Abstract
Corynebacterium glutamicum GlxR is a cyclic AMP (cAMP) receptor protein-type regulator. Although over 200 GlxR-binding sites in the C. glutamicum genome are predicted in silico, studies on the physiological function of GlxR have been hindered by the severe growth defects of a glxR mutant. This study identified the GlxR regulon by chromatin immunoprecipitation in conjunction with microarray (ChIP-chip) analyses. In total, 209 regions were detected as in vivo GlxR-binding sites. In vitro binding assays and promoter-reporter assays demonstrated that GlxR directly activates expression of genes for aerobic respiration, ATP synthesis, and glycolysis and that it is required for expression of genes for cell separation and mechanosensitive channels. GlxR also directly represses a citrate uptake gene in the presence of citrate. Moreover, ChIP-chip analyses showed that GlxR was still able to interact with its target sites in a mutant with a deletion of cyaB, the sole adenylate cyclase gene in the genome, even though binding affinity was markedly decreased. Thus, GlxR is physiologically functional at the relatively low cAMP levels in the cyaB mutant, allowing the cyaB mutant to grow much better than the glxR mutant.
INTRODUCTION
Cyclic AMP (cAMP) receptor proteins (CRPs) are global transcriptional regulators that are broadly distributed in bacteria (44). CRP family proteins have diverse cellular functions, including in carbohydrate metabolism (44, 91), development of competence for transformation (16), growth phase-dependent regulation of gene expression (1), modulation of virulence gene expression and pathogenesis (20, 71, 87), resuscitation (64), and germination and morphological development (21, 22).
The best-studied member of the family is the CRP of Escherichia coli. E. coli CRP activated by cAMP specifically binds to the consensus sequence 5′-TGTGA-N6-TCACA-3′ in target promoters, thereby recruiting RNA polymerase and promoting transcription at the promoter via protein-protein interactions (13). Intracellular cAMP levels in E. coli cells increase in response to glucose starvation via activation of adenylate cyclase, an enzyme which catalyzes the formation of cAMP from ATP. The interaction between the phosphotransferase system for glucose uptake and adenylate cyclase is involved in the control of synthesis of cAMP (44, 46). The presence of glucose lowers intracellular cAMP levels and deactivates CRP (44), allowing CRP to mediate preferential utilization of the fast-metabolizable carbon source, a phenomenon called carbon catabolite repression (23). Based on transcriptome or in silico analyses (29, 65, 75, 89, 90), E. coli CRP directly affects the expression of up to 200 genes.
Corynebacterium glutamicum is a Gram-positive bacterium with a high G+C content which has been used in the biotechnological production of amino acids, organic acids, and alcohols (35, 36, 39, 48, 72, 85). In addition, because it is nonpathogenic and grows fast, C. glutamicum is an emerging model system for members of the Corynebacterineae, an Actinomycetes suborder, including Mycobacterium tuberculosis, the causative agent of tuberculosis. In the course of extensive genome-wide analyses, a transcriptional regulatory network model composed of an increasing number of known regulators has been constructed and updated (8, 9, 56, 68). GlxR, which is a CRP-type transcriptional regulator, is one of the most important regulators of C. glutamicum. First characterized as a factor repressing the promoter activity of a gene coding for a glyoxylate pathway enzyme (41), GlxR is involved in the regulation of several other genes. Like E. coli CRP, it binds to the consensus site 5′-TGTGA-N6-TCACA-3′ in a cAMP-dependent manner in vitro (32, 33, 40, 41, 49). In silico analyses have detected more than 200 potential binding sites for GlxR in the C. glutamicum genome, and binding to 72 of the sites has been verified by in vitro binding assays, revealing that the regulon includes genes for carbon metabolism, nitrogen metabolism, respiration, resuscitation, cell wall formation, and cell division (42, 43). However, whether GlxR acts as a transcriptional activator or repressor of most of these genes is difficult to evaluate, not only because construction of a glxR deletion mutant is difficult but because any mutant that has been successfully constructed shows severe growth defects (41, 49, 59, 79). The role of GlxR has been assessed only by in vitro binding assays and overexpression studies in vivo (14, 32, 33, 40, 49), although GlxR-dependent repression of genes involved in the glyoxylate pathway and glutamate uptake system was recently confirmed by experiments using a glxR mutant (59). Therefore, the physiological function of and environmental signal(s) sensed by GlxR remain poorly understood.
In contrast to the case for E. coli, cAMP levels in C. glutamicum are higher during growth on glucose than during growth on acetate (41). In addition, C. glutamicum can simultaneously utilize different carbon sources with glucose (11, 18, 24, 77, 86), apart from glutamate and ethanol, consumption of which is almost completely repressed in the presence of glucose (2, 45, 47). That the repressed expression of a glutamate transporter gene in the presence of glucose is relieved in a glxR mutant suggests the involvement of GlxR in carbon catabolite repression (59). However, cAMP levels in cells grown in the presence of glutamate or ethanol have not been determined, and effects of cAMP levels on GlxR function in vivo are not known. Although the C. glutamicum genome carries only a single putative adenylate cyclase gene, cyaB, cAMP is still detected in a cyaB mutant (15). In addition, the cyaB mutant is able to grow on any carbon source much better than a glxR mutant (15, 59), implying that GlxR may be physiologically functional even in the cyaB mutant background.
Chromatin immunoprecipitation in conjunction with microarray (ChIP-chip) analysis has been used to identify in vivo target sites and physiological states of bacterial transcriptional regulators in recent years (12, 17, 27, 30, 31, 52, 60, 66). The regions bound by the transcriptional regulator are enriched by ChIP, and the degrees of the enrichment reflect the ability of the regulator to bind to the regions in the cells under the conditions tested. Therefore, in response to an environmental signal that alters binding of the regulator, the degree of enrichment of target regions in ChIP-chip analysis is changed (17, 25, 27, 66). Addition of glucose to the culture eliminates enrichment of target regions by CRP in E. coli (31).
In this study, we employed ChIP-chip analyses to investigate the target genes and binding ability of GlxR in vivo at the genome-wide level. The binding sites identified are validated by in vitro binding assays and in vivo promoter assays, demonstrating that GlxR directly activates expression of genes with various cellular functions. Furthermore, we used ChIP-chip analyses with a cyaB mutant to show that even in the absence of a known adenylate cyclase, GlxR still binds to the sites in vivo, albeit with lower affinity than in the wild type, suggesting that GlxR in the cyaB mutant is physiologically active.
MATERIALS AND METHODS
Bacterial strains, plasmids, oligonucleotides, and growth conditions.
The strains and plasmids used in this study are listed in Table 1. The oligonucleotides used are listed in Table S1 in the supplemental material. For genetic manipulation, E. coli strains were grown at 37°C in Luria-Bertani (LB) medium. C. glutamicum strains were grown at 33°C in nutrient-rich A medium (37) with 4% glucose. When appropriate, the media were supplemented with antibiotics. The final antibiotic concentrations for E. coli were 50 μg of ampicillin ml−1 and 50 μg of kanamycin ml−1; for C. glutamicum, kanamycin (50 μg ml−1) was used. For promoter assays, C. glutamicum strains chromosomally carrying a promoter-lacZ fusion were grown in A medium containing 1% glucose or acetate to the stationary phase (8 h). When citH promoter activity was determined, A medium containing 5 mM CaCl2 was used as basal medium for efficient uptake of citrate (11).
Table 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| JM109 | recA1 endA1 gyrA96 thi hsdR17 supE44 relA1 Δ(lac-proAB)/F′ (traD36 proAB+lacIqlacZΔM15) dam dcm supE44 hsdR17 thi leu rpsL | Takara |
| JM110 | lacY galK galT ara tonA thr tsx Δ(lac-proAB)/F′ (traD36 proAB+lacIqlacZΔM15) | 67 |
| BL21(DE3) | F−ompTgaldcmlonhsdSB (rB− mB−) λ(DE3) | 74 |
| C. glutamicum | ||
| R | Wild-type strain | 88 |
| KT7 | R with deletion in glxR | 79 |
| KT23 | R with Strep-tag II-tagged glxR | This study |
| ΔcyaB mutant | R with deletion in cyaB | 57 |
| KT25 | ΔcyaB mutant with Strep-tag II-tagged glxR | This study |
| Plasmids | ||
| pCold | Apr; cold-inducible expression | Takara |
| pGEM T-Easy | Vector | Promega |
| pCRA725 | Kmr; suicide vector containing the B. subtilissacB gene | 36 |
| pCRA741 | Kmr; pCRA725 with a 2.0-kb PCR fragment from strain-specific island 7 and a 3.1-kb PCR fragment containing the E. colilacZ gene | 38 |
| pCRC619 | Kmr; pCRA725 with a 2.4-kb fragment containing a Strep-tag II-tagged glxR gene | This study |
| pCRC620 | Apr: pColdI with a 684-bp fragment containing the glxR gene | This study |
Construction of a GlxR-Strep-tag II strain.
