Abstract
Background
Glucagon-like peptide-2(GLP-2) induces small intestine mucosal epithelial cell (EC) proliferation; and may have benefit for patients suffering from short bowel syndrome (SBS). However, GLP-2 is rapidly inactivated in vivo by dipeptidyl peptidase IV (DPPIV). Therefore, we hypothesized that selectively inhibiting DPPIV would prolong the circulating life of GLP-2 and lead to increased intestinal adaptation after development of SBS.
Methods
8-week old C57BL/6J mice underwent a 50% proximal small bowel resection and were treated with either sitagliptin, a DPPIV-inhibitor (DPPIV-I), starting 1 day before surgery versus placebo. DPPIV-I efficacy was assessed 3 days after resection, including intestinal morphology, EC apoptosis and EC proliferation. Adaptive mechanisms were assessed with quantitative real-time PCR, and plasma bioactive GLP-2 was measured by radioimmunoassay.
RESULT
Body weight loss and peripheral blood glucose levels did not change compared to SBS controls. DPPIV-I treatment led to significant increases in villus height and crypt depth. DPPIV-I treatment did not significantly change EC apoptosis rates, but significantly increased crypt EC proliferation versus placebo-SBS controls. DPPIV-I treatment markedly increased mRNA expression of β-catenin and c-myc in ileal mucosa. Plasma GLP-2 levels significantly increased(~40.9%) in DPPIV-I-SBS mice.
Conclusions
DPPIV- I treatment increased SBS adaptation, and may potentially be useful for SBS patients.
INTRODUCTION
Short bowel syndrome (SBS) is a highly disabling condition in which patients have insufficient small bowel length for proper nutritional absorption, and can lead to serious life-threatening complications1. While advancements have been made in the treatment of SBS, currently, therapeutic options available for treatment of SBS remain limited. Bowel growth and regeneration is regulated by a range of nutritional and non-nutritional factors. Non-nutritional factors suggested to be important stimulators of intestinal adaptation include endogenous secretions, peptide hormones, growth factors, neurovascular components and mesenchymal interactions2. Peptide hormones and growth factors, which can drive epithelial cell (EC) proliferation, can be produced locally in the bowel or derived from extra-gastrointestinal sites of synthesis. Adaptation is a compensatory response of the small intestine that occurs following massive loss of mucosal surface area3. Recent studies have shown glucagon-like peptide-2 (GLP-2) is a pleiotropic hormone that affects multiple facets of intestinal physiology, including growth, barrier function, digestion, absorption, motility, and blood flow. GLP-2 can augment intestinal adaptation, and is a potential treatment for gastrointestinal disorders associated with insufficient mucosal function 4; and clinical trials are underway for patients with intestinal failure5. Evidence from both animal studies and clinical trials demonstrate that GLP-2 is a trophic hormone that plays an important role in controlling intestinal adaptation. Furthermore, clinical trials demonstrate that GLP-2 is safe, well-tolerated, and promotes intestinal growth in SBS patients5, 6. However, GLP-2 can be cleaved by the endogenous serine protease dipeptidyl peptidase IV (DPP IV), and rapidly inactivated7; resulting a short 7 minute half-life for native GLP-25. Due to rapid cleavage and inactivation, therapy with native GLP-2 administered parenterally is not practical for SBS treatment, and thus incretin mimetics, such as teduglutide, that are resistant to DPPIV cleavage have been developed. Such therapies, however, are highly expensive and must be given chronically5.
DPP-IV is a serine protease cleaving dipeptides from the N-terminal end of polypeptides with l-proline or l-alanine at the penultimate position and occurs as a soluble as well as membrane-bound form in many tissues and body fluids8. DPP-IV either alters or abolishes many circulating biological peptides including glucose-dependent insulinotropic polypeptide (GIP). However, DPPIV has high affinity for GLP-1 and GLP-2, inactivating them and thereby reducing their half-life9.
The DPPIV inhibitor (DPPIV-I), sitagliptin (Januvia®, Merk& CO., Inc, Whitehouse Station, NJ, USA), is a first-in-class drug for diabetes. The drug prevents breakdown of the incretins GLP-1 and GLP-210. Sitagliptin is an orally active, potent, selective DPPIV-I with excellent oral bioavailability11. Therefore, we hypothesized that DPPIV-I would prolong the circulating life of the GLP-2, and lead to a greater degree of intestinal adaptation in SBS than what is achieved normally. We further investigated the mechanisms which effect these changes. The data suggest a novel approach to SBS adaptation, and also demonstrate the safety with its usage in an SBS model.
