Abstract
Under pathophysiological conditions such as obesity, excessive oxidation of nutrients may induce mitochondrial stress, leading to mitochondrial unfolded protein response (UPRmt) and initiation of a retrograde stress signaling pathway. Defects in the UPRmt and the retrograde signaling pathways may disrupt the integrity and homeostasis of the mitochondria, resulting endoplasmic reticulum stress and insulin resistance. Improving the capacity of mitochondria to reduce stress may be an effective approach to improve mitochondria function and to suppress obesity-induced metabolic disorders such as insulin resistance and type 2 diabetes.
Keywords: insulin resistance, diabetes, mitochondria, ER stress, unfolded protein response
1. Introduction
Metabolic syndrome, which is characterized by a combination of several metabolic risk factors including abdominal obesity, insulin resistance, hypertension, and atherogenic dyslipidemia, is one of the most common health problems in the modern society. The causes of metabolic syndrome are complicated involving in interaction of various genetic and environmental components [1–3] and changes of life style [4]. However, the fundamental mechanisms underlying metabolic syndrome remain largely uncertain.
Metabolic homeostasis is largely dependent on mitochondria. Known as “cellular power plants” for most eukaryotic cells, mitochondria metabolize nutrients to produce the “energy currency” ATP for normal cell function. Changes in mitochondrial activity, which could be induced by genetic and external factors such as nutrients, hormones, temperature, exercise, hypoxia and aging, may have a great impact on various cellular activities such as growth, proliferation, and survival [5–8]. Mitochondrial dysfunction is central to many chronic diseases, ranging from major metabolic disorders such as obesity, insulin resistance, type 2 diabetes to cardiovascular disease, cancer and aging-associated neurodegenerative diseases [6, 8–11]. However, whether mitochondrial dysfunction is a cause or a consequence of these diseases remains elusive.
In this review, we have summarized our current understanding on the potential role of mitochondrial dysfunction in metabolic disorders, highlighting mitochondrial stress as a potential mechanism linking mitochondrial dysfunction to metabolic diseases.
2. Mitochondrial dysfunction is closely associated with metabolic disorders
As a cellular power plant, the central and most important function of mitochondria is the synthesis of ATP by oxidative phosphorylation (OXPHOS), a process by which mitochondria generate energy through oxidation of nutrients such as free fatty acids to create an electron chemical gradient across the mitochondrial inner membrane. This electron chemical gradient is used as a source of potential energy to generate ATP, transport substrates or ions, or produce heat [6]. Oxygen radicals, e.g. Reactive oxygen species (ROS), are also generated during OXPHOS as a toxic by-product in mitochondria, which may damage the mitochondrial and cellular DNA, proteins, lipids, and other molecules, leading to oxidative stress and mitochondrial dysfunction. Mitochondrial dysfunction has been shown to be associated with insulin resistance in various tissues including skeletal muscle, liver, fat, heart, and pancreas [12–17]. In skeletal muscle, decreased mitochondrial respiration capacity, reduced ATP production rate and increased ROS levels have been implicated in the pathogenesis of insulin resistance and type 2 diabetes [12, 18], although it remains to be established whether mitochondrial dysfunction is the consequence or the cause of insulin resistance [19, 20]. Impaired mitochondrial β-oxidation is found in patients with nonalcoholic fatty liver disease (NAFLD), a potential cause of hepatic steatosis and liver injury [21–23]. New evidence shows that hepatocyte mitochondrial dysfunction plays an important role in the early stages of liver fibrosis [24]. In adipose tissue, mitochondria provide key intermediates for the synthesis of triglycerides (TGs) and are critical for lipogenesis [17, 25]. Adipose mitochondria are also important for lipolysis through β-oxidation of fatty acids, which constitutes an important source of energy for ATP production to meet the energy requirement for normal cell function. Mitochondrial dysfunction is linked to reduced fatty acid oxidation and increased cytosolic free acid levels that result in insulin resistance and obesity/diabetes [17, 26, 27].
