Abstract
Streptococcus pyogenes (group A streptococcus [GAS]) responds to environmental changes in a manner that results in an adaptive regulation of the transcriptome. The objective of the present study was to understand how two global transcriptional regulators, CodY and CovRS, coordinate the transcriptional network in S. pyogenes. Results from expression microarray data and quantitative reverse transcription-PCR (qRT-PCR) showed that the global regulator CodY controls the expression of about 250 genes, or about 17% of the genome of strain NZ131. Additionally, the codY gene was shown to be negatively autoregulated, with its protein binding directly to the promoter region with a CodY binding site. In further studies, the influence of codY, covRS, and codY-covRS mutations on gene expression was analyzed in growth phase-dependent conditions using C medium, reported to mimic nutritional abundance and famine conditions similar to those found during host GAS infection. Additional biological experiments of several virulence phenotypes, including pilin production, biofilm formation, and NAD glycohydrolase activity, demonstrated the role that both CodY and CovRS play in their regulation. Correlation analysis of the overall data revealed that, in exponentially growing cells, CodY and CovRS act in opposite directions, with CodY stimulating and CovRS repressing a substantial fraction of the core genome, including many virulence factors. This is the first report of counteractive balancing of transcriptome expression by global transcription regulators and provides important insight into how GAS modulates gene expression by integrating important extracellular and intracellular information.
INTRODUCTION
Bacterial infection is a dynamic process requiring coordination of virulence factor gene expression. This task is achieved at the cellular level by a hierarchical transcriptional network control. An epistatic regulator usually controls the expression of several virulence-associated genes of a transcriptional network as a response to changes in the environment. These changes are commonly recognized by specific signal transduction systems able to process an intra- or extracellular stimulus (42, 50, 61). Once a specific stimulus is perceived, the signal is transferred to an intracellular transcription regulator, thus enabling the cell to respond with adequate changes in the transcriptional profile (5, 45). A signal transduction system usually responds to only one specific stimulus, but the cellular response to a changing host environment might require integration of several stimuli. The cell therefore faces the challenge of coordinating several transcriptional networks (33).
One well-studied regulatory system responding to environmental changes during the course of infection is the covRS (csrRS) two-component signal (TCS) transduction regulatory systems in the group A streptococcus (GAS) Streptococcus pyogenes (14, 22, 35). GAS causes a wide range of strictly human diseases from more superficial, self-limiting infections such as pharyngitis to life-threatening invasive diseases like toxic shock syndrome and necrotizing fasciitis (16, 20). CovRS directly regulates the expression of major virulence factor genes and, overall, about 15% of the S. pyogenes genome (24). The operon organization of covRS indicates a typical TCS: CovS being the sensor kinase, which upon stimulus perception and subsequent autophosphorylation can transfer the phosphoryl group to the receiver domain of CovR, increasing its affinity to specific DNA binding sites (60). In addition, the individual components seem to take on regulatory functions independent of the presence of the cognate partner. For example, CovR can still exert some of its regulatory functions even in the absence of CovS. Transphosphorylation by other TCSs or acetyl phosphate has been discussed as a potential mechanism but not corroborated by in vivo evidence. The fact that CovS can act as a phosphatase increases the complexity of this regulatory system. Environmental stresses can lead to dephosphorylation of CovR-P by CovS and enable expression of CovR-P-repressed genes required to adapt to the respective stress conditions (reviewed in reference 14). Interestingly, there is evidence that the CovRS system undergoes mutation during the course of infection, thus rendering the CovS part inactive (21, 31, 63). Kansal et al. recently provided genetic evidence that a covS mutation is responsible for the development of a hypervirulence phenotype using a mouse model of infection (31). Under these conditions, the CovS mutation seems to be beneficial for GAS invasion and virulence. This hypervirulence phenotype has even more profound consequences, since it may be associated with a resurgence of invasive disease by GAS of a new M type 1 strain over the last 3 decades (4). In addition to its regulatory function, CovRS might be a major control point for the evolutionary development of invasive strains.
Several genes regulated by CovRS are under the control of additional global regulators. With the occurrence of CovS mutations during invasive infection, those regulators might become more important and shift the gene regulation network response to a different set of environmental stimuli. One of the global regulators overlapping in regulation with CovRS is CodY (38, 57). Our previous work provided evidence that CovRS and CodY show a wide overlap of action in sharing multiple virulence genes as direct or indirect targets (39). For example, the has operon (encoding proteins required for the synthesis of the hyaluronic acid capsule) or ska gene (encoding streptokinase) among others are controlled by both transcriptional regulators.
The essential role of CodY in the relA-independent response to branched-chain amino acid (BCAA) starvation in GAS was first demonstrated for the oligopeptide permease operon opp (39). During amino acid deprivation, this operon is strongly upregulated, most likely to replenish intracellular levels of amino acids after pepB-dependent processing of oligopeptides; pepB is also upregulated under starvation conditions. Further examination identified CodY as a pleiotropic transcriptional regulator affecting the expression of additional genes (38). The CodY homologs in Lactococcus lactis and Bacillus subtilis have been shown to respond to BCAA in a similar manner, pointing to the importance of this regulator under starvation conditions in Gram-positive bacteria (6, 27, 28). Direct interaction of a conserved domain of CodY with BCAA has recently been confirmed in B. subtilis (34), making it very likely that GAS CodY functions in a similar way.
The fact that CodY influences the expression of several other transcriptional regulators, including covRS, suggests that the CodY regulatory network may interfere with the control of virulence development during host invasion controlled by CovRS. Thus, its role might become even more important when GAS cells persist in the invasive state with a mutated CovRS system.
In this report, we investigated the protein-encoding transcriptome controlled by CodY by using expression microarray technology. Furthermore, we investigated the influence of a covRS and a codY-covRS mutation on gene expression to define the gene network controlled by both global regulators. Cells were subjected to different growth conditions to mimic nutritional abundance and famine conditions, as can be found during host GAS infection. The results of these experiments reveal a counteractive balancing of transcriptome expression by CodY to modulate CovRS-controlled regulation according to the nutritional status of the cell. Additionally, biofilm formation, pilus production, and NAD glycohydrolase activity were phenotypically characterized to demonstrate epistatic and independent regulation by CodY and/or CovRS.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
S. pyogenes strain NZ131 (M serotype 49) was used in this study (43). Routine culturing of S. pyogenes was done in brain heart infusion (BHI) broth (Difco) or on BHI agar plates with incubation at 37°C in sealed tubes without agitation or in candle jars when cells were grown on agar plates. Growth of S. pyogenes for Western blotting, RNA preparation for microarray, and real-time PCR used C medium, which was reported to mimic in vivo gene expression (36). Briefly, cells from frozen stocks were incubated on agar plates supplemented with erythromycin (2.5 μg/ml) or spectinomycin (100 μg/ml) for the mutant strains. Several colonies were used to inoculate 5 ml of C medium with or without antibiotics. After overnight culture, 50 ml of C medium was inoculated, and growth was monitored periodically using a spectrophotometer (A600). Cells were grown until mid-exponential (ME) or early stationary (ST) phase and used for the respective experiments when grown in the absence of antibiotics. Escherichia coli strain JM109 was used for cloning procedures and grown in LB broth (Difco) at 37°C aerobically.
Construction of codY1 and covR mutants.
Construction and characteristics of the codY1 and covR mutants (henceforth referred to as codY and covRS, respectively) were described previously (39, 58). Briefly, both are insertion mutations disrupting the wild-type genes at codons 197 and 174, respectively. While codY was nonpolar, covR was polar and also prevented the expression of the downstream covS gene, ruling out the possibility that expression results were confounded by CovS involvement in cross talk with other regulators. The codY-covRS double mutant was constructed by transformation of the codY mutant (erythromycin resistant at 2.5 μg/ml), with plasmid pFC1 used to disrupt covR (39), selecting transformants for resistance to 100 μg/ml spectinomycin.
Growth kinetics.
The growth of wild-type and mutant strains was monitored using a Bioscreen C analyzer, version 2.4 (Oy Growth Curves AB Ltd., Finland), which measures the turbidity in multiple cultures in parallel. Growth kinetics were monitored at 37°C aerobically in C medium.
