Abstract
Kaposi's sarcoma-associated herpesvirus (KSHV) latency-associated nuclear antigen (LANA) is a 1,162-amino-acid protein that acts on viral terminal repeat (TR) DNA to mediate KSHV episome persistence. The two essential components of episome persistence are DNA replication prior to cell division and episome segregation to daughter nuclei. These functions are located within N- and C-terminal regions of LANA. N- and C-terminal regions of LANA are sufficient for TR DNA replication. In addition, N- and C-terminal regions of LANA tether episomes to mitotic chromosomes to segregate episomes to progeny cell nuclei. To generate a tethering mechanism, N-terminal LANA binds histones H2A/H2B to attach to mitotic chromosomes, and C-terminal LANA binds TR DNA and also associates with mitotic chromosomes. Here, we test the importance of the internal LANA sequence for episome persistence. We generated LANA mutants that contain N- and C-terminal regions of LANA but have most of the internal sequence deleted. As expected, the LANA mutants bound mitotic chromosomes in a wild-type pattern and also bound TR DNA as assayed by electrophoretic mobility shift assays (EMSA). The mutants mediated TR DNA replication, although with reduced efficiency compared with LANA. Despite the ability to replicate DNA and exert the chromosome and DNA binding functions necessary for segregating episomes to daughter nuclei, the mutants were highly deficient for the ability to mediate both short- and long-term episome persistence. These data indicate that internal LANA sequence exerts a critical effect on its ability to maintain episomes, possibly through effects on TR DNA replication.
INTRODUCTION
Kaposi's sarcoma-associated herpesvirus (KSHV), also termed human herpesvirus 8, is a gamma-2-herpesvirus that is tightly associated with Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease (10, 11, 49, 63). KSHV infection is predominantly latent, and during latency, only a small subset of viral genes is expressed. Latently infected cells have multiple copies of the viral genome maintained as extrachromosomal circular DNA (episomes) in the nuclei of cells (10, 14). Latency-associated nuclear antigen (LANA) is one of the viral genes expressed during latency and is necessary and sufficient for episome persistence in the absence of other viral genes (3, 4).
LANA permits episome persistence by mediating the replication of, and tethering of the KSHV terminal repeats (TRs) to, host cell chromosomes. Both the N- and C-terminal regions of LANA are essential for episome maintenance. The C-terminal region of LANA (herein termed C-terminal LANA) binds to two adjacent sites in each TR, and this binding is essential for TR DNA replication and episome persistence (4, 13, 16, 21, 22, 24, 26, 33, 45). LANA also associates with mitotic chromosomes (3, 34, 55, 66) through two independent chromosome binding regions located within N- and C-terminal LANA (5, 30, 31, 34, 43, 55, 71) (Fig. 1). N-terminal LANA binds to mitotic chromosomes by directly interacting with histones H2A/H2B, and this interaction is important for DNA replication and essential for episome maintenance (5, 6, 24, 26, 42, 43, 45, 69). C-terminal LANA binds to pericentromeric and peritelomeric regions of mitotic chromosomes, and this binding also contributes to episome persistence, although the effect can be detected only when N-terminal LANA chromosome binding is compromised (30–32).
Fig. 1.
Schematic diagram of KSHV LANA and LANA deletion mutants used in this investigation. Indicated are the proline-rich region (P), the aspartate- and glutamate-rich region (DE), the glutamine- and glutamate-rich region (Q) and the putative leucine zipper (LZ). The DE, Q, and LZ regions all contain repeat elements. The shaded region represents the N-terminal nuclear localization (NLS) signal. C-terminal LANA can also localize to nuclei but an NLS has not been precisely mapped. Amino acids 5 to 13 mediate chromosome association through interaction with histones H2A/H2B. Amino acids 996 to 1139 contain the TR DNA binding, self-association, and chromosome association functions. Capabilities for TR DNA binding, chromosome association, DNA replication, and episome persistence for each of the constructs are summarized at the right. Fractions are the numbers of G418-resistant cell lines containing episomes over the total number of G418-resistant cell lines assayed by Gardella analysis, and percentages are shown in parentheses.
Fusion of N-terminal LANA with C-terminal LANA is predicted to be capable of episome maintenance. For episomes to persist, they must replicate with each cell division and segregate to progeny nuclei. N- and C-terminal regions of LANA are expected to provide both of these functions. Fusion of at least the N-terminal 23 amino acids to the C-terminal LANA DNA binding domain is sufficient for LANA-mediated TR DNA replication (24, 26, 42, 43, 45, 69). In addition, in some reports C-terminal LANA alone is capable of DNA replication, although at a reduced level (26, 69). Further, N-terminal chromosome binding and C-terminal TR DNA binding are the critical components for tethering KSHV episomes to chromosomes and therefore are expected to segregate DNA to daughter nuclei (5, 33).
This work investigates the importance of the LANA internal regions for episome persistence. We generated fusions of N- and C-terminal regions of LANA which have the internal LANA regions deleted. Results indicate that although the N- and C-terminal LANA fusions can maintain KSHV episomes, they do so with greatly reduced efficiency. Therefore, the internal LANA domains exert an important role in episome maintenance.
MATERIALS AND METHODS
Cell lines.
BJAB cells were maintained in RPMI medium containing 10% bovine growth serum (BGS) (HyClone) or Fetalplex (Gemini) and 15 μg/ml gentamicin. KSHV-infected BCBL-1 cells were maintained in RPMI medium containing 20% BGS or Fetalplex and 15 μg/ml gentamicin.
Plasmids.
pSG5 oligonucleotide was generated by inserting a linker into pSG5 (Strategene) between EcoRI and BamHI. The linker was generated using oligonucleotides pSG5 oligo-F and pSG5 oligo-R, listed in Table 1; this linker adds restriction sites SacI, NotI, HpaI, and EcoRV. pT7 was generated by removing the enhanced green fluorescence protein (EGFP) gene from pEGFP-c1 (Clonetech) by using NheI and BspeI and inserting an oligonucleotide encoding the T7 phage promoter sequence, a Kozak consensus sequence, and the T7 epitope tag sequence (Table 1, oligonucleotides pT7-F and pT7-R). pT7 LANA was generated by inserting an SmaI restriction fragment from GFP LANA (5) into the SmaI site from pT7, which leaves the multicloning site from pEGFP-c1 intact between the T7 epitope tag and the SmaI site in LANA and results in the deletion of the first four LANA amino acids and the loss of GFP. T7 epitope-tagged LANA mutants were generated by PCR using the oligonucleotides listed in Table 1 and contained a four-alanine-residue linker in place of the deletions. T7LANA 5 to 32 (T7LANA 5-32) was amplified from pT7LANA using the primers NotI T7 tag-F and LANA 32 Ala-R. LANA 930-1162, LANA 950-1162, and LANA 900-1162 were amplified with the corresponding forward primers and LANA 1162 EcoRV-R. LANA 889-1162 and LANA 900-1162 were amplified using the same forward primer (LANA 900Ala-F), which falls within a repeat sequence and therefore was able to prime both amplifications. The products of the PCR for the N- and C-terminal LANAs were combined and amplified with NotI T7 tag-F and LANA 1162 EcoRV-R to generate T7LANAΔ33-929, T7LANAΔ33-949, T7LANAΔ33-888, and T7LANAΔ33-899. The PCR products were then digested and ligated into the NotI and EcoRV restriction sites of pSG5 oligonucleotide to generate pSG5 oligonucleotide T7LANAΔ33-929, pSG5 oligonucleotide T7LANAΔ33-949, pSG5 oligonucleotide T7LANAΔ33-888, and pSG5 oligonucleotide T7LANAΔ33-899. LANA sequences were all confirmed. pSG5 oligonucleotide T7LANA was constructed by subcloning the HindIII/NruI fragment from pT7LANA into the HindIII/NruI restriction sites of a pSG5 oligonucleotide T7LANA N- and C-terminal fusion mutant. pSG5 oligonucleotide T7LANAΔ465-497 was constructed by digesting pSG5 oligonucleotide T7LANA with PstI and religating the fragments. p8TR contains eight copies of the KSHV terminal repeat unit (TR) cloned into pRep9 (Invitrogen), which was modified by deleting the sequence between ClaI and KpnI (3, 5).
Table 1.
Oligonucleotides used for cloning
| Oligonucleotide | Sequence |
|---|---|
| pSG5 oligo-F | AATTCAGAGCTCATCGCGGCCGCGTTAACAGATATCAAG |
| pSG5 oligo-R | GATCCTTGATATCTGTTAACGCGGCCGCGATGAGCTCTG |
| pT7-F | CTAGCTAATACGACTCACTATAGGGAGACCACCATGGCATCGATGACAGGTGGCCAACAGATGGGTT |
| pT7-R | CCGGAACCCATCTGTTGGCCACCTGTCATCGATGCCATGGTGGTCTCCCTATAGTGAGTCGTATTAG |
| NotI T7 tag-F | ATAAGAATGCGGCCGCCCACCATGGCATCGATGACAGGTGGC |
| LANA 1162 EcoRV-R | TGATATCTTATGTCATTTCCTGTGGAGAGTCCC |
| LANA 32Ala-R | TGCTGCTGCTGC TCTTTCCGGAGACCTGTTTCG |
| LANA 950Ala-F | GCAGCAGCAGCAGTGGATTACCCTGTTGTTAGCACACATGAA |
| LANA 930Ala-F | GCAGCAGCAGCA CAGGAGACGGTGGAAGAGC |
| LANA 900Ala-F | GCAGCAGCAGCAGAGCAGGAGTTAGAGGAGGTGGAA |
Generation of BJAB cells stably expressing LANA proteins.
BJAB cells were transfected in 400 μl of RPMI medium at 200 V and 960 μF in a 0.4-cm-gap cuvette with a Bio-Rad electroporator (3). pSG5 oligonucleotide plasmids encoding T7LANA and the T7LANA deletion mutants were cotransfected with a plasmid carrying the hygromycin resistance gene downstream of a simian virus 40 promoter into BJAB cells. After 48 h, cells were seeded into 96-well plates and selected for hygromycin resistance. Clones resistant to hygromycin were screened for LANA expression.