The Strep-tag II-coding sequence was introduced into the 3′ end of the glxR gene by overlapping PCR using overlapping primer pair glxRstrepFW-glxRstrepRV, together with primers glxRFW and glxRRV and genomic DNA from strain R as a template. The overlapping PCR products were purified and used as a template for PCR using primers glxRFW and glxRRV. The resulting fragment was cloned into pCRA725, a suicide vector for markerless gene disruption (36), yielding pCRC619. This was isolated as nonmethylated DNA from E. coli JM110 for efficient gene introduction into C. glutamicum R (84). C. glutamicum R (wild type) (79) and the cyaB mutant strain (57) were transformed by electroporation with pCRC619, and screening for the mutants was performed as described previously (36). Introduction of the tag into the glxR gene on the chromosome was confirmed by direct sequencing of a PCR product, which was amplified using primers glxRFW and glxRRV and genomic DNA extracted from the strains obtained as a template.
ChIP-chip analysis.
Exponentially growing C. glutamicum cultures (35 ml) at an optical density at 610 nm (OD610) of up to 2.5 were treated with formaldehyde (at a final concentration of 1%) and incubated for 20 min at room temperature. The cross-linking was quenched by addition of glycine (at a final concentration of 125 mM) and incubation for 10 min at room temperature. Cells were then collected by centrifugation, washed twice with Tris-buffered saline (20 mM Tris-HCl [pH 7.5], 150 mM NaCl), and stored at −80°C. Pellets were resuspended in 2 ml IP buffer (50 mM HEPES-KOH [pH 7.5], 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% sodium deoxycholate, 0.1% SDS, Roche Antiprotease mini). The cells were mechanically disrupted using a FastPrep FP120 instrument (Bio 101, Thermo Savant) as described previously (78), and the supernatant after centrifugation was sonicated on ice to shear DNA to an average size of 600 to 1,000 bp. A 50-μl fraction of the supernatant was saved for later analysis (reference DNA). The remainder was subjected to immunoprecipitation with 100 μl of magnetic beads coated with protein G (Invitrogen), which was coupled to the monoclonal anti-Strep-tag II antibody (Qiagen). The mixture was incubated overnight on a rotating platform at 4°C. The beads were washed once with IP buffer, twice with IP buffer containing 400 mM NaCl, eight times with radioimmunoprecipitation assay (RIPA) buffer (50 mM HEPES [pH 7.6], 500 mM LiCl, 1 mM EDTA, 1% NP-40, 0.7% sodium deoxycholate), and twice with Tris-EDTA (TE) buffer with 50 mM NaCl. Immunoprecipitated complexes were eluted from the beads by treatment with 210 μl elution buffer (50 mM Tris-HCl [pH 8.0], 10 mM EDTA, 1% SDS) at 65°C for 20 min. Cross-links of immunoprecipitated samples and of total DNA samples were reversed by incubation overnight at 65°C. Samples were then treated with RNase A and proteinase K for 2 h at 55°C. DNA was extracted with phenol-chloroform and purified with a QIAquick PCR purification minElute kit (Qiagen). DNA samples were blunted with T4 DNA polymerase, ligated to linkers, and amplified by PCR. Amplified DNAs from reference DNA and immunoprecipitated DNA were differentially labeled with Cy3 and Cy5, respectively, by using a CGH labeling kit (Invitrogen) according to the manufacturer's instructions. Hybridization to microarrays and array scanning were done as described previously (38). The arrays were spotted with 3,056 duplicate PCR products corresponding to C. glutamicum open reading frames (ORFs) (38). Data were normalized so that the mean of the Cy5/Cy3 intensity ratio of all of the features except the flagged ones was equal to 1, using GenePix 5.0 software. The enrichment factor for a given gene was calculated as the log2 ratio of hybridization of immunoprecipitated DNA to reference DNA. The entire procedure was carried out at least three times, and the results were averaged. A result was considered significant when the mean value was higher than 0.5 and with an associated P value of lower than 0.05.
Overexpression and purification of His-tagged GlxR protein.
The glxR gene was amplified from chromosomal DNA of C. glutamicum R by PCR with primers GlxRHisFW and GlxRHisRV. The PCR product was cloned into the expression vector pColdI (Takara), yielding pCRC620. His-tagged GlxR was overexpressed in E. coli BL21(DE3) by cold shock and purified by affinity chromatography as described previously (80). The concentration of the purified protein was determined by a Bio-Rad protein assay (Bio-Rad Laboratories) using bovine serum albumin (BSA) as a standard.
EMSA.
DNA fragments of interest were obtained by PCR amplification using promoter-lacZ fusion plasmids, which were constructed as described below, as templates and cloned into pGEM-T Easy (Promega). The sequence and direction of the cloned fragments were confirmed, and the cloned fragments were labeled with Cy3 by PCR amplification using primers SP6Cy3 and T7 (see Table S1 in the supplemental material). The probe containing the gapA promoter region was prepared by PCR amplification using primers PgapA RV3Cy3 and PgapAFW5NaeI (see Table S1 in the supplemental material). The resulting fragments were purified with the QIAquick PCR purification kit (Qiagen). Electrophoretic mobility shift assay (EMSA) was performed as described previously (80), except that His-tagged GlxR was incubated with 0.5 mM cAMP in EMSA buffer for 10 min before addition of the labeled DNA fragment. DNA and DNA-protein complexes were visualized with a Typhoon Trio variable-mode imager (GE Healthcare Bioscience).
Construction of promoter-lacZ fusions.
The regions containing putative GlxR targets were amplified by PCR from C. glutamicum R chromosomal DNA using primers listed in Table S1 in the supplemental material. Mutations in GlxR-binding sites were introduced by PCR using mutated primers (indicated with “mut” in Table S1 in the supplemental material). When the GlxR-binding site was located within 200 bp from the initiation codon of the target gene, mutations were introduced by overlapping PCR as described previously (78). Fragments were phosphorylated and cloned upstream of the lacZ gene in pCRA741 (38). The direction and sequence of the inserted fragment were confirmed by DNA sequencing. The plasmids isolated as nonmethylated DNA from E. coli JM110 were introduced into C. glutamicum R and were subsequently integrated into strain-specific island 7 (SSI7) on the chromosome of C. glutamicum R by markerless gene insertion methods as described previously (36).
β-Galactosidase assay.
C. glutamicum cells carrying the promoter-lacZ fusion were harvested, washed once with Z buffer (51), resuspended with the same buffer, and treated with toluene. The permeabilized cells were then incubated with o-nitrophenyl-β-galactoside, and activity was measured in Miller units as previously described (51).
5′-RACE-PCR.
For the identification of the transcriptional start points (TSPs) of mscL and cgR_1596, 5′ rapid amplification of cDNA ends (RACE)-PCR analyses were carried out. Total RNA was extracted using the RNeasy Minikit (Qiagen) from wild-type C. glutamicum grown in A medium containing 1% glucose and treated with DNase I (Takara) as described previously (38). The RNA extracted was poly(A) tailed using a poly(A) tailing kit (Life Technologies) according to the manufacturer's instructions. After phenol-chloroform extraction, the RNA with a poly(A) tail was purified by ethanol precipitation. cDNA was synthesized using the SMARTer RACE cDNA amplification kit (Clontech, CA) with a supplied oligo(dT)-anchored primer and 1 μg of the tailed RNA prepared as described above. The cDNA was amplified with universal primer A (supplied with the kit) and gene-specific primers (see Table S1 in the supplemental material). The resulting PCR products were cloned into a pGEM-T Easy vector (Promega). At least 10 clones for each 5′ RACE-PCR product were sequenced.
cAMP assays.
Intracellular cAMP levels were measured using the Amersham cAMP Biotrak enzyme immunoassay (EIA) system (GE Healthcare). Wild-type C. glutamicum and a cyaB deletion mutant were grown in A medium with 1% glucose or acetate at 33°C. Cells equivalent to an OD610 of 1.5 to 3 were harvested, resuspended in lysis buffer 1B (supplied with the kit) (200 μl for the wild type and 300 μl for the cyaB mutant), and incubated at 100°C for 15 min with intermittent mixing. The supernatants after centrifugation were stored at −20°C. Samples from the wild type were appropriately (from 5- to 40-fold) diluted in lysis buffer 1B, while those from the cyaB mutant were directly assayed. One hundred microliters of the samples from three independent experiments was assayed in duplicate according to the manufacturer's instructions.
Microarray data accession number.
The ChIP-chip data from this study have been deposited at the Gene Expression Omnibus database under accession number GSE26870.
RESULTS
Identification of binding sites for GlxR in vivo.
To elucidate the in vivo role of GlxR, we performed ChIP-chip analyses. We constructed strain KT23 whose glxR gene was modified to encode GlxR with a C-terminal Strep-tag II. Western blot analyses of total protein from strain KT23 and its parent C. glutamicum wild-type strain, probed with anti-Strep-tag II, detected the tagged GlxR protein with the expected size from only strain KT23 (data not shown). Although an in-frame deletion mutant of glxR is known to show severe growth defects (59, 79), strain KT23 grew as well as the wild type did (see Fig. S1 in the supplemental material), demonstrating that the tagged GlxR is as functional in vivo as the native one. Strain KT23 cells were grown in the presence of glucose for ChIP-chip analyses, bearing in mind that intracellular cAMP levels in C. glutamicum during growth on glucose are higher than that those during growth on acetate (41). ChIP and microarray analyses were performed as described in Materials and Methods. Genes exhibiting enrichment factors of higher than 0.5 with associated P values lower than 0.05 were chosen as possible targets of GlxR. Complete data sets are shown in Table S2 in the supplemental material. Since the microarrays were spotted with PCR products corresponding to coding sequences but not intergenic regions, it was not necessarily apparent which ORF was under the control of GlxR, especially in the case of the GlxR-binding site located between divergently transcribed genes. Therefore, when divergently transcribed ORFs were identified in ChIP-chip analyses, we regarded the intergenic region and the intragenic region of the gene with a higher enrichment factor as one putative GlxR-binding region (e.g., cgR_1326 and cgR_1327) (Table 2). When two consecutive genes transcribed in the same direction were identified, the intergenic region and the intragenic region of the upstream gene were considered one GlxR-binding region (e.g., cgR_1636 and cgR_1637) (Table 2). In this way, 268 ORFs which corresponded to 209 regions of the chromosome were identified as possible GlxR target regions in vivo under the conditions used.
