Abstract
2'-3-dimethyl-4-aminoazobenzene (ortho-aminoazotoluene, OAT) is an azo dye and a rodent carcinogen that has been evaluated by the International Agency for Research on Cancer (IARC) as a possible (class 2B) human carcinogen. Its mechanism of action remains unclear. We examined the role of the xenobiotic receptor Constitutive Androstane Receptor (CAR, NR1I3) as a mediator of the effects of OAT. We found that OAT increases mouse CAR (mCAR) transactivation in a dose-dependent manner. This effect is specific because another closely related azo dye, 3'-methyl-4-dimethyl-aminoazobenzene (3'MeDAB), did not activate mCAR. Real-time Q-PCR analysis in wild-type C57BL/6 mice revealed that OAT induces the hepatic mRNA expression of the following CAR target genes: Cyp2b10, Cyp2c29, Cyp3a11, Ugt1a1, Mrp4, Mrp2 and c-Myc. CAR-null (Car−/−) mice showed no increased expression of these genes following OAT treatment, demonstrating that CAR is required for their OAT dependent induction. The OAT-induced CAR-dependent increase of Cyp2b10 and c-Myc expression was confirmed by Western blotting. Immunohistochemistry analysis of wild-type and Car−/− livers showed that OAT did not acutely induce hepatocyte proliferation, but at much later time points showed an unexpected CAR-dependent proliferative response. These studies demonstrate that mCAR is an OAT xenosensor, and indicate that at least some of the biological effects of this compound are mediated by this nuclear receptor.
Keywords: Ortho-Aminoazotoluene (OAT), Constitutive Androstane Receptor (CAR), CYP450s, c-Myc, hepatocyte proliferation
Introduction
Ortho-Aminoazotoluene (OAT, 2'-3-dimethyl-4-aminoazobenzene, 2-amino-5-azotoluene; also known as Solvent yellow 3, Garnet G base and Fast Garnet GB base) is an anthropogenic compound used for coloring oils, fats, and waxes (National Toxicology Program, 2002), as well as for pharmaceutical purposes. It is also used as a synthetic intermediate in the production of other dyes (HSDB, 2001). In 1994 estimates, the world production of azo dyes was around 0.5 million tons (Stolz, 2001; Pandey et al., 2007). OAT and other azo dyes can be released to the environment in wastewater and other emissions during the manufacturing process. Historically, OAT was the first model of experimental chemical carcinogenesis as demonstrated by Yoshida in the 1930's (Cook, 1947). Since that time, a large amount of data has confirmed the carcinogenic activity of azo dyes. Specifically, OAT is known as a mouse carcinogen (Kaledin et al., 1978; Kaledin et al., 1985), and has been evaluated by the International Agency for Research on Cancer (IARC) as a possible (class 2B) human carcinogen (IARC, 1975; National Toxicology Program, 2002). Despite more than a half a century of studies on OAT induced carcinogenesis, its mechanism of action is still unknown.
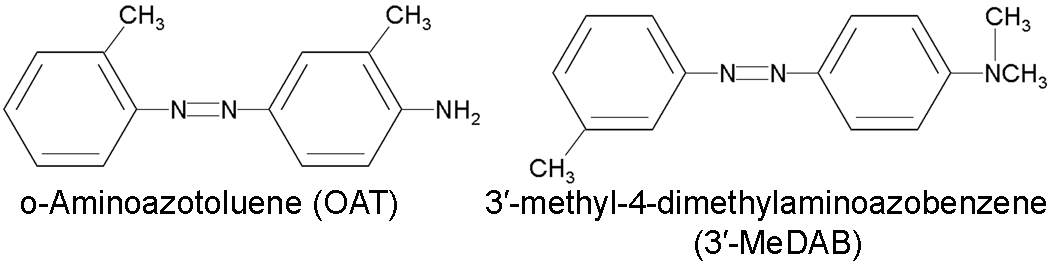
Research interest in azo dyes has focused on significant species- and strain-specific differences in response to the carcinogenic activity of these compounds (Merkulova et al., 2005). This allows OAT to be used as a tool to study the mechanisms of genetic predisposition to the tumor development. OAT is associated with a high incidence of liver tumors in a number of mouse strains (CBA, SWR, DBA/2, A/He, DD) but has little effect in other strains (AKR and CC57Br) and in rats (Kaledin and Zakharova, 1984; Kaledin et al., 1990; Zacharova et al., 2003). On the other hand, the structural analogue of OAT (the isomeric DAB) 3'-methyl-4-dimethylaminoazobenzene (3'-MeDAB), which is hepatocarcinogenic to rats, rarely causes tumors in mice (Merkulova et al., 2005). Although the mechanisms of hepatocarcinogenicity of these compounds remain unclear, it is known that they can activate drug metabolism, including both phase I xenobiotic-metabolizing enzymes (CYP450s) and phase II enzymes (such as UGTs and SULTs) and transporters (Mikhailova et al., 2005). Xenobiotic-inducible expression of hepatic biotransformation enzymes and transporters is regulated by the xenobiotic-responsive transcription factors Aryl-hydrocarbon receptor (Ah-R), Pregnane X Receptor (PXR), Constitutive Androstane Receptor (CAR), and others (Xu et al., 2005; Stanley et al., 2006; Pascussi et al., 2008).
We have previously shown that one of these receptors, CAR, is activated strongly by OAT and much more weakly by 3'-MeDAB in mice (Pakharukova et al., 2007). Thus, CAR DNA-binding activity is induced 3-fold in nuclear extracts from the mouse liver after OAT administration and far less so after 3'-MeDAB administration. CAR (NR1I3) is known as a ‘xenobiotic sensor’, and is a member of the nuclear hormone receptor superfamily (Choi et al., 1997). CAR activity can be modulated by a broad array of structurally diverse compounds (Chang and Waxman, 2006). Our previous results also demonstrated that OAT competed with the known ligand androstenol in a CAR binding assay using cytosol isolated from mouse liver cells. Based on these observations, we hypothesized that OAT could activate mouse CAR and, that at least some of its effects would be mediated by this nuclear receptor.
In the current study, we investigated the effects of OAT and 3'MeDAB on the activity of mCAR using transient transfections with an appropriate reporter system. Studies in WT (Car+/+) and Car−/− mice examined the effect of OAT and 3'-MeDAB on the expression of CAR target genes. In order to determine a possible role of CAR in the development of liver tumors caused by OAT, we explored hepatocyte proliferation at both relatively short times and much longer times after OAT treatment of WT and CAR KO mice. We observed that OAT specifically activated CAR target genes in the short term, but did not induce hepatocyte proliferation. However, OAT did induce CAR-dependent hepatocyte proliferation at much later times. Overall, these studies demonstrate that CAR mediates biological effects of OAT.
Materials and methods
Caution
Azo dyes used in this study are toxic and should be handled with care.
Chemicals
Fast Garnet GBC base (Ortho-Aminoazotoluene / OAT), (97% purity) (CAS number 97-56-3) and 1,4-bis-[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP) (CAS number 76150-91-9) were purchased from Sigma-Aldrich Inc. (St. Louis, MO). 3′-Methyl-4-dimethylaminoazobenzene (98%) (CAS number 55-80-1) was purchased from ABCR GmbH&Co.KG (Germany). 5α-Androstan-3α-ol (CAS number 1224-92-6) was purchased from Steraloids Inc. (Newport, RI). Solvents were obtained from Fisher Scientific (Houston, TX) unless otherwise noted.
Transactivation assay
The mouse CAR (mCAR) expression plasmid was described previously in Forman et al., 1998, and the CAR reporter (LXRE-TK-Luc reporter plasmid) was described in Tzameli et al., 2000. All the procedures were carried out according to Hernandez et al., 2007. Briefly, transactivation assays were performed in HepG2 human hepatoma cells (ATCC, Rockville, MD) cultured in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum and 1% Penicillin/Streptomycin (Invitrogen), under 5% CO2 at 37°C. Cells were plated in 24-well plates at 1×105 cells per well. Twenty-four hours after plating, cells were transfected with 200 ng of CAR expression vector (pCMX-mCAR) or the pCMX vector alone and cotransfected with 200 ng of LXRE-TK-Luc reporter using “lipofectamine 2000” reagent as per company protocol (GIBCO BRL). 100 ng of pCMX-β-galactosidase (Promega, Madison, WI) was cotransfected to normalize transfection efficiency. Twelve hours following transfection, cells were treated with different concentrations of OAT or 3′-MeDAB in combination with 10 µM androstanol (CAR’s inverse agonist which represses its constitutive activity and increases the sensitivity of the assay (Forman et al., 1998)) or vehicle control, and incubated for 12 hours. 0.1% DMSO was used as a vehicle for all chemicals and 500 nM TCPOBOP was used as a positive control for CAR activation. Subsequently, cell lysates were assayed for firefly luciferase activities and normalized to β-galactosidase activities using the Dual-Luciferase reporter assay system (Promega, Madison, WI-not promega) according to the manufacturer’s instructions. All experiments were done in quadruplicate, and the data is presented as mean ± S.D from two to three separate assays.
Animals
All experiments were done upon approval of the protocol by the Baylor College of Medicine Institutional Animal Care and Use Committee (IACUC). Mice were maintained in a pathogen-free Baylor College of Medicine Transgenic Mice Facility under a constant 12-h light/dark cycle and fed standard rodent chow and water ad libitum. Wild type (WT) C57BL/6 mice (sensitive to hepatic tumor induction with OAT (Zacharova et al., 2003)) and CAR KO (Car−/−) mice (Wei et al., 2000) (>10 backcrosses to C57BL/6 background) were used in the experiments.
