Abstract
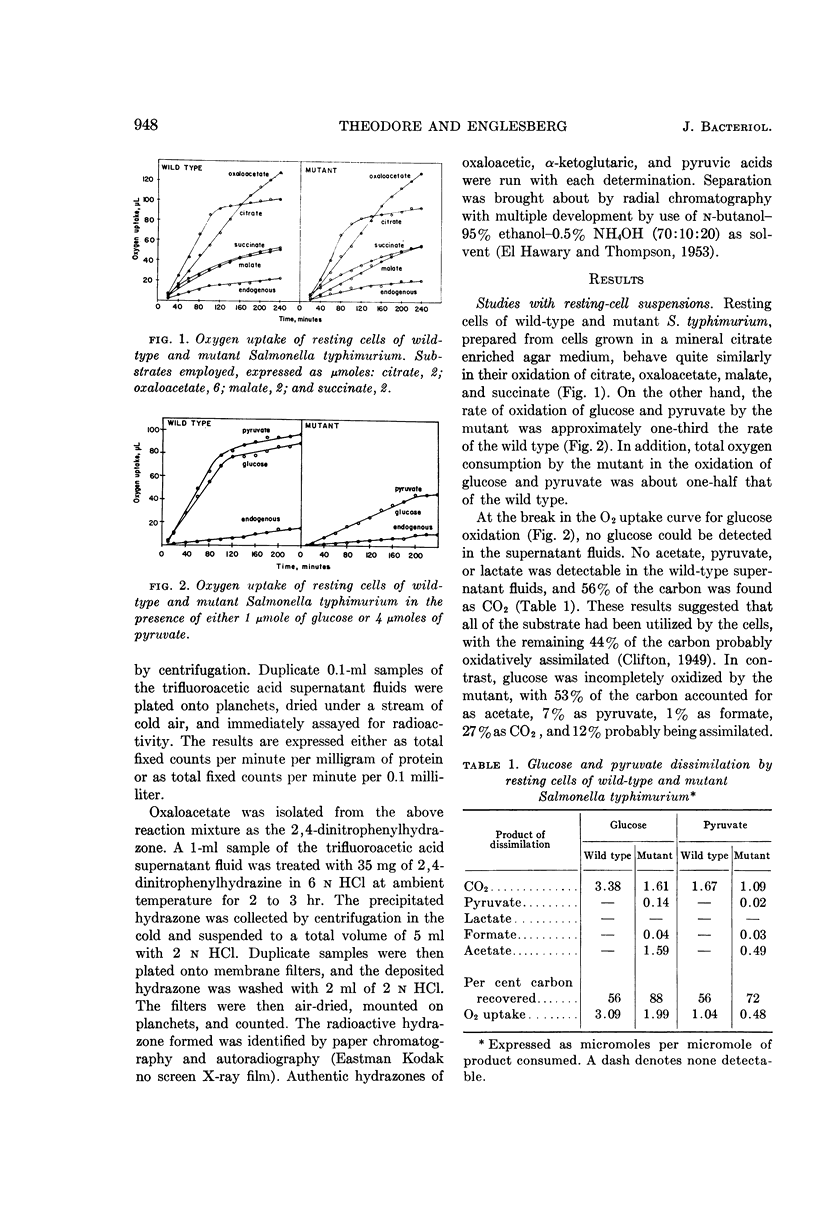
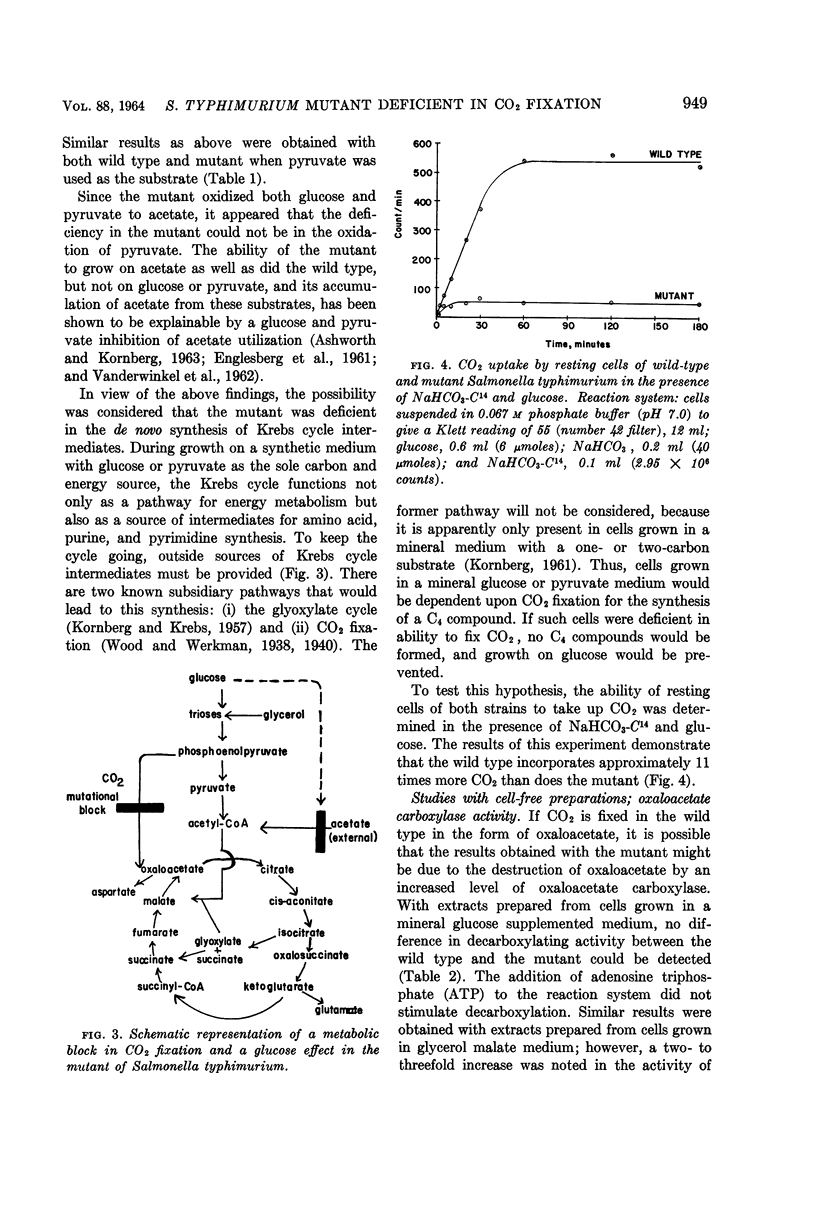
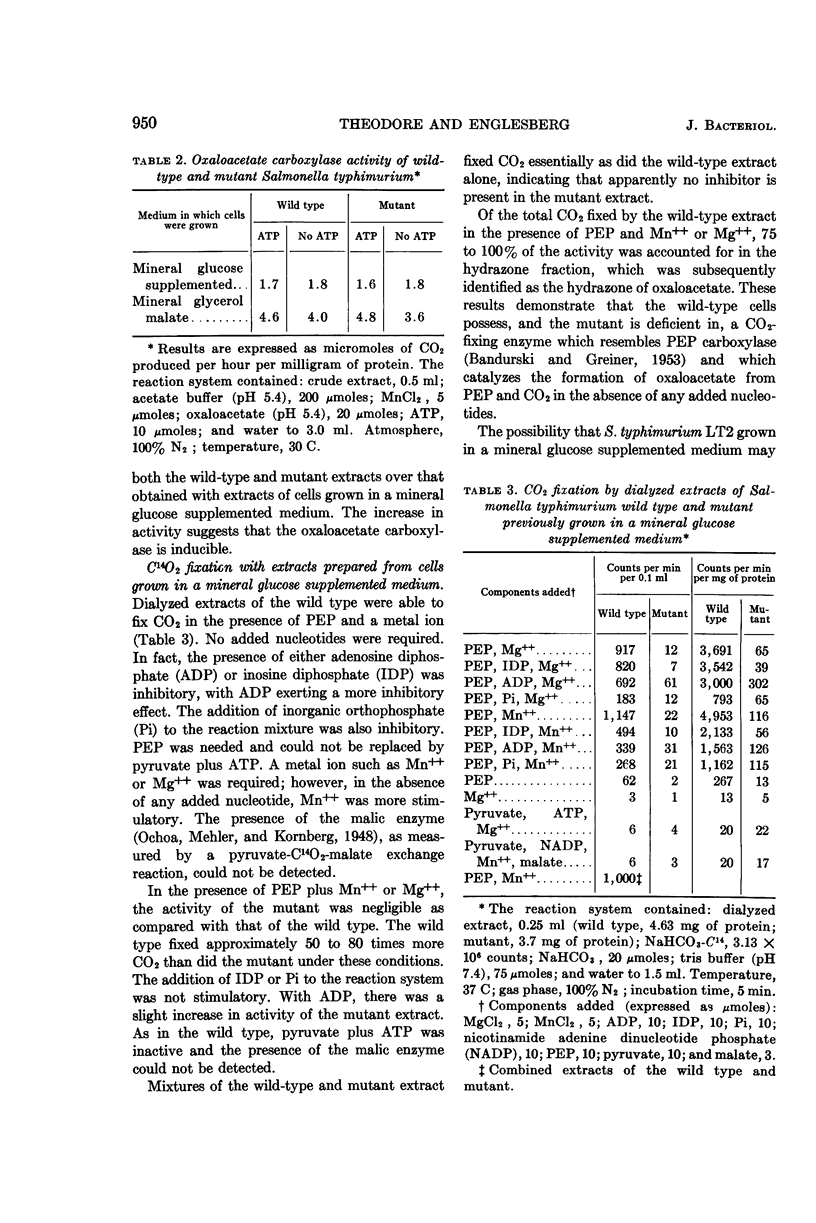
Theodore, Theodore S. (University of Pittsburgh, Pittsburgh, Pa.), and Ellis Englesberg. Mutant of Salmonella typhimurium deficient in the carbon dioxide-fixing enzyme phosphoenolpyruvic carboxylase. J. Bacteriol. 88:946–955. 1964.—Resting cells of Salmonella typhimurium wild type (C+dgs) and the C-dgs mutant characterized by impaired glucose and glycerol metabolism are able to oxidize Krebs cycle intermediates to the same extent. The wild type oxidized glucose and pyruvate “completely,” and the mutant oxidized these substrates at a reduced rate and incompletely, with the accumulation of acetate. Resting cells of the wild type in the presence of NaHCO3-C14 and glucose incorporated 11 times more CO2 than did similar suspensions of the mutant. Extracts prepared from cells previously grown in a mineral glucose supplemented medium revealed that the mutant was deficient in the CO2-fixing enzyme phosphoenolpyruvic carboxylase (PEP carboxylase). This enzyme was found to be present in the wild-type extracts. It catalyzes the formation of oxaloacetate from phosphoenolpyruvate and CO2 in the presence of Mn++ or Mg++. No added nucleotides are required for its activity. Since only low levels of phosphoenolpyruvic carboxykinase activity are present in