RNA isolation, real-time qRT-PCR.
Overnight cultures of S. pyogenes strains were diluted 1:40 in fresh C medium and grown at 37°C without shaking to the desired growth phase. Streptococcal cells were harvested by centrifugation (5,000 × g, 5 min, 4°C). Cell pellets were resuspended in TRIzol (Invitrogen) and stored at −80°C. To isolate RNA, cells were disrupted three times for 30 s each using Lysing Matrix B (MP Biomedicals, Solon, OH) in a FastPrep FP210 homogenizer (Thermo Scientific). Total RNA isolation was carried out according to the manufacturer's instructions for isolation of total RNA using TRIzol (Invitrogen). RNA samples were treated with Turbo DNase (Ambion) to remove traces of chromosomal DNA. RNeasey MiniElute cleanup kit (Qiagen) was used to purify RNA samples after DNase treatment. cDNA was synthesized from 1 μg of total RNA by using the iScript cDNA synthesis kit (Bio-Rad). Quantitative real-time PCR (qRT-PCR) was carried out as described previously (39). Using the comparative threshold cycle (CT) method with fluorescein-spiked SYBR green as fluorophore (Bio-Rad, Hercules, CA) and the gyrA gene as an endogenous reference, fold changes (FC) of the mutant genes of interest were determined relative to the calibrator cDNA produced from wild-type NZ131 grown under identical conditions applying the ΔΔCT method. For the 68 selected genes of interest, qRT-PCR was performed at least in duplicate on RNA purified from 2 to 4 independently grown cultures, with oligonucleotide primers as specified in Table S1 in the supplemental material. To assess the significance of FC comparisons, the relative expression software tool (REST) (48) was applied, considering P values of ≤0.05 significant (see Table S2 in the supplemental material).
Microarray.
A whole-genome custom GeneChip antisense expression microarray was designed in collaboration with Affymetrix (Santa Clara, CA) essentially as described earlier for Streptococcus mutans (2). The microarray protocol described was also followed as described previously (2). Briefly, RNA extraction was performed and followed by DNase I treatment to remove traces of chromosomal DNA. Total RNA (15 μg) was used for subsequent cDNA synthesis with SuperScript II reverse transcriptase (Invitrogen, Carlsbad, CA). cDNA was fragmented with Roche DNase I (0.06 U per 1 μg of cDNA) at 37°C for 10 min and fragmentation evaluated using the Bioanalyzer 6000 (Agilent Technologies), followed by labeling with biotin-ddUTP using the BioArray terminal labeling kit (Enzo, New York, NY). Hybridization, washing, and scanning of the custom S. pyogenes microarrays were performed according to the procedures described by Affymetrix. Microarray experiments were repeated 5 times using independently grown cultures on different days to generate reproducible and statistically significant data.
Microarray data analysis.
Microarray data processing and analysis employed the GeneChip operating software (GCOS) version 1.4 analysis program (Affymetrix). The data sets were compared using GCOS batch analyses. Normalization of all probe sets was done by GCOS.
Significance analysis of microarrays.
The signal log2 ratios generated by the analysis of the comparison of the mutant to the wild type from all five independent experiments were converted to fold changes and subjected to significance analysis of microarrays (SAM) (65), using medium centering of the arrays for normalization of the data from different experiments. The one-class response type of the software was applied to test whether the mean gene expression of the mutant differed from that of the wild type. Missing data identified by absent (A) calls in the comparison analysis were imputed by SAM using K-Nearest-Neighbor averaging (64), offered by the software (65).
Biofilm formation and quantification.
Biofilm formation was measured using a modification of the crystal violet (CV) microtiter assay as previously reported (3). In brief, microtiter wells (Falcon Microtest 96; Becton Dickinson) were inoculated from overnight C medium cultures diluted 1:60 in fresh C medium. The cells were grown for 16 h, and the medium was removed by inverting the dish with shaking. The remaining cells were stained with 150 μl per well of CV (2.3% [wt/vol]; Accustain crystal violet solution; Sigma Diagnostics) for 15 min, and the microtiter dish was subsequently washed twice with water and air dried. Biofilms were solubilized with 150 μl of 95% ethanol per well and quantified by measuring the released CV stain in 100 μl at 570 nm with a microplate reader (model 680; Bio-Rad).
CLSM.
Biofilms for confocal laser scanning microscopy (CLSM) imaging were essentially grown as described above. The cell suspensions were inoculated into the Lab-TekII chamber slide system (Nalge Nunc International, Naperville, IL), where the objective slide was replaced with a thin cover slide to accommodate the working distance of the microscope objective. Biofilms were grown for 6 h and 18 h at 37°C aerobically. Cells were stained with CellTracker Orange CMTMR (Molecular Probes, Eugene, OR) according to the manufacturer's recommendations. CLSM was performed using an LSM-510META laser scanning confocal microscope (Carl Zeiss, Jena, Germany) equipped with detectors and filter sets for visualizing red fluorescence. Images were obtained with a 63× water (1.2 numerical aperture [NA]) objective.
Preparation of cell wall fractions and Western blot analysis.
S. pyogenes cells were grown in C medium. After growth to mid-exponential phase (optical density at 600 nm [OD600] = 0.4), cells were collected from 50 ml bacterial culture by centrifugation (5,000 × g, 10 min). Cell pellets were washed twice in phosphate-buffered saline (PBS) and resuspended in 1 ml PBS. Cells were disrupted by bead beating with zirconia/silica beads in a FastPrep system. After bead beating, the mixture was briefly centrifuged (15,000 × g, 5 min) to precipitate cell debris and zirconia/silica beads. The supernatant fluid, which contained the intracellular fraction of S. pyogenes proteins, was transferred to a clean tube and frozen at −80°C immediately. The cell debris, which contained the cell wall fraction of S. pyogenes proteins, was washed three times with PBS to remove any residual intracellular proteins. The cell debris was then suspended in 100 μl lysis buffer (10 units/μl mutanolysin in 0.1 M potassium phosphate buffer, pH 6.2) and incubated at 37°C for 1 h to release cell wall-anchored proteins. After incubation, the cell debris was centrifuged again and the supernatant was saved at −80°C for further analysis.
For Western blotting, proteins were separated by SDS-polyacrylamide gel electrophoresis through a 10% gel. Proteins were transferred to nitrocellulose membranes. The membranes were blocked in 5% skim milk for 1 h, incubated overnight with mouse anti-FctA antiserum diluted 1:2,000 in 3% bovine serum albumin (BSA), washed three times in PBS containing 0.2% Tween 20 (PBST), and then incubated for 1 h with horseradish peroxidase-conjugated goat anti-mouse secondary antibody (Pierce) diluted 1:100. Following the washing steps, the membranes were developed with ECL Western blot reagents (Pierce).
NADase assay.
NADase activity was determined as previously described by Bricker et al. (10). Briefly, a bacterial supernatant was obtained from a mid-exponential-phase GAS culture by centrifugation at 10,000 × g for 5 min. Supernatant fluids were serially diluted 2-fold in a 96-well microtiter plate. NAD+ (Sigma) in PBS was added to each well to a final concentration of 0.67 mM. The plate was incubated at 37°C for 1 h. NaOH was then added to 2 N, and the plates were incubated in the dark at room temperature for 1 h. The reactions were visualized by exciting the samples with 360 nm UV light. Results were reported as the greatest dilution of supernatant that exhibited no fluorescence, indicating that NADase had converted all fluorescent NAD+ substrate to nonfluorescent products.
Overexpression of S. pyogenes CodY in E. coli and purification of CodY.
The codY gene was PCR amplified with primers CodY-Bam-Fw (5′-AAAAAAGGATCCATGCCTAACTTATTAG-3′) and CodY-Hind-Rv (5′-AAAAAAAAGCTTTTAAAATTCTTTTAATTT-3′). The PCR product was cloned into the BamHI/HindIII sites of pQE30, a vector for expression of N-terminal His6-tagged proteins (Qiagen). The resulting plasmid, namely, pQE-codY, was transformed into E. coli BL21. The codY coding sequence was confirmed by sequencing. Purification of His6-CodY was performed by means of Ni affinity chromatography (Invitrogen). Imidazole was removed by passing the eluate through a PD-10 desalting column (Amersham Biosciences). The purified protein was stored at −80°C until further use. The preparation was free of contaminating proteins, as determined by Coomassie blue staining of an SDS-PAGE gel. The protein concentration was 1 mg/ml, as determined by the bicinchoninic acid assay (Bio-Rad).