Fluorescence microscopy.
For metaphase spreads, 0.5 × 106 cells/ml were incubated overnight in 1 μg/ml of Colcemid (Calbiochem). Colcemid-treated cells were swollen in hypotonic buffer for 5 min (1% sodium citrate, 10 mM CaCl2, 10 mM MgCl2), spread onto slides by cytospin (Thermoshandon), and fixed for 10 min in 4% paraformaldehyde (Polysciences) in phosphate-buffered saline. To detect LANA or the LANA mutants, cell spreads were incubated with anti-T7-tag monoclonal antibody (Novagen) or anti-LANA monoclonal antibody (ABI). For secondary antibody, anti-mouse Alexa Fluor 488 or anti-rat Alexa Fluor 488 (Molecular Probes) was used. Cells were counterstained with propidium iodide (Molecular Probes) (1 μg/ml), and coverslips were applied with Aqua-Poly mount (Polysciences). Microscopy was performed with a Zeiss Axioskop, PCM2000 hardware, and C-imaging software (Compix, Inc.).
DNA replication assay.
For DNA replication assays, 10 × 106 cells were transfected with 5 μg of p8TR, using Amaxa nucleofactor program O-17 and solution V. After transfection, cells were seeded into 1 well of a 6-well plate in 5 ml of medium. Twenty-four hours after transfection, cells were transferred to 25-cm2 flasks and placed at 0.4 × 106 cells/ml. Seventy-two hours posttransfection, low-molecular-weight DNA was harvested from cells by the Hirt method (25). Five micrograms of Hirt DNA was digested overnight with 40 U of BglII, and 30 μg of DNA was overdigested overnight with 100 U of BglII and 80 U of DpnI. Digested DNA was resolved in a 0.7% agarose gel, and Southern blotting was performed with a 32P-labeled TR probe.
EMSA.
For electrophoretic mobility shift assays (EMSA), LANA and LANA mutants were in vitro translated using TNT Quick-coupled reticulocyte lysate systems (Promega). Similar amounts of in vitro-translated proteins, as determined by Western blotting, were incubated in DNA binding buffer [20 mM Tris (pH 7.5), 10% glycerol, 50 mM KCl, 0.1 mM dithiothreitol, 10 mM MgCl2, 1 mM EDTA, 20 μg/ml of poly(dI-dC)] with 50,000 counts per minute of 32P-labeled TR-13 or 32P-labeled Ti7 oligonucleotide for 30 min at room temperature. TR-13 is a 20-nucleotide LANA binding sequence (4), and Ti7 is a mutated LANA binding site that varies from TR-13 by a single C→T transition that abolishes LANA binding (64). For electrophoretic mobility supershift assays, after the 30-min incubation, samples were incubated for 15 min at room temperature with 1 μg of anti-T7 tag antibody (Novagen) or 1 μg of mouse IgG as an isotype-matched control. Bound complexes were resolved on a 4% nondenaturing polyacrylamide gel. The signal was detected by autoradiography.
Selection of G418-resistant cells and Gardella gel analyses.
Control BJAB cells and BJAB cells stably expressing T7LANA or T7LANA deletion mutants were cotransfected with 5 μg GFP and 30 μg p8TR by using a Bio-Rad electroporator as described above. Forty-eight to 72 h posttransfection, LANA or mutated LANA protein expression was assayed by Western blotting, and cells were seeded in 96-well plates at 1,000 cells/well in medium containing G418 (600 μg/ml) (Gibco or Gemini). G418-resistant cells were then expanded.
Gardella analysis was performed on G418-resistant clones. Cells were loaded into loading gel wells made of agarose containing DNase-free protease (Sigma) and sodium dodecyl sulfate (23) and in situ lysis of cells occurs as electrophoresis in Tris-borate-EDTA buffer begins. DNA was transferred to a nylon membrane, and KSHV DNA was detected using a 32P-labeled TR probe.
For bulk culture Gardella analyses, cells were transfected with 10 μg p8TR using Amaxa nucleofection as described above. Amaxa was used since cell survival was higher than after electroporation. After transfection, cells were seeded into 1 well of a 6-well plate in 5 ml of medium. Twenty-four hours after transfection, cells were transferred to 175-cm2 flasks, and cells were kept in log phase by seeding them at 0.3 × 106 cells/ml and then reseeding at 0.3 × 106 cells/ml 48 h posttransfection. Seventy-two hours posttransfection, the medium was changed to RPMI with G418 (600 μg/ml); cells were then fed with G418-containing medium every other day, and cells were reseeded to 0.3 × 106 cells/ml with each feeding. In some cases where cells were not growing, or were growing only slowly, cells required centrifugation and resuspension in smaller volumes for seeding at 0.3 × 106 cells/ml. Cells were initially maintained in 100 to 150 ml of medium in 175-cm2 flasks until the growth rate increased, at which point volumes of 50 to 100 ml were maintained. Gardella analyses were performed with cells harvested at different time points. The cells for Gardella analysis were either used directly for Gardella analysis or stored frozen at −80°C prior to analysis. Cell pellets were frozen after centrifugation and aspiration of medium. Pellets were frozen at 4, 8, 12, and 16 days under G418 selection prior to Gardella analysis (see Fig. 6C to E and G) but not frozen at other time points. Freezing cells had no effect on Gardella results as evidenced by the lack of detectable difference after loading live PEL cells and loading the same PEL cell line that had been frozen for up to 13 days (data not shown).
Fig. 6.
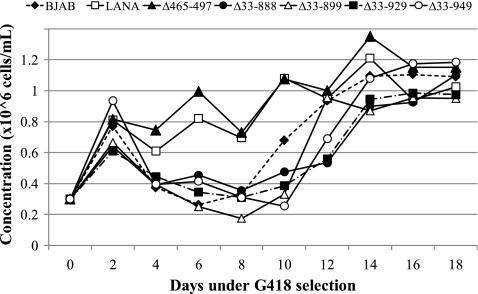
Outgrowth of p8TR-transfected samples in bulk culture. BJAB (control) and BJAB cells stably expressing LANA and LANA deletion mutants were transfected with p8TR using Amaxa nucleofection. After transfection, cells were seeded in 6-well plates. Twenty-four hours posttransfection, cells were transferred to 175-cm2 flasks and seeded at 0.3 × 106 cells/ml. Seventy-two hours posttransfection, cells were placed under G418 selection (600 μg/ml) at a concentration of 0.3 × 106 cells/ml. Cells were then fed every other day and placed at 0.3 × 106 cells/ml with each feeding. Cell concentration was recorded before the cells were cut back to 0.3 × 106 cells/ml in fresh RPMI with G418. The data are an average of two experiments and include the experiment from Fig. 7.
RESULTS
N- and C-terminal LANA fusions retain the ability to associate with mitotic chromosomes.
Both N- and C-terminal regions of LANA are essential for episome maintenance, but the importance of the internal LANA regions for episome persistence is not known. In order to stably persist in cells, KSHV episomes must replicate and efficiently segregate to daughter cell nuclei during each cell division. Efficient segregation is achieved by LANA-mediated tethering of the KSHV episome to the host chromosome; therefore, mitotic chromosome association is an essential process for KSHV episome maintenance. N-terminal LANA is the dominant effector for chromosome association, and C-terminal LANA also has a role in chromosome binding (5, 6, 30–32, 34, 40, 55, 71). C-terminal LANA also binds specific sequence in the TR, and this binding is essential for LANA-mediated DNA replication and tethering episomes to mitotic chromosomes (4, 13, 16, 21, 22, 24, 26, 33, 45).
In order to investigate whether the essential LANA N- and C-terminal regions are also sufficient for episome persistence, we generated a panel of LANA mutants containing only N- and C-terminal regions of LANA and termed LANAΔ33–888, LANAΔ33–899, LANAΔ33–929, and LANAΔ33–949 (Fig. 1). All have the same N-terminal region, but different C-terminal fusions were used in order to test the importance of LANA residues 889 to 949 at the N-terminal end of the C-terminal domain. As a control, we generated a smaller (33-amino-acid) deletion in the glutamine-rich repeat region, termed LANAΔ465–497. These mutants all contain the LANA chromosome association and DNA binding regions. BJAB cells stably expressing each of the LANA mutants were generated.
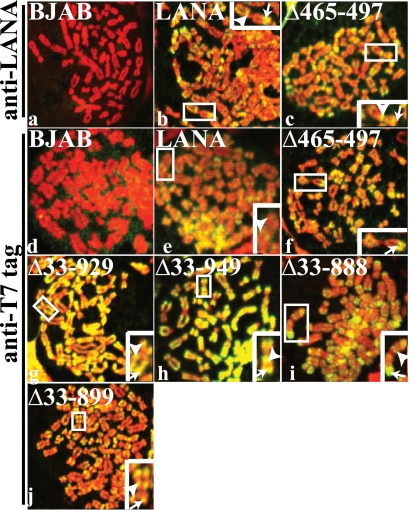
Although all mutants contain the known LANA chromosome binding regions, we wished to ensure that large internal deletions did not disrupt chromosome binding. Control BJAB cells or BJAB cells stably expressing the different LANA mutants (Fig. 1) were metaphase arrested by overnight treatment with colcemid, and LANA subcellular localization was analyzed by confocal microscopy. LANA (green) was detected with anti-LANA or anti-T7 epitope tag antibody; mitotic chromosomes were detected with propidium iodide (red). No LANA staining was present in control BJAB cells (Fig. 2a and d). Consistent with previous data (32), LANA associated with mitotic chromosomes (colocalization of green and red results in yellow) with concentration near the telemores and centromeres (Fig. 2b, arrow, peritelomeric staining; arrowhead, pericentromeric staining). LANAΔ465–497 (Fig. 2c and f), LANAΔ33–929 (Fig. 2g), LANAΔ33–949 (Fig. 2h), LANAΔ33–888 (Fig. 2i), and LANAΔ33–899 (Fig. 2j) also associated with mitotic chromosomes in a similar pattern, demonstrating that deletion of the internal domains and fusion of the N- and C-terminal regions of LANA did not affect LANA's ability to interact with chromosomes.
