at pH 6.8/37°C served as controls in this analysis (Fig. 5B, lanes 1 and 9, respectively). For medium preparations that required increased concentrations of key ingredients, we first prepared conventional BSK-II medium at pH 6.8 and added increasing amounts of various components and assessed the change in the pH (Table 4), as this could have a significant bearing on the outcome of levels of several borrelial proteins analyzed in this study. We did not observe any drastic changes in the pH of the medium supplemented with sodium bicarbonate equilibrated with 1% CO2 for a period of 72 h (data not shown).
Fig. 5.
Increased levels of yeastolate and sodium bicarbonate in the BSK-II growth medium contribute to restoration of select borrelial proteins in the bba34 mutant. Total protein lysates were prepared from wild-type (wt), mutant (mt), or complemented (ct) strains after growth in conventional BSK-II growth medium or upon supplementation with 2× or 5× concentration of yeastolate, 2× or 5× NaHCO3, 12% rabbit serum, or 10% BSA as described in Materials and Methods. (A) The protein samples were separated on a 12.5% SDS-PAGE gel and stained with Coomassie blue. Lanes: 1, wt (control); 2, mt (control); 3, mt (2× yeastolate); 4, mt (5× yeastolate); 5, mt (2× NaHCO3); 6, mt (5× NaHCO3); 7, mt (12% rabbit serum); 8, mt (10% BSA); 9, ct (control). The numbers to the left indicate the molecular size standards in kilodaltons. All strains were grown under fed-tick conditions (pH 6.8/37°C). (B) Proteins separated on 12.5% SDS-PAGE gels as described above were transferred to PVDF membranes and probed with anti-BBA34 serum, anti-A64 serum, anti-RpoS serum, and anti-OppA1 and -OppA4 sera. The blots were developed using appropriate secondary antibodies conjugated to HRP in conjunction with the Enhanced Chemiluminescence System.
Table 4.
Effect of different concentrations of yeastolate, sodium bicarbonate, and sodium acetate (dialyzed yeastolate)a
| Expt no. | pH of BSK-II medium with: |
|||||||
|---|---|---|---|---|---|---|---|---|
| Yeastolate |
Sodium bicarbonate |
Sodium acetate (dialyzed yeastolate) |
Sodium bicarbonate (dialyzed yeastolate) |
|||||
| 4 g/liter (2×) | 10 g/liter (5×) | 4.4 g/liter (2×) | 11 g/liter (5×) | 1.36 g/liter (10 mM) | 4.08 g/liter (30 mM) | 4.4 g/liter (2×) | 11 g/liter (5×) | |
| 1 | 7.06 | 7.07 | 7.24 | 7.46 | 6.99 | 7.03 | 7.21 | 7.36 |
| 2 | 7.07 | 7.08 | 7.23 | 7.46 | 7.01 | 7.01 | 7.23 | 7.36 |
| 3 | 7.06 | 7.06 | 7.21 | 7.45 | 7.06 | 7.01 | 7.22 | 7.35 |
The initial pH was 6.8 prior to the addition of the indicated component.
Increased supplementation of dialyzed yeastolate does not restore RpoS in the bba34 mutant.
In order to further determine the components that could restore the in vitro wild-type phenotype in the bba34 mutant, we supplemented the BSK-II growth medium with yeastolate preparation that had been dialyzed to remove compounds smaller than 10,000 Da. As shown in Fig. 6A, increased supplementation of dialyzed yeastolate at either 2× or 5× normal levels failed to restore the levels of OspC (Fig. 6A, Dialyzed Yeastolate, mt) in the mutant, while similar levels of regular yeastolate restored OspC in the bba34 mutant to levels observed in the control strains (Fig. 6A, Yeastolate, mt). Immunoblot analysis also confirmed that the levels of RpoS and BBA64 were lower in the mutant grown in higher concentrations of dialyzed yeastolate than in the mutant grown in regular yeastolate. These observations suggested that components smaller than 10,000 Da present in yeastolate modulate the levels of RpoS, OspC, and BBA64 in the bba34 mutant.
Fig. 6.
Increased levels of dialyzed yeastolate in the BSK-II growth medium fail to restore levels of select borrelial proteins in the bba34 mutant. Total protein lysates were prepared from wild-type (wt), mutant (mt), or complemented (ct) strains after growth in conventional BSK-II growth medium or upon supplementation with a 2× or 5× concentration of yeastolate or dialyzed yeastolate (10,000-Da cutoff) as described in Materials and Methods. (A) The protein samples were separated on a 12.5% SDS-PAGE gel and stained with Coomassie blue. The numbers to the left indicate the molecular size standards in kilodaltons. All strains were grown under fed-tick conditions (pH 6.8/37°C). (B) Proteins separated on 12.5% SDS-PAGE gels as described above were transferred to PVDF membranes and probed with anti-BBA34, anti-RpoS, or anti-A64 serum. The blots were developed using appropriate secondary antibodies conjugated to HRP in conjunction with the Enhanced Chemiluminescence System.
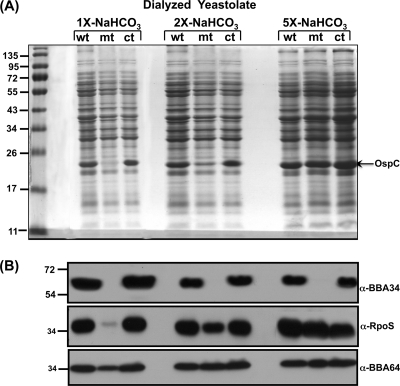
Sodium bicarbonate and sodium acetate restore levels of RpoS, OspC, and BBA64 in the bba34 mutant.