MATERIAL AND METHODS
Chemical
Sitagliptin phosphate, an orally-active inhibitor of the DPPIV enzyme (DPPIV-I), is described chemically as 7-[(3R)-3-amino-1-oxo-4-(2,4,5- trifluorophenyl)butyl]-5,6,7,8-tetrahydro-3-(trifluoromethyl)-1,2,4-triazolo[4,3-a]pyrazine phosphate (1:1) monohydrate. The empirical formula is C16H15F6N5O·H3PO4·H2O and the molecular weight is 523.32.
Animals
Specific pathogen free, 8-week-old, 20–22g C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) were used and maintained in a 12-h day-night rhythm at 23°C and a relative humidity of 40–60%. Forty eight hours before surgery standard chow was exchanged to micro-stabilized rodent liquid diet (TestDiet, Richmond, IN) and mice were maintained thereafter on liquid diet until harvest. All experiments were approved by the University Committee on Use and Care of Animals at the University of Michigan.
Experimental design
Mice were randomly assigned to three groups: 1) Sham mice underwent laparotomy, bowel transection and re-anastomosis (Sham, n = 5); 2) SBS mice underwent a 50% proximal small bowel resection and were treated with oral phosphate-buffered saline as a placebo (SBS, n = 6); and 3) SBS mice treated with DPP IV-I underwent a similar bowel resection and were treated with Sitagliptin orally every eight hours (50mg/kg/dose/0.2ml, SBS+DPPIV-I, n=6). Dosing was based on previously reported studies in animal models, and approximated a mid-range of the drug dosing 12. Placebo and DPPIV-I were started 1 day before surgery. Body weight was followed daily. Peripheral blood for glucose levels was taken daily by tail vein.
Short bowel syndrome (SBS) model
Mice underwent a 50% proximal bowel resection or sham (transection and re-anastomosis only) as previously reported3, 13. Anesthesia was induced and maintained by inhalational administration of isoflurane. The SBS model consisted of a resection of small bowel between 3.0 cm distal to the ligament of Treitz and 12.0 cm proximal to the ileocecal valve, and was followed by an end-to-end jejuno-ileal anastomosis with 9–0 suture (Ethilon, Ethicon Corporation, USA). The abdominal wall was closed in two layers. At the end of surgery, mice were resuscitated with a 2.0 ml subcutaneous injection of warmed saline and allowed free access to water and liquid chow.
Harvesting
Mice were killed using CO2 3 days post-initial surgery, and the intestine harvested. Small-bowel segments (0.5 cm) were preserved in 10% buffered formalin. Jejunal and ileal tissues were taken 2 cm distal to ligament of Treitz and 3 cm proximal to the ileocecal junction. Small bowel within 0.5 cm of the anastomotic sites was discarded. The remaining small bowel was immediately processed for mucosal cell isolation using previously described methods14.
Plasma GLP-2 determination
Near time of sacrifice, and 2 hours from final administration of DPPIV-I, blood was obtained via cardiac puncture and collected into MiniCollect® EDTA tube (Greiner Bio-One, North Carolina US) for analysis of circulating GLP-2 concentration. Tubes were immediately cooled on ice and centrifuged for 20 min at 1200 g and 4 °C. Plasma for GLP-2 analysis was stored at −80°C, and measured by radioimmunoassay using an antibody specific to the NH2 terminus of GLP-2; results were expressed as pmol/L15, 16.
Morphological analysis
Jejunum and ileum were fixed for 24 h in 10% formalin, and transverse 5 μm sections were stained with hematoxylin and eosin staining. Villus height and crypt depth were measured using light microscopy based on the mean of 20 well-oriented villi and crypts. All readings were performed in a masked fashion, where the researcher was blinded to the study group at the time of readings.
Proliferation/Apoptosis
Epithelial Cell Proliferation
To examine changes in intestinal EC crypt proliferation incorporation of 5-bromo-2-deoxyuridine (BrdU) was used as previously described13, 17. Mice were injected with BrdU (50 mg/kg, i.p., Roche Diagnostic, Indianapolis, IN) 2 h before harvest. An index of crypt EC proliferation rate was calculated using the ratio of the number of crypt cells incorporating BrdU to the total number of crypt cells (crypt proliferation index: CPI, counted at 20× magnification).