extracts prepared from both kinds of cells grown in a mineral glucose supplemented medium, and perhaps only trace amounts of the malic enzyme, phosphoenolpyruvic carboxytransphosphorylase, and of the enzyme requiring pyruvate, CO2, and adenosine triphosphate are present, it was concluded that PEP carboxylase is required for CO2, fixation in this organism. The loss of this enzyme prevents the growth of the mutant in a mineral-glucose or mineral-glycerol medium, and results in the incomplete oxidation of glucose and pyruvate with the accumulation of acetate. This is the first demonstration of the essential role of this particular enzyme in CO2 fixation in chemoorganotrophs.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHWORTH J. M., KORNBERG H. L. FINE CONTROL OF THE GLYOXYLATE CYCLE BY ALLOSTERIC INHIBITION OF ISOCITRATE LYASE. Biochim Biophys Acta. 1963 Jul 9;73:519–522. doi: 10.1016/0006-3002(63)90457-8. [DOI] [PubMed] [Google Scholar]
- BANDURSKI R. S., GREINER C. M. The enzymatic synthesis of oxalacetate from phosphoryl-enolpyruvate and carbon dioxide. J Biol Chem. 1953 Oct;204(2):781–786. [PubMed] [Google Scholar]
- CANNATA J., STOPPANI A. O. Adenosine polyphosphate requirement of baker's yeast phosphopyruvate carboxylase. Biochim Biophys Acta. 1959 Mar;32(1):284–285. doi: 10.1016/0006-3002(59)90591-8. [DOI] [PubMed] [Google Scholar]