We propagated all three strains in BSK-II medium supplemented with increasing concentrations of sodium bicarbonate at pH 6.8/37°C and analyzed levels of RpoS, OspC, and BBA64. As shown in Fig. 7A, the levels of OspC synthesized in the bba34 mutant increased with higher concentrations of sodium bicarbonate in medium prepared with dialyzed yeastolate (Fig. 7A, 2× and 5× sodium bicarbonate, mt). The levels of OspC were barely detectable in the mutant grown at the conventional 1× concentration of sodium bicarbonate. On the other hand, the levels of OspC were higher in wild-type and complemented strains grown in medium prepared with dialyzed yeastolate (Fig. 7A, 2× and 5× sodium bicarbonate, wt, ct). In addition, both RpoS and BBA64 levels were also elevated and restored in the mutant to levels similar to those observed in the control strains (Fig. 7B) with increasing concentrations of sodium bicarbonate (Fig. 7B, 2× and 5× sodium bicarbonate, α-RpoS, α-BBA64, wt, mt, and ct). The levels of OspC, RpoS, and BBA64 were much lower when the mutant was propagated in BSK-II medium with the conventional 1× concentration of sodium bicarbonate (Fig. 7A and B, 1×-NaHCO3, mt). Consistent with our prior observations, the levels of BBA34 were comparable in the parental and complemented strains and absent from the bba34 mutant.
Fig. 7.
Increased supplementation of sodium bicarbonate in the BSK-II growth medium prepared with dialyzed yeastolate restores levels of select borrelial proteins in the bba34 mutant. Total protein lysates were prepared from wild-type (wt), mutant (mt), or complemented (ct) strains after growth in BSK-II growth medium prepared with dialyzed yeastolate (10,000-Da cutoff) with a 1×, 2×, or 5× concentration of sodium bicarbonate described in Materials and Methods. (A) The protein samples were separated on a 12.5% SDS-PAGE gel and stained with Coomassie blue. The numbers to the left indicate the molecular size standards in kilodaltons. All strains were grown under fed-tick conditions (pH 6.8/37°C). (B) Proteins separated on 12.5% SDS-PAGE gels as described above were transferred to PVDF membranes and probed with anti-BBA34, anti-RpoS, or anti-A64 serum. The blots were developed using appropriate secondary antibodies conjugated to HRP in conjunction with the Enhanced Chemiluminescence System.
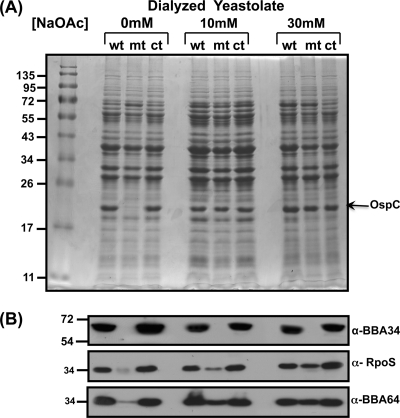
We further examined if levels of sodium acetate could also contribute to the restoration of the levels of proteins involved in vertebrate host-specific adaptation in the bba34 mutant. As shown in Fig. 8A, the levels of OspC in the mutant propagated in medium supplemented with higher levels of sodium acetate (Fig. 8A, 10 mM, 30 mM, mt) were comparable to the levels in the control strains (Fig. 8A, 10 mM, 30 mM, wt, ct), whereas there were significantly lower levels of OspC when no additional sodium acetate was added to the BSK-II medium (Fig. 8A, 0 mM, mt). It should be pointed that the major components used to make the BSK-II medium, such as CMRL (0.61 mM) and normal rabbit serum, do contribute to nominal levels of sodium acetate for the growth of B. burgdorferi (88). Based on these in vitro phenotypic analyses, it appears that BBA34 mediates transport of select solutes, such as sodium bicarbonate and sodium acetate. While supplementation with several other compounds, such as sodium pyruvate, l-proline, d-glucose, Casamino Acids, and sodium citrate, in the growth medium did not result in changes in the levels of OspC (data not shown), it appears that there is some selectivity for substances transported by BBA34.
Fig. 8.
Increased supplementation of sodium acetate in the BSK-II growth medium prepared with dialyzed yeastolate restores levels of select borrelial proteins in the bba34 mutant. Total protein lysates were prepared from wild-type (wt), mutant (mt), or complemented (ct) strains after growth in BSK-II growth medium prepared with dialyzed yeastolate (10,000-Da cutoff) with no additional (0 mM) or additional (10 mM or 30 mM) sodium acetate as described in Materials and Methods. (A) The protein samples were separated on a 12.5% SDS-PAGE gel and stained with Coomassie blue. The molecular size standards in kilodaltons are indicated to the left. All strains were grown under fed-tick conditions (pH 6.8/37°C). (B) Proteins separated on 12.5% SDS-PAGE gels as described above were transferred to PVDF membranes and probed with anti-BBA34, anti-RpoS, or anti-A64 serum. The blots were developed using appropriate secondary antibodies conjugated to HRP in conjunction with the Enhanced Chemiluminescence System.
DISCUSSION
A large number of studies have demonstrated the importance of vertebrate host-specific factors associated with the incoming blood meal in the midgut of ticks in modulating adaptive gene expression in B. burgdorferi (20, 22, 25, 39, 71, 72, 77). The resultant phenotypic changes in the spirochetes, in turn, contribute to their transmission from the tick vector and subsequently facilitate colonization of the vertebrate hosts (28, 32, 35, 36, 63). A major focus of recent investigations has been on the mechanistic basis of how multiple external signals are perceived and connected to induce appropriate transcriptional or translational changes leading to host-adapted spirochetes capable of establishing infection (10, 14–17, 29, 37, 46, 56–58, 66, 69, 81, 90, 92). Moreover, B. burgdorferi has limited metabolic capabilities with (i) few ORFs annotated as regulators of gene expression; (ii) no apparent biosynthetic pathways for synthesis of various amino acids, fatty acids, and nucleotides; and (iii) limited transport systems for acquiring key nutrients from the external environment. We therefore decided to investigate the role of one of the five paralogs of oligopeptide permease A proteins, BBA34, in vertebrate host-specific adaptation since it is (i) preferentially upregulated upon propagation of B. burgdorferi under conditions mimicking the mammalian host, (ii) incapable of binding oligopeptides, (iii) immunogenic, and (iv) comprised of a solute binding domain capable of binding/transporting key solutes (85).