Epithelial cell apoptosis
Cleaved-caspase-3 immunostaining (1:40 rabbit anti-cleaved caspase-3 Asp175, 5A1E antibody, Cell signaling, MA, USA) was used assess for EC apoptosis. Expression of apoptotic activity was performed by counting all apoptotic cells from both crypts and villi per all counted ECs, and is expressed as the apoptotic index per crypt–villus complex (API) (read at 40× magnification).
Real time polymerase chain reaction (RT-PCR)
Mucosal scrapings were placed in TRIzol (Invitrogen), homogenized, RNA extracted and purified as previously described14. All primers for selected gene sequences (SGLT-1, GLP-2R, β-catenin and c-myc) were designed using primer-BLAST software. Real-time PCR (RT-PCR) was performed using a Rotor-Gene 6000 (Corbett Life Science, Sydney, Australia) and β-actin was used as an internal control for normalization. Fold changes of target genes were calculated using comparative quantification to β-actin.
Statistical analysis
Data are expressed as the mean ± SEM. Statistical analysis between the three groups used one-way analysis of variance (ANOVA) followed by post-hoc analysis by Bonferroni test (Prism software; GraphPad Software, Inc., San Diego, CA). A value of P<0.05 was considered to be statistically significant.
RESULTS
Body weight changes
All mice were active and healthy throughout the study. There were no differences in survival (>90%) between groups. Both Sham and SBS mice showed some degree of weight loss following surgery; however, loss in each of these plateaued by the latter 2 days after the surgery period. The loss of body weight in the SBS group was significantly greater than the Sham (percent change from weight at surgery: −13.06 ± 1.10% versus −6.22 ± 1.69%, respectively; P<0.05). However, this loss was attenuated in the SBS+DPPIV-I group (−10.31 ± 1.05%), but differences were not significant.
Peripheral blood glucose level
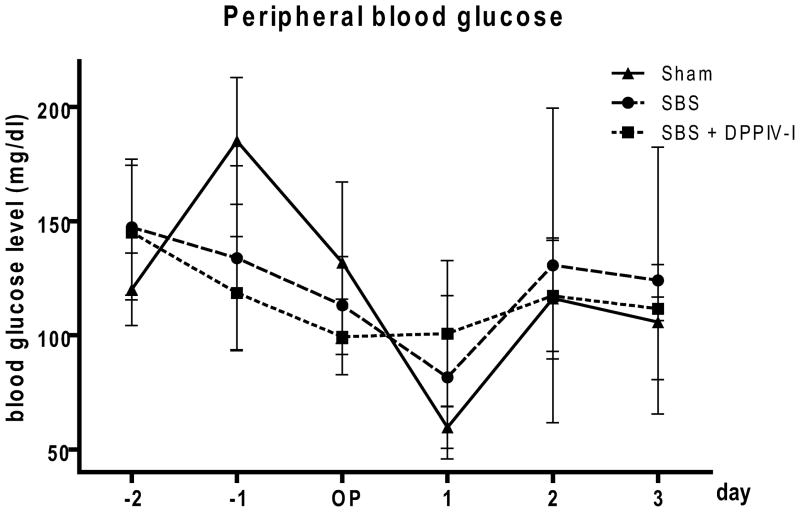
The average peripheral blood glucose levels over the experimental period did not significantly differ between the three groups (Sham 119.8± 16.58 mg/dl, SBS 121.7±9.26 mg/dl, SBS+DPP IV-I 115.4±6.78 mg/dl, P=0.92). (Figure 1).
Figure 1.
Time course of peripheral blood glucose levels in each study group. Peripheral blood for measuring glucose level was taken daily from the tail vein. Values are means ± SEM. N=5–6.
DPPIV-I significantly altered mucosal morphometry
The adaptive morphological responses after formation of the SBS model are shown in Table 1. Increases in villus height and crypt depth occurred after SBS as compared to the Sham group. DPPIV-I administration to SBS mice resulted in a significant increase in villus height and crypt depth beyond that seen in untreated SBS mice.
Table 1.