Fig. 2.
LANA and LANA deletion mutants associate with mitotic chromosomes. Control BJAB cells or BJAB cells stably expressing LANA, LANAΔ465–497, LANAΔ33–888, LANAΔ33–899, LANAΔ33–929, or LANAΔ33–949 were metaphase arrested with Colcemid and analyzed for LANA localization by confocal microscopy. LANA (green) was detected with anti-LANA antibody or anti-T7 epitope tag antibody. Chromosomes were counterstained with propidium iodide (red). The overlay of green and red generates yellow. Insets show enlargements of boxed chromosomes. Arrowheads indicate pericentromeric staining, and arrows indicate peritelomeric staining. Magnification, ×630.
N- and C-terminal LANA fusions retain the ability to bind KSHV TR DNA.
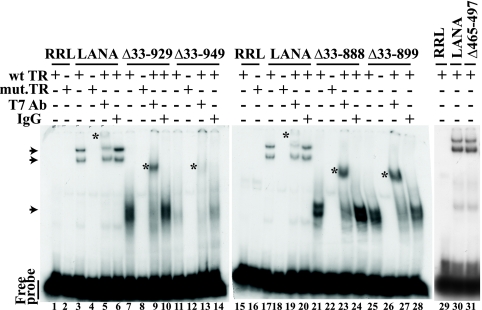
Since C-terminal LANA binding to KSHV TR DNA is essential for episome persistence, we investigated the ability of LANA mutants to bind TR DNA in electrophoretic mobility shift assays (EMSA). LANA, or each LANA mutant, was in vitro translated and incubated with radiolabeled oligonucleotide sequence containing the LANA TR binding site or with a probe containing a single-base-pair substitution that abolishes LANA binding. Consistent with previous results (4, 64), the incubation of LANA with TR resulted in two major complexes (Fig. 3, lanes 3, 17, and 30; upper two arrowheads indicate complexes). The intrinsic differences between these complexes are currently unknown. In contrast, incubation of LANA with the mutated TR sequence did not form specific complexes (Fig. 3, lanes 4 and 18), similar to incubation of the probe with rabbit reticulocyte lysate (RRL) (Fig. 3, lanes 1, 2, 15, 16, and 29). LANAΔ33–929 (Fig. 3, lane 7), LANAΔ33–949 (Fig. 3, lane 11), LANAΔ33–888 (Fig. 3, lane 21), LANAΔ33–899 (Fig. 3, lane 25), and LANAΔ465–497 (Fig. 3, lane 31) also formed complexes with TR. Interestingly, both LANAΔ33–888 and LANAΔ33–899 formed two closely migrating complexes, like LANA, while LANAΔ33–929 and LANAΔ33–949 formed only one broad complex, indicating that the formation of the two complexes is due to LANA amino acids 900 to 929. The signal from the LANAΔ33–949 complex was somewhat reduced compared to that from LANA and the other mutants, consistent with modestly reduced DNA binding despite the presence of all residues necessary for DNA binding (33). Incubation with the mutated TR probe also abolished specific complex formation with LANAΔ33–929 (Fig. 3, lane 8), LANAΔ33–949 (Fig. 3, lane 12), LANAΔ33–888 (Fig. 3, lane 22), and LANAΔ33–899 (Fig. 3, lane 26).
Fig. 3.
Deletion of the internal LANA regions does not abolish DNA binding as detected by electrophoretic mobility shift assays (EMSA). Radiolabeled wild-type TR DNA (TR-13) probe (wt TR) containing the LANA high-affinity binding site was incubated with rabbit reticulocyte lysate (RRL) (lanes 1, 15, 29) or with in vitro-translated LANA (lanes 3, 17, 30), LANAΔ33–929 (lane 7), LANAΔ33–949 (lane 11), LANAΔ33–888 (lane 21), LANAΔ33–899 (lane 25), or LANAΔ465–497 (lane 31). Radiolabeled mutated TR DNA (Ti7) probe (mut. TR), which differs from TR-13 by a single base pair and abolishes the interaction with LANA, was incubated with RRL (lanes 2 and 16), LANA (lanes 4, 18), LANAΔ33–929 (lane 8), LANAΔ33–949 (lane 12), LANAΔ33–888 (lane 22), or LANAΔ33–899 (lane 26). Supershift analyses were performed after incubation with anti-T7 epitope tag antibody with LANA (lanes 5 and 19), LANAΔ33–929 (lane 9), LANAΔ33–949 (lane 13), LANAΔ33–888 (lane 23), or LANAΔ33–899 (lane 27). Incubation with IgG isotype-matched control for anti-T7 antibody did not result in supershifts for LANA (lanes 6 and 20), LANAΔ33–929 (lane 10), LANAΔ33–949 (lane 14), LANAΔ33–888 (lane 24), or LANAΔ33–899 (lane 28). Free probe is shown. Arrowheads indicate LANA-TR complexes or mutated LANA-TR complexes. Asterisks indicate supershifted complexes.
To further confirm LANA DNA binding, supershift assays were performed after the incubation of complexes with anti-T7 antibody or isotype-matched control IgG. As expected, incubation with anti-T7 antibody resulted in a supershifted complex for LANA (Fig. 3, lanes 5 and 19, asterisks). Anti-T7 antibody also induced supershifted complexes for LANAΔ33–929 (Fig. 3, lane 9, asterisk), LANAΔ33–949 (Fig. 3, lane 13, asterisk), LANAΔ33–888 (Fig. 3, lane 23, asterisk), and LANAΔ33–899 (Fig. 3, lane 27, asterisk). Incubation with isotype-matched control antibody did not affect the migration of the complexes for LANA (Fig. 3, lanes 6 and 20), LANAΔ33–929 (Fig. 3, lane 10), LANAΔ33–949 (Fig. 3, lane 14), LANAΔ33–888 (Fig. 3, lane 24), and LANAΔ33–899 (Fig. 3, lane 28). Therefore, each mutant maintained the ability to specifically bind TR DNA. Further, the ability to form two separately migrating LANA complexes is due to the presence of amino acids 900 to 929.
LANA mutants mediate TR DNA replication.
Another essential aspect of KSHV episome maintenance is LANA-mediated replication of the KSHV episomes with each cell division. C-terminal LANA binds to the KSHV terminal repeat (TR) sequence to mediate replication (21, 24, 26, 33, 45). The N-terminal LANA chromosome binding region is also critical for DNA replication (5, 26, 40, 69). Previous work has shown that the fusion of N- and C-terminal LANAs is sufficient to mediate the replication of TR-containing plasmids after transient transfection (43, 45, 69). We investigated whether BJAB cells stably expressing LANA or the LANA mutants could mediate replication of a plasmid containing 8 copies of the KSHV TR sequence (p8TR). For this replication assay, a TR plasmid that has been purified from Dam methylase-positive bacteria is used, resulting in the DNA being susceptible to DpnI digestion, since DpnI requires Dam methylation for digestion. Mammalian cells lack Dam methylase, and, therefore, DNA that has undergone replication after transfection is resistant to DpnI digestion.
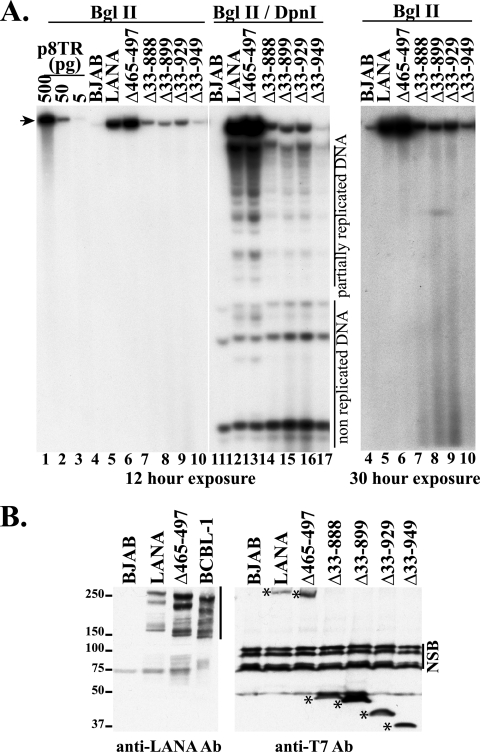
p8TR was transfected into control BJAB cells and BJAB cells expressing LANA, LANAΔ465–497, LANAΔ33–888, LANAΔ33–899, LANAΔ33–929, and LANAΔ33–949. Three days posttransfection, low-molecular-weight DNA was isolated using the Hirt method. Similar amounts of Hirt DNA were digested with BglII, which linearizes p8TR (Fig. 4A, lanes 4 through 10), or with BglII and DpnI to detect replicated DNA (Fig. 4A, lanes 11 through 17). As expected, DpnI-resistant p8TR DNA (replicated DNA) was detected in cells stably expressing wild-type (wt) LANA (Fig. 4A, lane 12, at level of arrowhead) but not in the negative control BJAB (Fig. 4A, lane 11). Replicated p8TR was also detected in cells stably expressing LANAΔ465–497 (Fig. 4A, lane 13). Cells stably expressing LANAΔ33–888 (Fig. 4A, lane 14), LANAΔ33–899 (Fig. 4A, lane 15), and LANAΔ33–929 (Fig. 4A, lane 16) had similar amounts of replicated p8TR DNA, but the amount detected was lower than that in cells expressing wild-type LANA or LANAΔ465–497 (Fig. 4A, compare lanes 14 through 16 to lanes 12 and 13). Cells expressing LANAΔ33–949 (Fig. 4, lane 17) had the least amount of replicated p8TR DNA. The lower levels of replicated p8TR DNA were not due to lower expression levels of the LANA mutants in these cell lines. LANAΔ33–888, LANAΔ33–899, LANAΔ33–929, and LANAΔ33–949 were each expressed at levels at least as high as those for LANA or LANAΔ465–497 (Fig. 4B). This higher level of expression is due to the deletion of the LANA central repeat elements, which inhibit its translation (36).