We undertook a genetic approach to assess the contribution of this lp54-encoded protein to vertebrate host-specific conditions by deleting bba34 in a noninfectious lp25-deficient strain ML23 (Fig. 1). Although bba34 was annotated as one of the five oligopeptide permeases in the borrelial genome, contrary to our expectations, we did not observe a detectable difference in the growth curves under vertebrate host-specific conditions (data not shown) even though increased levels of synthesis of BBA34 in the parental and complemented strains were observed only under these latter conditions (Fig. 2B). Based on this analysis, it appears that the function of BBA34 was dispensable for in vitro growth or was compensated by other oligopeptide permeases and/or other uncharacterized proteins.
Further in vitro phenotypic analysis of the bba34 mutant propagated under conditions that mimicked the vertebrate host revealed reduction in the levels of key regulators, such as CsrABb, RpoS, and BosR, compared to those of the parental and complemented strains (Fig. 2C). Consistent with this observation, we also noted that several lipoproteins (except for OspA) whose levels are modulated by these regulators were also reduced in the mutant compared to those in the control strains (Fig. 2D). The reduction in the protein levels of regulators and select lipoproteins appears to be at the transcriptional level, as the mRNA transcripts for the above-described genes were significantly lower in the bba34 mutant than in the parental and complemented strains (Fig. 3). Based on in silico analysis of bba34, we predicted that it could potentially bind/transport solutes/nutrients. We were surprised to note that these predicted functions could lead to alterations in the transcriptional levels of a variety of genes involved in the vertebrate host-specific adaptation. These in vitro observations provided key insights into the possibility of bba34 functionally contributing to the regulation of adaptive gene expression in B. burgdorferi and facilitated further in vivo analysis of the bba34 mutant.
Since several proteins, such as OspC, DbpA, and BBK32, that were significantly reduced in the bba34 mutant have been shown to contribute to the virulence of B. burgdorferi, we expected a significant attenuation in infectivity in the mouse model of Lyme disease. Contrary to our expectation, there was no difference between the ability of the bba34 mutant to colonize C3H/HeN mice following intradermal needle inoculation and that of the control strains (Table 3). The lack of a defect in the in vivo phenotype of bba34 mutant suggested that it was capable of expressing virulence-associated regulators and determinants at levels sufficient to facilitate the colonization of C3H/HeN mice. Based on these distinct differences between the in vitro and in vivo phenotypes, we hypothesized that bba34 is probably involved in facilitating binding/transport of one or more key factors that either are present only in the vertebrate host or are present in the in vivo environment at significantly higher levels. Alternatively, it was also possible that compensatory changes in the levels of other oligopeptide permeases (OppA1, OppA2, and OppA4) could functionally compensate for the lack of BBA34 under in vivo conditions. We observed no significant differences in the levels of OppA1, OppA2, and OppA4 between the mutant and control strains under conditions that mimic fed ticks (Fig. 2F), arguing for a lack of compensatory role for other OppA paralogs. While it is feasible that there may not be a compensatory increase in the levels of these proteins, there could a functional increase in their binding and transport of components specifically mediated by BBA34. Counter to this argument has been the observation that while OppA1 to OppA3 bound heptapeptides, OppA4 and OppA5 (BBA34) did not exhibit peptide-binding properties (84). The differences in the in vitro and in vivo phenotypic analysis provided us with a unique strategy to determine what growth condition(s) would restore the levels of regulators and mediators of vertebrate host-specific proteins in the bba34 mutant to that of control strains.
We therefore decided to test the hypothesis that bba34 is involved in facilitating the binding/transport of one or more key ingredients that are either present only in the vertebrate host or present at much higher levels than those of the in vitro growth medium. Our strategy was to supplement components of the in vitro growth medium at higher concentrations and determine what would partly or completely restore the levels of select proteins downregulated in the bba34 mutant to wild-type parental levels. We used immunoblot analysis to detect OspC, RpoS, and BBA64 levels to calibrate the levels of restoration of vertebrate host-specific adaptation in the bba34 mutant in response to an increase in the levels of select nutrients in the BSK-II growth medium.
We propagated the bba34 mutant in BSK-II growth medium with increased concentrations of select components used in its preparation, such as BSA, NRS, proteose peptone, yeastolate, and sodium bicarbonate (Table 4). Since alterations to growth medium could have significant effects on the pH, a critical signal-regulating gene expression in B. burgdorferi, we measured the change in the pH of growth medium following supplementation and found that it ranged from 6.8 (initial pH before supplementation) to 7.46 (Table 4). There were increased levels of synthesis of OspC, RpoS, and BBA64 in the mutant when it was propagated with increased levels of yeastolate (Fig. 5, lanes 3 and 4) and sodium bicarbonate (Fig. 5, lanes 5 and 6) compared to the levels observed on propagation in conventional BSK-II growth medium with 6% NRS at pH 6.8/37°C. There were no significant differences upon additional supplementation of either rabbit serum or BSA at levels higher than that present in conventional BSK-II growth medium (Fig. 5, lanes 7 and 8), indicating that BBA34 is probably involved in the binding and transport solutes rather than oligopeptides or other complex proteins. Since we observed a dramatic increase in the levels of OspC, RpoS, and BBA64 upon additional supplementation of yeastolate, we determined what subfraction of yeastolate could significantly contribute to the restoration of parental phenotype in the bba34 mutant. When we propagated the bba34 mutant in BSK-II medium supplemented with dialyzed yeastolate (10,000 Da cutoff) at higher concentrations (2× or 5× of normal levels) there was no increase in the levels of OspC, RpoS, and BBA64 compared to those in the control strains (Fig. 6). On the other hand, mutant propagated with increased concentrations of regular yeastolate exhibited normal levels of the above markers of vertebrate host adaptation (Fig. 6). These observations demonstrated that components smaller that 10,000 Da present in the yeastolate were the targets bound/transported by BBA34, which in turn probably facilitated vertebrate host-specific gene expression.