Animal treatment
For gene expression studies, eight- to ten- week old CAR KO and WT male mice (three per group) were injected with OAT or 3′-MeDAB (225 mg/kg of body weight) intraperitoneally (i.p.). Controls received an equivalent amount of the vehicle (corn oil). TCPOBOP (at a dosage of 3 mg/kg body weight, i.p.) was used for the positive and negative controls in WT and CAR KO mice respectively. Animals were sacrificed by cervical dislocation 3 h (for RNA extraction) and 6 h (for protein preparation) after dosing. Livers were removed, diced into several pieces and stored at −80°C.
For the first set of cell proliferation studies, eight- to ten- week old CAR KO and WT male mice (five per group) were administered by a single i.p. injection of OAT at a dose of 225 mg/kg of body weight. Controls (three mice per group) received an equivalent amount of the vehicle (corn oil). Animals were sacrificed by cervical dislocation 3 days and 1 week after dosing. Immediately after death, mice were weighed and the livers were excised and weighed. The liver index was determined by dividing the liver weight by the weight of the mouse. Liver sections were fixed in Histochoice MB Fixative (histology grade, Electron Microscopy Sciences, Hatfield, PA) and processed for immunohistochemistry.
For the second set of cell proliferation studies, thirteen-days old CAR KO and WT male mice (nine per group) were administered by a single i.p. injection of OAT at a dose of 225 mg/kg of body weight. According to previous publications, suckling mice were more sensitive to the hepatocarcinogenic effect of OAT compared to adult animals (Il'nitskaya et al., 2004). Controls (also thirteen-days old CAR KO and WT male mice, three per group) received an equivalent amount of the vehicle (corn oil). 7 months after the administrations, bromodeoxyuridine (BrdU, 50 mg/kg, Sigma Chemical Co., St. Louis, MO), which intercalates into double stranded DNA during replication thus identifying the cells in S-phase, was administered intraperitoneally 2 h before sacrificing the animals. Preparation and processing of the liver sections was as in the first set of cell proliferation studies.
Quantitative real-time polymerase chain reaction (Q-PCR)
To analyze messenger RNA (mRNA) levels, total RNA was isolated from homogenized livers using TRIzol (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. The concentration of total RNA in each sample was quantified spectrophotometrically at 260 nm. RNA integrity was confirmed by visualization of intact 18S and 28S rRNA under UV light. Then the cDNAs were synthesized using the SuperScript™ III RT (Invitrogen, Carlsbad, CA) in accordance with manufacturer’s instructions. Real-time quantitative PCR (RTQ-PCR) was performed using an ABI PRISM 7700 Sequence Detection System™ (Applied Biosystems, Inc., Foster City, CA) with SYBR Green or Taq Man Universal PCR Master Mix (Applied Biosystems). Primers and probes were designed using Primer Express Version 3.0 software (Applied Biosystems) and synthesized by Sigma-Genosys (Woodlands, TX) or purchased from Qiagen Inc. (Valencia, CA). Sequences of the primers (and probes) used in RTQ-PCR are given in Table 1. Each measurement was done in triplicate and normalized to mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a control using the standard curve method (Bookout et al., 2006).
Table 1.
CAR target genes chosen for the experiments.
| Gene | Primer (and probe) sequences | Process | Reference |
|---|---|---|---|
| Cytochrome P450 2B10 (Cyp2b10) | Fw. 5'-GACTTTGGGATGGGAAAGAG-3' Rev. 5'-CCAAACACAATGGAGCAGAT-3' Probe: 5'-[FAM]-GTAGTGGAGGAACTGCGGAAATCCC-<BHQ1>-3' |
Phase I drug metabolism (oxidative metabolism phase) |
Honkakoski et al., 1998 |
| Cytochrome P450 2C29 (Cyp2c29) | Fw. 5'-GGCCTCAAAGCCCTACTGTCA-3' Rev. 5'-AACGCCAAAACCTTTAATC-3' |
Hernandez et al., 2006; Ross et al., 2009 | |
| Cytochrome P450 3A11 (Cyp 3a11) | Fw. 5'-AGATTGGTTTTGATGCCTGGTT-3' Rev. 5'-GCAAATTTCCTGTGCTGTCACT-3' |
Xie et al., 2000; Smirlis et al., 2001; Wei et al., 2002; Assem et al., 2004 | |
| NADPH CYP450 oxidoreductase | Fw. 5'-GGGCACTCGAGTAGTTCAGC-3' Rev. 5'-TGCCCAGAATCTTGGAAATC-3' |
Maglich et al., 2002 | |
| UDP-glucuronosyltrans ferase 1A1 (Ugt1a1) | Fw. 5'-TGAGGCTTTGGGCAGAATTC-3' Rev.5'-CTTTGCAAGATTCGATGGTCTAGTT-3' |
Phase II drug metabolism (conjugation phase) | Maglich et al., 2002; Buckley and Klaassen, 2009 |
| Multidrug resistance-associated protein 2 (Mrp2) | Fw. 5'-CGCCCTCAACATCACACAAA-3' Rev. 5'-ATTCGCTCAACAGCCACGAT-3' |
Drug transport (transport phase) |
Kast et al., 2002; Urquhart et al., 2007 |
| Multidrug resistance-associated protein 4 (Mrp4) | Fw. 5'-GGGCACTCGAGTAGTTCAGC-3' Rev. 5'-TGCCCAGAATCTTGGAAATC-3' |
Assem et al., 2004; Urquhart et al., 2007 | |
| Mouse Double Minute 2 (Mdm2) | Fw. 5'-ATCACCGCGCTTCTCCTG-3' Rev. 5'-AGCACCCTCGGTAGACACAGA-3' Probe: 5'-[FAM]- CTCCAGGCCAATGTGCAATACCAACAT-<TAM>-3' |
Suppression of p53-dependent apoptosis, cell proliferation | Huang et al., 2005 |
| Cyclin D1 | Fw. 5'-GCACTTTCTTTCCAGAGTCATCAA-3' Rev. 5'-CTCCAGAAGGGCTTCAATCTGT-3' |
Cell cycle regulation, prolipheration | Ledda-Columbano et al., 2003; Columbano et al., 2005; Costa et al., 2005 |
| Cellular myelocytomatosis oncogene (c-Myc) | Fw. 5'-ACAGAGGGAGTGAGCGGACG-3' Rev. 5'-TTCACGTTGAGGGGCATCG-3' |
Prolipheration, cellular growth, apoptosis, angiogenesis, cell adhesion and differentiation | Blanco-Bose et al., 2008 |
Western blot analysis
Frozen liver samples were sonicated in ice-cold lysis buffer (25 mM Tris-HCl (pH 7.4), 10 mM sodium orthovanadate, 10 mM sodium pyrophosphate, 100 mM sodium fluoride, 10 mM EDTA, 10 mM EGTA, and 1 mM phenylmethylsulfonyl fluoride). Protein samples (30 µg each) were separated electrophoretically in a NuPAGE® Novex® 10 % Bis-Tris Mini Gel (Invitrogen, Carlsbad, CA), and transferred to a Hybond-P PVDF Transfer Membrane (Amersham Biosciences, Piscataway, NJ). The membrane was incubated with specific primary antibodies: rabbit polyclonal Cyp2b10 antibody (GenScript USA Inc., Piscataway, NJ) or mouse monoclonal c-Myc antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) diluted 1:450 and 1:500, respectively. Rabbit polyclonal β-actin antibody (Cell Signalling Technology, Danvers, MA) was used as internal control. Blots were washed and incubated with HRP-conjugated goat anti-rabbit or goat anti-mouse IgG (H+L) antibody (ZyMax™, San Francisco, CA), respectively, diluted 1:5000. Films were developed using SuperSignal® West Pico Chemiluminescent Substrate (Thermo Scientific, Rockford, IL). The films were subjected to a densitometry analysis using LabWorks image analysis software (UVP laboratory products, Upland, CA).
Immunohistochemistry
For determination of hepatocyte proliferation, we used mouse monoclonal antibromodeoxyuridine (Dako North America, Inc., Carpinteria, CA) as the primary antibody and biotinylated horse anti-mouse IgG (H+L) (Vector Laboratories, Inc., Burlingame, CA) as the secondary antibody. Staining for proliferation marker Ki67 was done using rabbit polyclonal anti-Ki67 (Abcam, Cambridge, MA) as the primary antibody and biotinylated horse anti-rabbit IgG (H+L) (Vector Laboratories, Inc., Burlingame, CA) as the secondary antibody. Paraffin-embedded liver tissue sections (5 µm thick) were deparaffinized and rehydrated. Vectastatin Elite ABC kit (Vector Laboratories, Inc., Burlingame, CA) was used to stain Ki67-positive and BrdU-positive hepatocytes. Endogenous peroxidase activity was quenched by incubating slides in methanol/H2O2. All procedures were carried out according to the manufacturer’s instructions. Immunohistochemical staining was observed at 20× with a Nikon Eclipse E400 light microscope equipped with a Sony ExWaveHAD 3CCD color video camera. The labeling index was expressed as number of Ki67-positive or BrdU-positive nuclei/100 nuclei.
Statistical analysis
Data are presented in all figures as mean ± S.D. The number of subjects is indicated by n. Figures shown are representative of consistent results. Statistical comparisons were carried out using two-tailed Student’s t-test, and statistical significance was assessed at 3 levels (*p<0.05, **p<0.01, ***p<0.001).