Table 2.
Binding sites identified by ChIP-chip which were used for validation in this study
| Promoter region detected | Gene ID | Gene name | Direction | Wild type |
cyaB mutant |
Function | Binding site (5′ → 3′) | Distanceb | Predictionc | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EFa | P value | EF | P value | ||||||||
| cgR_0087 and cgR_0088 | cgR_0087 | citH | − | 0.15 | 3.41E−01 | 0.01 | 9.59E−01 | Citrate transporter | CGTGACACACGCCACC | −76 (−20) | Novel |
| cgR_0088 | citA | + | 0.89 | 3.89E−03 | 0.59 | 3.60E−02 | Sensory histidine kinase | GGTGGCGTGTGTCACG | −132 | Novel | |
| cglR0323 and cgR_0324 | cgR_0323 | leuA | − | 0.55 | 1.79E−02 | 0.48 | 2.78E−02 | 2-Isopropylmalate synthase | TGTGATTTCAGACACA | −294 (−159) | Novel |
| cgR_0324 | + | 0.01 | 9.01E−01 | −0.05 | 6.78E−01 | Hypothetical protein | TGTGTCTGAAATCACA | −345 | Novel | ||
| cgR_0992 | cgR_0992 | mscL | − | 0.39 | 1.74E−01 | −0.14 | 5.29E−01 | Large-conductance mechanosensitive channel | TGTGACAAACGTCACT | −389 (−349) | Novel |
| cgR_0993 | − | 3.14 | 5.77E−03 | 2.28 | 2.99E−03 | Putative secreted protein | |||||
| cgR_1284 | cgR_5018 | − | 1.73 | 2.30E−04 | 0.83 | 5.01E−02 | Hypothetical protein | ||||
| cgR_1284 | atpB | + | 0.24 | 7.52E−02 | 0.06 | 5.15E−01 | FoF1 ATP synthase a chain | TGTGACACACATAACA | −326 (−118) | Novel | |
| cgR_1326 and cgR_1327 | cgR_1326 | − | 2.36 | 2.95E−03 | 1.74 | 2.58E−02 | Putative membrane protein | TGTGGTGTGCTGCACA | −79 | Predicted | |
| cgR_1327 | pfk | + | 1.26 | 4.24E−03 | 0.78 | 2.17E−03 | 6-Phosphofructokinase | TGTGCAGCACACCACA | −166 (−166) | Predicted | |
| cgR_1596 | cgR_1596 | − | 0.21 | 1.29E−01 | 0.51 | 1.62E−02 | Secreted cell wall-associated hydrolase | AGTGATAAACATCACA | −411 (−132) | Predicted | |
| cgR_1597 | − | 1.40 | 3.08E−03 | 0.92 | 1.04E−02 | Putative membrane protein | |||||
| cgR_1636 | cgR_1636 | gapA | − | 0.77 | 2.61E−03 | 0.68 | 3.10E−02 | Glyceraldehyde-3-phosphate dehydrogenase | TGTGAGTTGCATCACA | −427 (−244) | Predicted |
| cgR_1637 | − | 1.34 | 2.66E−03 | 0.77 | 2.96E−02 | Putative transcriptional regulator, WhiA homolog | |||||
| cgR_2076 and cgR_2077 | cgR_2076 | ctaC | − | 0.81 | 1.33E−02 | 0.74 | 2.95E−03 | Cytochrome c oxidase subunit II | TGTGACGTGGCATACA | −215 (−162) | Predicted |
| cgR_2077 | ltsA | + | 0.13 | 7.99E−03 | 0.29 | 2.28E−02 | Glutamine-dependent amidotransferase | TGTATGCCACGTCACA | −303 | Predicted | |
| cgR_2120 | cgR_2119 | − | 2.35 | 1.90E−02 | 1.54 | 1.45E−02 | Hypothetical protein | ||||
| cgR_2120 | aceE | + | −0.09 | 6.77E−02 | 0.09 | 1.46E−01 | Pyruvate dehydrogenase | TGAGAGAAACATCACA | −337 (−219) | Novel | |
| cgR_2431 | cgR_2431 | ctaD | − | 0.13 | 3.42E−01 | 0.23 | 1.34E−01 | Cytochrome c oxidase subunit I | TGTGAACCCCTTCACA | −227 (−171) | Predicted |
| cgR_5047 | − | 2.46 | 6.49E−03 | 1.77 | 1.77E−02 | Hypothetical protein | |||||
EF, enrichment factor in strain KT23 or strain KT25 (cyaB mutant); values are means from at least three independent experiments.
Distance from the translational start point of the gene to the center of the binding site identified. In parentheses, the distance from the transcriptional start point of the gene to the center of the binding site is indicated. Except for mscL and cgR_1596, information on the transcriptional start points was obtained from literature data.
Predicted, sites detected by both ChIP-chip and in silico analyses; novel, sites newly identified by ChIP-chip analysis.
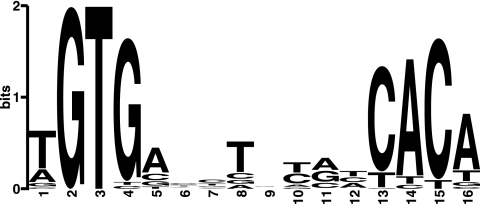
A MEME (Multiple Em for Motif Elicitation) (6) alignment of the upstream sequences of the genes with relatively high enrichment factors (≥1) produced a consensus sequence of 5′-TGTGNNNNNNNNCACA-3′ (Fig. 1). The consensus motif obtained is almost the same as the previously reported 5′-TGTGANNTANNTCACA-3′ (42, 43, 49). It was further confirmed that, with the notable exception of one region, the consensus sequence is present in all other regions with enrichment factors of between 0.5 and 1 (see Table S2 in the supplemental material).
Fig. 1.
GlxR consensus sequences. A conserved motif was identified using the MEME algorithm by analyzing the regions with enrichment factors of ≥1 in the ChIP-chip experiment. The size of each letter indicates the relative abundance at the respective position in the consensus matrix generated with MEME.
The regions identified by ChIP-chip analyses in this study were compared with the regions detected by previous in silico analyses in C. glutamicum ATCC 13032 (42, 43). The ChIP-chip analyses detected almost half of the sites predicted by in silico analyses (84/170). If the cutoff threshold was relaxed from 0.5 to 0.2, 34 sites were additionally identified. When the predicted sites that were not detected or were detected with low enrichment factors (<0.5) in the ChIP-chip analyses were compared with the motif obtained in the current study (Fig. 1), the GTG (position 2 to 4) and/or CAC (position 13 to 15) were not perfectly conserved (data not shown). In the motif obtained (Fig. 1), the sequences GTG and CAC are most conserved, implying that the nucleotides at these positions are important for the GlxR-DNA interactions.
Based on the results of ChIP-chip analyses and the consensus motif identified, 94 GlxR-binding regions that have not been previously predicted were newly identified here (Table 2; see Table S2 in the supplemental material). Of these, 53 sites are located in the coding region or the intergenic region between two convergently oriented genes.
Validation of the identified GlxR-binding sites.
To validate the GlxR-binding sites identified in the upstream regions of genes, we examined effects of mutations within the sites on the binding of GlxR in vitro. We exchanged the nucleotides corresponding to the positions of GTG and CAC in the consensus motif (Fig. 1) to CAC and GTG, respectively (5′-TGTG-N8-CACA-3′ → 5′-TCAC-N8-GTGA-3′). We chose the upstream regions of the genes involved in fundamental cellular functions for further investigation (Table 2): aerobic respiration (cytochrome c oxidase subunits, ctaC [cgR_2076] and ctaD [cgR_2431]), ATP synthesis (FoF1 ATPase subunit A, atpB [cgR_1284]), carbon metabolism (2-isopropylmalate synthase, leuA [cgR_0323]; phosphofructokinase, pfk [cgR_1327]; glyceraldehyde-3-phosphate dehydrogenase, gapA [cgR_1636]; and E1 component of pyruvate dehydrogenase, aceE [cgR_2120]), transport of carbon sources (citrate transporter, citH [cgR_0087]), cell division (secreted cell wall-associated hydrolase, cgR_1596), and the stress response (large-conductance mechanosensitive channel, mscL [cgR_0992]). The GlxR-binding sites located in the upstream regions of leuA, aceE, mscL, and citH were newly identified by ChIP-chip analyses in the current study.
