Abstract
Background
Subclinical hypothyroidism (SHT), characterized by normal thyroid hormone levels maintained by elevated thyrotropin (TSH), predisposes patients to health problems as they age. Some cases arise from mutations of the TSH receptor (TSHR) that confer TSH resistance. This resistance might be circumvented by TSHR agonists with different modes of binding compared with TSH. We hypothesized that the recently discovered small-molecule TSHR agonist C2, with its unique mode of receptor binding, would activate mutant TSHRs associated with SHT, facilitating their study.
Materials and Methods
HEK-EM293 cells transiently expressing TSHR variants—wild-type TSHR or mutants C41S, L252P, L467P, or C600R—were analyzed for TSH or C2-induced cyclic adenosine monophosphate (cAMP) signaling to establish C2 as a mutant TSHR agonist. These cells were also pretreated with TSH or C2 to characterize each mutant receptor's ability to maintain and desensitize cAMP signaling.
Results
We showed that C2 could activate the TSH-unresponsive TSHR ectodomain mutants C41S and L252P but had no effect on the serpentine mutant L467P. We found that TSH and C2 could acutely activate the serpentine mutant C600R. Preincubation with C2 caused persistent cAMP signaling and receptor desensitization in wild-type TSHR and cAMP signal persistence with no detectable desensitization in the cases of C41S and L252P.
Conclusions
The small-molecule agonist C2 is a useful pharmacological tool for the study of mutant TSHRs. It revealed that some naturally occurring TSH-insensitive mutants can mediate induction of cAMP elevation upon stimulation with C2 and that this signal is differentially maintained within cells.
Introduction
Thyroid function is modulated by thyrotropin (TSH) through its interaction with the TSH receptor (TSHR), a member of the glycoprotein hormone receptor family, which is a subclass of the G protein-coupled receptor superfamily. Binding of TSH to TSHR elicits activation of heterotrimeric G-proteins, predominantly those containing Gαs subunits. Their activation of transmembrane adenylyl cyclases cause an elevation in intracellular cyclic adenosine monophosphate (cAMP) (1). It is predominantly the downstream cohort of cAMP effectors that augment thyroid function in support of the changing needs of the body. This TSH signal is reversed in part by receptor desensitization involving G-protein receptor kinases and arrestins (2) as well as by second messenger-activated kinases such as cAMP-dependent protein kinase (PKA). Dysregulation of TSHR activity is the basis for metabolic disorders such as Graves' disease. Small-molecule modulators of the TSHR are being developed as pharmacological tools to elucidate TSHR function in both healthy and disease states.
Recently, our laboratory introduced the first small-molecule agonists of the TSHR. The agonist C2 (NCGC00161870) was shown to activate TSHR both in vitro and in vivo (3). Importantly, it does so with a mode of binding that differs from TSH. TSH binds primarily to the ectodomain of TSHR, which presumably then leads to the activating conformational change of the serpentine domain, whereas C2 induces an active conformation by directly binding in the serpentine region (3). This was demonstrated by C2 activation of the KFLR TSHR variant, which lacks the entire ectodomain of the TSHR (4). In addition, this receptor is poorly expressed at the cell surface, yet C2 was able to induce an increase of cAMP in cells transiently expressing KFLR (3).
Seeking to take advantage of the activation mechanism of C2, we sought mutant TSHRs that might require direct serpentine domain binding for activation. Several potential receptors were found in patients diagnosed with subclinical hypothyroidism (SHT). SHT is diagnosed in patients with normal circulating levels of thyroid hormone maintained by elevated TSH (5). Investigations on patients with nonautoimmune SHT have identified several rare but deleterious mutations of TSHR. Patients with these germline TSHR mutations are heterozygotes and show varying degrees of TSH resistance (6–9). This resistance occurs in part because many of these TSHR mutants exhibit impaired TSH binding and/or poorly localize to the cell surface because of protein misfolding and entrapment in the endoplasmic reticulum (ER) (6,8). Impeding normal receptor function further is the ability of some trafficking-deficient receptors to trap wild-type TSHR in the ER through receptor dimerization or oligomerization (10). These impairments of TSHR function yield SHT.
