Abstract
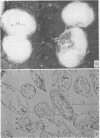
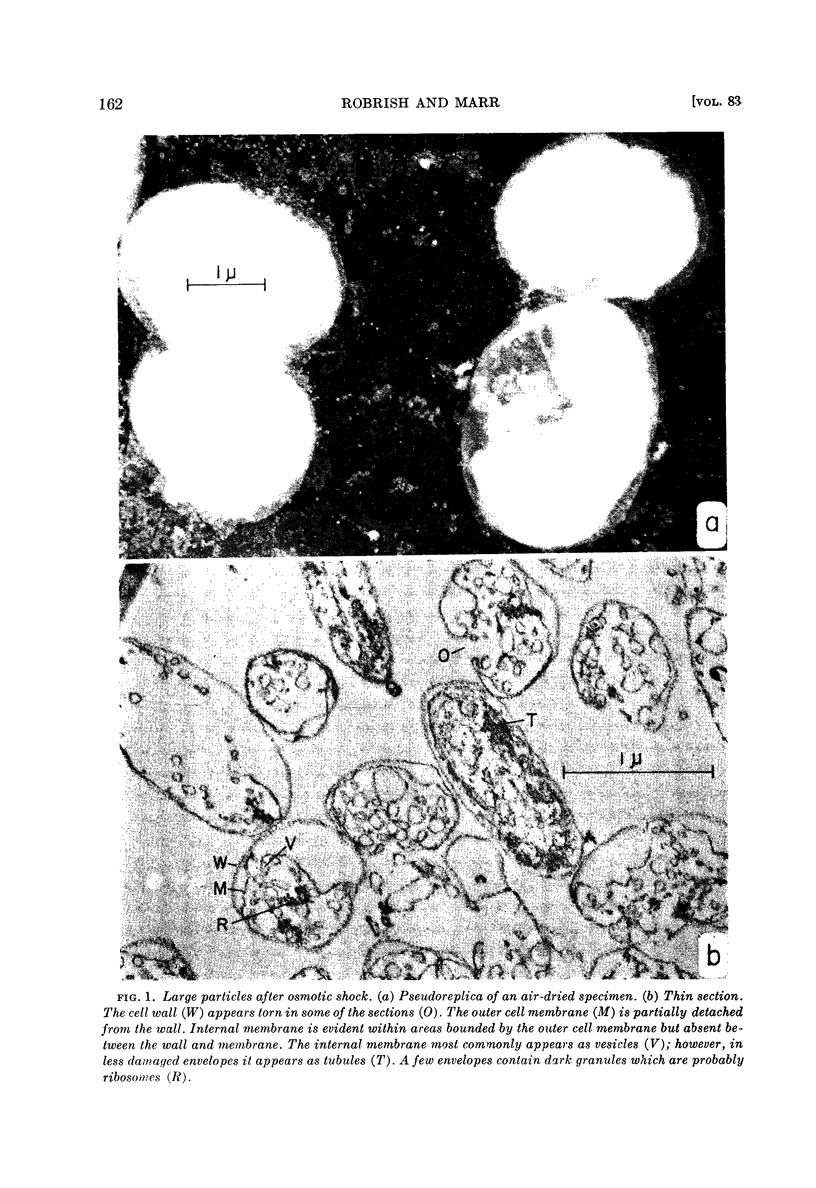
Robrish, Stanley A. (University of California, Davis) and Allen G. Marr. Location of enzymes in Azotobacter agilis. J. Bacteriol. 83: 158–168. 1962.—If the cells of Azotobacter agilis are disrupted by osmotic shock, respiratory enzymes and the compounds characteristic of cell wall and cytoplasmic membrane are recovered almost completely in large particles. The large particles obtained by osmotic shock were found by electron microscopy to consist of cell wall, cell membrane, and an internal membrane appearing as either vesicles or tubules in section. These envelopes are free of all the soluble cytoplasmic material and are essentially free of ribosomes.