In order to determine if this assumption was true, we supplemented BSK-II growth medium prepared with dialyzed yeastolate with increased concentrations of sodium bicarbonate and sodium acetate. As shown in Fig. 7 and 8, the levels of OspC, RpoS, and BBA64 in the bba34 mutant were restored to levels observed in the wild-type and complemented strains with increased amounts of the above solutes. Supplementation with increased amounts of pyruvate, l-proline, d-glucose, Casamino Acids, or sodium citrate did not result in changes in the levels of OspC in the bba34 mutant (data not shown), suggesting that BBA34 may specifically bind/transport sodium bicarbonate, sodium acetate, or both. While we have not determined the stoichiometry of the interactions of BBA34 with these solutes, the genetic analysis of the bba34 mutant has provided key details on the role of this protein as a solute transporter contributing to the adaptation of B. burgdorferi to the vertebrate host.
The mechanistic basis for the ability of increased levels of sodium bicarbonate to restore levels of OspC, BBA64, and RpoS in the bba34 mutant comparable to those of control strains remains to be determined. Increased levels of sodium bicarbonate in the growth medium could contribute to sufficient levels of bicarbonate ions in the cytosol of the bba34 mutant that may alter the functions of regulatory proteins, leading to a phenotype similar to that of the control strains. The aforementioned possibility is not without precedence, since virulence gene expression in Streptococcus pyogenes (18), enterohemorrhagic E. coli (1), Citrobacter rodentium (89), and Vibrio cholerae (3) has been reported to be altered by differences in the levels of bicarbonate ions. Bicarbonate ion bound to an arginine side chain has been implicated in the activation of PepA, a multifunctional protein that regulates the carboamoylphosphate synthetase operon and the stable inheritance of plasmids in E. coli (79, 80). The functions of ToxT, which directly activates the genes encoding for cholera toxin (CT), toxin-regulated pilus (TCP), and other virulence-related proteins in V. cholerae is enhanced in the presence of bicarbonate ions (3). Therefore, it is interesting to speculate that increased levels of bicarbonate ions could potentially alter the regulatory functions of proteins or alternatively modulate the efficiency of transport for key signals that modulate gene expression in B. burgdorferi.
Previously, it has been shown that propagation of B. burgdorferi strain B31 (MSK5) under anaerobic conditions with 5% CO2 resulted in the increased expression of pathogenesis-related proteins of B. burgdorferi compared to that of growth at anaerobic conditions without CO2 or at microaerophilic conditions with 1% CO2 (39). The same study reported that OspC, DbpA, and RpoS were not detected in media with conventional (25 mM) or decreasing levels of sodium bicarbonate under microaerophilic conditions with 1% CO2, suggesting that the levels of CO2 rather than levels of sodium bicarbonate were responsible for altering the levels of these pathogenesis-related proteins (39). Since there are no recognizable homologs of carbonic anhydrases in the genome of B. burgdorferi, the conversion of CO2 to bicarbonate ions is unclear (31). However, a homolog of adenylyl cyclase and a PAS (Per-Amt-Sim) domain in the histidine kinase (BB0764) capable of activating the response regulatory protein Rrp2 have been suggested as possible sensory proteins modulating gene expression in response to levels of CO2 (39). Levels of acetyl phosphate rather than the cognate histidine kinase have been recently shown to play a critical role in the activation of Rrp2, suggesting that multiple regulatory networks modulate gene expression in B. burgdorferi in response to intra- and extracellular signals (88). While we consistently observed detectable levels of OspC and RpoS in our parental strain (ML23) grown under conventional microaerophilic conditions with 1% CO2 (Fig. 2), these observations could partly be attributed to differences in strains used (Table 1, MSK5 versus ML23) or other unknown variations in our laboratory growth conditions used in the propagation of B. burgdorferi. We did not observe any dramatic change in the pH of the borrelial growth medium in the presence of increased levels of sodium bicarbonate used to grow the borrelial strains (Table 4), and therefore the observed changes in the levels of OspC, BBA64, and RpoS cannot be attributed to changes in the pH of the growth medium.
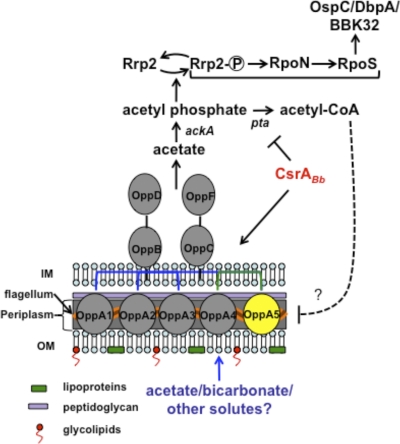
The aforementioned observations lead to the question of how levels of sodium acetate/sodium bicarbonate potentially regulate gene expression in B. burgdorferi. Based on past and recent studies, we propose the model shown in Fig. 9 as to how the concentration of acetyl phosphate or acetyl-CoA could contribute to the activation or dampening of the regulatory pathways for vertebrate host-specific adaptation in B. burgdorferi (27). We and others have recently shown that CsrABb is one of the key regulators involved in modulating vertebrate host-specific gene expression (41, 70, 82). While the deletion of csrABb reduced the levels of RpoS, BosR, and several virulence-related proteins, overexpression of CsrABb, on the other hand, increased levels of these proteins. Additional studies also showed that activation of the Rrp2-RpoN-RpoS pathway was mediated by increased levels of acetyl phosphate due to repression of phosphate acetyltransferase (Pta) by CsrABb (64, 67, 88). Since acetate is converted to acetyl phosphate by acetate kinase, the intracellular levels of acetate in B. burgdorferi could have significant bearing on the activation of the Rrp2-RpoN-RpoS pathway. It is therefore conceivable that the reduced binding/transport of acetate due to the absence of bba34 could result in levels of acetyl phosphate that are insufficient to activate the Rrp2-RpoN-RpoS pathway, with a concomitant decrease in the levels of OspC, BBA64, and other vertebrate host-specific determinants. When the bba34 mutant was propagated under increased levels of sodium acetate, it is feasible that sufficient levels of acetate accumulated intracellularly via unknown transporter(s), resulting in increased levels of RpoS, OspC, and BBA64. The differences in the levels of acetate in the vertebrate host compared to those in the in vitro growth medium have been given previously as one possible reason contributing to sufficient levels of acetyl phosphate leading to the activation of the Rrp2-RpoN-RpoS pathway under vertebrate host-specific conditions (88).