Based on these data, we hypothesized that C2 might serve as an agonist for the TSH binding–deficient TSHR mutants found in the above cases of SHT and so could be used as a pharmacological tool for their study. Here, we present that C2 is indeed an agonist for some of these naturally occurring TSHR mutants and can be used to reveal characteristics of their functionality, such as cAMP signal persistence over time.
Materials and Methods
Site-directed mutagenesis
Mutations were introduced into the human TSHR (hTSHR-pCDNA3.1) with the QuikChange XL site-directed mutagenesis kit (Stratagene, Cedar Creek, TX). All were sequence verified (MWG Biotech, Huntsville, AL).
Cell culture and transfection
HEK-EM293 cells were grown in DMEM supplemented with 10% FBS, 100 U/mL penicillin, and 10 μg/mL streptomycin (Life Technologies, Rockville, MD) at 37°C in a humidified 5% CO2 incubator. Cells were plated in 10-cm dishes or 12-well plates at amounts of 1 × 106 cells/plate and 15 × 104 cells/well, respectively. Cells were then transiently transfected with TSHR variants or empty pCDNA 3.1 using Fugene 6 (Roche, Indianapolis, IN) following the manufacturer's protocol.
Determination of TSHR cell surface expression
Cells were harvested at 48 hours after transfection by treatment with 1 mM EDTA/1 mM EGTA in PBS and transferred to Falcon 2058 tubes. Cells were washed once with PBS containing 0.1% BSA and 0.1% NaN3 (binding buffer) and then incubated in 1:200 dilutions of TSHR-2C11 antibody (Serotec, Oxford, United Kingdom) or TSHR-4C1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) for 30 minutes. Cells were then washed once more and incubated for 30 minutes with a 1:200 dilution of an Alexa-Fluor 488–conjugated F(ab′)2 fragment of goat anti-mouse IgG (Molecular Probes, Eugene, OR) in binding buffer. After a final wash, cells were fixed with 1% paraformaldehyde and analyzed 24 hours later by FACS (FACSCalibur; BD Biosciences, San Jose, CA). Receptor expression was determined by the percentage of analyzed singlet cells found in a gate that contained little to no positive cells during analysis of empty pCDNA3.1 transfectants. This percentage was then normalized to that of the wild-type TSHR.
Acute stimulation and intracellular cAMP measurement
After transfection, cells were moved from a 10-cm dish to 96-well plates provided in the cAMP Screen Direct Assay kit (Applied Biosystems, Carlsbad, CA) at a density of 7 × 104 cells/well. After 24 hours, growth media was replaced with Hank's balanced salt solution (HBSS) supplemented with 500 μM 3-isobutyl-1-methylxanthine (IBMX; Sigma, St. Louis, MO) and 10 mU/mL bovine TSH (Sigma) or 10 μM compound C2 (synthesized at the NIH Chemical Genomics Center at the National Institutes of Health, Rockville, MD) unless otherwise stated. Cells were stimulated for 1 hour followed by lysis and analysis of cAMP content according to the manufacturer's protocol.
Receptor desensitization and intracellular cAMP measurement
Transfected cells were seeded as earlier in 96-well plates with low-serum growth media containing only 2% FBS and supplemented with 30 μM C2 or 10 mU/mL TSH unless otherwise stated. After 18–20 hours of incubation, the medium was aspirated and replaced with normal growth media (10% FBS) for an additional 24 hours unless otherwise indicated. Then, the medium was aspirated and replaced with HBSS supplemented with 500 μM IBMX and either 30 μM C2 or 10 mU/mL TSH. After 1 hour, intracellular cAMP was assayed based on the manufacturer's protocol as mentioned earlier.