EMSA.
Gel mobility shift assay was performed essentially as described previously (18), with slight modification. Briefly, the 150-bp upstream region of the codY gene was PCR amplified with 5′ biotin-labeled primers PcodY-Fw (5′-AACAAGCTAGTGCTTATCTCCTTT-3′) and PcodY-Rv (5′-TCCTTTTCCTTTGAATTATTTGC-3′). One femtomole biotin-labeled DNA probes was added to 10 μl reaction mixture containing the binding buffer and purified CodY protein at a concentration of 0, 7, 13, 26, 53, 105, 210, 420, or 840 ng per reaction. The binding buffer was composed of 10 mM Tris-HCl (pH 7.5), 5% (vol/vol) glycerol, 5 mM MgCl2, 250 mM KCl, 1 mM dithiothreitol, and 50 μg/ml poly(dI-dC). Immediately after incubation for 20 min at room temperature, samples were loaded onto a 5% nondenaturing polyacrylamide gel. Gel electrophoresis was performed at 100 V for 60 min in 0.5× Tris-borate-EDTA (TBE) buffer. The binding reaction was transferred to a positively charged nylon membrane in a semidry transfer cell (Bio-Rad), and DNA was cross-linked to the membrane in a UV cross-linker (Stratagene). A LightShift chemiluminescent electrophoretic mobility shift assay (EMSA) kit (Pierce) was used for the detection of biotin-labeled DNA probes.
Microarray data accession numbers.
MIAME-compliant microarray data are available at the National Center for Biotechnology Information Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo) through accession number GSE26590.
RESULTS
Growth characteristics of covRS and codY mutants.
In agreement with earlier results obtained with blood cultures of strain NZ131 (38), the wild-type and the codY mutant strain showed similar generation times (71 ± 1 min versus 74 ± 2 min) in batch cultures. The covRS mutation decreased the final growth yield slightly, and the doubling time increased to 101 ± 3 min (data not shown). This result is similar to the growth impairment reported for a covRS mutant serotype M6 strain (22). The double mutant behaved similarly to the covRS mutant (96 ± 3 min). These observations suggest that the absence of CovRS imposed a stress situation for S. pyogenes, resulting in growth retardation under laboratory conditions.
Expression profile of codY and covRS in serotype M49.
To determine expression of codY and covRS in serotype M49 wild-type strain NZ131 over time, cDNA was synthesized from mRNA isolated during growth of batch cultures in C medium. The expression of covRS and codY was maximal during early logarithmic phase and decreased over time, with the lowest transcript levels measured in stationary cells (data not shown).
Molecular mechanism of CodY gene regulation.
While a detailed mechanistic analysis of CovR action on gene regulation has been performed (15), the molecular mechanism of CodY gene regulation activity in S. pyogenes has not been determined before. In silico analysis of the NZ131 genome sequence identified 15 potential binding sites for CodY (38) that were analogous to the high-affinity binding site for CodY identified in Lactococcus lactis (18). To verify regulation through DNA-promoter interactions, a C-terminal fusion protein including a 6× histidine tag was constructed (CodY-His6), expressed in E. coli, and purified. The purified CodY-His6 was used to test for DNA binding ability with EMSA using the codY promoter. As demonstrated in Fig. 1 A, increasing amounts of CodY were able to cause a shift in mobility of the codY promoter region but did not affect mobility of an unrelated DNA sequence (Fig. 1B). This result demonstrates that CodY is able to exert its regulatory function upon direct binding to DNA with a putative CodY binding box upstream of the open reading frame.
Fig. 1.
CodY-DNA binding assay using EMSA. (A) CodY was incubated at increasing concentrations (0, 7, 13, 26, 53, 105, 210, 420, or 840 ng per reaction) with the codYp including the predicted CodY-binding site. (B) Incubation of codYp and a DNA fragment not encoding a CodY binding site, sitBp from Streptococcus mutans, with and without CodY (250 ng).
Gene expression profile of the serotype M49 wild type and codY, covRS, and codY-covRS mutants.
Microarray analysis was performed to determine the gene expression regulatory network controlled by CodY and CovRS. Isogenic mutants were constructed to compare the gene expression profile in the wild type and the codY, covRS, and codY-covRS mutants. The transcriptome was analyzed during mid-exponential (ME) and stationary (ST) phases of growth, covering growth stages of high and low codY and covRS expression. To identify significantly changed genes, SAM software was used to perform data analysis using the one-class function. The SAM output generates a list of Δ values with false discovery rates (FDR). Table 1 lists example output Δ values for ME and ST cells with the wild type compared to the codY, covRS, and codY-covRS mutants; significantly changed genes should be identified by Δ values yielding low FDRs. Most notably, when comparing the ME and ST analysis, it is obvious that only the ME phase cells gave a high number of significantly changed genes yielding low FDRs. ST cells increase in FDRs up to 20% when a comparable number of differentially expressed genes were taken into consideration (Table 1). When using low FDR for ME cells, the number of genes yielding significant changes in expression was calculated to be 261 for the wild type versus the codY mutant, 305 for the wild type versus the covRS mutant, and 250 for the wild type versus the codY-covRS mutant. The number of significantly changed genes of 305 (representing about 17% of the genome) in the analysis of the wild type versus the covRS mutant is in agreement with previously published microarray results, demonstrating that about 15% of the genes are differentially expressed in a covR mutant (24). For ST phase cells, 244 genes for the wild type versus the codY mutant, 143 for the wild type versus the covRS mutant, and 179 for the wild type versus the codY-covRS mutant were significantly changed in expression (data not shown). Taking into consideration that codY and covRS are expressed significantly more in ME cells, the expression control of the gene network by both regulators comprises about 15% to 17% of genes in actively growing NZ131 cells.
Table 1.
Example Δ values generated by SAM of wild-type cells compared to codY, covRS, and codY-covRS mutant strains from ME and ST phases
| Correlation and phase | Δ value | No. of genes called | Median false positive rate (%) | False discovery rate (%) |
|---|---|---|---|---|
| Wild type vs codY mutant, ME | 0.97 | 945 | 90.1 | 9.53 |
| 1.54 | 515 | 13.8 | 2.68 | |
| 2.33 | 250 | 3.1 | 1.24 | |
| Wild type vs covRS mutant, ME | 0.97 | 784 | 46.9 | 5.98 |
| 1.52 | 496 | 5.1 | 1.03 | |
| 2.19 | 305 | 0.86 | 0.28 | |
| Wild type vs codY-covRS mutant, ME | 0.94 | 305 | 11 | 3.61 |
| 1.02 | 261 | 7 | 2.68 | |
| 1.93 | 164 | 0.7 | 0.43 | |
| Wild type vs codY mutant, ST | 0.32 | 655 | 318 | 48.55 |
| 0.45 | 244 | 52.3 | 21.43 | |
| 0.97 | 61 | 0.71 | 1.16 | |
| Wild type vs covRS mutant, ST | 0.1 | 182 | 71.77 | 39.43 |
| 0.12 | 143 | 33.52 | 23.44 | |
| 0.25 | 46 | 0.85 | 1.85 | |
| Wild type vs codY-covRS mutant, ST | 0.19 | 428 | 171.7 | 40.12 |
| 0.24 | 179 | 35.94 | 20.08 | |
| 0.36 | 66 | 2.8 | 4.24 |
Validation of microarray data by qRT-PCR.
Sixty-eight genes, including regulators, virulence factors, transporters, and metabolic enzyme genes were subjected to qRT-PCR. The averages of their relative transcript amounts were used in correlation analyses, with corresponding microarray data averaged from RNA isolated from five independent ME and ST phase cultures grown in unmodified C medium. Figure 2 and Table S2 in the supplemental material show that the great majority of these genes showed positive correlations between data obtained with the two techniques. These correlations were particularly strong for all three mutants grown to ME phase but generally weaker, although still highly significant, for ST phase cultures. Loss of steady-state growth conditions along with low expression levels in the ST phase were associated with increased biological variability and may be part of the reason for reduced correlation strength in ST phase conditions. In general, statistical significance was often lost for genes showing low differential expression values.