- EL HAWARY M. F. S., THOMPSON R. H. S. Separation and estimation of blood keto acids by paper chromatography. Biochem J. 1953 Feb;53(3):340–347. doi: 10.1042/bj0530340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGLESBERG E., WATSON J. A., HOFFEE P. A. The glucose effect and the relationship between glucose permease, acid phosphatase, and glucose resistance. Cold Spring Harb Symp Quant Biol. 1961;26:261–276. doi: 10.1101/sqb.1961.026.01.033. [DOI] [PubMed] [Google Scholar]
- Englesberg E. GLUCOSE INHIBITION AND THE DIAUXIE PHENOMENON. Proc Natl Acad Sci U S A. 1959 Oct;45(10):1494–1507. doi: 10.1073/pnas.45.10.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORNBERG H. L., KREBS H. A. Synthesis of cell constituents from C2-units by a modified tricarboxylic acid cycle. Nature. 1957 May 18;179(4568):988–991. doi: 10.1038/179988a0. [DOI] [PubMed] [Google Scholar]
- KORNBERG H. L. Selective utilization of metabolic routes by Escherichia coli. Cold Spring Harb Symp Quant Biol. 1961;26:257–260. doi: 10.1101/sqb.1961.026.01.032. [DOI] [PubMed] [Google Scholar]
- Markham R. A steam distillation apparatus suitable for micro-Kjeldahl analysis. Biochem J. 1942 Dec;36(10-12):790–791. doi: 10.1042/bj0360790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STADTMAN E. R., NOVELLI G. D., LIPMANN F. Coenzyme A function in and acetyl transfer by the phosphotransacetylase system. J Biol Chem. 1951 Jul;191(1):365–376. [PubMed] [Google Scholar]