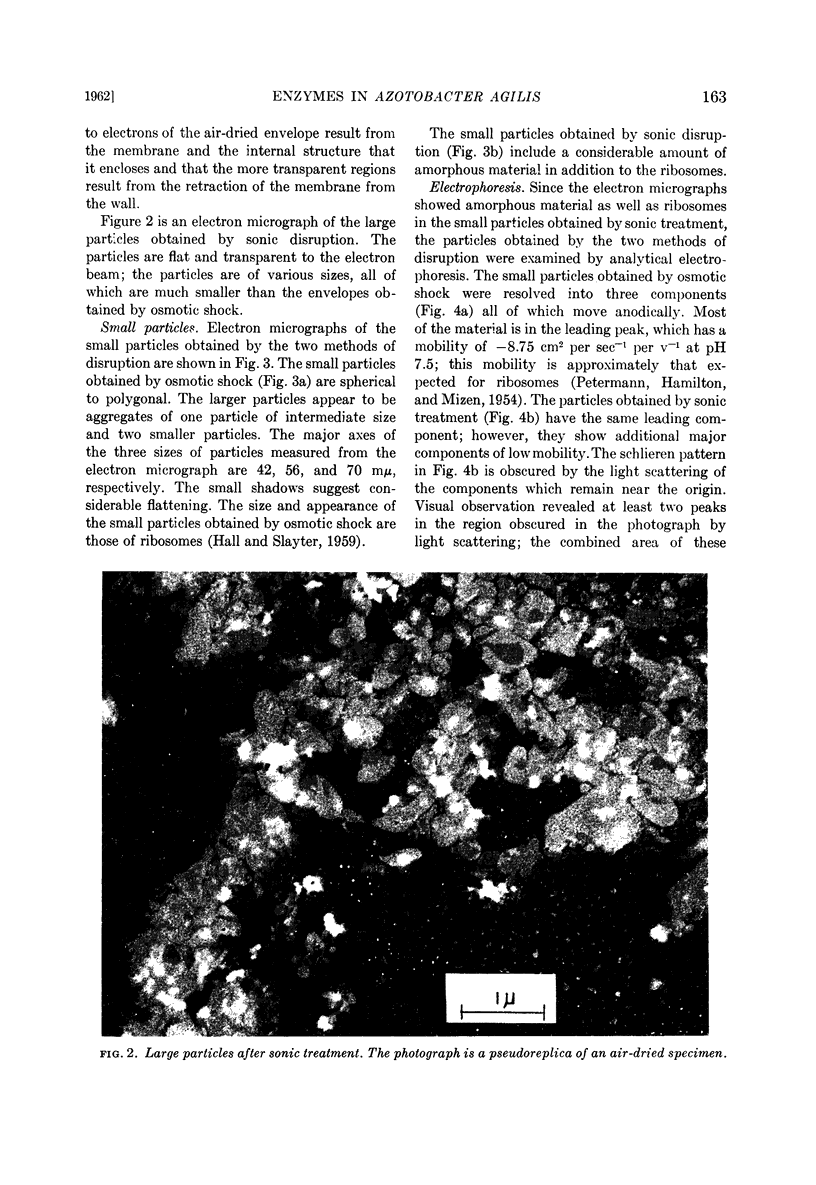
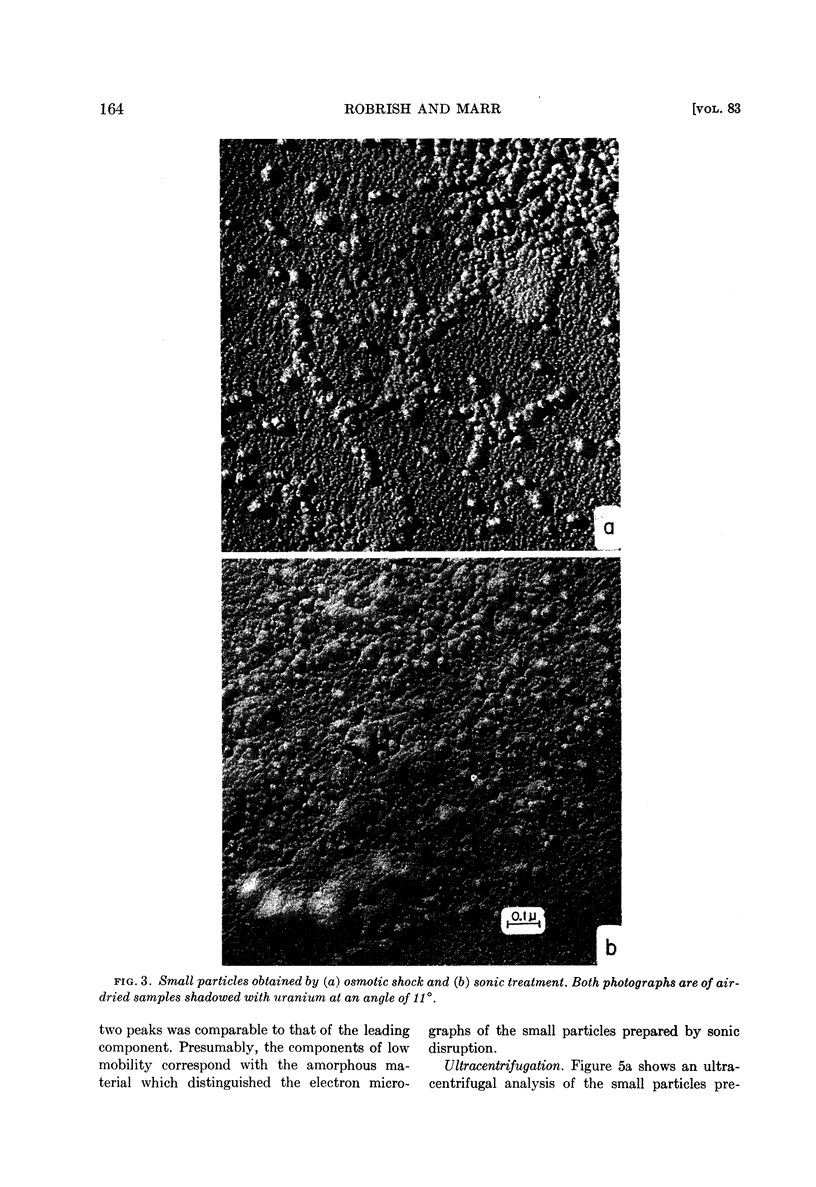
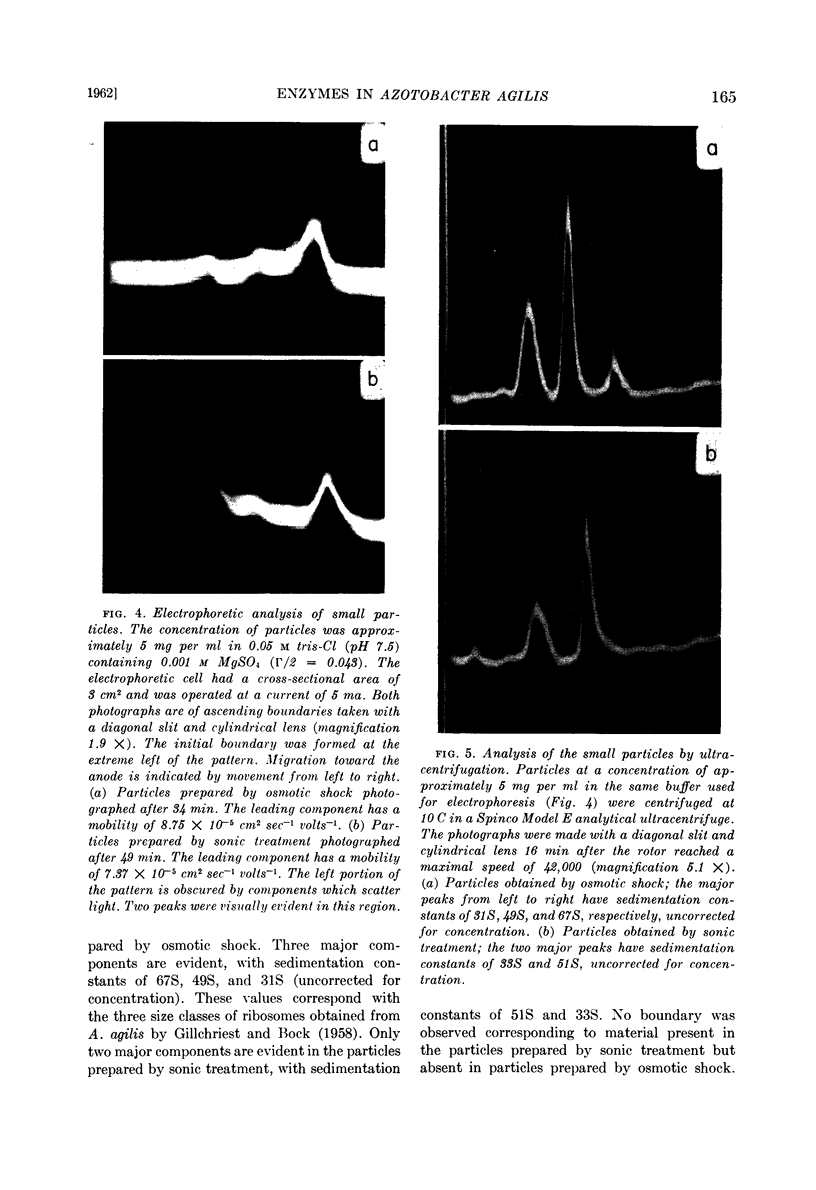
Small particles obtained by osmotic shock are ribosomes; small particles obtained by sonic oscillation consist of both ribosomes and amorphous material, presumably fragments of the envelope.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEXANDER M. Localization of enzymes in the microbial cell. Bacteriol Rev. 1956 Jun;20(2):67–93. doi: 10.1128/br.20.2.67-93.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen R. J. The estimation of phosphorus. Biochem J. 1940 Jun;34(6):858–865. doi: 10.1042/bj0340858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEER M. Disposition of membranes in Alcaligenes faecalis. J Bacteriol. 1960 Nov;80:659–664. doi: 10.1128/jb.80.5.659-664.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BILLEN D., VOLKIN E. The effect of x rays on the macromolecular organization of Escherichia coli. J Bacteriol. 1954 Feb;67(2):191–197. doi: 10.1128/jb.67.2.191-197.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOAS N. F. Method for the determination of hexosamines in tissues. J Biol Chem. 1953 Oct;204(2):553–563. [PubMed] [Google Scholar]