3. Quality control system in mitochondria
Under pathophysiological conditions such as obesity, nutrient overloading may lead to the accumulation of misfolded proteins in the endoplasmic reticulum (ER) causing ER stress. To maintain ER homeostasis, cells initiate an ER-specific unfolded protein response (UPRer) that alleviates ER stress through chaperon-mediated protein folding, protein quality control (QC), and misfolded protein degradation. Recent studies suggest that mitochondria have a similar unfolded protein response (UPRmt) and QC system to maintain the integrity of this organelle. The mitochondrial QC machinery is mainly composed of chaperones and proteases, which are up-regulated through a retrograde signaling pathway to the nucleus to degrade and remove damaged mitochondrial proteins maintaining mitochondrial homeostasis.
3.1. Molecular chaperons
A eukaryotic mitochondrion contains approximately 1,500 proteins [28]. About 98% of the mitochondrial proteins are encoded by nuclear genes, which are translocated from the cytosol to the mitochondrial matrix through the assistance of translocases using ATP and the membrane potential as energy sources. In the mitochondrial matrix, chaperones and auxiliary factors assist in proper folding and assembly of mitochondrial proteins into their native conformation [29].
Chaperone proteins have been initially identified by their ability to confer cellular resistance to various stress conditions. It has been reported that molecular chaperones participate in many other constitutive cellular processes in addition to assistance of newly synthesized proteins and degradation of misfolded and unfolded proteins [30–32]. The balance between unfolded/misfolded “client” protein levels and available chaperones determines the homeostasis in various cellular compartments. Increased levels of unfolded/misfolded protein initiate signal pathways and appropriately activation of nuclear chaperon genes targeted to the stressed organelles to restore homeostasis [33]. Mitochondria contain members of the major chaperone family, the heat-shock proteins (HSPs) family, that have important functions in maintaining mitochondrial function.
In eukaryotes, HSP 70 and 60 are the two major mitochondrial matrix chaperones activated by cellular stressors. HSP70 is a member of 70 kDa molecular chaperone family characterized by a conserved N-terminal ATPase domain and C-terminal substrate-binding domain [32]. In mitochondria, mitochondrial HSP70 (mtHsp70; Ssc1 in yeast, HSP 6 in C elegans) binds to newly imported and fully translocated polypeptides to assist their initial folding [34]. HSP70 has been shown to play important roles in protein synthesis, folding and the delivery of misfolded proteins to proteolytic enzymes in the mitochondrial matrix [32].
HSP60, also called chaperonin 60 (Cpn 60; GroEL in bacteria), is a member of the approximately 60 kDa molecular chaperone family. HSP60 proteins form large double-ring complexes with a central cavity that provides a protective environment for aggregation-prone polypeptides [32]. In the mitochondrial matrix, mitochondrial HSP60 (mtHSP60), which interacts with cofactors Hsp10 (or Cpn10; GroES in bacteria) to form large cage-like structures, interacts with partially folded polypeptides released from HSP70 to facilitate their further folding [34]. Although mtHSP60 and mtHSP70 act differently in mechanism, both of these chaperons promote the folding process through cycles of substrate binding and release controlled by their ATPase activity and by cofactor proteins in an ATP-dependent manner.
3.2. Proteases
There are two major ATP-dependent proteases in the mitochondrial matrix, Lon (Pim1 in yeast) and ClpXP. Lon is a serine protease that eliminates denatured or oxidatively damaged proteins in the matrix [35]. Down-regulation of Lon is associated with impairment of mitochondrial functions and cell death [36]. Decreased Lon is also linked to cellular aging since age-dependent progressive decline of Lon activity is accompanied by the accumulation of electron-dense aggregates in mitochondria [36]. Although the molecular basis of substrate recognition has not been well defined, it is proposed that Lon recognizes stably folded domains and selectively degrades misfolded and oxidized proteins [34]. In human cells Lon has been shown to initiate proteolysis at solvent-exposed sites of a misfolded protein [37].
The ATP-dependent protease ClpXP is a double-donut like ring complex formed by the interaction of the ClpX subunit with the ClpP proteolytic subunit in the presence of ATP [38]. In bacterial cells ClpP is involved in the proteolysis of diverse substrates, including transcription factors, metabolic enzymes, and proteins involved in the starvation and oxidative stress responses [39] and in C elegans, ClpP is involved in mitochondrial stress signaling [40], which will be discussed in detail below. Mitochondria also have two membrane-embedded metalloprotease complexes, namely m- and i-AAA proteases, which play important roles in mitochondrial QC [34]. The cooperation of chaperones and proteases in mitochondrial matrix is critical for maintaining mitochondrial protein homeostasis and function. Alterations of the cellular levels of these chaperone and protease and their interaction are likely to be associated with the mitochondrial dysfunction and onset of diseases.