Crypt–villus morphology
| Sham | SBS | SBS+DPPIV-I | |
|---|---|---|---|
| Villus height (μm) | |||
| Jejunum | 351.4±7.2 | 378.9±15.4 | 402.2±10.2* |
| Ileum | 231.3±2.9 | 264.1±7.4¶ | 302.9±3.5*# |
| Crypt depth(μm) | |||
| Jejunum | 73.9±1.2 | 98.1±2.1¶ | 118.9±2.4*# |
| Ileum | 76.9±0.9 | 95.9±1.7¶ | 106.6±2.2*# |
Data are expressed as mean ± SEM. Note significant difference in villus height and crypt depth between Sham and SBS with/without DPPIV-I treatment.
P<.05 Sham vs SBS,
P<.05 Sham vs SBS+DPPIV-I,
P<.05 SBS vs SBS+DPPIV-I
Effect of DPPIV-I on epithelial cell proliferation
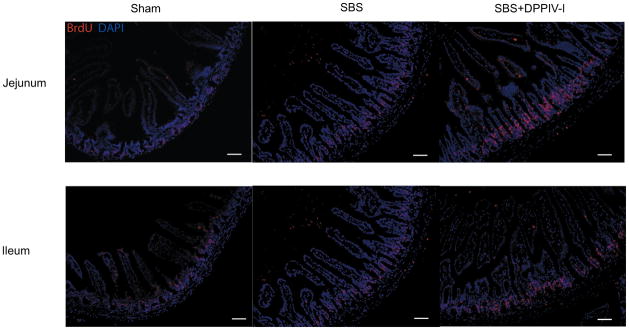
EC proliferation was assessed with BrdU incorporation (Figure 2). SBS led to significantly increased EC proliferation in both jejunum and ileum compared to Sham mice. DPPIV-I treatment, however, significantly increased this level of proliferation (P<0.05) compared to placebo treated SBS mice; and was true for ileum and jejunum.
Figure 2.
Representative immunofluorescent images of jejunum and ileum are shown (left panels) after undergoing BrdU/DAPI staining (20X magnification) 3 days after a 50% small bowel resection. Indices of crypt cell proliferation rates are shown in the graphs (right panels). Note that the proliferation index was significantly increased in SBS+DPPIV-I mice. Results are shown as the mean ± SEM. Statistical comparisons are made using ANOVA with post hoc Bonferroni test. N=5–6. Scale bars:50μm
Effect of DPPIV-I on epithelial apoptosis
Enterocyte API was higher in the SBS group (jejunum:0.90±0.14, ileum:0.42±0.05) versus the Sham group (jejunum:0.54±0.09, ileum:0.37±0.03), in agreement with previous studies13, but not significantly different. Administration of DPPIV-I brought EC apoptosis rates back to the range of the Sham group (jejunum: 0.57±0.12, ileum: 0.39±0.04).
Mechanisms contributing to improved adaptation with DPPIV-I treatment
Increase of plasma GLP-2 after oral DPPIV-I treatment
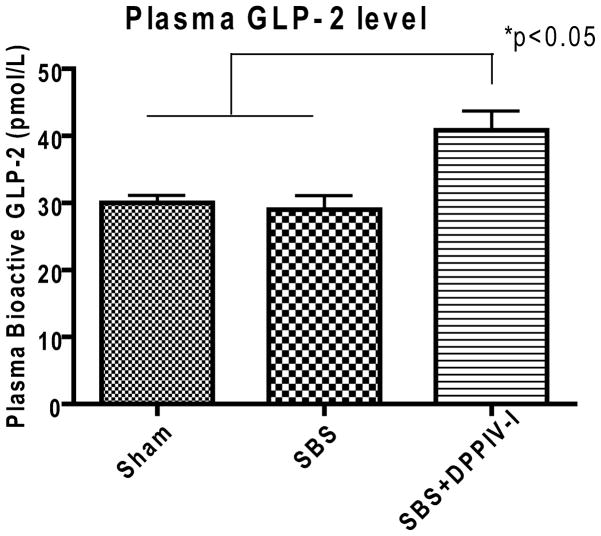
Sham and SBS groups showed similar concentrations of bioactive plasma GLP-2 levels (30.0±1.16 pmol/L and 29.0±2.12 pmol/L, respectively, P>0.05). However, bioactive plasma GLP-2 was significantly increased (~40.9%) in the DPPIV-I treatment group compared to the Sham and SBS groups (40.86±2.89 pmol/L, P<0.05). (Figure 3)
Figure 3.