Fig. 2.
Correlation analysis of qRT-PCR and DNA microarrays. Correlation of qRT-PCR and DNA microarray assays in ME and ST phase cells of the indicated mutants, including a selected 68-gene set. The fold changes in transcript levels obtained by both methods for ME and ST phase cells were log transformed, and the values were plotted against each other to evaluate their correlation.
Elucidation of the global gene transcription profiles using correlation analysis.
One of the most important results of this study concerns the relationship between the actions of CodY and CovRS. There was a clear negative correlation between the actions of these two regulators in ME phase cells when considering the selected 68-gene set, and this was evident for both the PCR data and the array data (Table 2). The observation that the correlation continued to be significantly negative in the double mutant suggests that, overall, the repressive action of CovRS was stronger than the expression-stimulating action of CodY. This is exemplified by a comparison of selected virulence genes in the respective mutant background (Fig. 3). A mutation in CovRS has an overall stronger effect on the fold difference in gene expression compared to the wild type than the CodY mutant.
Table 2.
Negative correlation between the action of CodY and CovRS in ME phase cells considering a 68-gene set in qRT-PCR and microarray assays
| Technique | Correlation coefficient r(66) | Correlation P value | Regression line equation |
|---|---|---|---|
| qRT-PCR, codY mutant vs covRS mutant | −0.57 | <0.0001 | y = −0.79x + 0.57 |
| qRT-PCR, codY mutant vs codY-covRS mutant | −0.29 | 0.0170 | y = −0.39x + 1.01 |
| Array, codY mutant vs covRS mutant | −0.61 | <0.0001 | y = −0.73x + 0.68 |
| Array, codY mutant vs codY-covRS mutant | −0.29 | 0.0182 | y = −0.41x + 0.70 |
Fig. 3.
Negative correlation of virulence gene transcript levels between the codY and covRS mutants. The expression fold changes were determined by qRT-PCR. Only virulence genes are presented with a significant change, as determined with the relative expression software tool (REST). P < 0.05.
It is possible that these observations were biased by the selection of the particular 68-gene set employed in this analysis. Therefore, a whole-genome approach was taken using the microarray data for all genes of the core genome, totaling 1,605 genes and excluding the three phage genomes. As shown in Fig. 4 for ME phase cells, the correlation between the action of CodY and CovRS continued to be significantly negative, although the r value was less negative than that observed with the 68-gene set [r(1603) = −0.40 compared to r(66) = −0.57]. Furthermore, the negative correlation between the actions of the two regulatory systems on the expression of the core genome was again relieved in the double mutant but stayed slightly negative (Fig. 4). It is also possible that inclusion in this analysis of many of the genes of the core genome, the expression of which was insignificantly influenced by the regulators, might lessen the physiological meaning of the observed opposite action of the two regulatory systems. To obviate this concern for genes to be included in the analysis, log2 ratio cutoffs of I ≥ 0.8 and D ≤ −0.8 were used from the comparison analysis of the arrays. As shown in Fig. 4 for ME phase cells, a strong negative correlation was maintained, with r values very similar to those found in the analysis of the 68-gene set (Table 2 and Fig. 4). Thus, the principal conclusion is that CodY and CovRS act on common target genes in opposite directions, with CodY stimulating and CovRS repressing a substantial fraction of the core genome, including many virulence factors.
Fig. 4.
Correlation analysis of CodY and CovRS action using whole-genome array data comparison and a subset of 68 selected genes. The correlation is based on gene expression profiles determined for cells grown to ME and ST phase. (i) Correlation analysis of ME and ST cells based on microarray data using log-transformed values from the covRS and codY mutants; (ii) correlation analysis of ME and ST cells based on microarray data using log-transformed values from the codY-covRS and codY mutants; (iii) correlation analysis of ME and ST cells based on qRT-PCR data using log-transformed values from the covRS and codY mutants; (iv) correlation analysis of ME and ST cells based on qRT-PCR data using log-transformed values from the codY-covRS and codY mutant.
To investigate whether or not ST phase cells behaved in a similar fashion to those in the ME phase, the former were subjected to correlation analyses identical to those reported above. Figure 4 shows that the highly significant negative correlation between the global actions of the two systems in ME phase ceased to operate in ST phase cells, an observation consistent with essentially all analytical parameters. In particular, the low r values observed under expression cutoff thresholds did not reach significance. This observation would appear to be consistent with the notion that the action of both systems becomes weaker, or ceases, in the stationary phase. Nevertheless, CodY continued to act to some extent in the ST phase, as seen by the shift of correlation in the positive direction in the double mutant (data not shown). In summary, CodY and CovRS were found to interact in a growth phase-dependent fashion, their balancing effect on core genome expression being largely confined to actively growing cells. When the three phage genomes of NZ131 were included in the whole-genome correlation analyses, extremely low degrees of correlation were observed under essentially all analytical parameters, with 50% of the P values not reaching significance (Table 3). The smoothing effect of the phage genes on the negative correlations in ME phase cells appeared to be attributable to their general repression in both the codY and the covRS mutants.
Table 3.
Correlation analysis between the action of CodY and CovRS using the whole-genome approach with phage genomes included
| Correlation | Growth phase | r (df)a | P value | Regression line equation |
|---|---|---|---|---|
| codY mutant vs covRS mutant | ME | −0.05 (1743) | 0.0535 | y = −0.07x + 0.07 |
| codY mutant vs codY-covRS mutant | ME | 0.14 (1743) | <0.0001 | y = 0.24x − 0.15 |
| codY mutant vs covRS mutant | ME | −0.01 (112) | 0.9124 | y = −0.01x − 0.13 |
| codY mutant vs codY-covRS mutant | ME | 0.26 (112) | 0.0048 | y = 0.40x + 0.10 |
| codY mutant vs covRS mutant | ST | 0.08 (1743) | 0.0016 | y = 0.15x + 1.00 |
| codY mutant vs codY-covRS mutant | ST | 0.16 (1743) | <0.0001 | y = 0.26x + 0.70 |
| codY mutant vs covRS mutant | ST | 0.03 (112) | 0.7310 | y = 0.05x + 1.19 |
| codY mutant vs codY-covRS mutant | ST | 0.17 (112) | 0.0769 | y = 0.24x + 1.18 |
Degree of freedom with all genes included(df = 1,743) or gene expression ratios cut off at I ≥ 0.8 and D ≤ −0.8(df = 112).
Pilin production.
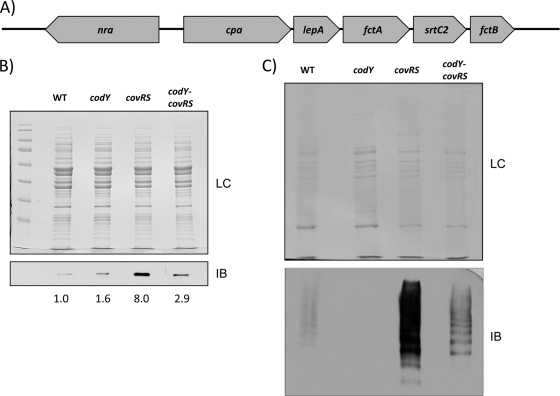
The microarray analysis showed a 2- to 3-fold increase in fctA expression in the FCT3 pathogenicity region in the covRS and codY-covRS mutants, while the codY mutant was only moderately affected (data not shown). The specific fibronectin-binding, collagen-binding, T-antigen (FCT3) chromosomal region (Fig. 5 A) in a serotype M49 strain has been shown to be involved in the formation of pilin structures (8, 46). Pilin cell surface proteins can facilitate the initial attachment process during biofilm formation and the attachment process to human cells and abiotic surfaces in S. pyogenes (41, 46). The fctA gene encodes the major pilin subunit, and to determine if increased fctA expression leads to more FctA abundance, detection of the major pilin subunit was therefore performed with mouse polyclonal antiserum against recombinant FctA (46). Immunoblot analysis with the cytoplasmic fraction for the detection of FctA monomers (about 35 kDA in size) showed an increase in FctA abundance in the cytoplasm of the covRS mutant (Fig. 5B). The FctA abundance in the codY-covRS mutant was also increased but less than in the covRS mutant. Analysis of band pixel intensities approximated the increase for the covRS mutant to be 8-fold compared to that for the wild type, while that for the double mutant increased about 3-fold. Although CodY was found not to influence significantly the expression of fctA, this result shows that CodY diminishes the effect of CovRS in line with its counteractive action, suggesting independent regulation of fctA expression (Fig. 5B).