- CHAPMAN G. B. Electron microscopy of ultrathin sections of bacteria. III. Cell wall, cytoplasmic membrane, and nuclear material. J Bacteriol. 1959 Jul;78(1):96–104. doi: 10.1128/jb.78.1.96-104.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COTA-ROBLES E. H., MARR A. G., NILSON E. H. Submicroscopic particles in extracts of Azotobacter agilis. J Bacteriol. 1958 Mar;75(3):243–252. doi: 10.1128/jb.75.3.243-252.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE DEKEN-GRENSON M., DE DEKEN R. H. Elimination of substances interfering with nucleic acids estimation. Biochim Biophys Acta. 1959 Jan;31(1):195–207. doi: 10.1016/0006-3002(59)90456-1. [DOI] [PubMed] [Google Scholar]
- GILBY A. R., FEW A. V., McQUILLEN K. The chemical composition of the protoplast membrane of Micrococcus lysodeikticus. Biochim Biophys Acta. 1958 Jul;29(1):21–29. doi: 10.1016/0006-3002(58)90141-0. [DOI] [PubMed] [Google Scholar]
- GLAUERT A. M., BRIEGER E. M., ALLEN J. M. The fine structure of vegetative cells of Bacillus subtilis. Exp Cell Res. 1961 Jan;22:73–85. doi: 10.1016/0014-4827(61)90087-8. [DOI] [PubMed] [Google Scholar]
- GLAUERT A. M., HOPWOOD D. A. A membranous component of the cytoplasm in Streptomyces coelicolor. J Biophys Biochem Cytol. 1959 Dec;6:515–516. doi: 10.1083/jcb.6.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN M., WILSON P. W. Hydrogenase and nitrogenase in Azotobacter. J Bacteriol. 1953 May;65(5):511–517. doi: 10.1128/jb.65.5.511-517.