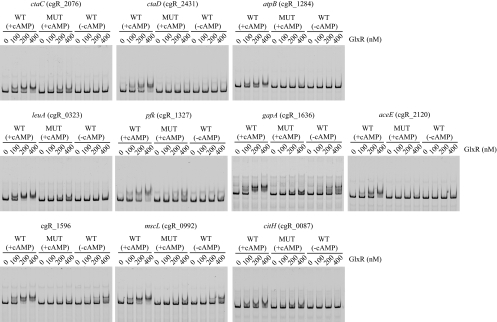
The results of EMSA showed that GlxR binds to the corresponding regions in a cAMP-dependent manner (Fig. 2). In addition, the mutations introduced abolished the GlxR binding (Fig. 2), demonstrating that the binding sites identified are essential for GlxR binding and that the ChIP-chip was able to detect interaction of GlxR with target sites at genome-wide level in vivo.
Fig. 2.
EMSAs of representative in vivo GlxR-binding promoter regions detected by ChIP-chip analyses. The regions containing a wild-type (WT) or mutated (MUT) GlxR site were amplified by PCR using primers listed in Table S1 in the supplemental material and were labeled with Cy3 as described in Materials and Methods. The DNA probe was incubated with the purified GlxR at the indicated concentrations in the presence (+cAMP) or absence (−cAMP) of 0.5 mM cAMP and analyzed by nondenaturing PAGE. The target genes are indicated at the tops of the gels. Each well contained 10 nM DNA fragment.
GlxR bound to the sites upstream of cgR_1596 and mscL in the absence of cAMP, although the affinity was much lower than that in its presence. The strong affinity of these two sites may be attributed to the fact that the two sites share the same nucleotides at 12 positions (5′-tGTGAnAAACnTCACa-3′; shared nucleotides are capitalized) out of 16 positions in the consensus motif (Fig. 1).
In vivo function of GlxR.
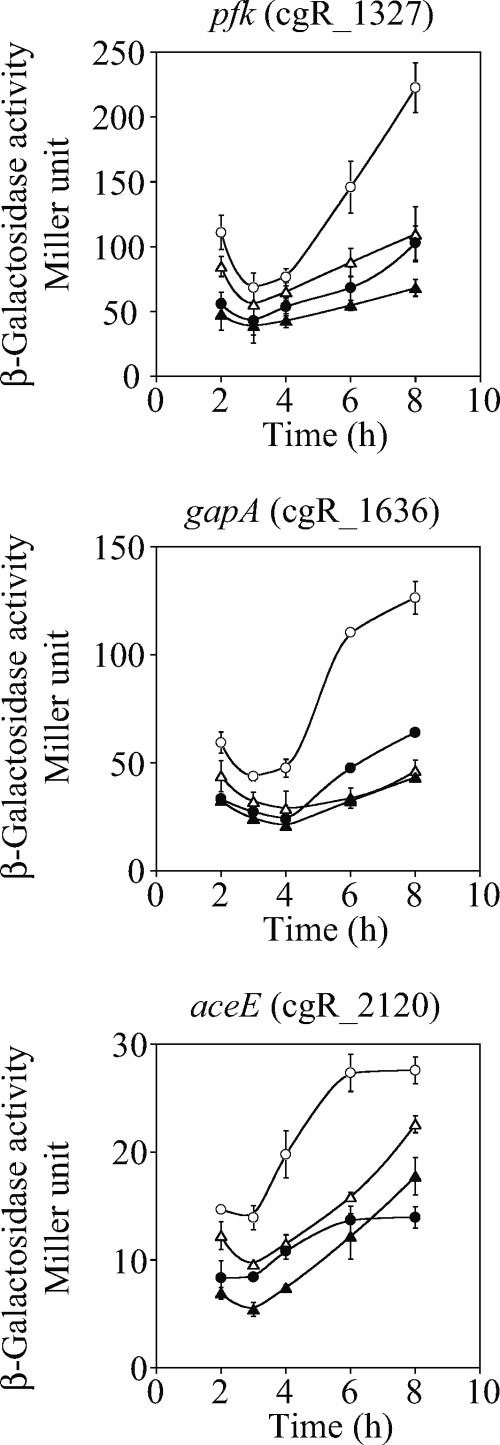
Even though some GlxR-binding sites listed in Table 2 have been previously found and shown to be bound by GlxR in vitro, those functions in vivo have not been investigated. To test the in vivo roles of GlxR in regulation of target gene expression, each of the same DNA fragments as those tested by EMSA was fused to the promoterless lacZ gene, and the resulting construct was integrated into the wild-type chromosome as described in a previous study (78). It should be noted that a strain chromosomally carrying an empty vector, pCRA741, had no detectable β-galactosidase activity. As we and others have shown that the expression levels of the glycolytic genes, pfk, gapA, and aceE, in cells grown on glucose are higher than those in cells grown on acetate (33, 34, 53), we first tested whether GlxR is involved in the carbon source-dependent regulation of these genes. The promoter activities of the pfk, gapA, and aceE genes exhibited similar patterns during growth in nutrient-rich A medium containing 1% either glucose or acetate; they were increased more than 2-fold at the onset of the stationary phase (6 h) of growth on glucose, while the increase was not as drastic on acetate (Fig. 3). Introduction of the mutations into the GlxR-binding sites in the promoters suppressed the increase of the activity on glucose, while the activity on acetate was slightly reduced by the mutations. Thus, GlxR activates genes for the glycolytic enzymes, especially during growth on glucose.
Fig. 3.
GlxR activates expression of pfk (cgR_1327), gapA (cgR_1636), and aceE (cgR_2120). The β-galactosidase activities of C. glutamicum strains chromosomally carrying promoters of the genes, which were fused with the lacZ reporter gene, were measured. Activities derived from the native (open symbols) or GlxR-binding site-mutated (closed symbols) promoters during growth in nutrient-rich A medium with 1% glucose (circles) or acetate (triangles) are shown. The activity is the mean value from at least three independent cultivations. Error bars indicate standard deviations.
The intracellular cAMP levels of C. glutamicum during growth on glucose are higher than those on acetate (15, 41), suggesting that the relatively high cAMP levels in the presence of glucose activate GlxR to increase the glycolytic gene expression. Under the growth conditions used in this study, the intracellular cAMP levels were comparable in glucose-grown cells and acetate-grown cells at the exponential phase, whereas, at the stationary phase, the cAMP levels in glucose-grown cells were up to 5-fold higher than those in acetate-grown cells (see Fig. S2A in the supplemental material). Thus, the increase in promoter activity of the glycolytic genes was likely to be correlated with the increase in the intracellular cAMP levels.
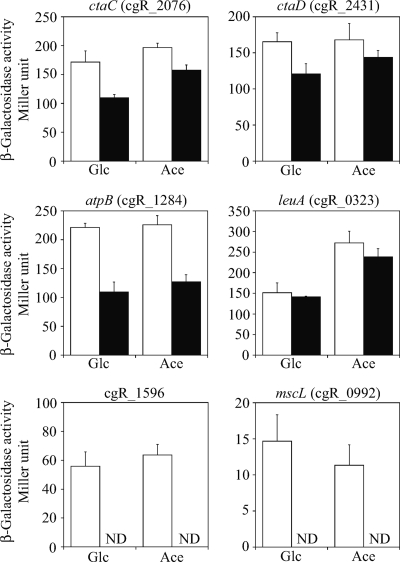
In contrast, promoter activity of the other genes, except citH, was not affected by the growth conditions examined (Fig. 4), implying that the changes in the intracellular cAMP levels had little effect on expression of these genes. However, mutations in the GlxR site resulted in a decrease of the activities of the ctaC, ctaD, and atpB promoters, with less effect on the ctaD promoter, indicating that GlxR activates expression from these promoters (Fig. 4). Moreover, promoter activity of cgR_1596 and mscL was lost by the mutation in the GlxR-binding site under the conditions used (Fig. 4), indicating that expression of these genes is highly dependent on GlxR. The TSPs of cgR_1596 and mscL were determined by 5′-RACE to be located at 279 nucleotides (nt) and 40 nt upstream of the start codon, respectively. Since the GlxR-binding sites are located at positions −132 and −349 with respect to the TSPs of cgR_1596 and mscL, respectively, the mutations introduced in the binding sites are unlikely to affect the basal promoter activity. Taken together, the results indicate that GlxR is important for upregulation of genes underlying various cellular functions but that the regulation is not entirely dependent on the cAMP levels.
Fig. 4.
Involvement of the GlxR-binding sites in regulation of in vivo expression of the target genes. The β-galactosidase activity of C. glutamicum strains chromosomally integrated with the target promoter fused with the lacZ reporter gene was measured. Activities derived from the native (white bars) or GlxR-binding site-mutated (black bars) promoters at the stationary phase of growth in nutrient-rich A medium with 1% glucose (Glc) or acetate (Ace) are shown. The activity is the mean value from at least three independent cultivations. Error bars indicate standard deviations. ND, not detected.