Fig. 4.
(A) Detection of p8TR replication mediated by LANA and LANA deletion mutants. Control BJAB cells, or BJAB cells stably expressing LANA or the LANA deletion mutants, were transfected with p8TR. Seventy-two hours posttransfection, low-molecular-weight DNA was isolated using the Hirt extraction method. Isolated DNA was digested with BglII (lanes 4 through 10) or BglII and DpnI (lanes 11 through 17), resolved in a 0.8% agarose gel, blotted onto nylon membrane, and detected by Southern blotting with radiolabeled TR probe. Lanes 1 through 3 contain the indicated amounts of p8TR plasmid digested with BglII. BJAB (lanes 4 and 11), LANA (lanes 5 and 12), LANAΔ465–497 (lanes 6 and 13), LANAΔ33–888 (lanes 7 and 14), LANAΔ33–899 (lanes 8 and 15), LANAΔ33–929 (lanes 9 and 16), and LANAΔ33–949 (lanes 10 and 17) cells are shown. The arrowhead indicates linearized p8TR. Nonreplicated (DpnI-sensitive) DNA and partially replicated DNA are indicated. Exposure to film at the left was for 12 h, and the panel at the right is the same blot for lanes 4 through 10, but after a 30-h exposure. This experiment is representative of two experiments. (B) Western blot for LANA and LANA mutants. BJAB cells, BCBL-1 PEL cells, or BJAB cells stably expressing LANA, LANAΔ465–497, LANAΔ33–888, LANAΔ33–899, LANAΔ33–929, or LANAΔ33–949 were assessed. LANA immune serum is more sensitive than T7 antibody, since it detects multiple LANA epitopes including within-repeat elements, so 150,000 cells per lane were used for this antibody, while 350,000 cells per lane were used for the T7 antibody blot. The vertical line indicates LANA, which includes full-length and also faster-migrating forms detected with LANA antibody. Asterisks indicate LANA and LANA mutants detected with T7 antibody. Nonspecific bands (NSB) are indicated.
Total p8TR plasmid linearized after digestion with BglII was also assessed and was present in all transfected cells (Fig. 4A, lanes 4 through 10). The LANA and LANAΔ465–497 mutants had the highest amounts of linearized DNA, the other LANA mutants had less linearized p8TR DNA (Fig. 4A, lanes 7 through 10), and non-LANA-expressing BJAB cells had the least (Fig. 4A, lane 4; longer exposure at right in Fig. 4A). The lower amounts of linearized p8TR DNA in the absence of LANA and for the LANA mutants were not due to decreased transfection efficiency. LANA's ability to reduce the rate of loss of TR DNA after transfection (62) accounts for the lower levels of p8TR DNA in the absence of LANA (Fig. 4, lane 4), and we have previously observed similar reductions at 72 h while performing DNA replication assays following nucleofection (15). LANA replicates DNA, adding to the total TR DNA present in cells. Further, LANA segregates TR DNA to the nucleus after cell division, thereby avoiding TR DNA destruction in the cytoplasm. The lower levels of linearized p8TR for LANAΔ33–888, LANAΔ33–899, LANAΔ33–929, and LANAΔ33–949 therefore are consistent with reduced DNA replication ability, as evidenced by the presence of less DpnI-resistant DNA for these transfections (Fig. 4A, lanes 14 through 17), and possibly are also related to deficiencies in segregation.
Deletion of internal LANA regions diminishes episome persistence.
We investigated whether LANA with deletion of its internal regions is capable of episome persistence. Control BJAB cells, or BJAB cells stably expressing LANA, LANAΔ465–495, LANAΔ33–888, LANAΔ33–899, LANAΔ33–929, and LANAΔ33–949, were electroporated with p8TR and selected for G418 resistance conferred by the plasmid vector. After 7 days of selection, >95% of the LANA and LANAΔ465–495 microtiter wells had robust, G418-resistant outgrowth that was macroscopically visible, and cells could be expanded to 24-well plates. These results indicated that the deletion of residues 465 to 495 did not reduce the persistence of p8TR DNA. In contrast, BJAB had G418-resistant outgrowth in fewer than 60% of microtiter wells, and cells could not be expanded until ∼12 to 14 days after G418 selection. The lower G418-resistant outgrowth for BJAB cells is due to the need for p8TR integration, which occurs at a very low frequency compared to LANA-mediated episome persistence. G418-resistant outgrowth for LANAΔ33–888, LANAΔ33–899, LANAΔ33–929, and LANAΔ33–949 cells occurred in fewer than 60% of wells, and cells could not be expanded until ∼12-14 days after G418 selection, similar to what was seen for BJAB. These differences were not due to reduced transfection efficiencies as assessed by cotransfection of GFP. Therefore, the G418-resistant outgrowth observed in the microtiter plates suggested that LANAΔ33–888, LANAΔ33–899, LANAΔ33–929, and LANAΔ33–949 either do not maintain episomes at all or persist at a rate much lower than that for LANA.
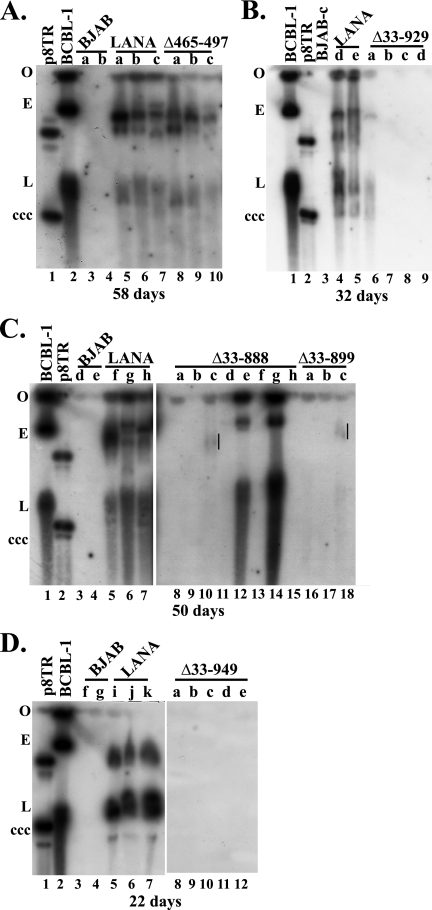
G418-resistant clones were analyzed by Gardella cell analysis (23) to detect the presence of episomes. In Gardella gels, cells are loaded into gel wells and lysed in situ. During electrophoresis, chromosomal DNA remains at the origin while extrachromosomal DNA (as large as several hundred kilobases) migrates into the gel. Analysis of BCBL-1, a KSHV-infected primary effusion lymphoma cell line, results in a slowly migrating band representing the viral episome (Fig. 5A, lane 2; B, lane 1; C, lane 1; and D, lane 2; E indicates episome) and a faster-migrating band from linear DNA that is associated with the lytic replication of the virus (Fig. 5A, lane 2; B, lane 1; C, lane 1; and D, lane 2; L indicates linear virus). As expected, BJAB cells did not contain extrachromosomal DNA (Fig. 5A, lanes 3 and 4; B, lane 3; C, lanes 3 and 4; and D, lanes 3 and 4), while LANA-expressing cells had episomes in all lanes (Fig. 5A, lanes 5 through 7; B, lanes 4 and 5; C, lanes 5 through 7; and D, lanes 5 through 7). Episomes were also present in all LANAΔ465–497 lanes (Fig. 5A, lanes 8 through 10). Of note, much of the episomal DNA migrated more slowly than the p8TR plasmid (Fig. 5A, lane 1; B, lane 2; C, lane 2), as we have previously observed (4). This slower migration is due to the duplication of TR elements and the arrangement of input plasmids into multimers and occurs over time as cells are carried in culture (4). For instance, after 22 days of G418 selection, most episomal DNA in LANA cells (Fig. 5D, lanes 5 through 7) migrates similarly to circular, covalently closed p8TR plasmid (Fig. 5D, lane 1, indicated by ccc), while after 58 days of selection most episomal DNA in LANA cells (Fig. 5A, lanes 5 through 7) migrates similarly to the ∼200-kb episomal BCBL-1 KSHV genome (Fig. 5A, lane 2, indicated by E).
Fig. 5.
Internal LANA domains are critical for efficient long-term episome persistence. Control BJAB cells, or BJAB cells stably expressing LANA or the different LANA deletion mutants, were transfected with p8TR. Seventy-two hours posttransfection, transfected cells were seeded into microtiter plates at 1,000 cells/well and selected for G418 resistance. Gardella gel analysis detected p8TR episomes from G418-resistant cell lines expanded from the microtiter plates. Cells (∼1 × 106 per lane) were loaded in Gardella gels. (A) Gardella gel containing naked p8TR plasmid (lane 1), BCBL-1 (KSHV-infected primary effusion cell line) (lane 2), p8TR-transfected G418-resistant BJAB cells (lanes 3 and 4), or p8TR-transfected G418-resistant BJAB cells expressing LANA (lanes 5 through 7) or LANAΔ465–497 (lanes 8 through 10). The Gardella gel analysis was done after 58 days of G418 selection. (B) Gardella gel containing BCBL-1 (lane 1), naked p8TR plasmid (lane 2), p8TR-transfected G418-resistant BJAB cells (lane 3), or p8TR-transfected G418-resistant BJAB cells expressing LANA (lanes 4 and 5), or LANAΔ33–929 (lanes 6 through 9). The Gardella analysis was done after 32 days of G418 selection. (C) Gardella gel containing BCBL-1 cells (lane 1), naked p8TR plasmid (lane 2), p8TR-transfected G418-resistant BJAB cells (lanes 3 and 4), or p8TR-transfected G418-resistant BJAB cells expressing LANA (lanes 5 through 7), LANAΔ33–888 (lanes 8 through 15), or LANAΔ33–899 (lanes 16 through 18). Vertical lines (lanes 10 and 18) indicate faint episomal signal. The Gardella gel analysis was done after 50 days of G418 selection. (D) Gardella gel containing naked p8TR plasmid (lane 1), BCBL-1 cells (lane 2), p8TR-transfected G418-resistant BJAB cells (lanes 3 and 4), and G418-resistant p8TR-transfected BJAB cells expressing LANA (lanes 5 through 7) or LANAΔ33–949 (lanes 8 through 12). The Gardella gel analysis was done after 22 days of G418 selection. Gel origin (O), BCBL-1 episomal (E) and linear (L) forms (due to KSHV lytic replication) and the p8TR circular, covalently closed (ccc) form are indicated.