Results
OAT activates mCAR in HepG2 cells
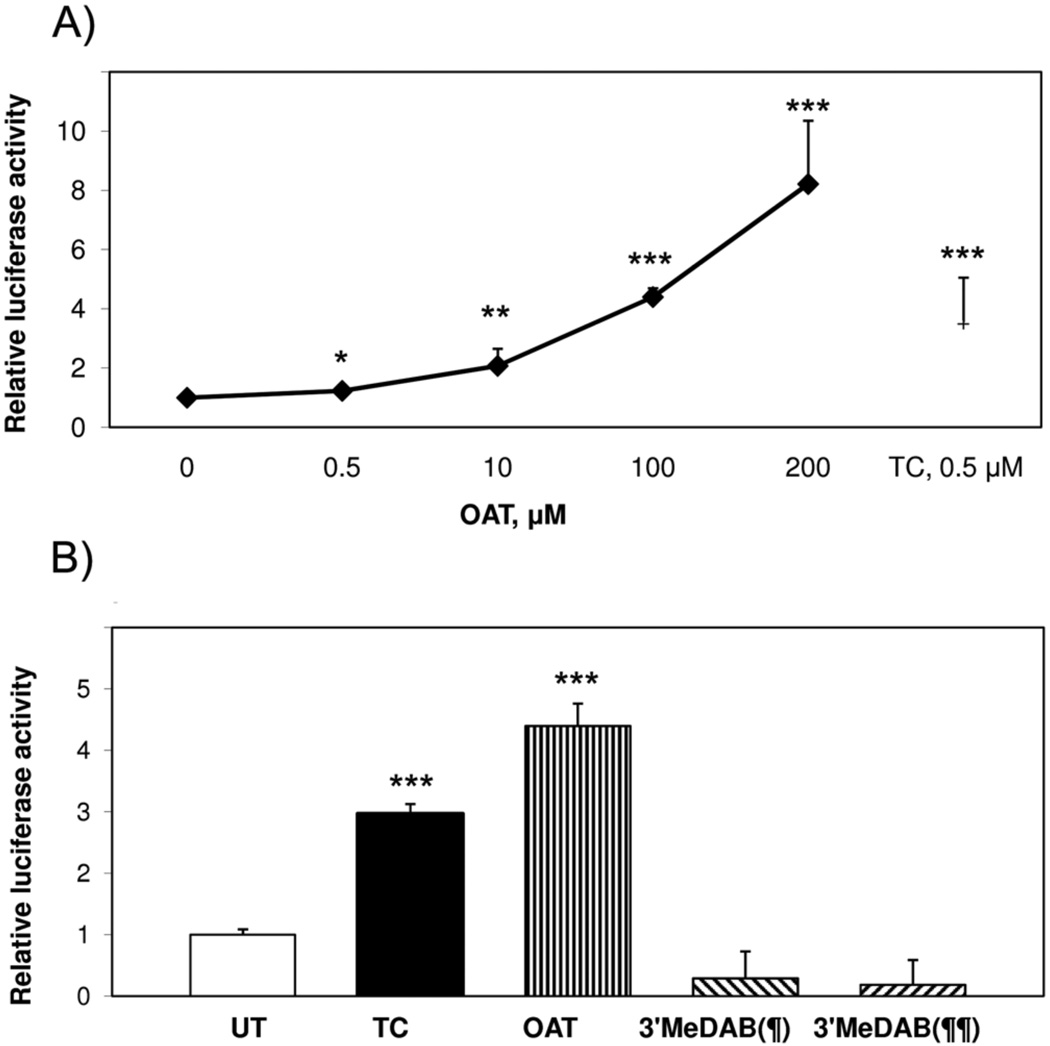
To determine whether OAT activates mCAR in vitro, HepG2 cells were cotransfected with an expression vector for full-length mCAR and a CAR-responsive reporter. OAT was used in concentrations ranging from 0.5 µM to 200 µM. As shown in Fig. 1A, OAT activated mCAR at 0.5 µM. With increasing OAT concentrations, mCAR transactivation gradually increased. Maximum mCAR activity at 200 µM OAT was 8 times higher than the untreated (UT) control. This activation was greater than that observed with a saturating dose (500 nM) of the known CAR agonist ligand TCPOBOP (TC), indicating that OAT is a very strong CAR activator. We also examined the effect of another azo dye 3′-MeDAB (Fig. 2), which is a structural analogue of OAT and a very weak carcinogen for mice compared to OAT. 3′-MeDAB did not activate mCAR at either 100 or 200 µM (Fig. 1B). Based on these responses, and our previous results showing that OAT competes with androstenol for CAR binding (Pakharukova et al., 2007) we conclude that OAT is a very effective activator of mCAR.
Fig. 1. OAT activates mCAR in HepG2 cells.
A) Dose-dependent activation of mCAR by OAT. B) OAT (100 µM), TCPOBOP (TC, 500 nM) and 3′-MeDAB (100 (¶) or 200 (¶¶) µM) effects on transactivation by mCAR. HepG2 cells were cotransfected with full-length mCAR expression plasmid together with a luciferase-coupled reporter plasmid containing LXRE and pCMX-β-gal. 24 h later transfected cells were treated with OAT (black diamonds in Fig. 1A; straight striped bar in Fig. 1B) or 3′-MeDAB (oblique striped bars in Fig. 1B) or TC (black square in Fig. 1A; black bar in Fig. 1B) or DMSO (UT, black diamond at 0 concentration in Fig. 1A, white bar in Fig. 1B). In 16–18 h cells were subjected to luciferase and β-gal assay. Transcriptional activation was assessed by the relative luciferase activity normalized by β-gal activity. The results are presented as fold induction compared to the UT (DMSO control), means ± SD (n = 4 wells). Asterisk-marked data were significantly different from the UT (*p<0.05, **p<0.01, ***p<0.001, Student's t-test).
Fig. 2. Structures of the used amino-azodyes.
OAT affects mRNA expression of a number of CAR target genes in mouse liver
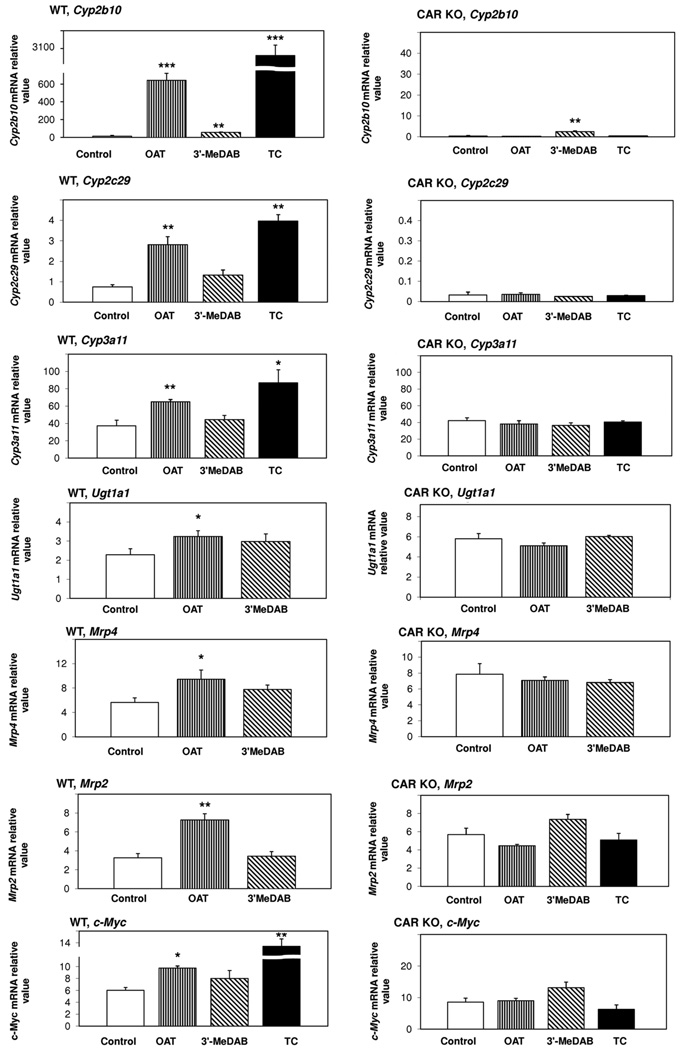
CAR regulates a number of genes in xenobiotic metabolism, cell cycle and proliferation (Swales and Negishi, 2004). Using quantitative real-time PCR we measured changes in expression of the following genes in response to OAT: cytochromes P450 (Cyp2b10, Cyp2c29 and Cyp3a11), UDP-glucuronosyltransferase 1A1 (Ugt1a1), NADPH CYP450 oxidoreductase, Multidrug resistance-associated proteins 2–4 (Mrp2, Mrp4), Mouse Double Minute 2 (Mdm2), Cyclin D1 and c-Myc (Table 1). Based on previous studies demonstrating relatively rapid responses to CAR activation (Kubitz et al., 1999; Locker et al., 2003; Kiyosawa et al., 2008; etc.), we chose a 3 hour treatment in order to focus on primary CAR targets.
The experiments were carried out in wild type (WT, Car+/+) C57BL/6 mice, susceptible to the hepatocarcinogenic effects of OAT (Zacharova et al., 2003), and CAR knock-out mice (CAR KO, Car−/−) of the same strain. Mice were treated either with OAT or 3′-MeDAB, and corn oil was used as vehicle and control. In some experiments, we used TC as a positive control in WT mice, as it induces the expression of CAR target genes; and we used TC treatment as a negative control in CAR KO mice (Wei et al., 2000; Maglich et al., 2002; Assem et al., 2004; etc.). For 7 out of 10 genes examined, we observed CAR-dependent induction of expression in response to OAT treatment. A particularly marked increase was observed for Cyp2b10 (Fig. 3, left panel). Although this response was less than that to TC, due perhaps due differences in delivery of the compounds in vivo relative to the cultured cells used in Fig. 1, OAT increased hepatic Cyp2b10 mRNA by approximately 40 fold in WT mice. 3′-MeDAB increased mRNA level of this gene only 4 fold. In CAR KO mice, OAT had no effect on Cyp2b10 mRNA expression (Fig. 3, right panel) whereas 3′-MeDAB increased it about 7 fold. Therefore, we concluded that OAT increases the expression of Cyp2b10 mRNA through CAR, and 3′-MeDAB effect on the mRNA expression of this gene is CAR-independent. After OAT treatment of WT mice, mRNA levels increased for the other investigated genes as follows: Cyp2c29 (4.3 fold), Cyp3a11 (1.8 fold), Ugt1a1 (1.4 fold), Mrp4 (1.7 fold) and Mrp2 (2.2 fold), c-Myc (1.6 fold) (Fig. 3, left panel). No induction of these genes in response to OAT was observed in CAR KO mice. In contrast to OAT, 3'-MeDAB had no effect on the expression of any of these genes (Fig. 3). The remaining genes (NADPH CYP450 oxidoreductase, Mdm2 and Cyclin D1) did not respond to OAT or 3'-MeDAB (data not shown).