Statistical analysis
All amplitudes that make up a given histogram bar were compared with the control for that experiment in a Student's t-test with two tails and homoscedastic relationship. Calculations were performed in Microsoft Excel. Labels were added to all histograms to represent statistical significance (*p ≤ 0.05 or **p ≤ 0.005). Histogram bars with no such labels are not statistically different from their controls.
Results
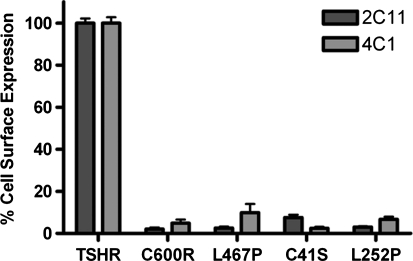
To begin, a panel of reported binding- and/or trafficking-deficient mutant TSHRs identified in patients with SHT was generated by mutagenesis. This panel was comprised of a series of single-site mutations including Cys41 to Ser (C41S) (9), Leu252 to Pro (L252P) (8), Leu467 to Pro (L467P) (6), and Cys600 to Arg (C600R) (6). To confirm the trafficking deficiency of these mutant receptors, HEK-EM293 cells transiently expressing one of these variants or wild-type TSHRs were analyzed by FACS for cell surface receptor expression using two TSHR antibodies, 2C11 and 4C1, specific for two distinct extracellular epitopes (2C11, amino acids 354–359; 4C1, amino acids 378–384). Figure 1 shows the reduced cell surface expression of all mutants, validating not only the previously published findings but also their presence at the cell surface under our experimental conditions.
FIG. 1.
Expression profiles of thyrotropin receptor (TSHR) variants. HEK-EM293 cells were transfected with plasmids encoding the listed TSHR variants. After 48 hours, all were probed with 2C11 or 4C1 antibody, both specific to two different epitopes within the extracellular portion of TSHR. All variants showed reduced cell surface presentation compared with wild-type TSHR. Bars represent mean ± standard error of the mean (SEM).
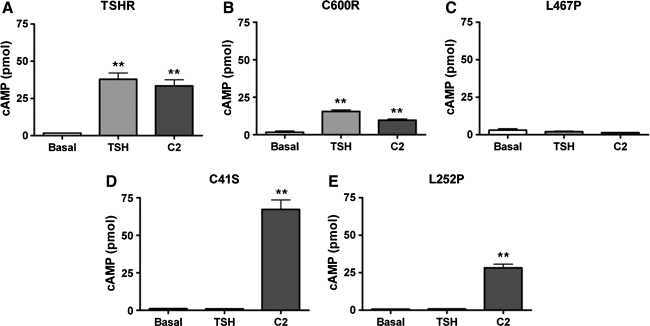
Several receptors in our panel have been noted in the literature to be TSH insensitive. We sought to confirm this and to also test whether our small-molecule agonist C2 could elicit a cAMP increase in cells expressing these mutants. HEK-EM293 cells were transiently transfected with vectors encoding the genes of these receptors, and after 48 hours, the cells were challenged with vehicle (DMSO), TSH, or C2 in the presence of the phosphodiesterase inhibitor IBMX. After stimulation for 1 hour, intracellular cAMP was measured. As shown in Figure 2, multiple ligand sensitivity profiles arose for this panel of receptors. The mutant C600R receptor (Fig. 2B) shows the same sensitivity profile as TSHR (Fig. 2A). Both receptors caused elevation of cAMP levels in cells treated with either TSH or C2. In contrast, L467P could not be activated by either TSH or C2 (Fig. 2C). The last profile was found in the cases of C41S (Fig. 2D) and L252P (Fig. 2E), which could not be activated by TSH but do increase cAMP levels in response to C2. Of note, neither TSH nor C2 elicited any response from cells expressing empty pCDNA3.1 vectors (data not shown). Most importantly, C2 is a functional agonist for the TSH-insensitive ectodomain mutants C41S and L252P. We highlight here experiments with similar response amplitudes to illustrate the differences and similarities between receptor ligand sensitivities. It should be noted that, however, compared with TSHR-expressing cells in the same experiment, mutant receptor-expressing cells performed more poorly at percentages of approximately 8%, 3%, and 15% of TSHR response amplitude for C41S, L252P, and C600R, respectively.