The leuA promoter showed higher activity during growth on acetate than during growth on glucose. The activity was not affected by the mutations in the GlxR-binding site under the experimental conditions used (Fig. 4). Promoter activity during growth in minimal medium containing either glucose or acetate was almost the same as that in nutrient-rich medium and was not affected by the mutations in the GlxR-binding site (data not shown). Three ORFs (cgR_0324, cgR_0325, and cgR_0326), which are not conserved in the C. glutamicum ATCC 13032 genome, are present upstream of leuA in the C. glutamicum R genome (88). The GlxR-binding site found in the upstream region of leuA is also not conserved. Therefore, GlxR binding to the site may affect expression of the cgR_0324 gene, which is transcribed divergently from leuA and encodes a protein of unknown function.
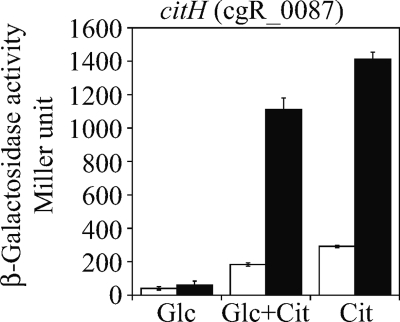
The citH gene and the tctCBA operon encode citrate transporters with different cation specificities, and their expression is induced by the CitAB two-component system in the presence of citrate (11). Here, the ChIP-chip analyses identified GlxR-binding sites upstream of both citH and tctC (cgR_2710), suggesting that GlxR is involved in regulation of citrate uptake. We tested how GlxR is involved in induction of citH expression in the presence of citrate. The activity of the citH promoter in the presence of citrate was 5-fold higher than that in its absence (Fig. 5, white bars). In the presence of both glucose and citrate, the citH promoter activity was slightly downregulated compared with that in the presence of citrate alone. Mutations introduced in the GlxR-binding site enhanced the promoter activity in the presence of citrate by 5-fold (Fig. 5, black bars). As they had no effect on the activity in the absence of citrate and did not affect the putative −10 and −35 regions (11), the possibility that the mutations affected the basal promoter activity was excluded. These results suggested that GlxR is involved in attenuation of induction of citH by CitAB.
Fig. 5.
GlxR attenuates upregulation of citH (cgR_0087) expression by CitAB in the presence of citrate. The β-galactosidase activities of C. glutamicum strains chromosomally integrated with the citH promoter fused with the lacZ reporter gene were measured. Activities derived from the native (white bars) or GlxR-binding site-mutated (black bars) promoters at the exponential phase of growth in nutrient-rich A medium with 1% glucose (Glc), citrate (Cit), or glucose-citrate (Glc+Cit) are shown. The activity is the mean value from at least three independent cultivations. Error bars indicate standard deviations.
Deletion of the cyaB gene decreases affinity of GlxR for the target sites in vivo.
Although the C. glutamicum genome carries only a single putative adenylate cyclase gene, cyaB, cAMP was still detected in a cyaB mutant (15). The cAMP levels in the cyaB mutant were 25- and 45-fold lower than those in the wild type at the exponential and the onset of the stationary phase of growth on glucose, respectively (see Fig. S2B in the supplemental material). To examine binding of GlxR in vivo in the cyaB mutant background, we performed ChIP-chip analyses using strain KT25 expressing a GlxR-Strep-tag II in the background of the cyaB deletion mutant as described above. The number of ORFs with an enrichment factor of ≥0.5 was reduced from 268 in the wild type to 108 in the cyaB mutant (see Table S2 in the supplemental material). In addition, the signal intensities of more than 80 probes which exhibited an enrichment factor of ≥0.5 in the wild type decreased more than 1.5-fold in the cyaB mutant background. These results indicated that affinity of GlxR for its target sites in vivo was decreased in the mutant but that the cyaB gene was dispensable for GlxR binding to many target regions.
DISCUSSION
In this study, we used ChIP-chip technology to investigate the genome-wide binding sites of GlxR in vivo. The ChIP-chip experiments identified 209 GlxR-binding regions. Of these, 84 regions have been previously predicted by in silico analyses (42, 43). Among the remaining 125 regions, 94 were newly identified, 31 of which are specific to C. glutamicum R. Fifty-two other regions predicted by previous in silico analyses were not detected by the ChIP-chip analyses in this study. These discrepancies are probably caused by differences in the methods used to detect the binding sites and by differences in the regions used to search for the binding sites. While in silico analyses can identify all the possible binding sites based on the sequence similarity, ChIP-chip can identify binding sites where a protein of interest, GlxR in this study, actually binds in vivo. As the cAMP levels in C. glutamicum are dependent on the carbon source used and growth phase (41), GlxR may be unable to interact with some sites at the sampling point under the conditions used in this study. While in silico analyses searched for putative binding sites within intergenic regions of annotated ORFs (42), the current study used microarrays which were spotted with PCR products corresponding to coding sequences but not intergenic regions. Therefore, the GlxR-binding sites located far from the coding region could not be detected by the microarray, as has been described by others (27, 52). False negatives may also occur in ChIP-chip experiments due to sequestration of the transcription factor in nucleoprotein complexes, rendering them inaccessible to the specific test antibody used for the IP reaction (12, 30). Of the binding sites not detected by ChIP-chip analyses in this study, 21 have been shown to bind to GlxR in EMSA (42, 43). However, GlxR did not bind in vitro to two sites predicted by in silico analyses (42), suggesting that GlxR does not actually bind to these sites in vivo.
The ChIP-chip analyses also identified binding sites located within coding regions. Although the binding sites in the regions may be fortuitous and have no regulatory role, the ChIP-chip study of CRP from E. coli has suggested that, in addition to regulating transcription initiation, CRP contributes to the compaction of the E. coli chromosome by binding throughout the genome (31). The analyses also suggested that GlxR is involved in expression of strain-specific genes. Of 60 strain-specific genes in the C. glutamicum R genome, 41, corresponding to 31 regions, were identified by the ChIP-chip analyses.
The results of promoter-reporter fusion studies indicated that GlxR positively regulates expression of genes for various cellular functions, although it has been characterized as a repressor for some genes, such as gltA, aceB, gluABCD, and sdhCAB, encoding citrate synthase, malate syntheses, the glutamate uptake system, and succinate dehydrogenase, respectively (14, 59, 83). ctaC and atpB are the first genes of operons coding for cytochrome c oxidase and ATP synthase, respectively. No transcriptional regulator has been reported to be involved in expression of these genes, although atpB may be activated by SigH, which is a sigma factor that promotes expression of genes in response to various stress involving heat and oxidative stress (26), under alkaline conditions (7). The activity of promoters of the genes was not affected by either the carbon source or growth phase (Fig. 4 and data not shown). The loss of the GlxR-binding site resulted in a decrease of the promoter activity regardless of carbon source used, suggesting that these promoters are constitutively activated by GlxR and not affected by changes in cAMP levels under the conditions used here.
In contrast, the promoter activities of gapA, pfk, and aceE, which are involved in central carbon metabolism, were enhanced by the presence of glucose and the GlxR-binding site. The intracellular cAMP levels that are increased by the presence of glucose are likely to stimulate GlxR activity. Previously, RamA and SugR have been shown to be involved in upregulation and downregulation, respectively, of gapA expression (78, 79). RamA is a global regulator which controls expression of a number of genes and is essential for acetate and ethanol utilization (2–4, 14, 19, 69, 83), while SugR is a sugar-responsive transcriptional regulator that negatively controls genes involved in sugar uptake and catabolism in the absence of sugar (27, 28, 76, 78, 81). Although the physiological signal sensed by RamA is unknown, RamA is required for upregulation of gapA expression regardless of the carbon source (79). These current and previous findings suggest that, besides basal upregulation by RamA, gapA expression is enhanced in the presence of glucose by inactivating SugR and activating GlxR with sugar metabolites and cAMP, respectively. While pfk is negatively regulated by SugR and RamA (4, 27), aceE is positively controlled by RamB, a repressor of acetate utilization genes, under nutrient-rich conditions (10). The binding sites of these regulators in the promoter regions of gapA, pfk, and aceE do not overlap the GlxR-binding sites, eliminating the possibility that the mutations in the GlxR-binding sites affect binding of these regulators. GlxR acts as a transcriptional repressor of genes encoding tricarboxylic acid (TCA) cycle enzymes, including gltA and sdhCAB (14, 83), whose expression is also controlled by RamA. Thus, it is likely that GlxR contributes to the synchronization of expression of the central metabolic enzymes in collaboration with other transcription factors.
Although the promoter activities of cgR_1596 and mscL were not affected by the carbon source, the GlxR-binding sites identified are likely essential for activity of these promoters (Fig. 3). C. glutamicum possesses at least two cell wall hydrolases (cgR_1596 and cgR_2070) involved in separation of two daughter cells during cell division (82) and two mechanosensitive channels (MscL [cgR_0992] and MscS [cgR_1346]). cgR_2070 encodes a protein carrying the same catalytic cell wall hydrolase domain as that of the cgR_1596 protein and plays a minor or supportive role in cell separation in addition to the cgR_1596 protein (82). In silico analyses detected the GlxR-binding site upstream of cgR_2070 (43), and the site is highly similar to that in the upstream region of cgR_1596 (see Table S2 in the supplemental material). Therefore, GlxR is probably involved in control of cgR_2070 expression. The low enrichment factor derived from the GlxR-binding site upstream of cgR_2070 is likely to be because the site is far from flanking ORFs (cgR_2070 and cgR_2071). MscS (cgR_1346) is a mechanosensitive channel with small conductance. MscL and MscS are required to adapt to hypoosomotic stress (58). MscS is also involved in glutamate excretion by C. glutamicum (55), suggesting the importance of these channels in view of biotechnological production. In silico analyses identified the GlxR-binding site in the upstream region of mscS only, while the ChIP-chip analyses identified the sites in the upstream regions of both mscL and mscS. Hence, both channels are likely to be under the control of GlxR. To our knowledge, this is the first report on transcriptional regulation of genes encoding cell wall hydrolases and mechanosensitive channels in C. glutamicum. Interestingly, E. coli CRP is also involved in regulation of one of the mechanosensitive channel genes, although binding to the promoter has not been examined (89). Taken together, these results indicate that the essential role of GlxR in expression of genes related to maintenance of cell morphology may be largely responsible for the severe growth defect of the glxR mutant.