Fig. 3. Effects of OAT and 3′-MeDAB on hepatic Cyp2b10, Cyp2c29, Cyp3a11, Ugt1a1, Mrp4, Mrp2 and c-Myc, mRNA expression in wild-type (left panels) vs. CAR knock-out (right panels) mice.
Mice were injected i.p. with a single dose of OAT (225 mg/kg) or 3′-MeDAB (225 mg/kg) and sacrificed 3 hours later. TCPOBOP (TC, 3 mg/kg) was used as a positive control in WT mice and as a negative control in CAR KO mice. Corn oil (control) was used as a solvent. Gene expression changes were measured by Real-time Q-PCR and standardized for GAPDH mRNA levels. White bars, control; straight striped bars, OAT; oblique striped bars, 3’MeDAB; black bars, TC (TCPOBOP). Bars, mean values from three animals per group, ± SD. Statistical analysis was performed by a Student’s t-test (treatment vs. control). Significant differences are indicated with asterisks (*p<0.05, **p<0.01, ***p<0.001).
Taken together, these data demonstrate that the induction of transcription of Cyp2b10, Cyp2c29, Cyp3a11, Ugt1a1, Mrp4, Mrp2 and c-Myc by OAT is CAR-dependent.
OAT induces hepatic Cyp2b10 and c-Myc protein expression in CAR-dependent manner
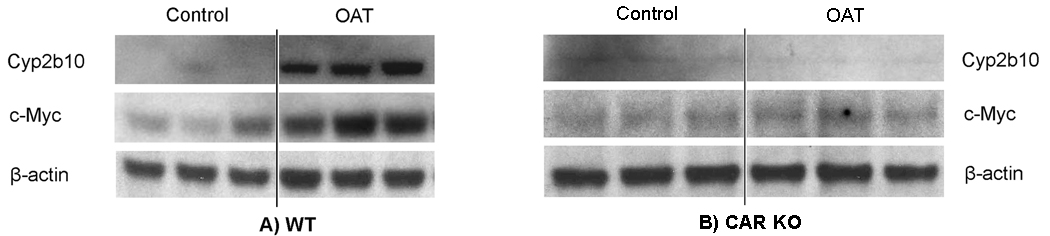
Next, we tested whether expression of Cyp2b10 (as a well-known marker to measure CAR activation) and c-Myc is also changed at the protein level in response to OAT in mouse liver. WT and CAR KO mice were injected with OAT (corn oil was used as a control), and 6 hours later livers were harvested and used for Western blot analysis. As shown in Fig. 4A, Cyp2b10 protein levels in the liver of WT mice treated with OAT were markedly increased compared to the untreated control (18 fold, as measured by densitometry). Thus, OAT treatment induced Cyp2b10 at both protein (Fig. 4A) and mRNA (Fig. 3, left panel) levels. Importantly, no change was observed in Cyp2b10 protein levels in the livers of CAR KO mice treated with OAT (Fig. 4B). Our results demonstrate that Cyp2b10 induction in response to OAT is CAR-dependent both at the mRNA and protein levels.
Fig. 4. OAT induces Cyp2B10 and c-Myc protein expression in wild-type mice (A) and has no effect in CAR-knockout (B) mice.
Mice were injected ip with OAT or corn oil (control) and were sacrificed 6 hours later. Western blots were preformed with hepatic whole cell lysates and antibodies against Cyp2b10, c-Myc and β-actin (as a loading control). The representative Western blot is shown, each lane means the sample of one mouse.
Similarly, in WT mice we observed a 3 fold increase of c-Myc protein levels in response to OAT, relative to control (Fig. 4A), which is consistent with the change of c-Myc transcript levels (Fig. 3, left panel). In the liver of CAR KO mice, OAT treatment did not alter c-Myc protein levels (Fig. 4B). We can conclude that the induction of c-Myc protein is CAR-dependent as in the case of its gene transcript (Fig. 3, right panel).
OAT effects on hepatocyte proliferation
Cell proliferation has often been implicated in the development of chemically induced cancers. The CAR activators phenobarbital and TCPOBOP are well known chemical mitogens, and CAR mediates liver tumor promotion by these compounds (Yamamoto et al., 2004; Huang et al., 2005). OAT can also behave like a classical tumor promoter, enhancing the effect of such tumor inducers as DENA and NEM, and increases tumor incidence 10 times (Baginskaya et al., 2007). Therefore it was interesting to find out whether OAT affects hepatocyte proliferation and whether that effect is CAR-dependent.
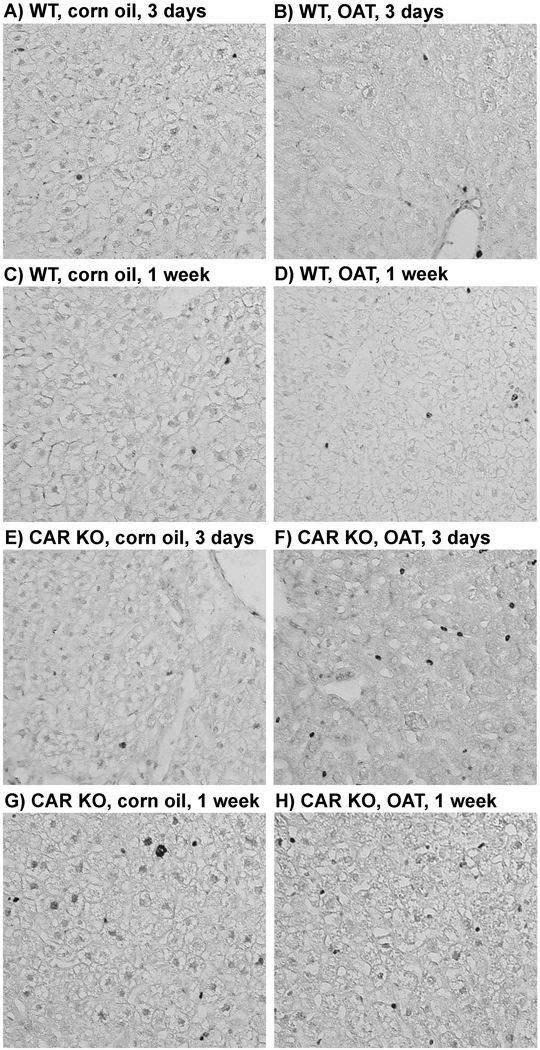
Using immunohistochemistry, we studied how OAT affects hepatocyte proliferation in WT mice, compared to CAR KO mice in short-term and much longer-term experiments. In short-term experiments we used Ki67 as a cellular marker for proliferation. In contrast to expectations based on acute gene expression, OAT did not increase hepatocyte proliferation in WT mice in short-term experiments (3 and 7 days after OAT administration) (Figure 5A–H, Table 2). CAR KO mice treated with OAT also showed no change in hepatocyte proliferation. Additionally, there was no change in liver index (liver weight to body weight ratio) after OAT treatment in both WT and CAR KO mice (Table 2).
Fig. 5. Hepatocyte proliferation in wild type (WT) and CAR-knockout (CAR KO) mice after short-term OAT treatment.
Mice were treated with OAT (single ip injection, dose 225 mg/kg) or corn oil and were sacrificed in 3 days or 1 week. Staining for proliferation marker Ki67 was performed for immunohistochemical analysis of liver sections in WT (A – D) and CAR KO (E – H) mice after corn oil or OAT treatment.
Table 2.
Liver weight to body weight ratios and the quantification of Ki67-positive cells per field.
| l/w : b/w ratios (a) | Ki67+ cells, % (b) | |
|---|---|---|
| WT, corn oil (3 days) | 0.0471 ± 0.0025 (3) | 0.0556 ± 0.1925 (3) |
| WT, corn oil (1 week) | 0.0510 ± 0.0030 (3) | 0.2500 ± 0.4949 (3) |
| WT, OAT (3 days) | 0.0505 ± 0.0027 (5) | 0.1167 ± 0.2709 (5) |
| WT, OAT (1 week) | 0.0553 ± 0.0060 (5) | 0.2000 ± 0.3134 (5) |
| CAR KO, corn oil (3 days) | 0.0339 ± 0.0033 (3) | 0.1111 ± 0.2171 (3) |
| CAR KO, corn oil (1 week) | 0.0408 ± 0.0025 (3) | 0.1388 ± 0.1716 (3) |
| CAR KO, OAT (3 days) | 0.0385 ± 0.0026 (5) | 0.0500 ± 0.1221 (5) |
| CAR KO, OAT (1 week) | 0.0454 ± 0.0021 (5) | 0.1000 ± 0.1567 (5) |
Liver weight to body weight ratios (a) and Ki67-positive cells (%) (b) were measured from 5 or 3 animals (the number is shown in paranthesis) per treatment group, ± SD.
Statistical analysis was performed by a Student’s t-test (CAR KO vs. WT of the same treatment and OAT vs. corn oil, no significant changes were observed).