In contrast to the LANA and the LANAΔ465–497 mutant, the mutants with deletion of the internal sequence, namely, the LANAΔ33–888, LANAΔ33–899, LANAΔ33–929, and LANAΔ33–949 mutants, were greatly diminished for episome persistence. LANAΔ33–929 had episomal DNA in only one of four lanes (Fig. 5B, lane 6) while the other lanes (Fig. 5B, lanes 7 through 9) did not contain episomes. LANAΔ33–888 had episomes in three lanes, (Fig. 5C, lanes 10, 12, and 14) but lacked extrachromosomal DNA in the other five lanes (Fig. 5C, lanes 8, 9, 11, 13, and 15). LANAΔ33–899 had episomes in one lane (Fig. 5C, lane 18) but not in other lanes (Fig. 5C, lanes 16 and 17). LANAΔ33–949 did not contain episomes in any lanes (Fig. 5D). From a total of eight experiments, LANA had episomes in 25/25 (100%), and from two experiments LANAΔ465–497 had episomes in 6/6 (100%) G418-resistant cell lines (Fig. 1). In contrast, the rate of episome persistence was much reduced after deletion of all internal LANA sequence. From a total of six experiments, LANAΔ33–929 had episomes in 3/44 (7%), LANAΔ33–949 had episomes in 0/28 (0%), LANAΔ33–888 had episomes in 9/36 (25%), and LANAΔ33–899 had episomes in 2/18 (11%) of G418-resistant cell lines (Fig. 1). Not only were the rates of episome persistence lower, but the amount of episomal DNA present was often low in the large deletion mutant lanes compared with that in LANA and LANAΔ465–497 lanes (Fig. 5 and data not shown). Therefore, LANA and LANAΔ465–497 maintained TR-containing episomes with high efficiency while N- and C-terminal LANA deletion mutants were greatly reduced for the ability to maintain episomes. These results indicate that the internal domains of LANA exert an important role in LANA-mediated episome persistence.
Deletion of internal LANA regions diminishes episome persistence at early time points.
Since the deletion of internal LANA regions reduced the efficiency of long-term episome persistence, we wished to investigate episome persistence at earlier time points. For instance, the internal regions may have a role in episome persistence only after long-term selection of several weeks or more. p8TR was transfected by Amaxa nucleofection into control BJAB cells or BJAB cells expressing LANA, LANAΔ465–497, LANAΔ33–888, LANAΔ33–899, LANAΔ33–929, or LANAΔ33–949. Three days posttransfection, cells were placed in bulk under G418 selection at 0.3 × 106 cells/ml. Every other day, cells were fed and resuspended at 0.3 × 106 cells/ml. Similar to the results in microtiter plates, G418-resistant cell outgrowth from the bulk cultures was also greater for LANA and the LANAΔ465–497 mutant than with the other LANA mutants. Prior to reseeding cells at 0.3 × 106 cells every other day, cell concentration was determined (Fig. 6). Both LANA and the LANAΔ465–497 mutant at least doubled in cell number (to over 0.6 × 106 cells) at every time point throughout the experiment. In contrast, control BJAB cells and BJAB cells expressing LANAΔ33–888, LANAΔ33–899, LANAΔ33–929, and LANAΔ33–949 exhibited little to no growth between days 4 and 8 and only began to grow comparably to BJAB cells expressing LANA and LANAΔ465–497 after day 10. The rapid G418-resistant outgrowth was consistent with efficient episome persistence for LANA and LANAΔ465–497, while the much slower G418-resistant outgrowth was consistent with a requirement for p8TR integration, a rare event, in the BJAB cells and either highly inefficient episome persistence or integration in the cell lines containing the large deletion mutants.
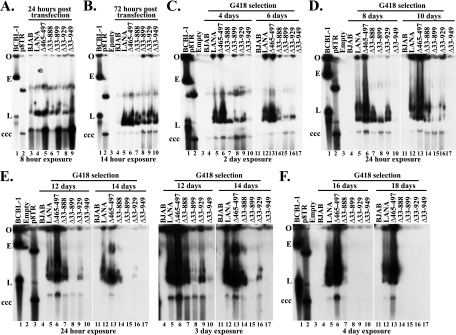
To assess the presence of p8TR episomes, Gardella cell analysis was performed from the bulk cultures at 24 h posttransfection, 3 days posttransfection, after 4 days of G418 selection, and thereafter every other day up to 18 days of G418 selection. Gardella cell analysis performed at 24 h posttransfection (Fig. 7A) shows that all transfected cell lines had detectable p8TR DNA in relatively large amounts that could easily be detected after 8 h of exposure to film (Fig. 7A, lanes 3 through 9). Interestingly, much of the DNA in the transfected cell lines had an intermediate migration rate, comigrating with a minor species of p8TR plasmid, between the circular, covalently closed, and nicked p8TR (Fig. 7A, lane 2, and better seen in Fig. 7B, C, D, and F, lanes 2, and E, lane 3).
Fig. 7.
Analysis of episome persistence at early time points indicates that the internal LANA domains exert a critical role. Control BJAB cells and BJAB cells stably expressing the different LANA constructs were transfected with p8TR and cultured as described in the legend to Fig. 6. Gardella cell analyses to detect the presence of episomal p8TR were performed at the indicated time points. Samples were resolved in 0.8% agarose gel followed by Southern blotting. DNA was detected using a 32P-labeled TR fragment probe, and the blot was exposed to film for the indicated times. In order to detect smaller amounts of DNA in control BJAB and in cells expressing LANA or LANA deletion mutants, twice as many cells were loaded per well (2 × 106 cells) in Gardella gels at 12 days under selection and onwards. Naked plasmid p8TR DNA was loaded in indicated lanes as a control. (A) Episomal p8TR DNA at 24 h posttransfection. BCBL-1 (lane 1), p8TR plasmid (lane 2), BJAB cells (lane 3), BJAB cells expressing LANA (lane 4), LANAΔ465–497 (lane 5), LANAΔ33–888 (lane 6), LANAΔ33–899 (lane 7), LANAΔ33–929 (lane 8), or LANAΔ33–949 (lane 9). (B) Episomal p8TR DNA at 72 h posttransfection. BCBL-1 (lane 1), p8TR (lane2), BJAB (lane 4), BJAB cells expressing LANA (lane 5), LANAΔ465–497 (lane 6), LANAΔ33–888 (lane 7), LANAΔ33–899 (lane 8), LANAΔ33–929 (lane 9), or LANAΔ33–949 (lane 10). (C) Episomal p8TR DNA at 4 days under G418 selection (lanes 4 through 10) and 6 days under G418 selection (lanes 11 through 17). Pellets from cells at 4 days under G418 selection were frozen until the day of the Gardella analysis at 6 days under selection. BCBL-1 (lane 1), p8TR (lane 2), lane 3 is empty, control BJAB cells (lanes 4 and 11), and BJAB cells expressing LANA (lanes 5 and 12), LANAΔ465–497 (lanes 6 and 13), LANAΔ33–888 (lanes 7 and 14), LANAΔ33–899 (lanes 8 and 15), LANAΔ33–929 (lanes 9 and 16), and LANAΔ33–949 (lanes 10 and 17). (D) Episomal p8TR DNA at 8 days under G418 selection (lanes 4 through 10) and 10 days under G418 selection (lanes 11 through 17). Pellets from cells at 8 days under G418 selection were frozen until the day of the Gardella analysis at 10 days under selection. BCBL-1 (lane 1), p8TR (lane 2), lane 3 is empty, control BJAB cells (lanes 4 and 11), and BJAB cells expressing LANA (lanes 5 and 12), LANAΔ465–497 (lanes 6 and 13), LANAΔ33–888 (lanes 7 and 14), LANAΔ33–899 (lanes 8 and 15), LANAΔ33–929 (lanes 9 and 16), and LANAΔ33–949 (lanes 10 and 17). (E) Episomal p8TR DNA at 12 days under G418 selection (lanes 4 through 10) and 14 days under G418 selection (lanes 11 through 17). Pellets from cells at 12 days under G418 selection were frozen until the day of the Gardella analysis at 14 days under selection. BCBL-1 (lane 1), lane 2 is empty, p8TR (lane 3), control BJAB cells (lanes 4 and 11), and BJAB cells expressing LANA (lanes 5 and 12), LANAΔ465–497 (lanes 6 and 13), LANAΔ33–888 (lanes 7 and 14), LANAΔ33–899 (lanes 8 and 15), LANAΔ33–929 (lanes 9 and 16), and LANAΔ33–949 (lanes 10 and 17). The panel on the right is the same blot but is after a 3-day exposure to film to better show episomal p8TR in the LANA deletion mutants. (F) Episomal p8TR DNA at 16 days under G418 selection (lanes 4 through 10) and 18 days under G418 selection (lanes 11 through 17). Pellets from cells at 16 days under G418 selection were frozen until the day of the Gardella analysis at 18 days under selection. BCBL-1 (lane 1), p8TR (lane 2), lane 3 is empty, control BJAB cells (lanes 4 and 11), and BJAB cells expressing LANA (lanes 5 and 12), LANAΔ465–497 (lanes 6 and 13), LANAΔ33–888 (lanes 7 and 14), LANAΔ33–899 (lanes 8 and 15), LANAΔ33–929 (lanes 9 and 16), and LANAΔ33–949 (lanes 10 and 17). This figure is representative of two experiments.