Fig. 9.
A model for the role of BBA34 in modulating the vertebrate host-specific adaptation in B. burgdorferi. We propose that BBA34/OppA5 facilitates the transport of acetate, which is converted to acetyl phosphate by acetate kinase. The increased accumulation of acetyl phosphate in turn facilitates the phosphorylation of Rrp2, leading to the activation of the Rrp2-RpoN-RpoS pathway under fed-tick or mammalian host-specific conditions (41, 70, 82, 88). Since CsrABb is upregulated under fed-tick conditions, leading to repression of phosphate acetyl transferase (Pta), there is increased accumulation of acetyl phosphate, resulting in sustained activation of the Rrp2-RpoN-RpoS pathway. Under conditions where the levels of CsrABb are reduced, there is a lack of repression of Pta, leading to the increased accumulation of acetyl-CoA, which in turn leads to reduced levels of expression of BBA34/OppA5. This latter proposition is based on our previous finding that the csrABb deletion mutant had reduced levels of BBA34, suggesting that acetyl-CoA could play a role as a key metabolite in modulating the pathways for the adaptation of spirochetes to vertebrate host-specific conditions. The model is skewed toward assuming acetyl phosphate as a major substrate contributing to the activation of the Rrp2-RpoN-RpoS pathway.
The ability to bind/transport sodium acetate could also partly explain why the levels of a variety of determinants involved in vertebrate host-specific adaptation were reduced in the bba34 mutant following in vitro growth conditions, while there was no defect in the capacity of the bba34 mutant to colonize C3H/HeN mice. It is feasible that the increased levels of acetate in the vertebrate host could contribute to sufficient activation of the Rrp2-RpoN-RpoS pathway, resulting in the expression of pathogenesis-related proteins, such that the in vivo phenotype for the bba34 mutant is indistinguishable from those of the control strains. It is therefore interesting to speculate that acetyl-CoA may be a key intracellular metabolite whose levels serve to determine the host-adapted state of the spirochetes (87). Additionally, we recently showed that the levels of BBA34 were reduced in the csrABb mutant, which also suggests that the accumulation of acetyl-CoA due to the lack of repression of Pta (as expected in the csrABb mutant) probably regulates the levels of this protein. Since BBA34 is localized in the periplasm of B. burgdorferi (Fig. 5), it is unclear how acetate is transported from the external environment into periplasm. It is conceivable that BBA34 could play a major role in the binding and transport of acetate across the inner membrane, leading to the activation of the vertebrate host-specific response. In the absence of bba34 and at higher concentrations of extracellular acetate, other low-affinity transporters could facilitate sufficient transport of acetate to mediate the activation of the vertebrate host-specific adaptation. Based on our genetic analyses, we believe that BBA34 serves as a key link connecting the extracellular environment with the intracellular signaling pathways that drive the adaptation of B. burgdorferi to highly disparate environments.
ACKNOWLEDGMENTS
We thank Darrin R. Akins for the anti-BBA64 serum used in the study, Frank C. Gheradini for anti-NapA serum, and Steven J. Norris for providing the plasmid pBBE22. We thank Ashlesh K. Murthy for critical reading of the manuscript. We also thank Rajesh G. Prabhu and Manasa Parvataneni for technical help.
This study was supported by Public Health Service grant SC1-AI-078559 from the National Institute of Allergy and Infectious Diseases (to J.S.), a predoctoral fellowship from the South Texas Center for Emerging Infectious Diseases (to T.A.V.L.), and a postdoctoral fellowship (AHA-0825175F) from the American Heart Association (to M.D.E.-G.).
Footnotes
Published ahead of print on 31 May 2011.
REFERENCES
- 1. Abe H., Tatsuno I., Tobe T., Okutani A., Sasakawa C. 2002. Bicarbonate ion stimulates the expression of locus of enterocyte effacement-encoded genes in enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 70:3500–3509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abouhamad W. N., Manson M., Gibson M. M., Higgins C. F. 1991. Peptide transport and chemotaxis in Escherichia coli and Salmonella typhimurium: characterization of the dipeptide permease (Dpp) and the dipeptide-binding protein. Mol. Microbiol. 5:1035–1047 [DOI] [PubMed] [Google Scholar]
- 3. Abuaita B. H., Withey J. H. 2009. Bicarbonate induces Vibrio cholerae virulence gene expression by enhancing ToxT activity. Infect. Immun. 77:4111–4120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Akins D. R., Bourell K. W., Caimano M. J., Norgard M. V., Radolf J. D. 1998. A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. J. Clin. Invest. 101:2240–2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alverson J., Bundle S. F., Sohaskey C. D., Lybecker M. C., Samuels D. S. 2003. Transcriptional regulation of the ospAB and ospC promoters from Borrelia burgdorferi. Mol. Microbiol. 48:1665–1677 [DOI] [PubMed] [Google Scholar]
- 6. Anderton J. M., et al. 2004. Whole-genome DNA array analysis of the response of Borrelia burgdorferi to a bactericidal monoclonal antibody. Infect. Immun. 72:2035–2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anguita J., Hedrick M. N., Fikrig E. 2003. Adaptation of Borrelia burgdorferi in the tick and the mammalian host. FEMS Microbiol. Rev. 27:493–504 [DOI] [PubMed] [Google Scholar]
- 8. Anguita J., et al. 2000. Borrelia burgdorferi gene expression in vivo and spirochete pathogenicity. Infect. Immun. 68:1222–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barbour A. G. 1984. Isolation and cultivation of Lyme disease spirochetes. Yale J. Biol. Med. 57:521–525 [PMC free article] [PubMed] [Google Scholar]