Fig. 5.
Pilin abundance in wild-type NZ131 and codY, covRS, and codY-covRS mutants. (A) Operon organization of the pilin-encoding FCT3 region. (B) Immunoblot analysis of cytoplasmatic FctA abundance. Pixel intensities of the bands were measured using ImageJ software (NIH). (C) Immunoblotting of cell wall fractions. Shown in panels B and C are the Coomassie-stained loading controls and the immunoblots after reaction with antiserum against FctA. LC, loading control; IB, immunoblot.
The abundance of surface-exposed FctA was assessed by immunoblot analysis with strain NZ131 cell wall fractions. Results from this analysis confirmed the increase in FctA abundance for the covRS mutant, showing that the intracellular increase leads to more FctA surface exposure (Fig. 5C). For the double mutant, a similar decrease was observed compared to the cytoplasmic FctA abundance. Interestingly, codY seemed to promote surface exposure of pilins, since the codY mutant had decreased FctA abundance compared to that of the wild type (Fig. 5C). The immunoblot results suggest that codY and covRS both regulate FCT3 expression, although in a differential mode: while covRS seemed to repress FCT3 expression, codY seemed to have an activating function.
Biofilm adhesion.
Earlier reports provided evidence that S. pyogenes was able to form a pilin-dependent biofilm-like structure on protein-coated and uncoated surfaces (46). Examination of S. pyogenes tissue-associated communities revealed a close resemblance to in vitro biofilm communities (13). In addition, biofilm cells from S. pyogenes reprogram their gene expression pattern compared to that of planktonic cultures (13). All these observations are indicative of the biofilm mode of growth as an important developmental stage for this bacterium. To determine whether increased pilus abundance influenced biofilm formation, biofilm adhesion capabilities of all strains were tested in a microtiter dish assay. After overnight incubation as static cultures, biofilm adhesion was determined. No differences between the wild type and the codY mutant were observed. However, the covRS and covRS-codY mutants increased in the adherent biofilm mass, and this difference was statistically significant (Fig. 6).
Fig. 6.
Quantification of attached biomass using a microtiter plate biofilm assay. The adherence of wild-type NZ131 and codY, covRS, and codY-covRS mutant strains to microtiter plates was quantified with crystal violet staining. Data presented are the averages and standard deviations of results from three independent experiments.
Biofilm architecture.
To further investigate the biofilm phenotype of the covRS mutant, the biofilm architecture was investigated using CLSM imaging. Biofilms were grown as static cultures on a coverslip glass surface. CLSM images were taken at two different growth stages to follow the developmental pattern. Consistent with the adhesion assay in the microtiter assay, the codY mutant was indistinguishable from the wild type at 6 and 18 h (Fig. 7 A). The biofilm phenotypes of the covRS and codY-covRS mutants showed an obvious difference compared to that of the wild type. Most noticeable after 6 h was the arrangement of chains attached to the glass surface. Compared to the wild type, the covRS and codY-covRS mutants seemed to attach evenly over the whole streptococcal chain in an almost ordered fashion. In contrast, only part of the chains of the wild type and the codY mutant seemed to attach to the surface, while the rest appeared to float in the medium (Fig. 7A).
Fig. 7.
CLSM analysis of static biofilm cultures. (A) CLSM images of wild-type NZ131 and codY, covRS, and codY-covRS mutant biofilms grown in the Lab-TekII Chamber SlideTM system for 6 h and 18 h. (B) Enlargement of the 18-h biofilms to visualize individual cell chains. Cells were stained with CellTracker Orange. Scale bar represents 100 μm.
The biofilm appearance changed after further incubation, and some morphological changes to the cells were visible for the covRS mutant. Compared to the 6-h-grown covRS mutant with a well-defined cell shape in the streptococcal chains, cells after 18 h seemed to lose their typical streptococcal morphology. These cells were surrounded by an extracellular component and increased in size. Interestingly, the codY-covRS mutant produced an intermediate phenotype more comparable to that of the wild type (Fig. 7B). These results suggest that biofilm development is influenced by covRS and that codY can counteract the covRS phenotype when cells are in the stationary phase.
NAD glycohydrolase activity.
The NAD glycohydrolase (Nga) is an important extracellular virulence factor (1). This enzyme has the capacity to gain access inside host cells through cytolysin-mediated translocation facilitated by streptolysin O (Slo) (37). Once inside the cytosol of the host cell, Nga exerts its action by modulating host-cell signaling and accelerating cell death (10). The role of covS and covR in the expression control of nga has been established (24, 44), but the influence of a covRS and a codY mutation on Nga activity has not been investigated. Furthermore, nga was differentially expressed during microarray and qRT-PCR analysis (see Table S2 in the supplemental material). Supernatants from cells grown to mid-logarithmic phase were used to determine Nga activity. The codY mutant showed slightly decreased activity compared to that of the wild type. Interestingly, CovRS seemed to repress Nga activity under the tested conditions, since the activity increased about 9-fold in the respective mutant. The codY-covRS double mutant was also increased in Nga activity compared to that of the covRS mutant (Fig. 8). These results suggest an epistatic role of CovRS in the control of Nga activity, confirming earlier reports for nga control by covR.
Fig. 8.
NAD glycohydrolase activity in wild-type NZ131 and codY, covRS, and codY-covRS mutants. The activity is presented relative to the wild-type control, which was set to 1. Presented are averages and standard deviations of results from two independent experiments.
DISCUSSION
In the present study, we sought to understand how two global transcriptional regulators, CodY and CovRS, coordinate the transcriptional network in S. pyogenes. We now demonstrate on a global scale that CodY and CovRS control separate and common targets, with the latter being subject to counteractive balancing of transcriptome expression. This approach is of particular interest for the following two reasons. (i) Bacterial virulence development requires a careful balance between energy-consuming virulence factor production and adaptation to a fluctuating host environment often sparse in nutritional content. Processing multiple environmental information results in a coordinated gene expression response best adapted for the particular infection state. (ii) The importance of individual global regulators might shift during the infection process. Although several regulators of virulence gene expression control have been investigated in great detail, only one particular study of GAS is available that reports how the interaction of global regulators influences gene expression during infection (52). Considering that a cellular response requires changes in the gene network controlled by multiple inputs, studying the effect of just one regulator at a time might miss crucial steps in virulence development of GAS.
CodY is an important global regulator of stationary-phase adaptation in low G-C Gram-positive bacteria, including S. pyogenes (55). CodY in S. pneumoniae has recently been shown to be essential (12), and a knockout mutant reported earlier (29) carried a compensatory mutation, which enabled growth. We did not observe any problems generating the codY mutant employed in these studies; however, we cannot exclude a similar adaptive mutation in the chromosome of NZ131.