The promoter assays in the current study indicated that GlxR attenuated the induction of citH expression by CitAB in response to the presence of citrate. This is in contrast to expression of the citrate transporter (CitS) of Klebsiella pneumoniae (50). K. pneumoniae citS is induced by the two-component system, CitAB, like citH of C. glutamicum but is positively regulated by the CRP homolog and repressed by the presence of glucose. The binding site of K. pneumoniae CRP in the citS promoter is centered at position −41.5 with respect to the citS TSP. This position was previously demonstrated to be one of the optimal sites for transcriptional activation by E. coli cAMP-CRP (13). The GlxR sites in the upstream regions of citH and tctCBA, another type of citrate transporter activated by CitAB, are located near positions −10 and +10 with respect to the TSP, respectively, implying that GlxR downregulates these genes. The attenuation by GlxR was similarly observed irrespective of the presence of glucose, indicating that GlxR does not mediate the glucose repression that is observed for K. pneumoniae CRP. This is supported by the facts that cAMP levels in citrate-grown cells are higher than those in glucose-grown cells (62) and that C. glutamicum is able to utilize glucose and citrate simultaneously (11). The attenuation of citH induction by GlxR may be required for achievement of an appropriate expression level.
In M. tuberculosis, which is phylogenetically closely related to C. glutamicum, a CRP homolog plays an important role in survival in macrophages and resuscitation (64). Despite its high amino acid sequence identity (79%) to GlxR, the M. tuberculosis CRP homolog, which is encoded by Rv3676, binds to DNA even in the absence of cAMP in vitro (5, 64), although its affinity to DNA is slightly increased upon forming a complex with cAMP (63, 73). Deletion of Rv3676 causes a growth defect and affects expression of several genes during exponential phase (64). The consensus binding site of Rv3676, 5′-GTGNNANNNNNCACA-3′, is almost identical to that for GlxR (5, 64). In silico analyses detected 55 intergenic regions that contained the consensus site (5). Of the genes with altered expression in the Rv3676 mutant or those flanking the consensus sites detected by in silico analyses, nearly 10 orthologs of C. glutamicum genes, including cgR_1596, rpfA (cgR_1010), and whiB1 (whcE), were detected in our ChIP-chip analyses. rpfA encodes resuscitation-promoting factor, which is involved in reviving dormant bacteria (54). The rpfA orthologs in M. tuberculosis and C. glutamicum are directly regulated by Rv3676 and GlxR, respectively (40, 64). This may suggest that the two CRP homologs are in part functionally conserved in the two bacteria. On the other hand, almost all the remaining genes in the putative Rv3676 regulon encode proteins specific to M. tuberculosis, suggesting that GlxR- and Rv3676-dependent regulatory systems have diversified to adapt to the habitat of each organism over the evolutionary history. This may also be represented by the low response of Rv3676 to cAMP and high intracellular cAMP levels in M. tuberculosis, possessing 17 adenylate cyclase genes in the genome (70).
The current study demonstrated that GlxR was able to bind to target sites in vivo in the background of the cyaB mutant. This is in agreement with the facts that cAMP was still detected in the cyaB deletion mutant and its growth phenotype was not consistent with that of the glxR mutant (see Fig. S2 in the supplemental material) (15, 59). In E. coli, a cAMP-free intracellular environment can be achieved by deletion of the sole adenylate cyclase gene, cya, and the phenotype of the cya mutant is basically comparable to that of a crp mutant (61). In addition, the binding of E. coli CRP to the genome in vivo was completely inhibited in the presence of glucose, which lowered the cAMP level (31). As cyaB is the sole gene annotated as encoding adenylate cyclase in the genome, C. glutamicum is likely to possess a novel enzyme and/or pathway to synthesize cAMP. However, it should be noted that the possibility that the GlxR binding detected in the cyaB mutant is derived from not only the cAMP-GlxR complex but also cAMP-free GlxR cannot be ruled out. The results of EMSAs showed that GlxR was able to bind to the upstream regions of cgR_1596, mscL, and gapA even in the absence of cAMP, with much lower affinity than in its presence (Fig. 2). Furthermore, the binding of GlxR was not evenly affected by the depletion of cAMP in the cyaB mutant (see Table S2 in the supplemental material). These results indicate that the cAMP levels required for GlxR to bind to DNA vary depending on the target site. Finding an additional enzyme(s) involved in synthesis of cAMP and conditions which deplete intracellular cAMP would provide additional clues to understand the highly versatile physiological function of GlxR.
Supplementary Material
ACKNOWLEDGMENTS
We thank Crispinus A. Omumasaba (RITE) for critical reading of the manuscript.
This work was partially supported by a grant from the New Energy and Industrial Technology Development Organization (NEDO).
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
Published ahead of print on 10 June 2011.
REFERENCES
- 1. Agari Y., Kashihara A., Yokoyama S., Kuramitsu S., Shinkai A. 2008. Global gene expression mediated by Thermus thermophilus SdrP, a CRP/FNR family transcriptional regulator. Mol. Microbiol. 70:60–75 [DOI] [PubMed] [Google Scholar]
- 2. Arndt A., Eikmanns B. J. 2007. The alcohol dehydrogenase gene adhA in Corynebacterium glutamicum is subject to carbon catabolite repression. J. Bacteriol. 189:7408–7416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Auchter M., Arndt A., Eikmanns B. J. 2009. Dual transcriptional control of the acetaldehyde dehydrogenase gene ald of Corynebacterium glutamicum by RamA and RamB. J. Biotechnol. 140:84–91 [DOI] [PubMed] [Google Scholar]
- 4. Auchter M., et al. 2011. RamA and RamB are global transcriptional regulators in Corynebacterium glutamicum and control genes for enzymes of the central metabolism. J. Biotechnol. 154:126–139 [DOI] [PubMed] [Google Scholar]
- 5. Bai G., McCue L. A., McDonough K. A. 2005. Characterization of Mycobacterium tuberculosis Rv3676 (CRPMt), a cyclic AMP receptor protein-like DNA binding protein. J. Bacteriol. 187:7795–7804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bailey T. L., Elkan C. 1994. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc. Int. Conf. Intell. Syst. Mol. Biol. 2:28–36 [PubMed] [Google Scholar]
- 7. Barriuso-Iglesias M., Barreiro C., Flechoso F., Martín J. F. 2006. Transcriptional analysis of the F0F1 ATPase operon of Corynebacterium glutamicum ATCC 13032 reveals strong induction by alkaline pH. Microbiology 152:11–21 [DOI] [PubMed] [Google Scholar]
- 8. Baumbach J. 2007. CoryneRegNet 4.0—a reference database for corynebacterial gene regulatory networks. BMC Bioinformatics 8:429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baumbach J., Apeltsin L. 2008. Linking Cytoscape and the corynebacterial reference database CoryneRegNet. BMC Genomics 9:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blombach B., Cramer A., Eikmanns B. J., Schreiner M. 2009. RamB is an activator of the pyruvate dehydrogenase complex subunit E1p gene in Corynebacterium glutamicum. J. Mol. Microbiol. Biotechnol. 16:236–239 [DOI] [PubMed] [Google Scholar]
- 11. Brocker M., Schaffer S., Mack C., Bott M. 2009. Citrate utilization by Corynebacterium glutamicum is controlled by the CitAB two-component system through positive regulation of the citrate transport genes citH and tctCBA. J. Bacteriol. 191:3869–3880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bucca G., et al. 2009. Development and application of versatile high density microarrays for genome-wide analysis of Streptomyces coelicolor: characterization of the HspR regulon. Genome Biol. 10:R5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Busby S., Ebright R. H. 1999. Transcription activation by catabolite activator protein (CAP). J. Mol. Biol. 293:199–213 [DOI] [PubMed] [Google Scholar]
- 14. Bussmann M., et al. 2009. Transcriptional control of the succinate dehydrogenase operon sdhCAB of Corynebacterium glutamicum by the cAMP-dependent regulator GlxR and the LuxR-type regulator RamA. J. Biotechnol. 143:173–182 [DOI] [PubMed] [Google Scholar]
- 15. Cha P. H., et al. 2010. Characterization of an adenylate cyclase gene (cyaB) deletion mutant of Corynebacterium glutamicum ATCC 13032. Appl. Microbiol. Biotechnol. 85:1061–1068 [DOI] [PubMed] [Google Scholar]