FIG. 2.
Ligand sensitivity profiles of TSHR variants: TSHR (A), C600R (B), L467P (C), C41S (D), and L252P (E). Transfected HEK-EM293 cells were stimulated with DMSO, 10 mU/mL TSH, or 10 μM C2 for 1 hour in the presence of 500 μM 3-isobutyl-1-methylxanthine (IBMX). After incubation with agonists, intracellular cyclic adenosine monophosphate (cAMP) was measured and three distinct profiles arose. Bars represent mean ± SEM. **p ≤ 0.005.
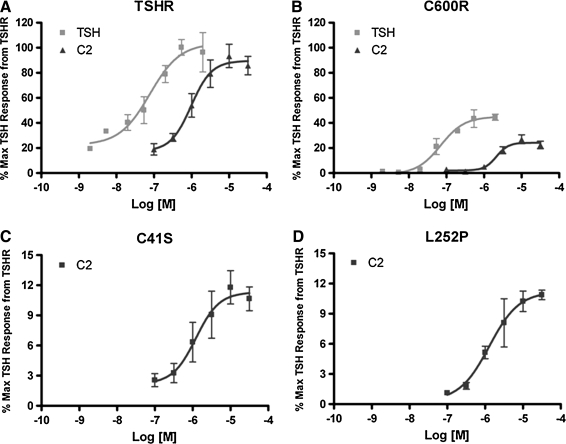
To evaluate whether C2 has similar pharmacological properties on all these TSHR variants, dose–response curves to TSH and C2 or C2 alone were generated. As shown in Figure 3, TSH is 10-fold more potent as a TSHR agonist compared with C2 in cells expressing TSHR. This difference is maintained in the case of cells expressing C600R. C2 also caused dose-dependent responses in cells expressing C41S and L252P. Importantly, the EC50s of C2 on all these receptors were comparable at approximately 1, 2, 1, and 1 μM for TSHR, C600R, C41S, and L252P, respectively.
FIG. 3.
Dose–response curves to TSHR variants TSHR (A), C600R (B), C41S (C), and L252P (D) to TSH and C2. Cells were stimulated for 1 hour in the presence of IBMX. All cAMP levels were normalized to the maximal TSH response from cells expressing TSHR. Please note the varying amplitudes of the y-axes. Bars represent mean ± SEM.
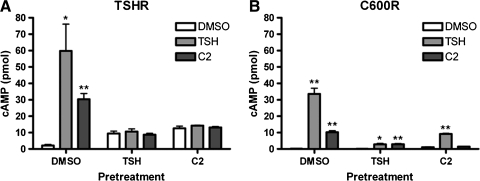
It is known that TSH activation of TSHR can initiate not only rapid cAMP production but also persistent cAMP signaling (11,12) followed by the activation of receptor desensitization machinery in HEK cells (2). We began our study by determining whether TSHR persistent signaling and desensitization could be caused by C2. We examined receptor activities of TSHR transiently expressed in HEK-EM293 cells after a 20-hour exposure to DMSO (vehicle for C2), TSH, or C2 followed by a 24-hour recovery period. As shown in Figure 4, TSHR-expressing cells pretreated only with the vehicle DMSO responded to stimulation with either TSH or C2 as expected. Pretreatment with C2, like TSH, caused persistent cAMP production in these cells, indicated by increased basal cAMP levels even after a 24-hour period to recover in the absence of agonist. TSHR desensitization was evident from the lack of response to TSH or C2 detected under pretreated conditions. Similar results were seen in cells expressing C600R (Fig. 4B). Thus, C2, like TSH, caused persistent cAMP signaling and TSHR desensitization.
FIG. 4.