- 10. Boardman B. K., et al. 2008. Essential role of the response regulator Rrp2 in the infectious cycle of Borrelia burgdorferi. Infect. Immun. 76:3844–3853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bono J. L., Tilly K., Stevenson B., Hogan D., Rosa P. 1998. Oligopeptide permease in Borrelia burgdorferi: putative peptide-binding components encoded by both chromosomal and plasmid loci. Microbiology 144:1033–1044 [DOI] [PubMed] [Google Scholar]
- 12. Brooks C. S., Hefty P. S., Jolliff S. E., Akins D. R. 2003. Global analysis of Borrelia burgdorferi genes regulated by mammalian host-specific signals. Infect. Immun. 71:3371–3383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burgdorfer W., et al. 1982. Lyme disease—a tick-borne spirochetosis? Science 216:1317–1319 [DOI] [PubMed] [Google Scholar]
- 14. Burtnick M. N., et al. 2007. Insights into the complex regulation of rpoS in Borrelia burgdorferi. Mol. Microbiol. 65:277–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Caimano M. J., Eggers C. H., Gonzalez C. A., Radolf J. D. 2005. Alternate sigma factor RpoS is required for the in vivo-specific repression of Borrelia burgdorferi plasmid lp54-borne ospA and lp6.6 genes. J. Bacteriol. 187:7845–7852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Caimano M. J., Eggers C. H., Hazlett K. R., Radolf J. D. 2004. RpoS is not central to the general stress response in Borrelia burgdorferi but does control expression of one or more essential virulence determinants. Infect. Immun. 72:6433–6445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Caimano M. J., et al. 2007. Analysis of the RpoS regulon in Borrelia burgdorferi in response to mammalian host signals provides insight into RpoS function during the enzootic cycle. Mol. Microbiol. 65:1193–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Caparon M. G., Geist R. T., Perez-Casal J., Scott J. R. 1992. Environmental regulation of virulence in group A streptococci: transcription of the gene encoding M protein is stimulated by carbon dioxide. J. Bacteriol. 174:5693–5701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carroll J. A., Cordova R. M., Garon C. F. 2000. Identification of 11 pH-regulated genes in Borrelia burgdorferi localizing to linear plasmids. Infect. Immun. 68:6677–6684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carroll J. A., Garon C. F., Schwan T. G. 1999. Effects of environmental pH on membrane proteins in Borrelia burgdorferi. Infect. Immun. 67:3181–3187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Casjens S., et al. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:490–516 [DOI] [PubMed] [Google Scholar]
- 22. Coburn J., Fischer J. R., Leong J. M. 2005. Solving a sticky problem: new genetic approaches to host cell adhesion by the Lyme disease spirochete. Mol. Microbiol. 57:1182–1195 [DOI] [PubMed] [Google Scholar]
- 23. de Silva A. M., Fikrig E. 1997. Arthropod- and host-specific gene expression by Borrelia burgdorferi. J. Clin. Invest. 99:377–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. De Silva A. M., Fikrig E. 1997. Borrelia burgdorferi genes selectively expressed in ticks and mammals. Parasitol. Today 13:267–270 [DOI] [PubMed] [Google Scholar]
- 25. Eggers C. H., Caimano M. J., Radolf J. D. 2006. Sigma factor selectivity in Borrelia burgdorferi: RpoS recognition of the ospE/ospF/elp promoters is dependent on the sequence of the −10 region. Mol. Microbiol. 59:1859–1875 [DOI] [PubMed] [Google Scholar]
- 26. Esteve-Gassent M. D., Elliott N. L., Seshu J. 2009. sodA is essential for virulence of Borrelia burgdorferi in the murine model of Lyme disease. Mol. Microbiol. 71:594–612 [DOI] [PubMed] [Google Scholar]
- 27. Fang F. C. 2005. Sigma cascades in prokaryotic regulatory networks. Proc. Natl. Acad. Sci. U. S. A. 102:4933–4934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fikrig E., Narasimhan S. 2006. Borrelia burgdorferi—traveling incognito? Microbes Infect. 8:1390–1399 [DOI] [PubMed] [Google Scholar]
- 29. Fisher M. A., et al. 2005. Borrelia burgdorferi sigma54 is required for mammalian infection and vector transmission but not for tick colonization. Proc. Natl. Acad. Sci. U. S. A. 102:5162–5167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Frank K. L., Bundle S. F., Kresge M. E., Eggers C. H., Samuels D. S. 2003. aadA confers streptomycin resistance in Borrelia burgdorferi. J. Bacteriol. 185:6723–6727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fraser C. M., et al. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580–586 [DOI] [PubMed] [Google Scholar]
- 32. Gautam A., Hathaway M., McClain N., Ramesh G., Ramamoorthy R. 2008. Analysis of the determinants of bba64 (P35) gene expression in Borrelia burgdorferi using a gfp reporter. Microbiology 154:275–285 [DOI] [PubMed] [Google Scholar]
- 33. Gilmore R. D., Jr., Howison R. R., Schmit V. L., Carroll J. A. 2008. Borrelia burgdorferi expression of the bba64, bba65, bba66, and bba73 genes in tissues during persistent infection in mice. Microb. Pathog. 45:355–360 [DOI] [PubMed] [Google Scholar]
- 34. Guo B. P., Brown E. L., Dorward D. W., Rosenberg L. C., Hook M. 1998. Decorin-binding adhesins from Borrelia burgdorferi. Mol. Microbiol. 30:711–723 [DOI] [PubMed] [Google Scholar]
- 35. He M., et al. 2008. Abrogation of ospAB constitutively activates the Rrp2-RpoN-RpoS pathway (sigmaN-sigmaS cascade) in Borrelia burgdorferi. Mol. Microbiol. 70:1453–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hojgaard A., Eisen R. J., Piesman J. 2008. Transmission dynamics of Borrelia burgdorferi s.s. during the key third day of feeding by nymphal Ixodes scapularis (Acari: Ixodidae). J. Med. Entomol. 45:732–736 [DOI] [PubMed] [Google Scholar]
