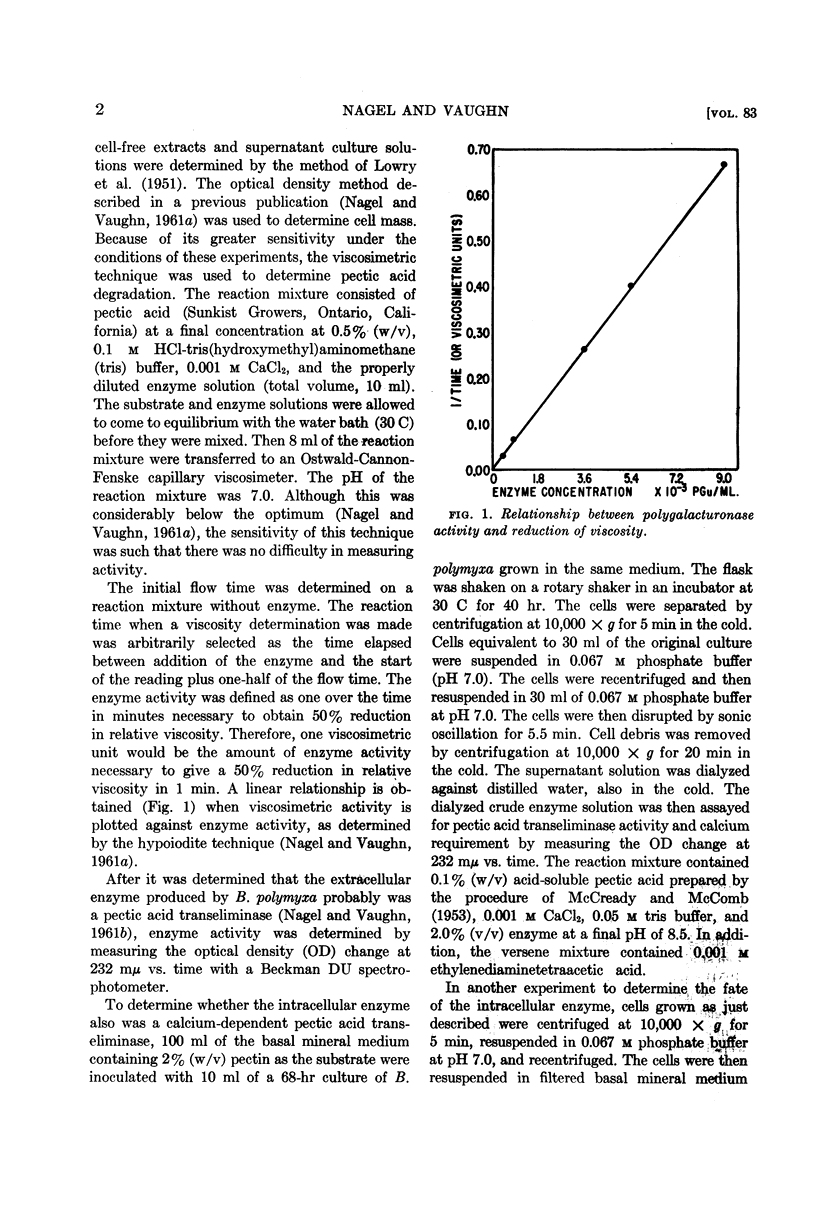
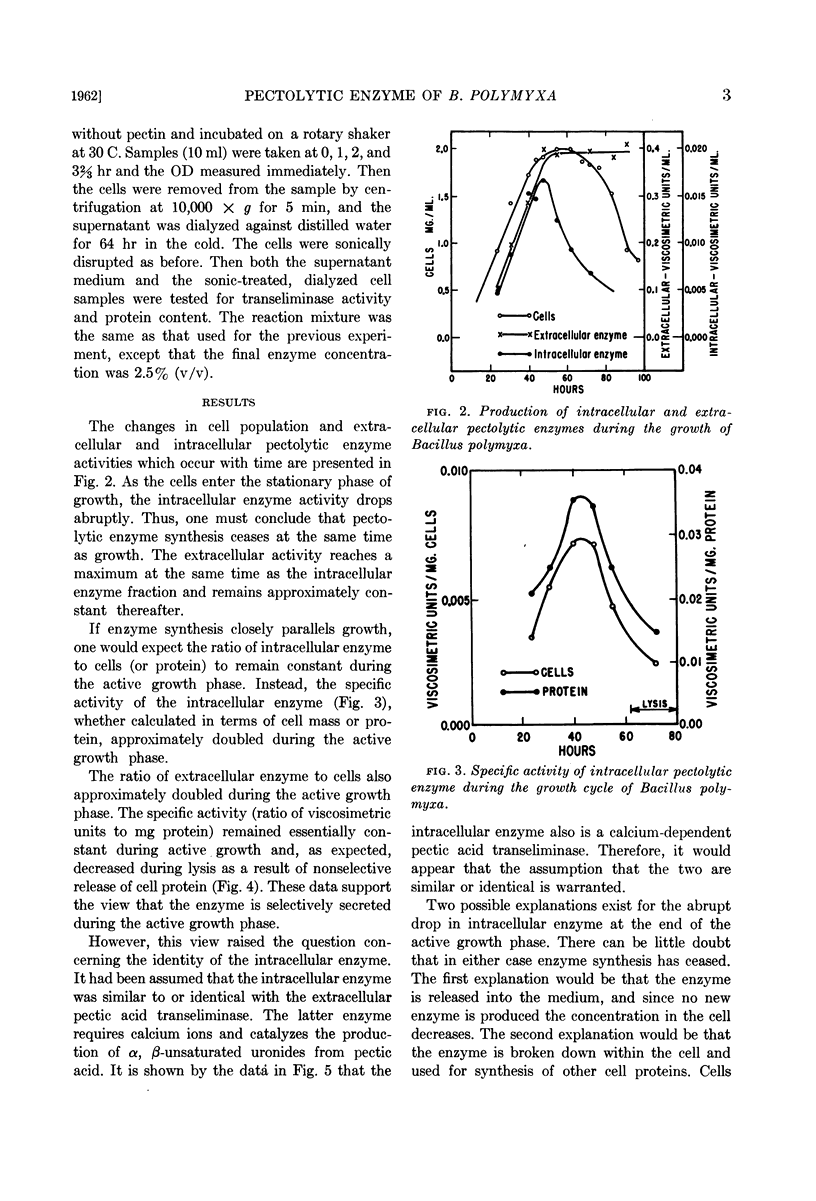
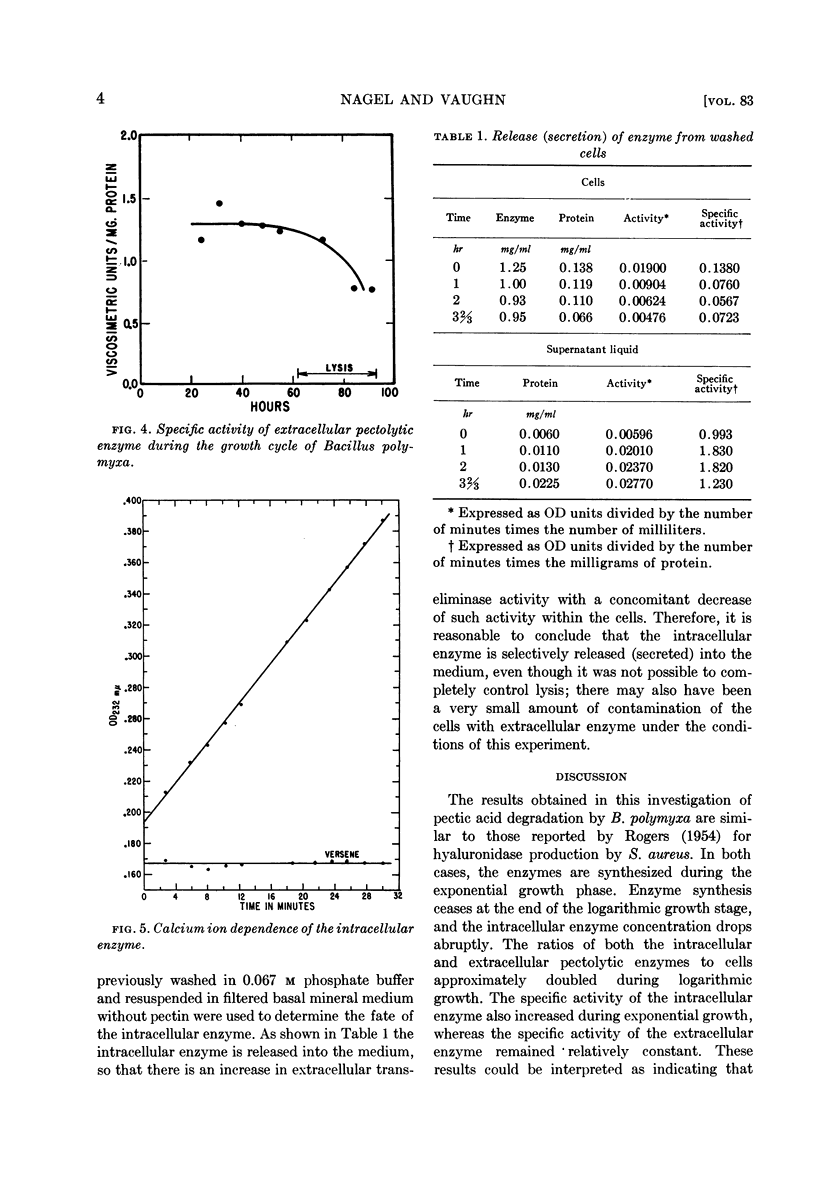
Abstract
Nagel, Charles W. (University of California, Davis) and Reese H. Vaughn. Comparison of growth and pectolytic enzyme production by Bacillus polymyxa. J. Bacteriol. 83:1–5. 1962.—Studies were made of pectolytic enzyme production by Bacillus polymyxa during growth. It was found that elaboration of enzyme occurred during logarithmic growth and ceased when the stationary phase was reached. The specific activity of the extra-cellular enzyme remained relatively constant until lysis occurred. The increased specific activity of the intracellular pectolytic enzyme may be explained if one assumes that the rate of secretion of the intracellular enzyme is dependent upon the concentration of the extracellular enzyme. The concentration of the intracellular