The infection process in general challenges cells with changing environmental and host conditions (66, 69). Expression of virulence factor genes presents a significant metabolic burden to the cell, especially when sources of nutrition are fluctuating. CodY could be the key regulator balancing the ability of GAS to express necessary virulence factors and to maintain the metabolic capabilities for successful infection of the host. CodY has been suggested to directly measure the nutritional status of the cell (56). Branched-chain amino acids (BCAA) are known effectors binding to CodY and increasing the affinity to promoter target sequences, as demonstrated in DNA binding experiments in several species (19, 27, 53). The in vivo activity of BCAA and CodY was recently confirmed in Bacillus subtilis engineered to increase the intracellular pool of BCAA (11). The present study demonstrates that CodY controls the expression of about 250 genes, or about 17% of the genome of strain NZ131. The genes of the codY regulon belong to several functional groups, including transcriptional regulators, transporters, and metabolic enzyme genes, and these results are consistent with reports found for other Gram-positive organisms (7, 29). CodY-regulated virulence genes, however, remain highly organism specific, as recently discussed (59). A full description of the CodY regulon will be the subject of a separate publication. The CovRS global regulator is one of the best-studied regulatory systems in GAS (14) and is known to respond to (i) the presence of cationic antimicrobial peptides (23, 26), (ii) low pH, (iii) high temperature, and (iv) changes in ion concentration (like the presence of Mg2+ or Fe) (17, 25). It has been suggested that the main environmental signal is envelope stress (23). A common route of GAS infection is invasion through the pharynx (51), where the cells are challenged with environmental fluctuations affecting not only the pH or ionic composition of the mucosal bathing saliva but also the availability of nutrients (9). Food intake is followed by a sharp decline in pH due to acid production by resident oral lactobacilli (49). GAS requires adaptation to effectively compete with the resident flora and initiate the infection process. This requires the integration of both environmental and metabolic information about the cells' capability to afford the infection process. Global regulators responding to separate environmental and cellular information could thus cooperate or counteract to cope with changing environmental conditions. A cooperative control of the regulatory network active during infection has recently been shown for GAS. Shelburne et al. (52) demonstrated the interaction of catabolite control protein A (CcpA) and CovRS in virulence network regulation. CcpA, a well-characterized global regulator able to monitor extracellular carbohydrate availability (68), also regulates several important virulence and metabolic genes controlled by CovR (52). For example, two important virulence genes are both repressed by CovR and CcpA; i.e., slo, encoding the pore-forming streptolysin O (Slo), and nga, encoding NAD glycohydrolase, which gains access to target cells facilitated by Slo (37). It appears that CovR exerts a greater repressive action on the expression of both genes since a ΔcovR mutant increased its expression about 12-fold for both genes, while a ΔccpA mutant increased expression only about 3-fold. Most importantly, if neither of the regulators were present, the genes increased in expression about 25- to 30-fold, clearly showing that CcpA and CovR have a similar regulatory effect on expression (52). CodY controls both genes in their expression, as we show in our study. Although the influence on the expression is not as dramatic compared to that of the control by CovR and CcpA, it demonstrates clearly how different environmental information is processed by S. pyogenes. Streptolysin O can lyse neighboring host cells, liberating nutrients for S. pyogenes, thus allowing a deeper penetration of underlying tissues (54). The current data suggest that during invasive infection with the occurrence of mutations in CovRS, CovR might not be actively repressing slo and nga. However, both slo and nga are repressed by CovR under noninvasive conditions (52). CcpA might therefore act as the main repressor for both genes. Low carbohydrate availability during the infectious process would therefore lift the repression, leading to an increased production of Slo and Nga. Such a circumstance would be favorable if the cells still have sufficient energy and resources to produce and secrete Slo and Nga. CodY measures the intracellular availability of BCAA (11), representing the major building blocks for successful production of Slo and Nga. If under invasive conditions BCAA levels are adequate, CodY would exert its activating function of Slo and Nga expression, therefore producing both proteins. We have confirmed in this study that the activating effect of CodY on Nga activity in NZ131 was at the protein level (Fig. 8). If the conditions were not permissive for the production of Nga and Slo, e.g., low levels of BCAA, CodY would not activate expression of the genes. Under noninvasive conditions, with an intact repressive function of CovRS, expression of major virulence factor genes would be counterproductive. In agreement with this hypothesis is the observation that CovRS is epistatic in the control of nga and slo expression and Nga protein activity, since the codY-covRS double mutant behaved like the CovRS mutant. This was observed as well for several other virulence factor genes, indicating that S. pyogenes monitors and integrates information about intracellular and extracellular conditions and adjusts the expression of key virulence factors in the most efficient way for the respective environment through CodY (Fig. 9).
Fig. 9.
Counteractive balancing of the transcriptome expression model. In the depicted situation, the cell monitors the extracellular availability of carbohydrate sources essential for all metabolic processes via the phospho-transferase system (PTS) and CcpA, as well as other extracellular host-derived information via CovRS. The cell uses extracellular information combined with the intracellular metabolic capability to adjust genome expression accordingly. If the cell is not able to provide enough amino acids (measured as a low BCAA concentration), CodY counteracts the activating function of CovRS and CcpA on selected genes. CodY therefore balances between costly virulence factor production and cell sustainability. At the same time, acquisition of amino acids is improved to allow gene expression required for successful colonization and persistence once the situation becomes favorable.
The counteractive balancing of transcriptome expression via metabolic-status monitoring of CodY is further supported by the observation that S. pyogenes tries to improve the metabolic status when the intracellular BCAA concentration is low. The acquisition of extracellular BCAA seems to be under the control of CodY, which represses the putative BCAA transporter gene braB and the oligopeptide permease genes oppA and oppB. Thus, if the cellular status would not allow for the activation of CodY-controlled virulence genes and CodY is not associated with BCAA, the repression of braB and oppAB would be lifted. This would eventually result in the acquisition of BCAA and therefore activation of virulence gene expression (Fig. 9).
Besides regulating its own and covRS expression, CodY controls the expression of other important transcriptional regulators. Notably are the two transcriptional regulators belonging to the RofA-like protein family of stand-alone response regulators, RofA and RivR, and the global regulator Mga (33, 42). RofA and RifR control by themselves several genes of the GAS transcriptome. Mga, one of the best-characterized transcriptional regulators, alone might control the expression of up to 10% of the GAS genome (30). In the current model, Mga activates several genes required for colonization, therefore preparing the cells for adherence to the host tissue with subsequent internalization. In this state, the cells are also able to evade the host immune system (33). After infection where the cells persist in the host, RofA and RifR downregulate mga expression and therefore the MgaA regulon. At the same time, RofA and RivR induce genes for persistent host-cell interactions (33). CodY exerts a significant activating function on Mga, RofA, and RivR (see Table S2 in the supplemental material), therefore fine-tuning the transition from a colonization/invasion phenotype into the persistence phenotype of GAS. The complexity of the regulatory function of the GAS genome illustrates that the integration of multiple signals and communication between different regulators is required for successful host colonization.
During this study, we observed that pilus gene expression was influenced by CovRS, since the covRS mutant increased pilus production about 8-fold as determined by the amount of intracellular FctA pilus monomer present. The intracellular increase was also reflected in the actual surface abundance of the exported and assembled pili. We speculated that the increase in surface pili would lead to a biofilm phenotype. As cell appendices, pili are important for the adherence of several bacterial species to host tissue and other surfaces (41, 47, 67) and also have been discussed as important in biofilm formation (32, 40). In agreement with our hypothesis, we detected a significant increase of biomass attached to the polystyrene surface by the covRS mutant in the standard crystal violet biofilm assay. Interestingly, Cho and Caparon and Sugareva et al. reported that individual CovR and CovS mutants in several different M types, including M49, are defective in biofilm formation (13, 62). The mutant we used in this study, defective in both CovR and CovS, might explain this discrepancy. The growth conditions selected to test for biofilm formation varied considerably in growth temperature and medium used between all the studies but were consistent in biofilm formation for the wild type, suggesting that S. pyogenes is able to form biofilms under several different conditions.
Additionally, there is a change in the biofilm architecture as determined by CLSM analysis. After 6 h of biofilm growth, there appeared to be a greater attachment of the cells to the glass surface of the chamber slide used to grow the biofilms. Further incubation resulted in changes in the morphologies of the individual S. pyogenes cells in the covRS mutant, leading to a more fuzzy appearance of the cells. A possible explanation for this phenotype is the increased coating of cells with surface pili. Interestingly, CodY seemed to influence this phenotype since the codY-covRS mutants showed a reversal of the abundance of pilus monomers both intracellularly and on the surface of the cells. This effect seemed time dependent, since the reversal was most obvious with the 18-h biofilms, where cell appearance was more comparable to that of the wild type. Interestingly, although we detected a reduction in pilus abundance in the double mutant, the biofilm phenotype was not affected. A possible explanation for these results is that the crystal violet assay used to study biofilm development tests for both substratum and intercellular adhesion. After a final wash step in this assay, the remaining cells are quantified, and although decreased in abundance, the pili could still provide a major adhesive advantage under the tested condition.