Plasma concentration of bioactive glucagon-like peptide-2 (GLP-2) levels. Values are means ± SEM. N=5–6. *P<0.05
Expression of GLP-2 receptor (GLP-2R)
GLP-2 mediates some of its actions via GLP-2R, a member of the glucagon/GLP-1R superfamily18. GLP-2R is predominantly expressed in the intestine, especially in the proximal small bowel. Although there is a wealth of information about the effects of GLP-2 on intestinal physiology and on the signaling mechanisms initiated by the GLP-2R, there are relatively few studies addressing how these two events are functionally connected19. To better understand the increased adaptation in DPPIV-I mice, we examined GLP-2R mRNA expression. Jejunum and ileum expression of GLP-2R showed no significant differences between all study groups (Table 2).
Table 2.
Abundance of mRNA from GLP-2R, SGLT-1, β-catenin and c-myc in the small intestinal mucosa (jejunum, ileum) of after 3 days small bowel resection.
| Sham | SBS | SBS+DPPIV-I | |
|---|---|---|---|
| GLP-2R | |||
| Jejunum | 0.97±0.15 | 1.61±0.33 | 1.32±0.21 |
| Ileum | 0.64±0.12 | 0.98±0.15 | 0.76±0.18 |
| SGLT-1 | |||
| Jejunum | 3113±331 | 2207±367 | 2561±357 |
| Ileum | 1512±247 | 1389±246 | 1343±104 |
| β-catenin | |||
| Jejunum | 38.5±2.79 | 38.9±4.73 | 38.5±1.96 |
| Ileum | 27.4±3.72 | 31.5±2.37 | 40.1±1.64 * |
| c-myc | |||
| Jejunum | 11.5±1.51 | 11.9±2.29 | 13.3±1.36 |
| Ileum | 8.08±1.38 | 8.52±2.32 | 10.3±1.58 |
Results are expressed as mean ± SEM. Results scaled by 103. Fold changes of target genes were calculated using comparative quantification to β-actin.
P<.05 Sham vs SBS+DPPIV-I
Expression of Sodium glucose co-transporter 1 (SGLT-1)
Nutrient transport is an essential function of the small intestine and must be retained after bowel resection. D-glucose is transported across the apical membrane by the Na+-dependent glucose transporter SGLT1 into the EC cytosol20. Relative mRNA expression of SGLT-1 was greater in the jejunum compared to the ileum, as shown previously20. However, SGLT-1 did not significantly differ between Sham (jejunum:3.11±0.33, ileum:1.55±0.25) and SBS groups (jejunum:2.21±0.37, ileum:1.72±0.38). Similarly, treatment with DPPIV-I did not significantly alter SGLT-1 mRNA abundance (jejunum:2.56±0.36, ileum:1.53±0.29).
β-catenin and cellular myelocytomatosis (c-myc)
mRNA abundance of β-catenin and c-myc were investigated based on previous studies which have shown that the regulation of crypt cell proliferation often involves these key factors21. Table 2 shows that treatment of DPPIV-I led to a markedly increased expression of both β-catenin and c-myc in ileal mucosa. However, these changes were only modest and non-significant in the jejunum.
DISCUSSION
GLP-2, derived from intestinal L-cells in response to food intake and intestinotrophic stimulation, is one of the strongest mediators of intestinal adaptation after intestinal resection 5, 6. However, precisely what drives secretion of GLP-2 is not fully know. A major counter-regulatory control mechanism for GLP-2 is its cleavage and inactivation by the serine protease dipeptidyl peptidase IV (DPP IV)7. Such action is rapid, resulting in a relatively short half-life of native GLP-2 to approximately seven minutes5. Therefore, alternative approaches have been developed including modification of GLP-2 to prevent such degradation. Teduglutide, is such a GLP-2 analog, with a substantially longer half-life; and has been developed as a candidate drug for the treatment of gastrointestinal diseases, including short bowel disease. However, teduglutide may have some shortcomings in clinical use including the need for chronic administration, extremely high cost, the need for parenteral injection, and possible antigenicity.