- 16. Chandler M. S. 1992. The gene encoding cAMP receptor protein is required for competence development in Haemophilus influenzae Rd. Proc. Natl. Acad. Sci. U. S. A. 89:1626–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cho B. K., Barrett C. L., Knight E. M., Park Y. S., Palsson B. Ø. 2008. Genome-scale reconstruction of the Lrp regulatory network in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 105:19462–19467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cocaign-Bousquet M., Monnet C., Lindley N. D. 1993. Batch kinetics of Corynebacterium glutamicum during growth on various carbon substrates: use of substrate mixtures to localise metabolic bottlenecks. Appl. Microbiol. Biotechnol. 40:526–530 [Google Scholar]
- 19. Cramer A., Gerstmeir R., Schaffer S., Bott M., Eikmanns B. J. 2006. Identification of RamA, a novel LuxR-type transcriptional regulator of genes involved in acetate metabolism of Corynebacterium glutamicum. J. Bacteriol. 188:2554–2567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Crecy-Lagard V., et al. 1990. A Xanthomonas campestris pv. campestris protein similar to catabolite activation factor is involved in regulation of phytopathogenicity. J. Bacteriol. 172:5877–5883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Derouaux A., et al. 2004. Crp of Streptomyces coelicolor is the third transcription factor of the large CRP-FNR superfamily able to bind cAMP. Biochem. Biophys. Res. Commun. 325:983–990 [DOI] [PubMed] [Google Scholar]
- 22. Derouaux A., et al. 2004. Deletion of a cyclic AMP receptor protein homologue diminishes germination and affects morphological development of Streptomyces coelicolor. J. Bacteriol. 186:1893–1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Deutscher J. 2008. The mechanisms of carbon catabolite repression in bacteria. Curr. Opin. Microbiol. 11:87–93 [DOI] [PubMed] [Google Scholar]
- 24. Dominguez H., Cocaign-Bousquet M., Lindley N. D. 1997. Simultaneous consumption of glucose and fructose from sugar mixtures during batch growth of Corynebacterium glultamicum. Appl. Microbiol. Biotechnol. 47:600–603 [Google Scholar]
- 25. Efromovich S., Grainger D., Bodenmiller D., Spiro S. 2008. Genome-wide identification of binding sites for the nitric oxide-sensitive transcriptional regulator NsrR. Methods Enzymol. 437:211–233 [DOI] [PubMed] [Google Scholar]
- 26. Ehira S., Teramoto H., Inui M., Yukawa H. 2009. Regulation of Corynebacterium glutamicum heat shock response by the extracytoplasmic-function sigma factor SigH and transcriptional regulators HspR and HrcA. J. Bacteriol. 191:2964–2972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Engels V., Lindner S. N., Wendisch V. F. 2008. The global repressor SugR controls expression of genes of glycolysis and of the l-lactate dehydrogenase LdhA in Corynebacterium glutamicum. J. Bacteriol. 190:8033–8044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gaigalat L., et al. 2007. The DeoR-type transcriptional regulator SugR acts as a repressor for genes encoding the phosphoenolpyruvate:sugar phosphotransferase system (PTS) in Corynebacterium glutamicum. BMC Mol. Biol. 8:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gosset G., Zhang Z., Nayyar S., Cuevas W. A., Saier M. H., Jr 2004. Transcriptome analysis of Crp-dependent catabolite control of gene expression in Escherichia coli. J. Bacteriol. 186:3516–3524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grainger D. C., Aiba H., Hurd D., Browning D. F., Busby S. J. 2007. Transcription factor distribution in Escherichia coli: studies with FNR protein. Nucleic Acids Res. 35:269–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grainger D. C., Hurd D., Harrison M., Holdstock J., Busby S. J. W. 2005. Studies of the distribution of Escherichia coli cAMP-receptor protein and RNA polymerase along the E. coli chromosome. Proc. Natl. Acad. Sci. U. S. A. 102:17693–17698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Han S. O., Inui M., Yukawa H. 2008. Effect of carbon source availability and growth phase on expression of Corynebacterium glutamicum genes involved in the tricarboxylic acid cycle and glyoxylate bypass. Microbiology 154:3073–3083 [DOI] [PubMed] [Google Scholar]
- 33. Han S. O., Inui M., Yukawa H. 2007. Expression of Corynebacterium glutamicum glycolytic genes varies with carbon source and growth phase. Microbiology 153:2190–2202 [DOI] [PubMed] [Google Scholar]
- 34. Hayashi M., et al. 2002. Transcriptome analysis of acetate metabolism in Corynebacterium glutamicum using a newly developed metabolic array. Biosci. Biotechnol. Biochem. 66:1337–1344 [DOI] [PubMed] [Google Scholar]
- 35. Hermann T. 2003. Industrial production of amino acids by coryneform bacteria. J. Biotechnol. 104:155–172 [DOI] [PubMed] [Google Scholar]
- 36. Inui M., Kawaguchi H., Murakami S., Vertès A. A., Yukawa H. 2004. Metabolic engineering of Corynebacterium glutamicum for fuel ethanol production under oxygen-deprivation conditions. J. Mol. Microbiol. Biotechnol. 8:243–254 [DOI] [PubMed] [Google Scholar]
- 37. Inui M., et al. 2004. Metabolic analysis of Corynebacterium glutamicum during lactate and succinate productions under oxygen deprivation conditions. J. Mol. Microbiol. Biotechnol. 7:182–196 [DOI] [PubMed] [Google Scholar]
- 38. Inui M., et al. 2007. Transcriptional profiling of Corynebacterium glutamicum metabolism during organic acid production under oxygen deprivation conditions. Microbiology 153:2491–2504 [DOI] [PubMed] [Google Scholar]
- 39. Inui M., Terasawa M., Yukawa H. 1999. Encyclopedia of bioprocess technology: fermentation, biocatalysis, and bioseparation. Wiley, New York, NY [Google Scholar]
- 40. Jungwirth B., et al. 2008. Triple transcriptional control of the resuscitation promoting factor 2 (rpf2) gene of Corynebacterium glutamicum by the regulators of acetate metabolism RamA and RamB and the cAMP-dependent regulator GlxR. FEMS Microbiol. Lett. 281:190–197 [DOI] [PubMed] [Google Scholar]
- 41. Kim H. J., Kim T. H., Kim Y., Lee H. S. 2004. Identification and characterization of glxR, a gene involved in regulation of glyoxylate bypass in Corynebacterium glutamicum. J. Bacteriol. 186:3453–3460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kohl T. A., Baumbach J., Jungwirth B., Pühler A., Tauch A. 2008. The GlxR regulon of the amino acid producer Corynebacterium glutamicum: in silico and in vitro detection of DNA binding sites of a global transcription regulator. J. Biotechnol. 135:340–350 [DOI] [PubMed] [Google Scholar]
- 43. Kohl T. A., Tauch A. 2009. The GlxR regulon of the amino acid producer Corynebacterium glutamicum: detection of the corynebacterial core regulon and integration into the transcriptional regulatory network model. J. Biotechnol. 143:239–246 [DOI] [PubMed] [Google Scholar]
- 44. Kolb A., Busby S., Buc H., Garges S., Adhya S. 1993. Transcriptional regulation by cAMP and its receptor protein. Annu. Rev. Biochem. 62:749–795 [DOI] [PubMed] [Google Scholar]
- 45. Kotrbova-Kozak A., Kotrba P., Inui M., Sajdok J., Yukawa H. 2007. Transcriptionally regulated adhA gene encodes alcohol dehydrogenase required for ethanol and n-propanol utilization in Corynebacterium glutamicum R. Appl. Microbiol. Biotechnol. 76:1347–1356 [DOI] [PubMed] [Google Scholar]
- 46. Krin E., Sismeiro O., Danchin A., Bertin P. N. 2002. The regulation of enzyme IIAGlc expression controls adenylate cyclase activity in Escherichia coli. Microbiology 148:1553–1559 [DOI] [PubMed] [Google Scholar]
- 47. Kronemeyer W., Peekhaus N., Krämer R., Sahm H., Eggeling L. 1995. Structure of the gluABCD cluster encoding the glutamate uptake system of Corynebacterium glutamicum. J. Bacteriol. 177:1152–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kumagai H. 2000. Microbial production of amino acids in Japan. Adv. Biochem. Eng. Biotechnol. 69:71–85 [DOI] [PubMed] [Google Scholar]
- 49. Letek M., et al. 2006. Characterization and use of catabolite-repressed promoters from gluconate genes in Corynebacterium glutamicum. J. Bacteriol. 188:409–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Meyer M., Dimroth P., Bott M. 2001. Catabolite repression of the citrate fermentation genes in Klebsiella pneumoniae: evidence for involvement of the cyclic AMP receptor protein. J. Bacteriol. 183:5248–5256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Miller J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 52. Molle V., et al. 2003. The Spo0A regulon of Bacillus subtilis. Mol. Microbiol. 50:1683–1701 [DOI] [PubMed] [Google Scholar]
- 53. Muffler A., et al. 2002. Genome-wide transcription profiling of Corynebacterium glutamicum after heat shock and during growth on acetate and glucose. J. Biotechnol. 98:255–268 [DOI] [PubMed] [Google Scholar]
- 54. Mukamolova G. V., et al. 2002. The rpf gene of Micrococcus luteus encodes an essential secreted growth factor. Mol. Microbiol. 46:611–621 [DOI] [PubMed] [Google Scholar]