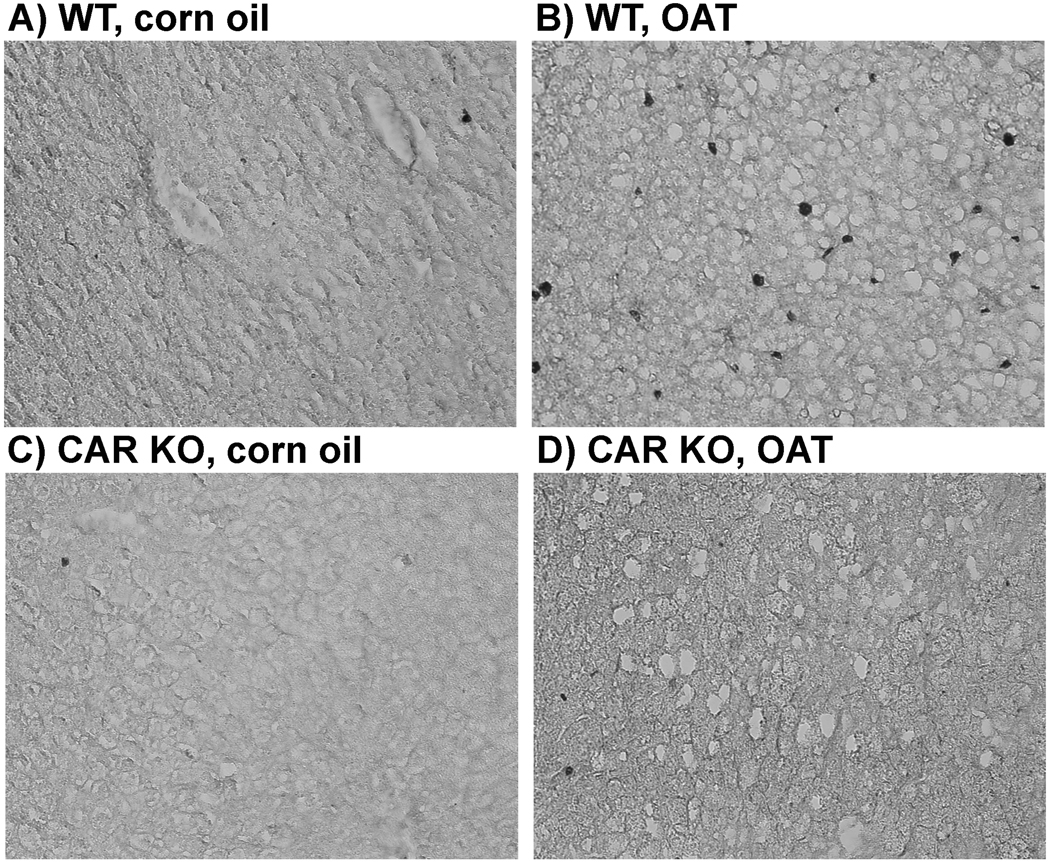
In long-term experiments, 7 months after a single administration of OAT to mice, we injected bromodeoxyuridine solution (BrdU) to identify cells in S-phase. The results shown in Figure 6A–D demonstrate that in WT mice OAT increases the number of hepatocytes in S phase ~ 7 fold (Table 3). In CAR KO mice treated with OAT, no change in proliferation of hepatocytes was observed. BrdU incorporation in liver cells of OAT treated CAR KO mice was comparable to that in non-treated WT mice. We conclude that a single administration of OAT did induce hepatocyte proliferation in WT mice in contrast to CAR KO mice, and, therefore, that CAR is responsible for this process. We also observed a significant, albeit modest, increase in liver index affected by OAT in WT mice vs. CAR KO mice (Table 3). In addition, one OAT-induced hepatic tumor was obtained in the wild type mouse, no tumors were found in CAR knock-out mice.
Fig. 6. The difference in hepatocyte proliferation between wild type (WT) and CAR-knockout (CAR KO) mice after long-term OAT treatment.
Mice were treated with OAT (single ip injection, dose 225 mg/kg) or corn oil and were sacrificed 7 months later. BrdU incorporation was assayed by immunohistochemical analysis of liver sections in WT (A, B) and CAR KO (C, D) mice after corn oil (A, C) or OAT (B, D) treatment.
Table 3.
Liver weight to body weight ratios and the quantification of BrdU-positive cells per field.
| WT, corn oil | WT, OAT | CAR KO, corn oil | CAR KO, OAT | |
|---|---|---|---|---|
| l/w : b/w ratios (a) | 0.0461 ± 0.0028 (3) | 0.0476 ± 0.0032 (9) | 0.0390 ± 0.0010 (3) | 0.0441 ± 0.0027 (9)* |
| BrdU+ cells, % (b) | 0.7809 ±0.4610 (3) | 5.3368 ± 2.8209 (9)# | 0.5196 ± 0.1134 (3) | 0.6543 ± 0.1581 (9)** |
Liver weight to body weight ratios (a) and BrdU-positive cells (%) (b) were measured from 9 or 3 animals (the number is shown in paranthesis) per treatment group, ± SD.
Statistical analysis was performed by a Student’s t-test (CAR KO vs. WT of the same treatment: *p<0.05, **p<0.01; OAT vs. corn oil: #p<0.05, ##p<0.01).
Discussion
Azo dyes, which are aromatic compounds with one or more –N=N– groups, constitute the largest class of synthetic dyes used in commercial applications. In 1994 estimates the world production of azo dyes was around 0.5 million tons (Stolz, 2001; Pandey et al., 2007). Depending on the class of the dye, loss in wastewaters can vary from 2% for basic dyes to as high as 50% for reactive dyes, leading to severe contamination of surface and ground waters in the vicinity of dyeing industries (Pandey et al., 2007). Biologically, the most relevant route of potential exposure of humans to azo colorants are dermal contact and inhalation. There are azo compounds for which human carcinogenicity has already been proved. For example, benzidine has been estimated by IARC as a Group 1 carcinogen. In a Japanese study, the risk of bladder cancer among benzidine-based dye applicators (kimono painters) was 6.8 times the expected rate (Bolt, 1995). In a British study, workers performing the dyeing of textiles (and not exposed to benzidine itself) had a higher risk of bladder cancer (RR=3.4) than expected (Bolt, 1995).
No adequate human studies of the relationship between exposure to o-aminoazotoluene and human cancer have been reported (National Toxicology Program, 2002). However, studies of carcinogenesis in response to OAT in laboratory animals showed nodules with several morphologic, biochemical, and molecular similarities to neoplastic and dysplastic nodules preceding the development of human HCC (Ohsawa et al. 2000).
OAT carcinogenicity is highly dependent on the route of ingestion. Azo dyes were originally given to animals with food in doses of 0.3–3 mg/ml for 150 days. About 95% of OAT was found in the liver, and tumors were formed by one year. However, this treatment was associated with significant mortality in the first month because of toxicity (Kinosita, 1940). When the carcinogen was applied on wounds, mortality rates among animals dropped significantly, but it also accumulated in the liver and showed carcinogenic effects. In later studies i.p. injections became widely used because this route of administration is covenient and less toxic, and leads to azo dye accumulation in the liver and liver tumor formation (Samuels et al., 1983; Delclos et al, 1984). In order to induce tumors previous studies have used OAT in a wide range of doses: from 10–40 mg/kg of body weight as Cheung et al. (1994) to 600 mg/kg of body weight as Ohsawa et al. (2000). In the present experiments a relatively moderate dose of OAT (225 mg/kg = 30% of LD50) was used. To induce tumors in adult mice, OAT is administered 6–10 times biweekly or monthly. However, 12–15 day old mice are more susceptible to carcinogenic actions of azo dyes (Delclos et al., 1984; Vesselinovitch et al., 1984), with a single OAT injection showing the same carcinogenic effect as compared to four times injections within the first month after birth (Delclos et al., 1984). Thus, we chose 12–15 day old mice mice for our long-term experiments.
Until recently, studies on the mechanisms of action of azo dyes have focused on the xenosensor Aryl-hydrocarbon receptor (AhR) (Beischlag et al., 2008), because the chemical structure of azo dyes is similar to the structure of classical activators and ligands of AhR – polycyclic aromatic hydrocarbons (PAH). Other xenobiotic receptors, which could also be potential xenosensors of azo dyes, remained outside the research scope until recently, when our laboratory demonstrated OAT-induced DNA-binding activity of CAR in mouse liver (Pakharukova et al., 2007). Moreover, OAT competed with the known ligand androstenol in an in vitro CAR binding assay using mouse liver cell extracts. These results strongly suggested that OAT functions as a ligand of mCAR.
mCAR activity can be modulated by a broad array of structurally diverse compounds (Chang and Waxman, 2006). Among these are agonists, such as 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP) (Tzameli et al., 2000), meclizine (Huang et al., 2004), clotrimazole and chlorpromazine (Makinen et al., 2003)), as well as indirect activators (namely, phenobarbital (Honkakoski et al., 1998; Kawamoto et al., 1999), acetaminophen (Zhang et al., 2002), phenytoin (Jackson et al., 2004; Wang et al., 2004), bilirubin (Huang et al., 2003), and nonylphenol (Hernandez et al., 2007) and etc.). Among the substances that modify mCAR activity, there are also inverse agonists. Two androstane metabolites, 5α-androst-16-en-3α-ol (androstenol) and 5α-androstan-3α-ol (androstanol) were identified as CAR ligands that repressed its constitutive activity (Forman et al., 1998). Similarly, progesterone and testosterone repress constitutive CAR activity, however, they do so at greater concentrations than the normal circulating levels of these hormones (Swales and Negishi, 2004).
Based on our earlier studies, we tested the possible involvement of CAR in the mechanism of action of OAT. First, transient transfection assays in HepG2 cells showed that OAT increases CAR transactivation in a dose-dependent manner, although the OAT concentration required was much higher than of the well studied high affinity CAR agonist TCPOBOP. The effect of OAT was quite specific since the structurally related azo dye 3′-MeDAB was not able to activate mCAR, even at high concentrations (10 mM).