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HICKMAN D. D., FRENKEL A. W. The structure of Rhodospirillum rubrum. J Biophys Biochem Cytol. 1959 Oct;6:277–284. doi: 10.1083/jcb.6.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOPWOOD D. A., GLAUERT A. M. The fine structure of the nuclear material of a blue-green alga, Anabaena cylindrica Lemm. J Biophys Biochem Cytol. 1960 Dec;8:813–823. doi: 10.1083/jcb.8.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT A. L., RODGERS A., HUGHES D. E. Sub-cellular particles and the nicotinic acid hydroxylase system in extracts of. Biochim Biophys Acta. 1959 Aug;34:354–372. doi: 10.1016/0006-3002(59)90288-4. [DOI] [PubMed] [Google Scholar]
- MARR A. G., COTA-ROBLES E. H. Sonic disruption of Azotobacter vinelandil. J Bacteriol. 1957 Jul;74(1):79–86. doi: 10.1128/jb.74.1.79-86.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARR A. G. Enzyme localization in bacteria. Annu Rev Microbiol. 1960;14:241–260. doi: 10.1146/annurev.mi.14.100160.001325. [DOI] [PubMed] [Google Scholar]
- McQuillen K., Roberts R. B., Britten R. J. SYNTHESIS OF NASCENT PROTEIN BY RIBOSOMES IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1959 Sep;45(9):1437–1447. doi: 10.1073/pnas.45.9.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERMANN M. L., HAMILTON M. G., MIZEN N. A. Electrophoretic analysis of the macromolecular nucleoprotein particles of mammalian cytoplasm. Cancer Res. 1954 Jun;14(5):360–366. [PubMed] [Google Scholar]