- 55. Nakamura J., Hirano S., Ito H., Wachi M. 2007. Mutations of the Corynebacterium glutamicum NCgl1221 gene, encoding a mechanosensitive channel homolog, induce l-glutamic acid production. Appl. Environ. Microbiol. 73:4491–4498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Neuweger H., et al. 2007. CoryneCenter—an online resource for the integrated analysis of corynebacterial genome and transcriptome data. BMC Syst. Biol. 1:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nishimura T., Teramoto H., Toyoda K., Inui M., Yukawa H. 2011. Regulation of nitrate reductase operon narKGHJI by cyclic AMP-dependent regulator GlxR in Corynebacterium glutamicum. Microbiology 157:21–28 [DOI] [PubMed] [Google Scholar]
- 58. Nottebrock D., Meyer U., Krämer R., Morbach S. 2003. Molecular and biochemical characterization of mechanosensitive channels in Corynebacterium glutamicum. FEMS Microbiol. Lett. 218:305–309 [DOI] [PubMed] [Google Scholar]
- 59. Park S. Y., Moon M. W., Subhadra B., Lee J. K. 2010. Functional characterization of the glxR deletion mutant of Corynebacterium glutamicum ATCC 13032: involvement of GlxR in acetate metabolism and carbon catabolite repression. FEMS Microbiol. Lett. 304:107–115 [DOI] [PubMed] [Google Scholar]
- 60. Partridge J. D., Bodenmiller D. M., Humphrys M. S., Spiro S. 2009. NsrR targets in the Escherichia coli genome: new insights into DNA sequence requirements for binding and a role for NsrR in the regulation of motility. Mol. Microbiol. 73:680–694 [DOI] [PubMed] [Google Scholar]
- 61. Perrenoud A., Sauer U. 2005. Impact of global transcriptional regulation by ArcA, ArcB, Cra, Crp, Cya, Fnr, and Mlc on glucose catabolism in Escherichia coli. J. Bacteriol. 187:3171–3179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Polen T., Schluesener D., Poetsch A., Bott M., Wendisch V. F. 2007. Characterization of citrate utilization in Corynebacterium glutamicum by transcriptome and proteome analysis. FEMS Microbiol. Lett. 273:109–119 [DOI] [PubMed] [Google Scholar]
- 63. Reddy M. C., et al. 2009. Structural insights into the mechanism of the allosteric transitions of Mycobacterium tuberculosis cAMP receptor protein. J. Biol. Chem. 284:36581–36591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rickman L., et al. 2005. A member of the cAMP receptor protein family of transcription regulators in Mycobacterium tuberculosis is required for virulence in mice and controls transcription of the rpfA gene coding for a resuscitation promoting factor. Mol. Microbiol. 56:1274–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Robison K., McGuire A. M., Church G. M. 1998. A comprehensive library of DNA-binding site matrices for 55 proteins applied to the complete Escherichia coli K-12 genome. J. Mol. Biol. 284:241–254 [DOI] [PubMed] [Google Scholar]
- 66. Sala C., et al. 2009. Genome-wide regulon and crystal structure of BlaI (Rv1846c) from Mycobacterium tuberculosis. Mol. Microbiol. 71:1102–1116 [DOI] [PubMed] [Google Scholar]
- 67. Sambrook J., Fritsch E. F., Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 68. Schröder J., Tauch A. 2010. Transcriptional regulation of gene expression in Corynebacterium glutamicum: the role of global, master and local regulators in the modular and hierarchical gene regulatory network. FEMS Microbiol. Rev. 34:685–737 [DOI] [PubMed] [Google Scholar]
- 69. Seibold G. M., et al. 2010. The transcriptional regulators RamA and RamB are involved in the regulation of glycogen synthesis in Corynebacterium glutamicum. Microbiology 156:1256–1263 [DOI] [PubMed] [Google Scholar]
- 70. Shenoy A. R., Sivakumar K., Krupa A., Srinivasan N., Visweswariah S. S. 2004. A survey of nucleotide cyclases in Actinobacteria: unique domain organization and expansion of the class III cyclase family in Mycobacterium tuberculosis. Comp. Funct. Genomics 5:17–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Skorupski K., Taylor R. K. 1997. Cyclic AMP and its receptor protein negatively regulate the coordinate expression of cholera toxin and toxin-coregulated pilus in Vibrio cholerae. Proc. Natl. Acad. Sci. U. S. A. 94:265–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Smith K. M., Cho K. M., Liao J. C. 2010. Engineering Corynebacterium glutamicum for isobutanol production. Appl. Microbiol. Biotechnol. 87:1045–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Stapleton M., et al. 2010. Mycobacterium tuberculosis cAMP receptor protein (Rv3676) differs from the Escherichia coli paradigm in its cAMP binding and DNA binding properties and transcription activation properties. J. Biol. Chem. 285:7016–7027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Studier F. W., Moffatt B. A. 1986. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol. 189:113–130 [DOI] [PubMed] [Google Scholar]
- 75. Tan K., Moreno-Hagelsieb G., Collado-Vides J., Stormo G. D. 2001. A comparative genomics approach to prediction of new members of regulons. Genome Res. 11:566–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tanaka Y., Teramoto H., Inui M., Yukawa H. 2008. Regulation of expression of general components of the phosphoenolpyruvate:carbohydrate phosphotransferase system (PTS) by the global regulator SugR in Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 78:309–318 [DOI] [PubMed] [Google Scholar]
- 77. Teramoto H., Inui M., Yukawa H. 2009. Regulation of expression of genes involved in quinate and shikimate utilization in Corynebacterium glutamicum. Appl. Environ. Microbiol. 75:3461–3468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Toyoda K., Teramoto H., Inui M., Yukawa H. 2008. Expression of the gapA gene encoding glyceraldehyde-3-phosphate dehydrogenase of Corynebacterium glutamicum is regulated by the global regulator SugR. Appl. Microbiol. Biotechnol. 81:291–301 [DOI] [PubMed] [Google Scholar]
- 79. Toyoda K., Teramoto H., Inui M., Yukawa H. 2009. Involvement of the LuxR-type transcriptional regulator, RamA, in regulation of expression of the gapA gene encoding glyceraldehyde-3-phosphate dehydrogenase of Corynebacterium glutamicum. J. Bacteriol. 191:968–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Toyoda K., Teramoto H., Inui M., Yukawa H. 2009. The ldhA gene, encoding fermentative l-lactate dehydrogenase of Corynebacterium glutamicum, is under the control of positive feedback regulation mediated by LldR. J. Bacteriol. 191:4251–4258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Toyoda K., Teramoto H., Inui M., Yukawa H. 2009. Molecular mechanism of SugR-mediated sugar-dependent expression of the ldhA gene encoding l-lactate dehydrogenase in Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 83:315–327 [DOI] [PubMed] [Google Scholar]
- 82. Tsuge Y., Ogino H., Teramoto H., Inui M., Yukawa H. 2008. Deletion of cgR_1596 and cgR_2070, encoding NlpC/P60 proteins, causes a defect in cell separation in Corynebacterium glutamicum R. J. Bacteriol. 190:8204–8214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. van Ooyen J., et al. 2011. Citrate synthase in Corynebacterium glutamicum is encoded by two gltA transcripts which are controlled by RamA, RamB, and GlxR. J. Biotechnol. 154:140–148 [DOI] [PubMed] [Google Scholar]
- 84. Vertès A. A., Inui M., Kobayashi M., Kurusu Y., Yukawa H. 1993. Presence of mrr- and mcr-like restriction systems in coryneform bacteria. Res. Microbiol. 144:181–185 [DOI] [PubMed] [Google Scholar]
- 85. Wendisch V. F., Bott M., Eikmanns B. J. 2006. Metabolic engineering of Escherichia coli and Corynebacterium glutamicum for biotechnological production of organic acids and amino acids. Curr. Opin. Microbiol. 9:268–274 [DOI] [PubMed] [Google Scholar]
- 86. Wendisch V. F., de Graaf A. A., Sahm H., Eikmanns B. J. 2000. Quantitative determination of metabolic fluxes during coutilization of two carbon sources: comparative analyses with Corynebacterium glutamicum during growth on acetate and/or glucose. J. Bacteriol. 182:3088–3096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wolfgang M. C., Lee V. T., Gilmore M. E., Lory S. 2003. Coordinate regulation of bacterial virulence genes by a novel adenylate cyclase-dependent signaling pathway. Dev. Cell 4:253–263 [DOI] [PubMed] [Google Scholar]
- 88. Yukawa H., et al. 2007. Comparative analysis of the Corynebacterium glutamicum group and complete genome sequence of strain R. Microbiology 153:1042–1058 [DOI] [PubMed] [Google Scholar]
- 89. Zhang Z., et al. 2005. Functional interactions between the carbon and iron utilization regulators, Crp and Fur, in Escherichia coli. J. Bacteriol. 187:980–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zheng D., Constantinidou C., Hobman J. L., Minchin S. D. 2004. Identification of the CRP regulon using in vitro and in vivo transcriptional profiling. Nucleic Acids Res. 32:5874–5893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zubay G., Schwartz D., Beckwith J. 1970. Mechanism of activation of catabolite-sensitive genes: a positive control system. Proc. Natl. Acad. Sci. U. S. A. 66:104–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.