Second, our results demonstrate that OAT induces the expression of a number of CAR target genes in a CAR-dependent manner: Cyp2b10, Cyp2c29, Cyp3a11, Ugt1a1, Mrp4, Mrp2 and c-Myc. Induction of these genes in response to OAT in the wild type mice was abolished in CAR-knockout mice. However, several other reported CAR target genes, including NADPH CYP450 oxidoreductase, Mdm2 and Cyclin D1 either did not respond to OAT, or their induction was CAR-independent. This suggests that OAT acts as a selective modulator of CAR activation. Consistent with this, similar differences in the responses of CAR target genes to various inducers of CAR were observed by others. Thus, TCPOBOP strongly induces both CYP2B10 and CYP3A11 expression but chlorpromazine induces only the former (Wei et al., 2002). Likewise, phenobarbital (PB) induces Cyp2f2, whereas TCPOBOP does not (Braeuning et al., 2009).
In addition to detoxification, CAR regulates many other processes in cells, and its effect on proliferation and apoptosis in the liver is well recognized (Ledda-Columbano et al., 2003; Beilke et al., 2009). In particular, CAR is required for the potent tumor promoter effects of its activators (Yamamoto et al., 2004; Ross et al., 2009). In contrast to rodents, humans are not sensitive to the tumorigenic effects of phenobarbital and other known CAR activators. In human subjects there is no evidence of increased liver tumor risk after many years of phenobarbital administration (Olsen et al., 1995), although it is known that hCAR is activated in response to phenobarbital (Ross et al., 2010). Additionally, transgenic mice whose Car gene was replaced with the human analog do not show hepatocyte proliferation and liver hyperplasia in response to mitogenic stimuli (Ross et al., 2010).
Unlike phenobarbital, TCPOBOP and other CAR activators, the acute CAR response to OAT did not include induction of cyclin D1 or increased liver size. Consistent with this selective response, acute CAR activation also failed to induce hepatocyte proliferation. In accordance with the well established hepatocarcinogenicity of OAT in mice, however, we did observe significant CAR-dependent proliferation in mice 7 months after a single OAT treatment. Increased liver cell proliferation creates a favorable environment for preneoplastic cell proliferation, and it is likely that this increased proliferation of liver cells is associated with the carcinogenic effects of OAT.
The basis for the delayed, CAR-dependent mitogenic effects of OAT remains an intriguing question. Although most of the OAT is lost from the liver within 30 days after a single administration, a residual amount was present at 84 days (Lawson, 1970). It is possible that secondary effects of CAR activation occurring within this extended period could contribute to the deferred proliferative response. Therein lies the interest of our results which are consistent with other researchers’ data on carcinogenic effects of azo dyes. German researchers observed a very long latency period before the time of the tumor formation in humans after exposure to azo dyes (Bolt, 1995). Thus, our results may be especially important for human risk assessement, and our data may provide the impetus for the study of mechanisms of deferred carcinogenic action. Another possibility is suggested by the action of longer term cumulative effects of c-Myc as a transcription factor directly involved in epigenetic remodeling of chromatin (Lin et al., 2009; Louis et al., 2005) and increasing the methylation of histone target genes. c-Myc also enhances remodeling of chromatin by interaction directly with histone acetylase / deacetylase (HAT) complex and with ATP-dependent chromatin remodeling complex SWI-SNF (Amati et al., 2001; Frank et al., 2001). c-Myc in collaboration with other transcription factors can reprogram mouse and human cells (Park et al., 2008). Thus the impact of chronic activation of c-Myc on epigenetic chromatin remodeling could also be involved in long-term effects that we observe in response to OAT.
Overall, we conclude that the azo dye and mouse carcinogen ortho-aminoazotoluene (but not azo dye 3'-MeDAB) is a selective mCAR activator that activates a specific subset of target genes and induces an unexpectedly deferred, but CAR-dependent liver cell proliferation. These findings provide important insights into the role of mCAR in biological responses to an established mouse hepatocarcinogen.
Highlights.
The azo dye and mouse carcinogen OAT is a very effective mCAR activator. > OAT increases mCAR transactivation in a dose-dependent manner. > OAT CAR-dependently increases the expression of a specific subset of CAR target genes. > OAT induces an unexpectedly deferred, but CAR-dependent hepatocyte proliferation.
Acknowledgements
Research support for this study was provided by Fulbright grant (IIE Grantee ID: 15073775 – M.A. Smetanina). D.D. Moore is supported by NIH grant R01-DK46546 and by Chair Grant No. Q-0022 from the Welch Foundation.
Abbreviations
- OAT
Ortho-Aminoazotoluene, 2'-3-dimethyl-4-aminoazobenzene
- 3'-MeDAB
3'-methyl-4-dimethylaminoazobenzene
- CAR
Constitutive Androstane Receptor
- CYP450s
Cytochromes P450
- TCPOBOP
1,4-bis[2-(3,5-dichloropyridyloxy)]benzene
- BrdU
bromodeoxyuridine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Policy
The authors declare that there are no conflicts of interest.
References
- Amati B, Frank SR, Donjerkovic D, Taubert S. Function of the c-Myc oncoprotein in chromatin remodeling and transcription. Biochim Biophys Acta. 2001;1471:M135–M145. doi: 10.1016/s0304-419x(01)00020-8. [DOI] [PubMed] [Google Scholar]
- Assem M, Schuetz EG, Leggas M, Sun D, Yasuda K, Reid G, Zelcer N, Adachi M, Strom S, Evans RM, Moore DD, Borst P, Schuetz JD. Interactions between hepatic Mrp4 and Sult2a as revealed by the constitutive androstane receptor and Mrp4 knockout mice. J. Biol. Chem. 2004;279:22250–22257. doi: 10.1074/jbc.M314111200. [DOI] [PubMed] [Google Scholar]
- Baginskaya NV, Il'nitskaya SI, Nikitenko EV, Kaledin VI. Promoting effect of o-aminoazotoluene on hepatocarcinogenesis is accompanied by the increase in inflammatory and proliferative processes in liver tissue and decrease in the concentration of free thyroxin in the blood. Bull. Exp. Biol. Med. 2007;144:821–824. doi: 10.1007/s10517-007-0440-0. [DOI] [PubMed] [Google Scholar]
- Beilke LD, Aleksunes LM, Olson ER, Besselsen DG, Klaassen CD, Dvorak K, Cherrington NJ. Decreased apoptosis during CAR-mediated hepatoprotection against lithocholic acid-induced liver injury in mice. Toxicol. Lett. 2009;188:38–44. doi: 10.1016/j.toxlet.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beischlag TV, Morales JL, Hollingshead BD, Perdew GH. The Aryl Hydrocarbon Receptor complex and the control of gene expression. Crit. Rev. Eukaryot. Gene Expr. 2008;18:207–250. doi: 10.1615/critreveukargeneexpr.v18.i3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Bose WE, Murphy MJ, Ehninger A, Offner S, Dubey C, Huang W, Moore DD, Trumpp A. C-Myc and its target FoxM1 are critical downstream effectors of constitutive androstane receptor (CAR) mediated direct liver hyperplasia. Hepatology. 2008;48:1302–1311. doi: 10.1002/hep.22475. [DOI] [PubMed] [Google Scholar]
- Bolt HM. Special points in the toxicity assessment of colorants (dyes and pigments) In: Thomas H, Hess H, Waechter F, editors. Toxicology of Industrial Compounds. London: Taylor & Francis Ltd.; 1995. pp. 301–308. [Google Scholar]
- Bookout AL, Cummins CL, Mangelsdorf DJ. High-throughput real-time quantitative reverse transcription PCR. Curr Protoc Mol Biol. 2006;15:15.8. doi: 10.1002/0471142727.mb1508s73. [DOI] [PubMed] [Google Scholar]
- Braeuning A, Sanna R, Huelsken J, Schwarz M. Inducibility of drugmetabolizing enzymes by xenobiotics in mice with liver-specific knockout of Ctnnb1. Drug Metab. Dispos. 2009;37:1138–1145. doi: 10.1124/dmd.108.026179. [DOI] [PubMed] [Google Scholar]
- Buckley DB, Klaassen CD. Induction of mouse UDP-glucuronosyltransferase mRNA expression in liver and intestine by activators of aryl-hydrocarbon receptor, constitutive androstane receptor, pregnane X receptor, peroxisome proliferator-activated receptor alpha, and nuclear factor erythroid 2-related factor 2. Drug Metab. Dospos. 2009;37:847–856. doi: 10.1124/dmd.108.024190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TK, Waxman DJ. Synthetic drugs and natural products as modulators of constitutive androstane receptor (CAR) and pregnane X receptor (PXR) Drug Metab. Rev. 2006;38:51–73. doi: 10.1080/03602530600569828. [DOI] [PubMed] [Google Scholar]
- Cheung YL, Puddicombe SM, Gray TJ, Ioannides C. Mutagenicity and CYP1A induction by azobenzenes correlates with their carcinogenicity. Carcinogenesis. 1994;15:1257–1263. doi: 10.1093/carcin/15.6.1257. [DOI] [PubMed] [Google Scholar]
- Choi H, Chung M, Tzameli I, Simha D, Lee YK, Seol W, Moore DD. Differential transactivation by two isoforms of the orphan nuclear hormone receptor CAR. J. Biol. Chem. 1997;272:23565–23571. doi: 10.1074/jbc.272.38.23565. [DOI] [PubMed] [Google Scholar]
- Columbano A, Ledda-Columbano GM, Pibiri M, Cossu C, Menegazzi M, Moore DD, Huang W, Tian J, Locker J. Gadd45beta is induced through a CAR-dependent, TNF-independent pathway in murine liver hyperplasia. Hepatology. 2005;42:1118–1126. doi: 10.1002/hep.20883. [DOI] [PubMed] [Google Scholar]
- Cook JW. Azo-dyes and experimental liver tumours. Br. J. Nutr. 1947;1:245–253. doi: 10.1079/bjn19470033. [DOI] [PubMed] [Google Scholar]
- Costa RH, Kalinichenko VV, Tan Y, Wang IC. The CAR nuclear receptor and hepatocyte proliferation. Hepatology. 2005;42:1004–1008. doi: 10.1002/hep.20953. [DOI] [PubMed] [Google Scholar]
- Delclos KB, Tarpley WG, Miller EC, Miller JA. 4-aminoazobenzene and N,N-dimethyl-4-aminoazobenzene as equipotent hepatic carcinogens in male C57BL/6 X C3H/He F1 mice and characterization of N-(Deoxyguanosin-8-yl)-4-aminoazobenzene as the major persistent hepatic DNA-bound dye in these mice. Cancer Res. 1984;44:2540–2550. [PubMed] [Google Scholar]
- Forman BM, Tzameli I, Choi HS, Chen J, Simha D, Seol W, Evans RM, Moore DD. Androstanemetabolites bind to and deactivate the nuclear receptorCAR. Nature. 1998;395:612–615. doi: 10.1038/26996. [DOI] [PubMed] [Google Scholar]
- Frank SR, Schroeder M, Fernandez P, Taubert S, Amati B. Binding of c-Myc to chromatin mediates mitogen-induced acetylation of histone H4 and gene activation. Genes Dev. 2001;15:2069–2082. doi: 10.1101/gad.906601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin B, Moore JT. CAR: detailing new models. Trends Pharmacol. Sci. 2004;25:437–441. doi: 10.1016/j.tips.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Graham MJ, Lake BG. Induction of drug metabolism: Species differences and toxicological relevance. Toxicology. 2008;254:184–191. doi: 10.1016/j.tox.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Hazardous Substances Data Base (HSDB) National Library of Medicine. 2001 available at http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB.