In summary, we have demonstrated a major influence of the transcriptional regulator CodY on global gene expression of S. pyogenes. Both CodY and the major global regulator CovRS regulate several genes. Our main conclusion is that CodY and CovRS act in opposite directions, with CodY stimulating and CovRS repressing a substantial fraction of the core genome, including many virulence factor genes. To our knowledge, this counteractive balancing of transcriptome expression has not been reported before for GAS. These results suggest that virulence gene regulation control in pathogens can be fully appreciated only if all control elements have been identified and their actions investigated in the context of their global regulatory potential.
Supplementary Material
ACKNOWLEDGMENTS
We thank Masanobu Nakata (Osaka University Graduate School of Dentistry) for providing the antiserum against FctA and William M. McShan (University of Oklahoma Health Sciences Center) for designing the expression microarray layout.
Support through NIH/NIDCR grant 4R00DE018400 to J.K. is greatly acknowledged.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
Published ahead of print on 24 June 2011.
REFERENCES
- 1. Ajdic D., McShan W. M., Savic D. J., Gerlach D., Ferretti J. J. 2000. The NAD-glycohydrolase (nga) gene of Streptococcus pyogenes. FEMS Microbiol. Lett. 191:235–241 [DOI] [PubMed] [Google Scholar]
- 2. Ajdic D., Pham V. T. 2007. Global transcriptional analysis of Streptococcus mutans sugar transporters using microarrays. J. Bacteriol. 189:5049–5059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ashby M. T., Kreth J., Soundarajan M., Sivuilu L. S. 2009. Influence of a model human defensive peroxidase system on oral streptococcal antagonism. Microbiology 155:3691–3700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aziz R. K., Kotb M. 2008. Rise and persistence of global M1T1 clone of Streptococcus pyogenes. Emerg. Infect. Dis. 14:1511–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beier D., Gross R. 2006. Regulation of bacterial virulence by two-component systems. Curr. Opin. Microbiol. 9:143–152 [DOI] [PubMed] [Google Scholar]
- 6. Belitsky B. R., Sonenshein A. L. 2008. Genetic and biochemical analysis of CodY-binding sites in Bacillus subtilis. J. Bacteriol. 190:1224–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bennett H. J., et al. 2007. Characterization of relA and codY mutants of Listeria monocytogenes: identification of the CodY regulon and its role in virulence. Mol. Microbiol. 63:1453–1467 [DOI] [PubMed] [Google Scholar]
- 8. Bessen D. E., Kalia A. 2002. Genomic localization of a T serotype locus to a recombinatorial zone encoding extracellular matrix-binding proteins in Streptococcus pyogenes. Infect. Immun. 70:1159–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bowden G. H., Hamilton I. R. 1998. Survival of oral bacteria. Crit. Rev. Oral Biol. Med. 9:54–85 [DOI] [PubMed] [Google Scholar]
- 10. Bricker A. L., Cywes C., Ashbaugh C. D., Wessels M. R. 2002. NAD+-glycohydrolase acts as an intracellular toxin to enhance the extracellular survival of group A streptococci. Mol. Microbiol. 44:257–269 [DOI] [PubMed] [Google Scholar]
- 11. Brinsmade S. R., Kleijn R. J., Sauer U., Sonenshein A. L. 2010. Regulation of CodY activity through modulation of intracellular branched-chain amino acid pools. J. Bacteriol. 192:6357–6368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Caymaris S., et al. 2010. The global nutritional regulator CodY is an essential protein in the human pathogen Streptococcus pneumoniae. Mol. Microbiol. 78:344–360 [DOI] [PubMed] [Google Scholar]
- 13. Cho K. H., Caparon M. G. 2005. Patterns of virulence gene expression differ between biofilm and tissue communities of Streptococcus pyogenes. Mol. Microbiol. 57:1545–1556 [DOI] [PubMed] [Google Scholar]
- 14. Churchward G. 2007. The two faces of Janus: virulence gene regulation by CovR/S in group A streptococci. Mol. Microbiol. 64:34–41 [DOI] [PubMed] [Google Scholar]
- 15. Churchward G., Bates C., Gusa A. A., Stringer V., Scott J. R. 2009. Regulation of streptokinase expression by CovR/S in Streptococcus pyogenes: CovR acts through a single high-affinity binding site. Microbiology 155:566–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cunningham M. W. 2000. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 13:470–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dalton T. L., Scott J. R. 2004. CovS inactivates CovR and is required for growth under conditions of general stress in Streptococcus pyogenes. J. Bacteriol. 186:3928–3937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. den Hengst C. D., et al. 2005. The Lactococcus lactis CodY regulon: identification of a conserved cis-regulatory element. J. Biol. Chem. 280:34332–34342 [DOI] [PubMed] [Google Scholar]
- 19. Dineen S. S., Villapakkam A. C., Nordman J. T., Sonenshein A. L. 2007. Repression of Clostridium difficile toxin gene expression by CodY. Mol. Microbiol. 66:206–219 [DOI] [PubMed] [Google Scholar]
- 20. Dmitriev A. V., Chaussee M. S. 2010. The Streptococcus pyogenes proteome: maps, virulence factors and vaccine candidates. Future Microbiol. 5:1539–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Engleberg N. C., Heath A., Miller A., Rivera C., DiRita V. J. 2001. Spontaneous mutations in the CsrRS two-component regulatory system of Streptococcus pyogenes result in enhanced virulence in a murine model of skin and soft tissue infection. J. Infect. Dis. 183:1043–1054 [DOI] [PubMed] [Google Scholar]
- 22. Federle M. J., McIver K. S., Scott J. R. 1999. A response regulator that represses transcription of several virulence operons in the group A streptococcus. J. Bacteriol. 181:3649–3657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Froehlich B. J., Bates C., Scott J. R. 2009. Streptococcus pyogenes CovRS mediates growth in iron starvation and in the presence of the human cationic antimicrobial peptide LL-37. J. Bacteriol. 191:673–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Graham M. R., et al. 2002. Virulence control in group A streptococcus by a two-component gene regulatory system: global expression profiling and in vivo infection modeling. Proc. Natl. Acad. Sci. U. S. A. 99:13855–13860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gryllos I., et al. 2007. Mg(2+) signalling defines the group A streptococcal CsrRS (CovRS) regulon. Mol. Microbiol. 65:671–683 [DOI] [PubMed] [Google Scholar]
- 26. Gryllos I., et al. 2008. Induction of group A Streptococcus virulence by a human antimicrobial peptide. Proc. Natl. Acad. Sci. U. S. A. 105:16755–16760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guedon E., Serror P., Ehrlich S. D., Renault P., Delorme C. 2001. Pleiotropic transcriptional repressor CodY senses the intracellular pool of branched-chain amino acids in Lactococcus lactis. Mol. Microbiol. 40:1227–1239 [DOI] [PubMed] [Google Scholar]
- 28. Guedon E., Sperandio B., Pons N., Ehrlich S. D., Renault P. 2005. Overall control of nitrogen metabolism in Lactococcus lactis by CodY, and possible models for CodY regulation in Firmicutes. Microbiology 151:3895–3909 [DOI] [PubMed] [Google Scholar]
- 29. Hendriksen W. T., et al. 2008. CodY of Streptococcus pneumoniae: link between nutritional gene regulation and colonization. J. Bacteriol. 190:590–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hondorp E. R., McIver K. S. 2007. The Mga virulence regulon: infection where the grass is greener. Mol. Microbiol. 66:1056–1065 [DOI] [PubMed] [Google Scholar]
- 31. Kansal R. G., et al. 2010. Dissection of the molecular basis for hypervirulence of an in vivo-selected phenotype of the widely disseminated M1T1 strain of group A Streptococcus bacteria. J. Infect. Dis. 201:855–865 [DOI] [PubMed] [Google Scholar]