- SUZUKI I., WERKMAN C. H. Chemoautotrophic carbon dioxide fixation by extracts of Thiobacillus thiooxidans. I. Formation of oxalacetic acid. Arch Biochem Biophys. 1958 Jul;76(1):103–111. doi: 10.1016/0003-9861(58)90124-3. [DOI] [PubMed] [Google Scholar]
- TCHEN T. T., VENNESLAND B. Enzymatic carbon dioxide fixation into oxal-acetate in wheat germ. J Biol Chem. 1955 Apr;213(2):533–546. [PubMed] [Google Scholar]
- UTTER M. F., KURAHASHI K. Mechanism of action of oxalacetic carboxylase. J Biol Chem. 1954 Apr;207(2):821–841. [PubMed] [Google Scholar]
- WORONICK C. L., JOHNSON M. J. Carbon dioxide fixation by cell-free extracts of Aspergillus niger. J Biol Chem. 1960 Jan;235:9–15. [PubMed] [Google Scholar]
- Wood H. G., Werkman C. H. The relationship of bacterial utilization of CO(2) to succinic acid formation. Biochem J. 1940 Feb;34(2):129–138. doi: 10.1042/bj0340129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood H. G., Werkman C. H. The utilisation of CO(2) in the dissimilation of glycerol by the propionic acid bacteria. Biochem J. 1936 Jan;30(1):48–53. doi: 10.1042/bj0300048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood H. G., Werkman C. H. The utilization of CO(2) by the propionic acid bacteria. Biochem J. 1938 Jul;32(7):1262–1271. doi: 10.1042/bj0321262. [DOI] [PMC free article] [PubMed] [Google Scholar]