In this study, we investigated whether the dipeptidyl peptidase IV inhibitor, Sitagliptin, would preserve GLP-2 and augment levels after SBS formation. We found that oral treatment with DPPIV-I significantly augmented the process of intestinal adaptation.
Exogenous administration of GLP-2 promotes growth of the small and large intestinal epithelium in part via stimulation of crypt EC proliferation and inhibition of EC apoptosis, leading to an increase in mucosal surface area22. These physiological effects of GLP-2 have been shown to lead to a reduction in epithelial damage, augmentation of endogenous adaptation and prevention of intestinal atrophy. Clinical studies indicate that GLP-2 enhances energy absorption and reduces fluid loss in subjects with SBS, suggesting that GLP-2 functions as a key regulator of mucosal integrity, permeability, and nutrient absorption in humans5. Furthermore, the regulation of crypt cell proliferation involves several key mediators, most notably the canonical wingless (Wnt)/β-catenin signaling system. A recent study has demonstrated that GLP-2 is a novel activator of β-catenin signaling in intestinal crypts, through a mechanism requiring insulin-like growth factor (IGF) signaling through the IGF-I receptor23. The activation of β-catenin transcriptional signaling by Wnt proteins or insulin-like growth factor I (IGF-I) occurs through prevention of constitutive β-catenin degradation, thereby allowing its translocation to the nucleus and activation of tcf-419. One role of β-catenin in the intestine is to drive the transcription of several genes required for proliferation, such as c-myc24, while inhibiting genes involved in terminal cell differentiation. This maintains cells within the active cell cycle, thereby increasing the overall numbers of proliferating cells.
In this study, we demonstrated that DPPIV-I markedly increased plasma GLP-2 levels and led to increased EC proliferation compared to untreated SBS mice. Furthermore, the mRNA abundance of β-catenin and c-myc were also up-regulated in the SBS+DPPIV-I treated group. This observation supports the fact that increased amounts of GLP-2 may further drive early adaptation after SBS. The study further suggests that one mechanism responsible for this is the activation of β-catenin signaling in the intestinal crypts. However, other mechanisms are clearly involved potentially including epidermal growth factor (EGF) or IGF-I signaling through the IGF-I receptor4–6.
The DPPIV enzyme is well known to be involved in the suppression of certain malignancies, particularly in limiting the tissue invasion of these tumors25. Inhibiting DPPIV enzymes may allow some cancers to progress25. This may be especially important in IBD patients, who already possess an increased risk of gastrointestinal cancer due to chronic inflammation. Therefore, although this approach may have future clinical applications, careful dose regulation and monitoring for gastrointestinal dysplasia must be considered with any clinical use of DPP IV-I. As GLP-2 also appears dependent on EGF signaling another high risk group may well be those patients who may have family histories of epithelial malignancies, such as breast carcinoma.
In this study, we focused on the early post-massive small bowel resection period, as the greatest adaptation occurs quite rapidly after a SBS in mice3, 4. While we do not know whether these precise signals are equivalent to human small bowel adaptation, mouse models of SBS show similar responses to GLP-226–28, and numerous mechanistic pathways which have been identified in mouse small bowel after resection appear to have relevance to humans 4. Therefore we based our selection of a 3 day course of DPPIV-I treatment due to this rapid adaptive period. It is interesting to note that DPPIV expression levels decrease two days after bowel resection, and further decreased to almost half the normal levels after seven days7. The decrease in levels of this enzyme following bowel resection might facilitate the trophic activity of GLP-2 during intestinal adaptation29. The dosing of DPPIV-I in the present study was given at 3-fold higher doses than typically given systemically; however, we did not identify any side-effects from its use. In particular, we failed to identify any hypoglycemia or substantial changes in body weight.
In our study we did not find any differences in SLGT-1 among our study groups. However, long term investigation will be needed, particularly in examining body weight gain and the potential for malignancy development. This is important as this short time period may not have allowed for full maturation of epithelial cell function.
In conclusion, treatment with DPPIV- I following a 50% small bowel resection led to an accelerated process of intestinal adaptation, potentially by augmenting GLP-2 levels. These results suggest the feasibility of using oral administered DPPIV-I following massive bowel resection on a clinical level; and such approach may maximize the growth potential of the residual intestine. It is important to emphasize that the use of DPPIV-I relies on the patient’s endogenous production of GLP-2, those patients with major losses of the distal small bowel and colon, may have insufficient levels of GLP-2 to gain any benefit from the use of these peptidase inhibitors. Further studies will be necessary to clarify the relationship between GLP-2 stimulation and the action of DPPIV- I on the gastrointestinal tract.