- 32. Kreikemeyer B., et al. 2010. Genomic organization, structure, regulation and pathogenic role of pilus constituents in major pathogenic streptococci and enterococci. Int. J. Med. Microbiol. 301:240–251 [DOI] [PubMed] [Google Scholar]
- 33. Kreikemeyer B., McIver K. S., Podbielski A. 2003. Virulence factor regulation and regulatory networks in Streptococcus pyogenes and their impact on pathogen-host interactions. Trends Microbiol. 11:224–232 [DOI] [PubMed] [Google Scholar]
- 34. Levdikov V. M., Blagova E., Joseph P., Sonenshein A. L., Wilkinson A. J. 2006. The structure of CodY, a GTP- and isoleucine-responsive regulator of stationary phase and virulence in Gram-positive bacteria. J. Biol. Chem. 281:11366–11373 [DOI] [PubMed] [Google Scholar]
- 35. Levin J. C., Wessels M. R. 1998. Identification of csrR/csrS, a genetic locus that regulates hyaluronic acid capsule synthesis in group A Streptococcus. Mol. Microbiol. 30:209–219 [DOI] [PubMed] [Google Scholar]
- 36. Loughman J. A., Caparon M. 2006. Regulation of SpeB in Streptococcus pyogenes by pH and NaCl: a model for in vivo gene expression. J. Bacteriol. 188:399–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Magassa N., Chandrasekaran S., Caparon M. G. 2010. Streptococcus pyogenes cytolysin-mediated translocation does not require pore formation by streptolysin O. EMBO Rep. 11:400–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Malke H., Ferretti J. J. 2007. CodY-affected transcriptional gene expression of Streptococcus pyogenes during growth in human blood. J. Med. Microbiol. 56:707–714 [DOI] [PubMed] [Google Scholar]
- 39. Malke H., Steiner K., McShan W. M., Ferretti J. J. 2006. Linking the nutritional status of Streptococcus pyogenes to alteration of transcriptional gene expression: the action of CodY and RelA. Int. J. Med. Microbiol. 296:259–275 [DOI] [PubMed] [Google Scholar]
- 40. Mandlik A., Swierczynski A., Das A., Ton-That H. 2008. Pili in Gram-positive bacteria: assembly, involvement in colonization and biofilm development. Trends Microbiol. 16:33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Manetti A. G., et al. 2007. Streptococcus pyogenes pili promote pharyngeal cell adhesion and biofilm formation. Mol. Microbiol. 64:968–983 [DOI] [PubMed] [Google Scholar]
- 42. McIver K. S. 2009. Stand-alone response regulators controlling global virulence networks in streptococcus pyogenes. Contrib. Microbiol. 16:103–119 [DOI] [PubMed] [Google Scholar]
- 43. McShan W. M., et al. 2008. Genome sequence of a nephritogenic and highly transformable M49 strain of Streptococcus pyogenes. J. Bacteriol. 190:7773–7785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Minami M., et al. 2010. Clindamycin-induced CovS-mediated regulation of the production of virulent exoproteins streptolysin O, NAD glycohydrolase, and streptokinase in Streptococcus pyogenes. Antimicrob. Agents Chemother. 54:98–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mitrophanov A. Y., Groisman E. A. 2008. Signal integration in bacterial two-component regulatory systems. Genes Dev. 22:2601–2611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nakata M., et al. 2009. Mode of expression and functional characterization of FCT-3 pilus region-encoded proteins in Streptococcus pyogenes serotype M49. Infect. Immun. 77:32–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Okahashi N., et al. 2010. Pili of oral Streptococcus sanguinis bind to fibronectin and contribute to cell adhesion. Biochem. Biophys. Res. Commun. 391:1192–1196 [DOI] [PubMed] [Google Scholar]
- 48. Pfaffl M. W., Horgan G. W., Dempfle L. 2002. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 30:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schachtele C. F., Jensen M. E. 1982. Comparison of methods for monitoring changes in the pH of human dental plaque. J. Dent. Res. 61:1117–1125 [DOI] [PubMed] [Google Scholar]
- 50. Seshasayee A. S., Bertone P., Fraser G. M., Luscombe N. M. 2006. Transcriptional regulatory networks in bacteria: from input signals to output responses. Curr. Opin. Microbiol. 9:511–519 [DOI] [PubMed] [Google Scholar]
- 51. Shaikh N., Leonard E., Martin J. M. 2010. Prevalence of streptococcal pharyngitis and streptococcal carriage in children: a meta-analysis. Pediatrics 126:e557–e564 [DOI] [PubMed] [Google Scholar]
- 52. Shelburne S. A., et al. 2010. A combination of independent transcriptional regulators shapes bacterial virulence gene expression during infection. PLoS Pathog. 6:e1000817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shivers R. P., Sonenshein A. L. 2004. Activation of the Bacillus subtilis global regulator CodY by direct interaction with branched-chain amino acids. Mol. Microbiol. 53:599–611 [DOI] [PubMed] [Google Scholar]
- 54. Sierig G., Cywes C., Wessels M. R., Ashbaugh C. D. 2003. Cytotoxic effects of streptolysin O and streptolysin S enhance the virulence of poorly encapsulated group a streptococci. Infect. Immun. 71:446–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sonenshein A. L. 2005. CodY, a global regulator of stationary phase and virulence in Gram-positive bacteria. Curr. Opin. Microbiol. 8:203–207 [DOI] [PubMed] [Google Scholar]
- 56. Sonenshein A. L. 2007. Control of key metabolic intersections in Bacillus subtilis. Nat. Rev. Microbiol. 5:917–927 [DOI] [PubMed] [Google Scholar]
- 57. Steiner K., Malke H. 2002. Dual control of streptokinase and streptolysin S production by the covRS and fasCAX two-component regulators in Streptococcus dysgalactiae subsp. equisimilis. Infect. Immun. 70:3627–3636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Steiner K., Malke H. 2000. Life in protein-rich environments: the relA-independent response of Streptococcus pyogenes to amino acid starvation. Mol. Microbiol. 38:1004–1016 [DOI] [PubMed] [Google Scholar]
- 59. Stenz L., et al. 2011. The CodY pleiotropic repressor controls virulence in Gram-positive pathogens. FEMS Immunol. Med. Microbiol. 62:123–139 [DOI] [PubMed] [Google Scholar]
- 60. Stock A. M., Robinson V. L., Goudreau P. N. 2000. Two-component signal transduction. Annu. Rev. Biochem. 69:183–215 [DOI] [PubMed] [Google Scholar]
- 61. Stock J. B., Stock A. M., Mottonen J. M. 1990. Signal transduction in bacteria. Nature 344:395–400 [DOI] [PubMed] [Google Scholar]
- 62. Sugareva V., et al. 2010. Serotype- and strain- dependent contribution of the sensor kinase CovS of the CovRS two-component system to Streptococcus pyogenes pathogenesis. BMC Microbiol. 10:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sumby P., Whitney A. R., Graviss E. A., DeLeo F. R., Musser J. M. 2006. Genome-wide analysis of group A streptococci reveals a mutation that modulates global phenotype and disease specificity. PLoS Pathog. 2:e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Troyanskaya O., et al. 2001. Missing value estimation methods for DNA microarrays. Bioinformatics 17:520–525 [DOI] [PubMed] [Google Scholar]
- 65. Tusher V. G., Tibshirani R., Chu G. 2001. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. U. S. A. 98:5116–5121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Virtaneva K., et al. 2005. Longitudinal analysis of the group A Streptococcus transcriptome in experimental pharyngitis in cynomolgus macaques. Proc. Natl. Acad. Sci. U. S. A. 102:9014–9019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. von Ossowski I., et al. 2010. Mucosal adhesion properties of the probiotic Lactobacillus rhamnosus GG SpaCBA and SpaFED pilin subunits. Appl. Environ. Microbiol. 76:2049–2057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Warner J. B., Lolkema J. S. 2003. CcpA-dependent carbon catabolite repression in bacteria. Microbiol. Mol. Biol. Rev. 67:475–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yoon H., McDermott J. E., Porwollik S., McClelland M., Heffron F. 2009. Coordinated regulation of virulence during systemic infection of Salmonella enterica serovar Typhimurium. PLoS Pathog. 5:e1000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.