At 3 days posttransfection, differences in p8TR persistence between LANA and the deletion mutants became evident. LANA (Fig. 7B, lane 5) and LANAΔ465–497 (Fig. 7B, lane 6) cells had similarly high levels of DNA, while LANAΔ33–888, LANAΔ33–899, LANAΔ33–929, and LANAΔ33–949 cells (Fig. 7B, lanes 7 through 10) had less episomal DNA. Notably, BJAB (Fig. 7B, lane 4) had already lost most p8TR DNA at this time point. After 4 or 6 days of G418 selection (Fig. 7C), differences in episome persistence efficiency between LANA deletion mutants became evident. LANA (Fig. 7C, lanes 5 and 12) and LANAΔ465–497 (Fig. 7C, lanes 6 and 13) cells had similarly strong signals, while LANAΔ33–888, LANAΔ33–899, and LANAΔ33–929 cells (Fig. 7C, lanes 7 through 9 and 14 through 16) had less episomal DNA, and LANAΔ33–949 cells (Fig. 7C, lanes 10 and 17) had the least amount of episomal DNA. BJAB cells had even less p8TR DNA, which was barely detectable (Fig. 7C, lanes 4 and 11). After 8 or 10 days of G418 selection, LANA (Fig. 7D, lanes 5 and 12) and LANAΔ465–495 (Fig. 7D, lanes 6 and 13) cells continued to have similarly strong signals, but differences emerged with less episomal DNA in LANAΔ33–899 (Fig. 7D, lanes 8 and 15) than in LANAΔ33–888 (Fig. 7D, lanes 7 and 14) and LANAΔ33–929 (Fig. 7D, lanes 9 and 16) cells. LANAΔ33–949 cells barely had any signal detected (Fig. 7D, lanes 10 and 17) and BJAB cells had lost all p8TR DNA (Fig. 7D, lanes 4 and 11) at these time points. (Longer exposure of the blot showed faint p8TR signal for LANAΔ33–949 cells but not for control BJAB cells [data not shown].) By 12 or 14 days of selection, differences were evident between LANAΔ33–888 (Fig. 7E, lanes 7 and 14) and LANAΔ33–929 (Fig. 7E, lanes 9 and 16) cells, with more episomal signal present for LANAΔ33–888 cells. LANAΔ33–899 (Fig. 7E, lanes 8 and 15) and LANAΔ33–949 (Fig. 7E, lanes 10 and 17) cells had only very faint p8TR signal, which could be seen on a longer 3-day exposure to film, while both LANA (Fig. 7E, lanes 5 and 12) and LANAΔ465–495 (Fig. 7E, lanes 6 and 13) cells continued to have strong signal. After 16 or 18 days of G418 selection, episomal DNA could no longer be detected for LANAΔ33–899 (Fig. 7F, lanes 8 and 15), LANAΔ33–929 (Fig. 7F, lanes 9 and 16), and LANAΔ33–949 (Fig. 7F, lanes 10 and 17) cells. Faint episomal signal was present for LANAΔ33–888 cells at 16 days under selection (Fig. 7F, lane 7) but was lost after 18 days of G418 selection (Fig. 7F, lane 14). LANA (Fig. 7F, lanes 5 and 12) and LANAΔ465–497 (Fig. 7F, lanes 6 and 13) cells continued to have strong signal.
Therefore, cell lines expressing the large LANA deletion mutants all lost episomal DNA by 18 days of G418 selection. In contrast, LANA and LANAΔ465–497 cells maintained episomes at robust levels throughout the experiment. The rate of loss of episomal DNA for the mutants was greatest for the LANAΔ33–949 mutant, followed in order by the LANAΔ33–899, LANAΔ33–929, and LANAΔ33–888 mutants. Although these mutants were highly deficient compared to LANA and LANAΔ465–497 cells, they retained low-level episome maintenance function since their expression reduced the rate loss when compared with BJAB cells.
DISCUSSION
This work demonstrates that the LANA internal regions exert a critical effect on episome maintenance. In both short- and long-term episome maintenance assays, LANA mutants with the internal sequence deleted were highly deficient for episome persistence (Fig. 5 and 7). The deficiencies in episome persistence were not due to unexpected loss of function in the essential N-terminal chromosome binding region or the C-terminal DNA binding domain, both of which were present in all mutants. The abilities to bind to mitotic chromosomes and KSHV TR DNA are each required for LANA to tether episomes to chromosomes for efficient segregation of KSHV DNA to daughter cell nuclei. Like LANA, each mutant bound to mitotic chromosomes in a wild-type pattern (Fig. 2), with preferential binding to pericentromeric and peritelomeric regions. The pericentromeric and peritelomeric pattern of binding is likely an effect of C-terminal LANA, since C-terminal LANA targets these regions in the absence of N-terminal LANA (30–32). In contrast, N-terminal LANA targets chromosomes through binding histones H2A/H2B and broadly distributes over chromosomes in the absence of C-terminal LANA (5, 6, 55). Episome maintenance deficiency was also not merely due to reductions in LANA expression level (Fig. 4B). In addition, each mutant was capable of recognizing the LANA TR binding site, without any obvious loss of binding, as shown by EMSA (Fig. 3), indicating that C-terminal LANA remained functional.
Interestingly, the previously observed formation of multiple LANA complexes on EMSA (4) was found to map to residues 900 to 929. LANA typically produces two complexes on EMSA, and on longer gel runs, a third complex can be resolved (4). Two major complexes were present for LANAΔ33–888 and LANAΔ33–899, but LANAΔ33–929 and LANAΔ33–949 each only generated one complex. The origin of these different complexes remains obscure. The stoichiometry of LANA binding to DNA may vary for the different complexes. LANA self-associates, but whether LANA exists as a dimer or higher-order structure is not known. It is possible that this sequence may affect the degree of LANA self-association and allow it to increase into higher-order structures. Alternatively, this sequence may bind an additional protein partner that is present in the reticulocyte lysate used for the LANA in vitro translations.
It is possible that a deficiency in DNA replication may underlie the reduced episome maintenance of the LANA mutants. Previous results assessing the DNA replication of LANA mutants with the internal regions deleted have produced conflicting results. A mutant equivalent to LANAΔ333-929 had reduced replication of about 20% of LANA activity in 293 cells (26) but in other work, also in 293 cells, LANAΔ275-933 and LANAΔ331-972 replicated similarly to LANA (69). In other studies, LANAΔ91-949 replicated similarly to LANA in 293T cells (45) but had reduced replication in C33A cells (42). In addition, LANAΔ31-950 and LANAΔ23-950 replicated DNA more efficiently than LANAΔ91-950 in 293T cells (43). Notably, these experiments all differed from our experiments in that they used transient expression of LANA and LANA mutants, while we used cell lines stably expressing LANA.
In our results, although each LANA mutant was capable of replicating KSHV TR DNA, the amount of replicated DNA was lower for each of the mutants with deletion of the LANA internal regions than for LANA or LANAΔ465–497, which lacks only 33 amino acids and maintained episomes at wild-type levels. It is possible that the lower replication levels underlie the reduced episome persistence for the mutants. Notably, LANAΔ33–949, which never maintained long-term episomes, supported less DNA replication than the other mutants. It is possible that there is a threshold of DNA replication that is necessary for any long-term episome persistence and that LANAΔ33–949 falls below this threshold. Interestingly, the levels of linearized p8TR DNA (in the absence of DpnI digestion) correlated with the amount of replicated DpnI-resistant DNA for LANA and the mutants. LANA and LANAΔ465–497 cells had the highest linearized p8TR levels, the large deletion mutants had intermediate levels, and BJAB cells had the least (Fig. 4). We have observed similar findings in other experiments with LANA and mutated LANA (5) known to have reduced episome maintenance deficiency (15). The lower levels of linearized DNA are not due to differences in transfection efficiencies and can be attributed to at least two possible reasons. Less linearized p8TR DNA may be due to the addition of less replicated DNA by the deficient mutants (or no addition of replicated DNA for BJAB, which lacks LANA). In addition, if p8TR is not segregated to progeny nuclei after mitosis, it will be degraded in the cytoplasm. Such degradation is expected to occur in BJAB cells and may occur for the mutants if any unexpected deficiency in segregation is present, despite the abilities of all mutants to bind mitotic chromosomes and TR DNA.
The internal LANA regions may exert effects on other episome maintenance functions in addition to DNA replication. It is possible that episome segregation may be altered in the deficient mutants, despite the retained abilities to bind mitotic chromosomes and TR DNA. For instance, perhaps a cell protein interacting with the deleted sequence may be responsible for transporting LANA-episome complexes efficiently to mitotic chromosomes. It is also possible that there are additional unknown functions other than DNA replication and segregation exerted by the internal regions that are critical for episome persistence.
The wild-type function of LANAΔ465–497 was expected since this region deletes only 33 amino acids within a large glutamine- and acid-rich repeat region. LANA genes sequenced from different KSHV isolates show significant variation in repeat copy number within the repeat elements, although copy number tends to remain constant within any single isolate (20). In fact, the variation in repeat copy number is often significantly larger than the 33 residues deleted in LANAΔ465–497.
It is possible that cis changes in the episome compensate for LANAΔ33–888, LANAΔ33–899, and LANAΔ33–929 deficiencies in long-term G418-resistant cell lines containing episomes. In these cell lines, there may be strong selection for recombinant episomes that are enhanced for episome persistence. After transfection of TR-containing plasmids into LANA-expressing cell lines and selecting for G418 resistance, episomes increase in size over time. This increase in size is due to TR duplication and generation of input plasmids into multimers (4). It is likely that the TR duplication and rearrangement of plasmids occurs until the total TR copy number per episome is ∼40, similar to that of the KSHV genome (37). Consistent with such recombination events, in Fig. 5, all lanes containing episomes have signal that comigrates with episomal KSHV from BCBL-1, which is ∼200 kb and which migrates very slowly compared with the p8TR circular, covalently closed DNA. While selection for recombination occurs with LANA, such rearrangement, with higher TR copy number, may be even more favorable for the mutants lacking the internal regions. The additional TRs may compensate for the reduced abilities to replicate or segregate DNA in the LANA mutants. For instance, the increased TR number provides additional LANA binding sites and higher copy numbers of the essential replicative (RE) element adjacent to the binding sites and therefore provides more opportunities for binding of proteins, such as SSRP1, which enhance the efficiency of LANA DNA replication (27, 28).