- Hernandez JP, Chapman LM, Kretschmer XC, Baldwin WS. Gender-specific induction of cytochrome P450s in nonylphenol-treated FVB/NJ mice. Toxicol. Appl. Pharmacol. 2006;216:186–196. doi: 10.1016/j.taap.2006.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez JP, Huang W, Chapman LM, Chua S, Moore DD, Baldwin WS. The environmental estrogen, nonylphenol, activates the Constitutive Androstane Receptor. Toxicol. Sci. 2007;98:416–426. doi: 10.1093/toxsci/kfm107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsapple MP, Pitot HC, Cohen SM, Boobis AR, Klaunig JE, Pastoor T, Dellarco VL, Dragan YP. Mode of Action in Relevance of Rodent Liver Tumors to Human Cancer Risk. Toxicol. Sci. 2006;89:51–56. doi: 10.1093/toxsci/kfj001. [DOI] [PubMed] [Google Scholar]
- Honkakoski P, Zelko I, Sueyoshi T, Negishi M. The nuclear orphan receptor CAR-retinoid X receptor heterodimer activates the phenobarbital-responsive enhancer module of the CYP2B gene. Mol. Cell. Biol. 1998;18:5652–5658. doi: 10.1128/mcb.18.10.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Zhang J, Chua SS, Qatanani M, Han Y, Granata R, Moore DD. Induction of bilirubin clearance by the constitutive androstane receptor (CAR) Proc. Natl. Acad. Sci. U. S. A. 2003;100:4156–4161. doi: 10.1073/pnas.0630614100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Zhang J, Wei P, Schrader WT, Moore DD. Meclizine is an agonist ligand for mouse Constitutive Androstane Receptor (CAR) and an inverse agonist for human CAR. Mol. Endocrinol. 2004;18:2402–2408. doi: 10.1210/me.2004-0046. [DOI] [PubMed] [Google Scholar]
- Huang W, Zhang J, Washington M, Liu J, Parant JM, Lozano G, Moore DD. Xenobiotic stress induces hepatomegaly and liver tumors via the nuclear receptor constitutive androstane receptor. Mol. Endocrinol. 2005;19:1646–1653. doi: 10.1210/me.2004-0520. [DOI] [PubMed] [Google Scholar]
- Il'nitskaya SI, Vasil'eva ED, Kropachev KY, Kaledin VI. Relationship between high sensitivity of suckling mice to hepatocarcinogenic and antiglucocorticoid effects of o-aminoazotoluene and diethylnitrosamine. Bull. Exp. Biol. Med. 2004;137:548–550. doi: 10.1023/b:bebm.0000042708.97983.23. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (IARC) Vol. 8. Lyon, France: 1975. Some Aromatic Azo Compounds. IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans; p. 357. [Google Scholar]
- Jackson JP, Ferguson SS, Moore R, Negishi M, Goldstein JA. The constitutive active/androstane receptor regulates phenytoin induction of Cyp2c29. Mol. Pharmacol. 2004;65:1397–1404. doi: 10.1124/mol.65.6.1397. [DOI] [PubMed] [Google Scholar]
- Kaledin VI, Alekseeva GV, Volkova AI. Carcinogenicity of orthoaminoazotoluene for mouse intestines. Bull. Exp. Biol. Med. 1978;86:476–477. [PubMed] [Google Scholar]
- Kaledin VI, Zakharova NP. Investigation on Tumor Induction and Metastasizing in Experimental Animals. Novosibirsk: IC&G Press; 1984. Effect of hepatocarcinogens on hormonal induction of tyrosine aminotransferase in the liver of sensitive and resistant animals; pp. 146–185. [Google Scholar]
- Kaledin VI, Serova IA, Tsellarius IuG, Semenova LA. Ratio of spontaneous and o-aminoazotoluene-induced hepatocarcinogenesis in mice. Eksp. Onkol. 1985;7:23–26. [PubMed] [Google Scholar]
- Kaledin VI, Serova IA, Semenova LA. Different predisposition to the development of spontaneous and induced liver tumors in mice of different strains and their hybrids. Eksp. Onkol. 1990;12:28–30. [PubMed] [Google Scholar]
- Kast HR, Goodwin B, Tarr PT, Jones SA, Anisfeld AM, Stoltz CM, Tontonoz P, Kliewer S, Willson TM, Edwards PA. Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor and constitutive androstane receptor. J. Biol. Chem. 2002;277:2908–2915. doi: 10.1074/jbc.M109326200. [DOI] [PubMed] [Google Scholar]
- Kato TA, Matsuda T, Matsui S, Mizutani T, Saeki K. Activation of the aryl hydrocarbon receptor by methyl yellow and related congeners: structure-activity relationships in halogenated derivatives. Biol. Pharm. Bull. 2002;25:466–471. doi: 10.1248/bpb.25.466. [DOI] [PubMed] [Google Scholar]
- Kawamoto T, Sueyoshi T, Zelko I, Moore R, Washburn K, Negishi M. Phenobarbital-responsive nuclear translocation of the receptor CAR in induction of the CYP2B gene. Mol. Cell. Biol. 1999;19:6318–6322. doi: 10.1128/mcb.19.9.6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinosita R. Studies on the cancerogenic azo and related compounds. Yale J Biol Med. 1940;12:287–300. [PMC free article] [PubMed] [Google Scholar]
- Kiyosawa N, Kwekel JC, Burgoon LD, Williams KJ, Tashiro C, Chittim B, Zacharewski TR. o,p'-DDT elicits PXR/CAR-, not ER-, mediated responses in the immature ovariectomized rat liver. Toxicol. Sci. 2008;101:350–363. doi: 10.1093/toxsci/kfm275. [DOI] [PubMed] [Google Scholar]
- Kubitz R, Warskulat U, Schmitt M, Häussinger D. Dexamethasone- and osmolarity-dependent expression of the multidrug-resistance protein 2 in cultured rat hepatocytes. Biochem. J. 1999;340:585–591. [PMC free article] [PubMed] [Google Scholar]
- Lawson TA. The effect of prolonged feeding of ortho-aminoazotoluene on binding to cellular constituents in mouse liver. Chem Biol Interact. 1970;2:9–16. doi: 10.1016/0009-2797(70)90033-5. [DOI] [PubMed] [Google Scholar]
- Ledda-Columbano GM, Pibiri M, Concas D, Molotzu F, Simbula G, Cossu C, Columbano A. Sex difference in the proliferative response of mouse hepatocytes to treatment with the CAR ligand, TCPOBOP. Carcinogenesis. 2003;24:1059–1065. doi: 10.1093/carcin/bgg063. [DOI] [PubMed] [Google Scholar]
- Lin CH, Lin C, Tanaka H, Fero ML, Eisenman RN. Gene regulation and epigenetic remodeling in murine embryonic stem cells by c-Myc. PLoS One. 2009;4:e7839. doi: 10.1371/journal.pone.0007839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locker J, Tian J, Carver R, Concas D, Cossu C, Ledda-Columbano GM, Columbano A. A common set of immediate-early response genes in liver regeneration and hyperplasia. Hepatology. 2003;38:314–325. doi: 10.1053/jhep.2003.50299. [DOI] [PubMed] [Google Scholar]
- Louis SF, Vermolen BJ, Garini Y, Young IT, Guffei A, Lichtensztejn Z, Kuttler F, Chuang TC, Moshir S, Mougey V, Chuang AY, Kerr PD, Fest T, Boukamp P, Mai S. c-Myc induces chromosomal rearrangements through telomere and chromosome remodeling in the interphase nucleus. Proc Natl Acad Sci U S A. 2005;102:9613–9618. doi: 10.1073/pnas.0407512102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu TT, Makishima M, Repa JJ, Schoonjans K, Kerr TA, Auwerx J, Mangelsdorf DJ. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol. Cell. 2000;6:507–515. doi: 10.1016/s1097-2765(00)00050-2. [DOI] [PubMed] [Google Scholar]
- Maglich JM, Stoltz CM, Goodwin B, Hawkins-Brown D, Moore JT, Kliewer SA. Nuclear pregnane X receptor and constitutive androstane receptor regulate overlapping but distinct sets of genes involved in xenobiotic detoxification. Mol. Pharmacol. 2002;62:638–646. doi: 10.1124/mol.62.3.638. [DOI] [PubMed] [Google Scholar]
- Makinen J, Reinisalo M, Niemi K, Viitala P, Jyrkkärinne J, Chung H, Pelkonen O, Honkakoski P. Dual action of estrogens on the mouse constitutive androstane receptor. Biochem. J. 2003;376:465–472. doi: 10.1042/BJ20030553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masi A, Marinis E, Ascenzi P, Marino M. Nuclear receptors CAR and PXR: Molecular, functional, and biomedical aspects. Mol. Aspects Med. 2009;30:297–343. doi: 10.1016/j.mam.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Merkulova TI, Kropachev KY, Timofeeva OA, Vasiliev GV, Levashova ZB, Ilnitskaya SI, Kobzev VF, Pakharukova MY, Bryzgalov LO, Kaledin VI. Species-specific effects of the hepatocarcinogens 3'-methyl-4-dimethyl-aminoazobenzene and ortho-aminoazotoluene in mouse and rat liver. Mol. Carcinog. 2005;44:223–232. doi: 10.1002/mc.20090. [DOI] [PubMed] [Google Scholar]