- 37. Hubner A., et al. 2001. Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN-RpoS regulatory pathway. Proc. Natl. Acad. Sci. U. S. A. 98:12724–12729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hyde J. A., Shaw D. K., Smith III R., Trzeciakowski J. P., Skare J. T. 2009. The BosR regulatory protein of Borrelia burgdorferi interfaces with the RpoS regulatory pathway and modulates both the oxidative stress response and pathogenic properties of the Lyme disease spirochete. Mol. Microbiol. 74:1344–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hyde J. A., Trzeciakowski J. P., Skare J. T. 2007. Borrelia burgdorferi alters its gene expression and antigenic profile in response to CO2 levels. J. Bacteriol. 189:437–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Indest K. J., et al. 1997. Cell-density-dependent expression of Borrelia burgdorferi lipoproteins in vitro. Infect. Immun. 65:1165–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Karna S. L., et al. 2011. CsrA modulates levels of lipoproteins and key regulators of gene expression critical for pathogenic mechanisms of Borrelia burgdorferi. Infect. Immun. 79:732–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Labandeira-Rey M., Baker E. A., Skare J. T. 2001. VraA (BBI16) protein of Borrelia burgdorferi is a surface-exposed antigen with a repetitive motif that confers partial protection against experimental Lyme borreliosis. Infect. Immun. 69:1409–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Labandeira-Rey M., Seshu J., Skare J. T. 2003. The absence of linear plasmid 25 or 28-1 of Borrelia burgdorferi dramatically alters the kinetics of experimental infection via distinct mechanisms. Infect. Immun. 71:4608–4613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Labandeira-Rey M., Skare J. T. 2001. Decreased infectivity in Borrelia burgdorferi strain B31 is associated with loss of linear plasmid 25 or 28-1. Infect. Immun. 69:446–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lin B., Short S. A., Eskildsen M., Klempner M. S., Hu L. T. 2001. Functional testing of putative oligopeptide permease (Opp) proteins of Borrelia burgdorferi: a complementation model in opp(-) Escherichia coli. Biochim. Biophys. Acta 1499:222–231 [DOI] [PubMed] [Google Scholar]
- 46. Lybecker M. C., Abel C. A., Feig A. L., Samuels D. S. 2010. Identification and function of the RNA chaperone Hfq in the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 78:622–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lybecker M. C., Samuels D. S. 2007. Temperature-induced regulation of RpoS by a small RNA in Borrelia burgdorferi. Mol. Microbiol. 64:1075–1089 [DOI] [PubMed] [Google Scholar]
- 48. Manson M. D., Blank V., Brade G., Higgins C. F. 1986. Peptide chemotaxis in E. coli involves the Tap signal transducer and the dipeptide permease. Nature 321:253–256 [DOI] [PubMed] [Google Scholar]
- 49. Marconi R. T., Samuels D. S., Garon C. F. 1993. Transcriptional analyses and mapping of the ospC gene in Lyme disease spirochetes. J. Bacteriol. 175:926–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Maruskova M., Esteve-Gassent M. D., Sexton V. L., Seshu J. 2008. Role of the BBA64 locus of Borrelia burgdorferi in early stages of infectivity in a murine model of Lyme disease. Infect. Immun. 76:391–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Maruskova M., Seshu J. 2008. Deletion of BBA64, BBA65, and BBA66 loci does not alter the infectivity of Borrelia burgdorferi in the murine model of Lyme disease. Infect. Immun. 76:5274–5284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Medrano M. S., et al. 2007. Regulators of expression of the oligopeptide permease A proteins of Borrelia burgdorferi. J. Bacteriol. 189:2653–2659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Medrano M. S., Policastro P. F., Schwan T. G., Coburn J. 2010. Interaction of Borrelia burgdorferi Hbb with the p66 promoter. Nucleic Acids Res. 38:414–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nadelman R. B., Wormser G. P. 1998. Lyme borreliosis. Lancet 352:557–565 [DOI] [PubMed] [Google Scholar]
- 55. Ojaimi C., et al. 2003. Profiling of temperature-induced changes in Borrelia burgdorferi gene expression by using whole genome arrays. Infect. Immun. 71:1689–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ouyang Z., Blevins J. S., Norgard M. V. 2008. Transcriptional interplay among the regulators Rrp2, RpoN and RpoS in Borrelia burgdorferi. Microbiology 154:2641–2658 [DOI] [PubMed] [Google Scholar]
- 57. Ouyang Z., He M., Oman T., Yang X. F., Norgard M. V. 2009. A manganese transporter, BB0219 (BmtA), is required for virulence by the Lyme disease spirochete, Borrelia burgdorferi. Proc. Natl. Acad. Sci. U. S. A. 106:3449–3454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ouyang Z., et al. 2009. BosR (BB0647) governs virulence expression in Borrelia burgdorferi. Mol. Microbiol. 74:1331–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Podbielski A., Leonard B. A. 1998. The group A streptococcal dipeptide permease (Dpp) is involved in the uptake of essential amino acids and affects the expression of cysteine protease. Mol. Microbiol. 28:1323–1334 [DOI] [PubMed] [Google Scholar]
- 60. Probert W. S., Johnson B. J. 1998. Identification of a 47 kDa fibronectin-binding protein expressed by Borrelia burgdorferi isolate B31. Mol. Microbiol. 30:1003–1015 [DOI] [PubMed] [Google Scholar]
- 61. Purser J. E., et al. 2003. A plasmid-encoded nicotinamidase (PncA) is essential for infectivity of Borrelia burgdorferi in a mammalian host. Mol. Microbiol. 48:753–764 [DOI] [PubMed] [Google Scholar]
- 62. Purser J. E., Norris S. J. 2000. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc. Natl. Acad. Sci. U. S. A. 97:13865–13870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ramamoorthy R., Scholl-Meeker D. 2001. Borrelia burgdorferi proteins whose expression is similarly affected by culture temperature and pH. Infect. Immun. 69:2739–2742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rasis M., Segal G. 2009. The LetA-RsmYZ-CsrA regulatory cascade, together with RpoS and PmrA, post-transcriptionally regulates stationary phase activation of Legionella pneumophila Icm/Dot effectors. Mol. Microbiol. 72:995–1010 [DOI] [PubMed] [Google Scholar]