LANA residues 889 to 949 appear to exert a role in episome persistence. Twenty-five percent of G418-resistant LANAΔ33–888 cell lines had episomes; 11% and 7% of LANAΔ33–889 and LANAΔ33–929, respectively, had episomes; and LANAΔ33–949 had none. Further, in the bulk cultures, LANAΔ33–888 lost p8TR more slowly than the other deficient mutants, while LANAΔ33–949 lost p8TR at the fastest rate (Fig. 7). Therefore, the presence of residues 889 to 949 modestly enhanced the efficiency of episome persistence.
Notably, all the highly deficient mutants eventually lost episomal DNA by day 18 in the bulk cultures, in contrast to the results where episomes persisted for much longer periods of time in some G418-resistant cell lines after seeding in microtiter plates (Fig. 5). This finding is likely due to the low density of cell seeding at 1,000 cells per well in microtiter plates compared with the bulk cultures. Fewer than 60% of microtiter wells had G418-resistant growth, consistent with clonal outgrowth in many of these wells, assuming a Poisson distribution of events. In wells with clonal outgrowth, there is no competition with other G418-resistant cells, such as those containing integrated p8TR. However, in the bulk cultures, cells with integrated p8TR are also present in the culture, increasing the possibility of overgrowth of any episome-containing cells by cells with integrated p8TR.
It is likely that host protein partners that interact with the LANA internal regions exert a key functional role(s) for episome persistence. Over 50 cell proteins have been described to interact with LANA. The ORC proteins, which are involved with DNA replication licensing, associate with LANA, but these interact with C-terminal LANA (45, 68), which is present in these mutants. However, it is possible that an other cell protein(s) with a role in LANA DNA replication may interact with the internal LANA region. Proteins which have been mapped to regions overlapping with the internal domains fall into several functional categories, including transcription (1, 2, 8, 35, 38, 39, 41, 42, 44, 47, 50, 51, 53, 58, 60, 67, 70), chromosome structure or modification (12, 29, 34, 48, 61, 65, 66, 72), ubiquitin ligase activity (9), and cell growth control (7, 17–19, 46, 52, 54, 56, 57, 59, 61, 73). It is possible that one or more of these proteins, or an as yet unknown interacting protein, may be responsible for exerting the critical episome maintenance function(s).
ACKNOWLEDGMENTS
Takashi Komatsu generated the pSG5 oligonucleotide plasmid. Mary Ballestas constructed the pT7 plasmid. Andrew Barbera constructed the pT7LANA plasmid.
This work was supported by grants (K.M.K.) from the National Cancer Institute (CA082036 and CA082036S1) and from the U.S. Department of Defense (PR093491).
Footnotes
Published ahead of print on 18 May 2011.
REFERENCES
- 1. An J., Sun Y., Rettig M. B. 2004. Transcriptional coactivation of c-Jun by the KSHV-encoded LANA. Blood 103:222–228 [DOI] [PubMed] [Google Scholar]
- 2. Bajaj B. G., et al. 2006. KSHV encoded LANA upregulates Pim-1 and is a substrate for its kinase activity. Virology 351:18–28 [DOI] [PubMed] [Google Scholar]
- 3. Ballestas M. E., Chatis P. A., Kaye K. M. 1999. Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science 284:641–644 [DOI] [PubMed] [Google Scholar]
- 4. Ballestas M. E., Kaye K. M. 2001. Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen 1 mediates episome persistence through cis-acting terminal repeat (TR) sequence and specifically binds TR DNA. J. Virol. 75:3250–3258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barbera A. J., Ballestas M. E., Kaye K. M. 2004. The Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen 1 N terminus is essential for chromosome association, DNA replication, and episome persistence. J. Virol. 78:294–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barbera A. J., et al. 2006. The nucleosomal surface as a docking station for Kaposi's sarcoma herpesvirus LANA. Science 311:856–861 [DOI] [PubMed] [Google Scholar]
- 7. Bubman D., Guasparri I., Cesarman E. 2007. Deregulation of c-Myc in primary effusion lymphoma by Kaposi's sarcoma herpesvirus latency-associated nuclear antigen. Oncogene 26:4979–4986 [DOI] [PubMed] [Google Scholar]
- 8. Cai Q., et al. 2006. Kaposi's sarcoma-associated herpesvirus latent protein LANA interacts with HIF-1α to upregulate RTA expression during hypoxia: latency control under low oxygen conditions. J. Virol. 80:7965–7975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cai Q. L., Knight J. S., Verma S. C., Zald P., Robertson E. S. 2006. EC5S ubiquitin complex is recruited by KSHV latent antigen LANA for degradation of the VHL and p53 tumor suppressors. PLoS Pathog. 2:e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cesarman E., et al. 1995. In vitro establishment and characterization of two acquired immunodeficiency syndrome-related lymphoma cell lines (BC-1 and BC-2) containing Kaposi's sarcoma-associated herpesvirus-like (KSHV) DNA sequences. Blood 86:2708–2714 [PubMed] [Google Scholar]
- 11. Chang Y., et al. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865–1869 [DOI] [PubMed] [Google Scholar]
- 12. Cotter M. A., II, Robertson E. S. 1999. The latency-associated nuclear antigen tethers the Kaposi's sarcoma-associated herpesvirus genome to host chromosomes in body cavity-based lymphoma cells. Virology 264:254–264 [DOI] [PubMed] [Google Scholar]
- 13. Cotter M. A., II, Subramanian C., Robertson E. S. 2001. The Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen binds to specific sequences at the left end of the viral genome through its carboxy-terminus. Virology 291:241–259 [DOI] [PubMed] [Google Scholar]
- 14. Decker L. L., et al. 1996. The Kaposi sarcoma-associated herpesvirus (KSHV) is present as an intact latent genome in KS tissue but replicates in the peripheral blood mononuclear cells of KS patients. J. Exp. Med. 184:283–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. De Leon Vazquez E., Kaye K. M. 7 April 2011. Rapid and quantitative assessment of KSHV LANA-mediated DNA replication. Arch. Virol. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fejer G., et al. 2003. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus interacts preferentially with the terminal repeats of the genome in vivo and this complex is sufficient for episomal DNA replication. J. Gen. Virol. 84:1451–1462 [DOI] [PubMed] [Google Scholar]
- 17. Friborg J., Jr., Kong W., Hottiger M. O., Nabel G. J. 1999. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature 402:889–894 [DOI] [PubMed] [Google Scholar]
- 18. Fujimuro M., Hayward S. D. 2003. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus manipulates the activity of glycogen synthase kinase-3beta. J. Virol. 77:8019–8030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fujimuro M., et al. 2003. A novel viral mechanism for dysregulation of beta-catenin in Kaposi's sarcoma-associated herpesvirus latency. Nat. Med. 9:300–306 [DOI] [PubMed] [Google Scholar]
- 20. Gao S. J., et al. 1999. Molecular polymorphism of Kaposi's sarcoma-associated herpesvirus (Human herpesvirus 8) latent nuclear antigen: evidence for a large repertoire of viral genotypes and dual infection with different viral genotypes. J. Infect. Dis. 180:1466–1476(Erratum, 180:1756.) [DOI] [PubMed] [Google Scholar]
- 21. Garber A. C., Hu J., Renne R. 2002. Latency-associated nuclear antigen (LANA) cooperatively binds to two sites within the terminal repeat, and both sites contribute to the ability of LANA to suppress transcription and to facilitate DNA replication. J. Biol. Chem. 277:27401–27411 [DOI] [PubMed] [Google Scholar]
- 22. Garber A. C., Shu M. A., Hu J., Renne R. 2001. DNA binding and modulation of gene expression by the latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus. J. Virol. 75:7882–7892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gardella T., Medveczky P., Sairenji T., Mulder C. 1984. Detection of circular and linear herpesvirus DNA molecules in mammalian cells by gel electrophoresis. J. Virol. 50:248–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grundhoff A., Ganem D. 2003. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus permits replication of terminal repeat-containing plasmids. J. Virol. 77:2779–2783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hirt B. 1967. Selective extraction of polyoma DNA from infected mouse cell cultures. J. Mol. Biol. 26:365–369 [DOI] [PubMed] [Google Scholar]
- 26. Hu J., Garber A. C., Renne R. 2002. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus supports latent DNA replication in dividing cells. J. Virol. 76:11677–11687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hu J., Liu E., Renne R. 2009. Involvement of SSRP1 in latent replication of Kaposi's sarcoma-associated herpesvirus. J. Virol. 83:11051–11063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hu J., Renne R. 2005. Characterization of the minimal replicator of Kaposi's sarcoma-associated herpesvirus latent origin. J. Virol. 79:2637–2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kaul R., Verma S. C., Robertson E. S. 2007. Protein complexes associated with the Kaposi's sarcoma-associated herpesvirus-encoded LANA. Virology 364:317–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kelley-Clarke B., Ballestas M. E., Komatsu T., Kaye K. M. 2007. Kaposi's sarcoma herpesvirus C-terminal LANA concentrates at pericentromeric and peri-telomeric regions of a subset of mitotic chromosomes. Virology 357:149–157 [DOI] [PubMed] [Google Scholar]
- 31. Kelley-Clarke B., et al. 2007. Determination of Kaposi's sarcoma-associated herpesvirus C-terminal latency-associated nuclear antigen residues mediating chromosome association and DNA binding. J. Virol. 81:4348–4356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kelley-Clarke B., De Leon-Vazquez E., Slain K., Barbera A. J., Kaye K. M. 2009. Role of Kaposi's sarcoma-associated herpesvirus C-terminal LANA chromosome binding in episome persistence. J. Virol. 83:4326–4337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Komatsu T., Ballestas M. E., Barbera A. J., Kelley-Clarke B., Kaye K. M. 2004. KSHV LANA1 binds DNA as an oligomer and residues N-terminal to the oligomerization domain are essential for DNA binding, replication, and episome persistence. Virology 319:225–236 [DOI] [PubMed] [Google Scholar]