- Mikhailova ON, Vasyunina EA, Ovchinnikova LP, Gulyaeva LF, Timofeeva OA, Filipenko ML, Kaledin VI. o-Aminoazotoluene does induce the enzymes of its own mutagenic activation in mouse liver. Toxicology. 2005;211:132–138. doi: 10.1016/j.tox.2005.03.006. [DOI] [PubMed] [Google Scholar]
- National Toxicology Program. o-Aminoazotoluene. Rep Carcinog. 2002;10:3. [PubMed] [Google Scholar]
- Ohsawa K, Hirano N, Sugiura M, Nakagawa S, Kimura M. Genotoxicity of o-aminoazotoluene (AAT) determined by the Ames test, the in vitro chromosomal aberration test, and the transgenic mouse gene mutation assay. Mutat Res. 2000;471:113–126. doi: 10.1016/s1383-5718(00)00120-0. [DOI] [PubMed] [Google Scholar]
- Olsen JH, Schulgen G, Boice JD, Jr, Whysner J, Travis LB, Williams GM, Johnson FB, McGee JO. Antiepileptic treatment and risk for hepatobiliary cancer and malignant lymphoma. Cancer Res. 1995;55:294–297. [PubMed] [Google Scholar]
- Pakharukova MY, Smetanina MA, Kaledin VI, Kobzev VF, Romanova IV, Merkulova TI. Activation of constitutive androstane receptor under the effect of hepatocarcinogenic aminoazo dyes in mouse and rat liver. Bull. Exp. Biol. Med. 2007;144:338–341. doi: 10.1007/s10517-007-0327-0. [DOI] [PubMed] [Google Scholar]
- Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2007;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- Pandey A, Singh P, Iyengar L. Bacterial decolorization and degradation of azo dyes. Int Biodeterior Biodegradation. 2007;59:73–84. [Google Scholar]
- Pascussi J, Gerbal-Chaloin S, Duret C, Daujat-Chavanieu M, Vilarem MJ, Maurel P. The tangle of nuclear receptors that controls xenobiotic metabolism and transport: crosstalk and consequences. Annu. Rev. Pharmacol. Toxicol. 2008;48:1–32. doi: 10.1146/annurev.pharmtox.47.120505.105349. [DOI] [PubMed] [Google Scholar]
- Rafii F, Cerniglia CE. Reduction of azo dyes and nitroaromatic compounds by bacterial enzymes from the human intestinal tract. Environ Health Perspect. 1995;103:17–19. doi: 10.1289/ehp.95103s417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J, Plummer SM, Rode A, Scheer N, Bowers CC, Vogel O, Henderson CJ, Wolf CR, Elcombe CR. Human constitutive androstane receptor (CAR) and pregnane X receptor (PXR) support the hypertrophic but not the hyperplastic response to the murine nongenotoxic hepatocarcinogens phenobarbital and chlordane in vivo. Toxicol. Sci. 2010;116:452–466. doi: 10.1093/toxsci/kfq118. [DOI] [PubMed] [Google Scholar]
- Ross PK, Woods CG, Bradford BU, Kosyk O, Gatti DM, Cunningham ML, Rusyn I. Time-course comparison of xenobiotic activators of CAR and PPARα in mouse liver. Toxicol. Appl. Pharmacol. 2009;235:199–207. doi: 10.1016/j.taap.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels AR, Bhargava MM, Levine WG. Uptake and hepatobiliary fate of two hepatocarcinogens, N,N-dimethyl-4-aminoazobenzene and 3'-methyl-N,N-dimethyl-4-aminoazobenzene, in the rat. Cancer Res. 1983;43:4816–4821. [PubMed] [Google Scholar]
- Smirlis D, Muangmoonchai R, Edwards M, Phillips IR, Shephard EA. Orphan receptor promiscuity in the induction of cytochromes p450 by xenobiotics. J. Biol. Chem. 2001;276:12822–12826. doi: 10.1074/jbc.M005930200. [DOI] [PubMed] [Google Scholar]
- Stanley LA, Horsburgh BC, Ross J, Scheer N, Wolf CR. PXR and CAR: nuclear receptors which play a pivotal role in drug disposition and chemical toxicity. Drug Metab. Rev. 2006;38:515–597. doi: 10.1080/03602530600786232. [DOI] [PubMed] [Google Scholar]
- Stolz A. Basic and applied aspects in the microbial degradation of azo dyes. Appl Microbiol Biotechnol. 2001;56:69–80. doi: 10.1007/s002530100686. [DOI] [PubMed] [Google Scholar]
- Swales K, Negishi M. CAR, driving into the future. Mol. Endocrinol. 2004;18:1589–1598. doi: 10.1210/me.2003-0397. [DOI] [PubMed] [Google Scholar]
- Tsuda S, Matsusaka N, Madarame H, Ueno S, Susa N, Ishida K, Kawamura N, Sekihashi K, Sasaki YF. The comet assay in eight mouse organs: results with 24 azo compounds. Mutat Res. 2000;465:11–26. doi: 10.1016/s1383-5718(99)00199-0. [DOI] [PubMed] [Google Scholar]
- Tzameli I, Pissios P, Schuetz EG, Moore DD. The xenobiotic compound 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene is an agonist ligand for the nuclear receptor CAR. Mol. Cell. Biol. 2000;20:2951–2958. doi: 10.1128/mcb.20.9.2951-2958.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urquhart BL, Tirona RG, Kim RB. Nuclear receptors and the regulation of drug-Metabolizing enzymes and drug transporters: implications for interindividual variability in response to drugs. J. Clin. Pharmacol. 2007;47:566–578. doi: 10.1177/0091270007299930. [DOI] [PubMed] [Google Scholar]
- Vesselinovitch SD, Koka M, Mihailovich N, Rao KV. Carcinogenicity of diethylnitrosamine in newborn, infant, and adult mice. J Cancer Res Clin Oncol. 1984;108:60–65. doi: 10.1007/BF00390974. [DOI] [PubMed] [Google Scholar]
- Wada T, Gao J, Xie W. PXR and CAR in energy metabolism. Trends Endocrinol. Metab. 2009;20:273–279. doi: 10.1016/j.tem.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Wang H, Faucette S, Moore R, Sueyoshi T, Negishi M, LeCluyse E. Human constitutive androstane receptor mediates induction of CYP2B6 gene expression by phenytoin. J. Biol. Chem. 2004;279:29295–29301. doi: 10.1074/jbc.M400580200. [DOI] [PubMed] [Google Scholar]
- Wei P, Zhang J, Egan-Hafley M, Liang S, Moore DD. The nuclear receptor CAR mediates specific xenobiotic induction of drug metabolism. Nature. 2000;407:920–923. doi: 10.1038/35038112. [DOI] [PubMed] [Google Scholar]
- Wei P, Zhang J, Dowhan DH, Han Y, Moore DD. Specific and overlapping functions of the nuclear hormone receptors CAR and PXR in xenobiotic response. Pharmacogenomics J. 2002;2:117–126. doi: 10.1038/sj.tpj.6500087. [DOI] [PubMed] [Google Scholar]
- Xie W, Barwick JL, Simon CM, Pierce AM, Safe S, Blumberg B, Guzelian PS, Evans RM. Reciprocal activation of xenobiotic response genes by nuclear receptors SXR/PXR and CAR. Genes Dev. 2000;14:3014–3023. doi: 10.1101/gad.846800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Li CY, Kong AN. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch. Pharmacal Res. 2005;28:249–268. doi: 10.1007/BF02977789. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Moore R, Goldsworthy TL, Negishi M, Maronpot RR. The orphan nuclear receptor constitutive active/androstane receptor is essential for liver tumor promotion by phenobarbital in mice. Cancer Res. 2004;64:7197–7200. doi: 10.1158/0008-5472.CAN-04-1459. [DOI] [PubMed] [Google Scholar]
- Zacharova LY, Gulyaeva LF, Lyakhovich VV, Mikhailova ON, Timofeeva OA, Filipenko ML, Kaledin VI. Cytochrome P4501A1 and 1A2 gene expression in the liver of 3-methylcholanthrene- and o-aminoazotoluene-treated mice: a comparison between PAHresponsive and PAH-nonresponsive strains. Toxicol. Sci. 2003;73:108–113. doi: 10.1093/toxsci/kfg053. [DOI] [PubMed] [Google Scholar]
- Zhang J, Huang W, Chua SS, Wei P, Moore DD. Modulation of acetaminophen-induced hepatotoxicity by the xenobiotic receptor CAR. Science. 2002;298:422–424. doi: 10.1126/science.1073502. [DOI] [PubMed] [Google Scholar]