- 65. Revel A. T., Talaat A. M., Norgard M. V. 2002. DNA microarray analysis of differential gene expression in Borrelia burgdorferi, the Lyme disease spirochete. Proc. Natl. Acad. Sci. U. S. A. 99:1562–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rogers E. A., et al. 2009. Rrp1, a cyclic-di-GMP-producing response regulator, is an important regulator of Borrelia burgdorferi core cellular functions. Mol. Microbiol. 71:1551–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Romeo T. 1998. Global regulation by the small RNA-binding protein CsrA and the non-coding RNA molecule CsrB. Mol. Microbiol. 29:1321–1330 [DOI] [PubMed] [Google Scholar]
- 68. Samuels D. S. 1995. Electrotransformation of the spirochete Borrelia burgdorferi. Methods Mol. Biol. 47:253–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Samuels D. S., Radolf J. D. 2009. Who is the BosR around here anyway? Mol. Microbiol. 74:1295–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sanjuan E., Esteve-Gassent M. D., Maruskova M., Seshu J. 2009. Overexpression of CsrA (BB0184) alters the morphology and antigen profiles of Borrelia burgdorferi. Infect. Immun. 77:5149–5162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Schwan T. G., Piesman J., Golde W. T., Dolan M. C., Rosa P. A. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. U. S. A. 92:2909–2913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Seshu J., Boylan J. A., Gherardini F. C., Skare J. T. 2004. Dissolved oxygen levels alter gene expression and antigen profiles in Borrelia burgdorferi. Infect. Immun. 72:1580–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Seshu J., et al. 2004. A conservative amino acid change alters the function of BosR, the redox regulator of Borrelia burgdorferi. Mol. Microbiol. 54:1352–1363 [DOI] [PubMed] [Google Scholar]
- 74. Seshu J., et al. 2006. Inactivation of the fibronectin-binding adhesin gene bbk32 significantly attenuates the infectivity potential of Borrelia burgdorferi. Mol. Microbiol. 59:1591–1601 [DOI] [PubMed] [Google Scholar]
- 75. Steere A. C., Coburn J., Glickstein L. 2004. The emergence of Lyme disease. J. Clin. Invest. 113:1093–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Steere A. C., et al. 1983. The spirochetal etiology of Lyme disease. N. Engl. J. Med. 308:733–740 [DOI] [PubMed] [Google Scholar]
- 77. Stevenson B., Schwan T. G., Rosa P. A. 1995. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect. Immun. 63:4535–4539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Stewart P. E., Thalken R., Bono J. L., Rosa P. 2001. Isolation of a circular plasmid region sufficient for autonomous replication and transformation of infectious Borrelia burgdorferi. Mol. Microbiol. 39:714–721 [DOI] [PubMed] [Google Scholar]
- 79. Strater N., Sherratt D. J., Colloms S. D. 1999. X-ray structure of aminopeptidase A from Escherichia coli and a model for the nucleoprotein complex in Xer site-specific recombination. EMBO J. 18:4513–4522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Strater N., Sun L., Kantrowitz E. R., Lipscomb W. N. 1999. A bicarbonate ion as a general base in the mechanism of peptide hydrolysis by dizinc leucine aminopeptidase. Proc. Natl. Acad. Sci. U. S. A. 96:11151–11155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sultan S. Z., Pitzer J. E., Miller M. R., Motaleb M. A. 2010. Analysis of a Borrelia burgdorferi phosphodiesterase demonstrates a role for cyclic-di-guanosine monophosphate in motility and virulence. Mol. Microbiol. 77:128–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sze C. W., Li C. 2011. Inactivation of bb0184, which encodes carbon storage regulator A, represses the infectivity of Borrelia burgdorferi. Infect. Immun. 79:1270–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tokarz R., Anderton J. M., Katona L. I., Benach J. L. 2004. Combined effects of blood and temperature shift on Borrelia burgdorferi gene expression as determined by whole genome DNA array. Infect. Immun. 72:5419–5432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wang X. G., et al. 2004. Analysis of differences in the functional properties of the substrate binding proteins of the Borrelia burgdorferi oligopeptide permease (Opp) operon. J. Bacteriol. 186:51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wang X. G., Lin B., Kidder J. M., Telford S., Hu L. T. 2002. Effects of environmental changes on expression of the oligopeptide permease (opp) genes of Borrelia burgdorferi. J. Bacteriol. 184:6198–6206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Weerasuriya S., Schneider B. M., Manson M. D. 1998. Chimeric chemoreceptors in Escherichia coli: signaling properties of Tar-Tap and Tap-Tar hybrids. J. Bacteriol. 180:914–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wei B., Shin S., LaPorte D., Wolfe A. J., Romeo T. 2000. Global regulatory mutations in csrA and rpoS cause severe central carbon stress in Escherichia coli in the presence of acetate. J. Bacteriol. 182:1632–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Xu H., et al. 2010. Role of acetyl-phosphate in activation of the Rrp2-RpoN-RpoS pathway in Borrelia burgdorferi. PLoS Pathog. 6:e1001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Yang J., et al. 2008. Bicarbonate-mediated transcriptional activation of divergent operons by the virulence regulatory protein, RegA, from Citrobacter rodentium. Mol. Microbiol. 68:314–327 [DOI] [PubMed] [Google Scholar]
- 90. Yang X., et al. 2000. Interdependence of environmental factors influencing reciprocal patterns of gene expression in virulent Borrelia burgdorferi. Mol. Microbiol. 37:1470–1479 [DOI] [PubMed] [Google Scholar]
- 91. Yang X., Popova T. G., Goldberg M. S., Norgard M. V. 2001. Influence of cultivation media on genetic regulatory patterns in Borrelia burgdorferi. Infect. Immun. 69:4159–4163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Yang X. F., Alani S. M., Norgard M. V. 2003. The response regulator Rrp2 is essential for the expression of major membrane lipoproteins in Borrelia burgdorferi. Proc. Natl. Acad. Sci. U. S. A. 100:11001–11006 [DOI] [PMC free article] [PubMed] [Google Scholar]