C2 causes persistent cAMP signaling and desensitization of TSHR (A) and C600R (B). Cells were pretreated with DMSO, TSH, or C2 for 20 hours and then incubated without ligands for 24 hours. Bars represent mean ± SEM. *p ≤ 0.05; **p ≤ 0.005.
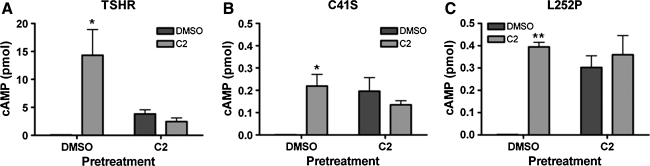
We next studied the TSHR variants C41S and L252P using the small-molecule agonist C2. Cells were transfected with TSHR, C41S, or L252P, pretreated for 20 hours with DMSO or C2, allowed a 24-hour recovery period, and then stimulated with either DMSO or C2. As previously shown, cells expressing TSHR (Fig. 5A) showed both persistent cAMP signaling and receptor desensitization to further agonist treatment. Cells expressing C41S (Fig. 5B) or L252P (Fig. 5C) responded to C2 albeit with lower amplitudes than TSHR. Those cells pretreated with C2 showed persistent cAMP production indicated by increased basal cAMP signaling, and interestingly, this persistent signal was the same amplitude as those found in cells acutely stimulated with C2. This is in contrast to TSHR, indicating that C41S and L252P do not undergo the same degree of desensitization as wild-type TSHR. Instead, they can maintain their small cAMP signals over the time tested.
FIG. 5.
Responses of pretreated TSHR variants C41S and L252P. Transfected HEK-EM293 cells were pretreated with DMSO or C2 (30 μM) for 18–20 hours. After an additional 24-hour recovery period, the cells were stimulated with DMSO or C2, and then intracellular cAMP levels were measured. TSHR (A) does show persistent signaling as well as desensitization, C41S (B), and L252P (C) show a strong persistence in cAMP signal. Bars represent mean ± SEM. Please note the varying amplitudes of the y-axes. *p ≤ 0.05; **p ≤ 0.005.
Discussion
Here, we describe the cAMP pathway signaling of a panel of mutant TSHR identified in patients with nonautoimmune SHT stimulated by TSH or a recently developed small-molecule agonist of TSHR C2. Parameters tested include not only rapid elevations of cAMP concentration elicited by acute agonist treatment but also two additional properties that have become clearly important for understanding TSHR function. The first, persistent cAMP signaling has been seen with several receptors including the sphingosine-1-phosphate receptor (13), parathyroid hormone receptor (14), and the TSHR (11,12). This signal persistence was shown to modify a response within TSHR-expressing cells (12). We show here that persistent cAMP signaling by TSHR can be elicited by C2 as well as TSH. The second additional parameter TSHR desensitization inhibits the reinitiation of the cAMP signal, yielding temporal control over cellular responses to TSH or C2. The effects of mutations on either of these properties could provide additional insight into not only mutant TSHR function but also that of wild-type TSHR.
All mutants have reduced cell surface expression as well as their own functional differences and the use of two TSHR agonists with different TSHR binding characteristics helped us to identify these. One TSHR variant with a site-specific mutation in transmembrane helix (TM)2 within the serpentine domain of the receptor, L467P, performed as previously reported. Its cell surface presentation was impaired relative to TSHR and neither TSH nor C2 could stimulate an elevation of cAMP in cells expressing this mutant. Although L467P could interfere with the binding pocket of C2, it is just as likely that this mutant has no signaling capacity. C600R, which contains a mutation in TM5, was previously found to be insensitive to TSH. Here, we demonstrated that it can respond to both TSH and C2. We cannot definitively explain the differences in these findings; however, they may have been caused by different levels of transfection efficiency leading to higher levels of expression in our cells. Thus, the TM5 mutation of C600R reduces but does not abolishes receptor functionality in this cellular context.