pectolytic enzyme dropped markedly at the end of the logarithmic growth phase; the enzyme was released into the medium during the stationary growth phase and subsequent lysis of the cells. It was shown that the intra- and extracellular enzymes were similar or identical in that both were calcium-dependent pectic acid transeliminases.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DWORSCHACK R. G., WICKERHAM L. J. Production of extracellular invertase by the yeast, Saccharomyces uvarum NRRL Y-972. Arch Biochem Biophys. 1958 Aug;76(2):449–456. doi: 10.1016/0003-9861(58)90170-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MANDELS G. R. Synthesis and secretion of invertase in relation to the growth of Myrothecium verrucaria. J Bacteriol. 1956 Jun;71(6):684–688. doi: 10.1128/jb.71.6.684-688.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGEL C. W., VAUGHN R. H. The characteristics of a polygalacturonase produced by Bacillus polymyxa. Arch Biochem Biophys. 1961 May;93:344–352. doi: 10.1016/0003-9861(61)90277-6. [DOI] [PubMed] [Google Scholar]
- NAGEL C. W., VAUGHN R. H. The degradation of oligogalacturonides by the polygalacturonase of Bacillus polymyxa. Arch Biochem Biophys. 1961 Aug;94:328–332. doi: 10.1016/0003-9861(61)90047-9. [DOI] [PubMed] [Google Scholar]
- ROGERS H. J. The rate of formation of hyaluronidase, coagulase and total extracellular protein by strains of Staphylococcus aureus. J Gen Microbiol. 1954 Apr;10(2):209–220. doi: 10.1099/00221287-10-2-209. [DOI] [PubMed] [Google Scholar]
- WICKERHAM L. J. Evidence of the production of extracellular invertase by certain strains of yeasts. Arch Biochem Biophys. 1958 Aug;76(2):439–448. doi: 10.1016/0003-9861(58)90169-3. [DOI] [PubMed] [Google Scholar]