Acknowledgments
Supported by NIH 2R01AI-44076-11(to DHT).
We wish to thank for the help the National Cancer Institute through the University of Michigan’s Cancer Center Support Grant (5 P03 CA46592) for processing histological samples.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spencer A, Safran J, Neeaga A, et al. Mortality and Outcomes of Pediatric Short Bowel Syndrome: Redefining Predictors of Success. Annals of Surgery. 2005;242:1–10. doi: 10.1097/01.sla.0000179647.24046.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolvekamp MC, Heineman E, Taylor RG, et al. Towards understanding the process of intestinal adaptation. Digestive diseases. 1996;14(1):59–72. doi: 10.1159/000171539. [DOI] [PubMed] [Google Scholar]
- 3.Helmrath M, Fong J, Dekaney C, et al. Rapid expansion of intestinal secretory lineages following a massive small bowel resection in mice. Am J Physiol Gastrointest Liver Physiol. 2007;292(1):G215–22. doi: 10.1152/ajpgi.00188.2006. [DOI] [PubMed] [Google Scholar]
- 4.Garrison A, Dekaney C, von Allmen D, et al. Early but not late administration of glucagon-like peptide-2 following ileo-cecal resection augments putative intestinal stem cell expansion. American journal of physiology: Gastrointestinal and liver physiology. 2009;296(3):G643–G650. doi: 10.1152/ajpgi.90588.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeppesen PB, Sanguinetti EL, Buchman A, et al. Teduglutide (ALX-0600), a dipeptidyl peptidase IV resistant glucagon-like peptide 2 analogue, improves intestinal function in short bowel syndrome patients. Gut. 2005;54(9):1224–1231. doi: 10.1136/gut.2004.061440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin GR, Beck PL, Sigalet DL. Gut hormones, and short bowel syndrome: the enigmatic role of glucagon-like peptide-2 in the regulation of intestinal adaptation. World J Gastroenterol. 2006;12(26):4117–29. doi: 10.3748/wjg.v12.i26.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunphy JL, Justice FA, Taylor RG, et al. mRNA levels of dipeptidyl peptidase IV decrease during intestinal adaptation. The Journal of surgical research. 1999;87(1):130–133. doi: 10.1006/jsre.1999.5735. [DOI] [PubMed] [Google Scholar]
- 8.Brandt I, Joossens J, Chen X, et al. Inhibition of dipeptidyl-peptidase IV catalyzed peptide truncation by Vildagliptin ((2S)-{[(3-hydroxyadamantan-1-yl)amino]acetyl}-pyrrolidine-2-carbonitrile) Biochemical pharmacology. 2005;70(1):134–143. doi: 10.1016/j.bcp.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Holst J, Deacon C. Glucagon-like peptide 1 and inhibitors of dipeptidyl peptidase IV in the treatment of type 2 diabetes mellitus. Current opinion in pharmacology. 2004;4(6):589–596. doi: 10.1016/j.coph.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Drucker D, Nauck M. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 11.Kim D, Wang L, Beconi M, et al. (2R)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl]-1-(2,4,5-trifluorophenyl)butan-2-amine: a potent, orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. Journal of medicinal chemistry. 2005;48(1):141–151. doi: 10.1021/jm0493156. [DOI] [PubMed] [Google Scholar]
- 12.Drab SR. Incretin-based therapies for type 2 diabetes mellitus: current status and future prospects. Pharmacotherapy. 2010;30(6):609–24. doi: 10.1592/phco.30.6.609. [DOI] [PubMed] [Google Scholar]
- 13.Koga H, Yang H, Haxhija EQ, et al. The role of angiotensin II type 1a receptor on intestinal epithelial cells following small bowel resection in a mouse model. Pediatr Surg Int. 2008;24(12):1279–86. doi: 10.1007/s00383-008-2277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spencer AU, Yang H, Haxhija EQ, et al. Reduced severity of a mouse colitis model with angiotensin converting enzyme inhibition. Dig Dis Sci. 2007;52(4):1060–70. doi: 10.1007/s10620-006-9124-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartmann B, Johnsen AH, Orskov C, et al. Structure, measurement, and secretion of human glucagon-like peptide-2. Peptides. 2000;21(1):73–80. doi: 10.1016/s0196-9781(99)00176-x. [DOI] [PubMed] [Google Scholar]