It was in the cases of the TSHR ectodomain variants that the most interesting ligand sensitivities were observed. L252P has been shown to respond, albeit poorly, to TSH, presumably owed to poor cell surface expression (8). Here, no TSH sensitivity was observed even though we found cell surface expression of L252P. However, we showed that L252P leads to an increase in cAMP in response to C2. Thus, although part of the reduced activity of L252P could stem from its poor membrane localization, it is also an inherent property of this deleterious ectodomain mutation. Position C41 participates in disulfide bonds that are essential to ectodomain structure and function and also receptor trafficking to the plasma membrane (15). Past works had found that there is no detectable antibody or TSH binding of C41S on the surface of cells (6). On the contrary, we showed that there is some localization of C41S to the surface of these HEK-EM293 cells via FACS analysis with two TSHR antibodies. In addition, C2 is capable of eliciting a cAMP response in cells expressing this variant. Although C41 is necessary for TSH-mediated TSHR activation, it is not necessary for activation by C2, which binds the serpentine domain of the receptor.
Neither C41S nor L252P showed detectable receptor desensitization. Perhaps the most straightforward explanation has to do with the amplitude of their response within the context of these HEK-EM293 cells. TSHR downregulation does not efficiently occur in this cell type (11). For this reason, the dominant role for cAMP signal reversal should fall to negative feedback mechanisms regulated by protein kinase A, such as activation of phosphodiesterases or inhibition of adenylyl cyclases (16). The small amount of cAMP produced by these mutants might not induce the same amount of negative feedback by PKA compared with other TSHR variants tested. Clearly, it is in the cases of these and other ectodomain mutants of the TSHR insensitive to TSH that C2 might find the most utility.
In conclusion, we present a previously discovered small-molecule agonist of TSHR as a useful pharmacological tool in the study of not only wild-type TSHR but also mutant TSHRs. C2 was successfully used to elucidate the differential signaling capacity and regulation of these naturally occurring mutants. Future studies will focus on the underlying mechanisms that make the cAMP signal from each mutant receptor unique.
Acknowledgment
This research was supported by the Intramural Research Program of the U.S. National Institutes of Health (1 Z01 DK011006).
Disclaimer
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of manuscript.
Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.Wilson B. Raghupat E. Tonoue T. Tong W. Tsh-like actions of dibutyryl-camp on isolated bovine thyroid cells. Endocrinology. 1968;83:877–884. doi: 10.1210/endo-83-4-877. [DOI] [PubMed] [Google Scholar]
- 2.Nagayama Y. Chazenbalk GD. Takeshita A. Kimura H. Ashizawa K. Yokoyama N. Rapoport B. Nagataki S. Studies on homologous desensitization of the thyrotropin receptor in 293 human embryonal kidney cells. Endocrinology. 1994;135:1060–1065. doi: 10.1210/endo.135.3.8070347. [DOI] [PubMed] [Google Scholar]
- 3.Neumann S. Huang W. Titus S. Krause G. Kleinau G. Alberobello AT. Zheng W. Southall NT. Inglese J. Austin CP. Celi FS. Gavrilova O. Thomas CJ. Raaka BM. Gershengorn MC. Small-molecule agonists for the thyrotropin receptor stimulate thyroid function in human thyrocytes and mice. Proc Natl Acad Sci U S A. 2009;106:12471–12476. doi: 10.1073/pnas.0904506106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vlaeminck-Guillem V. Ho SC. Rodien P. Vassart G. Costagliola S. Activation of the cAMP pathway by the TSH receptor involves switching of the ectodomain from a tethered inverse agonist to an agonist. Mol Endocrinol. 2002;16:736–746. doi: 10.1210/mend.16.4.0816. [DOI] [PubMed] [Google Scholar]