- 34. Krithivas A., Fujimuro M., Weidner M., Young D. B., Hayward S. D. 2002. Protein interactions targeting the latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus to cell chromosomes. J. Virol. 76:11596–11604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Krithivas A., Young D. B., Liao G., Greene D., Hayward S. D. 2000. Human herpesvirus 8 LANA interacts with proteins of the mSin3 corepressor complex and negatively regulates Epstein-Barr virus gene expression in dually infected PEL cells. J. Virol. 74:9637–9645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kwun H. J., et al. 2007. Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen 1 mimics Epstein-Barr virus EBNA1 immune evasion through central repeat domain effects on protein processing. J. Virol. 81:8225–8235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lagunoff M., Ganem D. 1997. The structure and coding organization of the genomic termini of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8). Virology 236:147–154 [DOI] [PubMed] [Google Scholar]
- 38. Lan K., Kuppers D. A., Robertson E. S. 2005. Kaposi's sarcoma-associated herpesvirus reactivation is regulated by interaction of latency-associated nuclear antigen with recombination signal sequence-binding protein Jkappa, the major downstream effector of the Notch signaling pathway. J. Virol. 79:3468–3478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lan K., et al. 2007. Kaposi's sarcoma herpesvirus-encoded latency-associated nuclear antigen stabilizes intracellular activated Notch by targeting the Sel10 protein. Proc. Natl. Acad. Sci. U. S. A. 104:16287–16292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lim C., Choi C., Choe J. 2004. Mitotic chromosome-binding activity of latency-associated nuclear antigen 1 is required for DNA replication from terminal repeat sequence of Kaposi's sarcoma-associated herpesvirus. J. Virol. 78:7248–7256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lim C., Gwack Y., Hwang S., Kim S., Choe J. 2001. The transcriptional activity of cAMP response element-binding protein-binding protein is modulated by the latency associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus. J. Biol. Chem. 276:31016–31022 [DOI] [PubMed] [Google Scholar]
- 42. Lim C., Lee D., Seo T., Choi C., Choe J. 2003. Latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus functionally interacts with heterochromatin protein 1. J. Biol. Chem. 278:7397–7405 [DOI] [PubMed] [Google Scholar]
- 43. Lim C., Seo T., Jung J., Choe J. 2004. Identification of a virus trans-acting regulatory element on the latent DNA replication of Kaposi's sarcoma-associated herpesvirus. J. Gen. Virol. 85:843–855 [DOI] [PubMed] [Google Scholar]
- 44. Lim C., Sohn H., Gwack Y., Choe J. 2000. Latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8) binds ATF4/CREB2 and inhibits its transcriptional activation activity. J. Gen. Virol. 81:2645–2652 [DOI] [PubMed] [Google Scholar]
- 45. Lim C., Sohn H., Lee D., Gwack Y., Choe J. 2002. Functional dissection of latency-associated nuclear antigen 1 of Kaposi's sarcoma-associated herpesvirus involved in latent DNA replication and transcription of terminal repeats of the viral genome. J. Virol. 76:10320–10331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu J., Martin H. J., Liao G., Hayward S. D. 2007. The Kaposi's sarcoma-associated herpesvirus LANA protein stabilizes and activates c-Myc. J. Virol. 81:10451–10459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lu F., Day L., Gao S. J., Lieberman P. M. 2006. Acetylation of the latency-associated nuclear antigen regulates repression of Kaposi's sarcoma-associated herpesvirus lytic transcription. J. Virol. 80:5273–5282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mattsson K., et al. 2002. Latent nuclear antigen of Kaposi's sarcoma herpesvirus/human herpesvirus-8 induces and relocates RING3 to nuclear heterochromatin regions. J. Gen. Virol. 83:179–188 [DOI] [PubMed] [Google Scholar]
- 49. Moore P. S., Chang Y. 1995. Detection of herpesvirus-like DNA sequences in Kaposi's sarcoma in patients with and without HIV infection. N. Engl. J. Med. 332:1181–1185 [DOI] [PubMed] [Google Scholar]
- 50. Murakami Y., et al. 2006. Ets-1-dependent expression of vascular endothelial growth factor receptors is activated by latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus through interaction with Daxx. J. Biol. Chem. 281:28113–28121 [DOI] [PubMed] [Google Scholar]
- 51. Muromoto R., et al. 2006. Physical and functional interactions between STAT3 and Kaposi's sarcoma-associated herpesvirus-encoded LANA. FEBS Lett. 580:93–98 [DOI] [PubMed] [Google Scholar]
- 52. Ottinger M., et al. 2006. Kaposi's sarcoma-associated herpesvirus LANA-1 interacts with the short variant of BRD4 and releases cells from a BRD4- and BRD2/RING3-induced G1 cell cycle arrest. J. Virol. 80:10772–10786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pan H. Y., et al. 2003. Identification of a novel cellular transcriptional repressor interacting with the latent nuclear antigen of Kaposi's sarcoma-associated herpesvirus. J. Virol. 77:9758–9768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Petre C. E., Sin S. H., Dittmer D. P. 2007. Functional p53 signaling in Kaposi's sarcoma-associated herpesvirus lymphomas: implications for therapy. J. Virol. 81:1912–1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Piolot T., Tramier M., Coppey M., Nicolas J. C., Marechal V. 2001. Close but distinct regions of human herpesvirus 8 latency-associated nuclear antigen 1 are responsible for nuclear targeting and binding to human mitotic chromosomes. J. Virol. 75:3948–3959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Platt G. M., Simpson G. R., Mittnacht S., Schulz T. F. 1999. Latent nuclear antigen of Kaposi's sarcoma-associated herpesvirus interacts with RING3, a homolog of the Drosophila female sterile homeotic (fsh) gene. J. Virol. 73:9789–9795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Radkov S. A., Kellam P., Boshoff C. 2000. The latent nuclear antigen of Kaposi sarcoma-associated herpesvirus targets the retinoblastoma-E2F pathway and with the oncogene Hras transforms primary rat cells. Nat. Med. 6:1121–1127 [DOI] [PubMed] [Google Scholar]
- 58. Sakakibara S., et al. 2004. Accumulation of heterochromatin components on the terminal repeat sequence of Kaposi's sarcoma-associated herpesvirus mediated by the latency-associated nuclear antigen. J. Virol. 78:7299–7310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sarek G., Ojala P. M. 2007. p53 reactivation kills KSHV lymphomas efficiently in vitro and in vivo: new hope for treating aggressive viral lymphomas. Cell Cycle 6:2205–2209 [DOI] [PubMed] [Google Scholar]
- 60. Shamay M., Krithivas A., Zhang J., Hayward S. D. 2006. Recruitment of the de novo DNA methyltransferase Dnmt3a by Kaposi's sarcoma-associated herpesvirus LANA. Proc. Natl. Acad. Sci. U. S. A. 103:14554–14559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Si H., Robertson E. S. 2006. Kaposi's sarcoma-associated herpesvirus-encoded latency-associated nuclear antigen induces chromosomal instability through inhibition of p53 function. J. Virol. 80:697–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Skalsky R. L., Hu J., Renne R. 2007. Analysis of viral cis elements conferring Kaposi's sarcoma-associated herpesvirus episome partitioning and maintenance. J. Virol. 81:9825–9837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Soulier J., et al. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 86:1276–1280 [PubMed] [Google Scholar]
- 64. Srinivasan V., Komatsu T., Ballestas M. E., Kaye K. M. 2004. Definition of sequence requirements for latency-associated nuclear antigen 1 binding to Kaposi's sarcoma-associated herpesvirus DNA. J. Virol. 78:14033–14038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Stuber G., et al. 2007. HHV-8 encoded LANA-1 alters the higher organization of the cell nucleus. Mol. Cancer 6:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Szekely L., et al. 1999. Human herpesvirus-8-encoded LNA-1 accumulates in heterochromatin-associated nuclear bodies. J. Gen. Virol. 80:2889–2900 [DOI] [PubMed] [Google Scholar]
- 67. Verma S. C., Borah S., Robertson E. S. 2004. Latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus up-regulates transcription of human telomerase reverse transcriptase promoter through interaction with transcription factor Sp1. J. Virol. 78:10348–10359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Verma S. C., Choudhuri T., Kaul R., Robertson E. S. 2006. Latency-associated nuclear antigen (LANA) of Kaposi's sarcoma-associated herpesvirus interacts with origin recognition complexes at the LANA binding sequence within the terminal repeats. J. Virol. 80:2243–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Viejo-Borbolla A., et al. 2005. Brd2/RING3 interacts with a chromatin-binding domain in the Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen 1 (LANA-1) that is required for multiple functions of LANA-1. J. Virol. 79:13618–13629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Watanabe A., et al. 2007. A novel KRAB-zinc finger protein interacts with latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus and activates transcription via terminal repeat sequences. Virus Genes 34:127–136 [DOI] [PubMed] [Google Scholar]
- 71. Wong L. Y., Matchett G. A., Wilson A. C. 2004. Transcriptional activation by the Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen is facilitated by an N-terminal chromatin-binding motif. J. Virol. 78:10074–10085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Xiao B., et al. 2010. Bub1 and CENP-F can contribute to Kaposi's sarcoma-associated herpesvirus genome persistence by targeting LANA to kinetochores. J. Virol. 84:9718–9732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. You J., et al. 2006. Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen interacts with bromodomain protein Brd4 on host mitotic chromosomes. J. Virol. 80:8909–8919 [DOI] [PMC free article] [PubMed] [Google Scholar]