- SALTON M. R. J., HORNE R. W. Studies of the bacterial cell wall. II. Methods of preparation and some properties of cell walls. Biochim Biophys Acta. 1951 Jul;7(2):177–197. doi: 10.1016/0006-3002(51)90017-0. [DOI] [PubMed] [Google Scholar]
- SAPERSTEIN S., STARR M. P. Association of carotenoid pigments with protein components in non-photosynthetic bacteria. Biochim Biophys Acta. 1955 Apr;16(4):482–488. doi: 10.1016/0006-3002(55)90267-5. [DOI] [PubMed] [Google Scholar]
- SCHACHMAN H. K., PARDEE A. B., STANIER R. Y. Studies on the macro-molecular organization of microbial cells. Arch Biochem Biophys. 1952 Jul;38:245–260. doi: 10.1016/0003-9861(52)90029-5. [DOI] [PubMed] [Google Scholar]
- SHATKIN A. J. A chlorophyll-containing cell fraction from the blue-green alga, Anabaena variabilis. J Biophys Biochem Cytol. 1960 Jun;7:583–584. doi: 10.1083/jcb.7.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUART D. C., Jr Fine structure of the nucleoid and internal membrane systems of Streptomyces. J Bacteriol. 1959 Aug;78:272–281. doi: 10.1128/jb.78.2.272-281.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIBULL C. Characterization of the protoplasmic constituents of bacillus megaterium. J Bacteriol. 1953 Dec;66(6):696–702. doi: 10.1128/jb.66.6.696-702.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]