- 5.Fatourechi V. Subclinical hypothyroidism: an update for primary care physicians. Mayo Clin Proc. 2009;84:65–71. doi: 10.4065/84.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alberti L. Proverbio MC. Costagliola S. Romoli R. Boldrighini B. Vigone MC. Weber G. Chiumello G. Beck-Peccoz P. Persani L. Germline mutations of TSH receptor gene as cause of nonautoimmune subclinical hypothyroidism. J Clin Endocrinol Metab. 2002;87:2549–2555. doi: 10.1210/jcem.87.6.8536. [DOI] [PubMed] [Google Scholar]
- 7.Russo D. Betterle C. Arturi F. Chiefari E. Girelli ME. Filetti S. A novel mutation in the thyrotropin (TSH) receptor gene causing loss of TSH binding but constitutive receptor activation in a family with resistance to TSH. J Clin Endocrinol Metab. 2000;85:4238–4242. doi: 10.1210/jcem.85.11.6985. [DOI] [PubMed] [Google Scholar]
- 8.Tonacchera M. Perri A. De Marco G. Agretti P. Banco ME. Di Cosmo C. Grasso L. Vitti P. Chiovato L. Pinchera A. Low prevalence of thyrotropin receptor mutations in a large series of subjects with sporadic and familial nonautoimmune subclinical hypothyroidism. J Clin Endocrinol Metab. 2004;89:5787–5793. doi: 10.1210/jc.2004-1243. [DOI] [PubMed] [Google Scholar]
- 9.de Roux N. Misrahi M. Brauner R. Houang M. Carel JC. Granier M. Le Bouc Y. Ghinea N. Boumedienne A. Toublanc JE. Milgrom E. Four families with loss of function mutations of the thyrotropin receptor. J Clin Endocrinol Metab. 1996;81:4229–4235. doi: 10.1210/jcem.81.12.8954020. [DOI] [PubMed] [Google Scholar]
- 10.Calebiro D. de Filippis T. Lucchi S. Covino C. Panigone S. Beck-Peccoz P. Dunlap D. Persani L. Intracellular entrapment of wild-type TSH receptor by oligomerization with mutants linked to dominant TSH resistance. Hum Mol Genet. 2005;14:2991–3002. doi: 10.1093/hmg/ddi329. [DOI] [PubMed] [Google Scholar]
- 11.Neumann S. Geras-Raaka E. Marcus-Samuels B. Gershengorn MC. Persistent cAMP signaling by thyrotropin (TSH) receptors is not dependent on internalization. FASEB J. 2010;24:3992–3999. doi: 10.1096/fj.10-161745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calebiro D. Nikolaev VO. Gagliani MC. de Filippis T. Dees C. Tacchetti C. Persani L. Lohse MJ. Persistent cAMP-signals triggered by internalized G-protein-coupled receptors. PLoS Biol. 2009;7:e1000172. doi: 10.1371/journal.pbio.1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mullershausen F. Zecri F. Cetin C. Billich A. Guerini D. Seuwen K. Persistent signaling induced by FTY720-phosphate is mediated by internalized S1P1 receptors. Nat Chem Biol. 2009;5:428–434. doi: 10.1038/nchembio.173. [DOI] [PubMed] [Google Scholar]
- 14.Ferrandon S. Feinstein TN. Castro M. Wang B. Bouley R. Potts JT. Gardella TJ. Vilardaga JP. Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat Chem Biol. 2009;5:734–742. doi: 10.1038/nchembio.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen CR. Tanaka K. Chazenbalk GD. McLachlan SM. Rapoport B. A full biological response to autoantibodies in Graves' disease requires a disulfide-bonded loop in the thyrotropin receptor N terminus homologous to a laminin epidermal growth factor-like domain. J Biol Chem. 2001;276:14767–14772. doi: 10.1074/jbc.M008001200. [DOI] [PubMed] [Google Scholar]
- 16.Murthy KS. Zhou H. Makhlouf GM. PKA-dependent activation of PDE3A and PDE4 and inhibition of adenylyl cyclase V/VI in smooth muscle. Am J Physiol Cell Physiol. 2002;282:C508–C517. doi: 10.1152/ajpcell.00373.2001. [DOI] [PubMed] [Google Scholar]