- 16.Koopmann M, Chen X, Holst J, et al. Sustained glucagon-like peptide-2 infusion is required for intestinal adaptation, and cessation reverses increased cellularity in rats with intestinal failure. American journal of physiology: Gastrointestinal and liver physiology. 2010;299(6):G1222–G1230. doi: 10.1152/ajpgi.00367.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haxhija E, Yang H, Spencer A, et al. Modulation of mouse intestinal epithelial cell turnover in the absence of angiotensin coverting enzyme. Amer J Physiol Gastrointestinal and Liver Physiology. 2008;295(1):G88–98. doi: 10.1152/ajpgi.00589.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drucker DJ. Biologic actions and therapeutic potential of the proglucagon-derived peptides. Nature clinical practice endocrinology & metabolism. 2005;1(1):22–31. doi: 10.1038/ncpendmet0017. [DOI] [PubMed] [Google Scholar]
- 19.Dub P, Brubaker P. Frontiers in glucagon-like peptide-2: multiple actions, multiple mediators. American journal of physiology: endocrinology and metabolism. 2007;293(2):E460–E465. doi: 10.1152/ajpendo.00149.2007. [DOI] [PubMed] [Google Scholar]
- 20.Dyer J, Al-Rammahi M, Waterfall L, et al. Adaptive response of equine intestinal Na+glucose co-transporter (SGLT1) to an increase in dietary soluble carbohydrate. Pflügers Archiv. 2009;458(2):419–430. doi: 10.1007/s00424-008-0620-4. [DOI] [PubMed] [Google Scholar]
- 21.Theodosiou N, Tabin C. Wnt signaling during development of the gastrointestinal tract. Developmental biology. 2003;259(2):258–271. doi: 10.1016/s0012-1606(03)00185-4. [DOI] [PubMed] [Google Scholar]
- 22.Tsai C, Hill M, Drucker D. Biological determinants of intestinotrophic properties of GLP-2 in vivo. American Journal of Physiology. 1997;272:G662–G668. doi: 10.1152/ajpgi.1997.272.3.G662. [DOI] [PubMed] [Google Scholar]
- 23.Dub P, Rowland K, Brubaker P. Glucagon-like peptide-2 activates beta-catenin signaling in the mouse intestinal crypt: role of insulin-like growth factor-I. Endocrinology. 2008;149(1):291–301. doi: 10.1210/en.2007-0561. [DOI] [PubMed] [Google Scholar]
- 24.Muncan V, Sansom O, Tertoolen L, et al. Rapid loss of intestinal crypts upon conditional deletion of the Wnt/Tcf-4 target gene c-Myc. Molecular and cellular biology. 2006;26(22):8418–8426. doi: 10.1128/MCB.00821-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kikkawa F, Kajiyama H, Shibata K, et al. Dipeptidyl peptidase IV in tumor progression. Biochim Biophys Acta. 2005;1751(1):45–51. doi: 10.1016/j.bbapap.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 26.Petersen Y, Burrin D, Sangild P. GLP-2 has differential effects on small intestine growth and function in fetal and neonatal pigs. Am J Physiol Regul Integr Comp Physiol. 2001;281(6):R1986–93. doi: 10.1152/ajpregu.2001.281.6.R1986. [DOI] [PubMed] [Google Scholar]
- 27.Warner BW. GLP-2 as therapy for the short-bowel syndrome.[comment] Gastroenterology. 2001;120(4):1041–3. doi: 10.1053/gast.2001.22560. [DOI] [PubMed] [Google Scholar]
- 28.Koopmann M, Nelson D, Murali S, et al. Exogenous glucagon-like peptide-2 (GLP-2) augments GLP-2 receptor mRNA and maintains proglucagon mRNA levels in resected rats. JPEN. 2008;32(3):254–65. doi: 10.1177/0148607108316198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baksheev L, Fuller PJ. Humoral factors in intestinal adaptation. Trends in Endocrinology and Metabolism. 2000;11(10):401–405. doi: 10.1016/s1043-2760(00)00307-6. [DOI] [PubMed] [Google Scholar]