4. Mitochondrial stress and unfolded protein response
Metabolic stimuli and other changes within mitochondria can result in broad changes in nuclear gene expression via retrograde mitochondrial to nuclear signaling. These responses are generally referred to as mitochondrial stress responses. Early studies found that mitochondrial stress is caused by altered mitochondrial membrane potential or uncoupling of OXPHOS [41]. Later studies show that accumulation of unfolded proteins in the organelle also triggers UPRmt [42–44]. A number of manipulations are shown to induce mitochondrial stress, including mtDNA mutation, heat, ethidium bromide treatment, electron transport chain (ETC) protein mutation or uncoupling of OXPHOS, and physiological stimuli-induced accumulation of ROS, all of which can either disrupt membrane potential or increase the load of misfolded proteins, leading to dysregulation of mitochondrial homeostasis and function.
Unlike ER stress, the stress signaling pathways in mitochondria has not been well characterized. However, there is evidence suggesting that the mitochondria-nucleus communication may play a key role in response to mitochondrial stress. Similar to the stress response in the ER, unfolded protein accumulation in mitochondria activates a signaling pathway defined as UPRmt, by which changes of the folding environment in the mitochondrial lumen selectively upregulate the expression of nuclear-encoded mitochondrial chaperones and proteases [40, 45–47]. The communication from mitochondria to the nucleus, known as mitochondrial retrograde signaling, facilitates the reestablishment of homeostasis by refolding or removing unfolded or misfolded proteins by the upregulation of mitochondrial chaperons and proteases.
By genomic RNAi screens, several important nuclear genes encoding UPRmt components have been identified including the putative transcription factor DVE-1, an ubiquitin-like protein UBL-5, a homologue of the E. coli ClpP protease CLPP-1, a putative ABC transporter HAF-1, and a bZIP transcription factor ZC376.7 [33, 40, 46]. Knockdown of either clpp-1 or haf-1 in C elegans aborted ATP-dependent release of peptides in mitochondria [40, 44, 47] suggesting that these proteins play an important role in regulating the initial step of the UPRmt pathway. Consistent with this, CLPP-1 has been found to localize at the mitochondrial matrix and degrade soluble proteins in an ATP-dependent manner [38, 40, 48]. Repressing the proteolytic activity of CLPP-1 disrupts UPRmt signaling in C elegens [40]. HAF-1 is also mitochondrial localized and potentially involved in peptide efflux. The sequence of HAF-1 is similar to that of the yeast ABC transporter Mdl1 [49], which functions as a transporter of degraded mitochondrial peptides in yeast. It is proposed that HAF-1 could also have a similar role in UPRmt [40, 49].
In mammalian cells, the signal pathway of UPRmt is largely unknown. Mitochondrial stress was first observed in rat hepatoma cells treated with ethidium bromide, which led to mtDNA depletion and selective upregulation of mitochondrial chaperones (mtHSP70 and mtHSP60) at both the transcript and protein levels [44, 50]. A number of genes encoding UPRmt-responsive proteins have been identified in mammalian cells including chaperones mtHSP60, mtHSP10, mitochondrial isoform of DnaJ (mtDnaJ), mitochondrial processing peptidase β subunit (MPPβ),and Trx 2 (mitochondrial thioredoxin), protease (ClpP, YME1L1), and other proteins including C/EBP homologous protein (CHOP), protein import component Tim17A, End G (Endonuclease G), NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 2 (NDUFB2), Cytochrome C reductase [43, 51].
The transcription factor CHOP plays a central role in UPRmt in mammalian cells [43, 44, 52]. CHOP regulates a number of genes in UPRmt including mtHSP60, mtHSP10, mtDnaJ, and ClpP [43, 53], all of which contain a CHOP element (GG/ATTGCA) in their promoters. Interestingly, genes encoding mitochondrial proteins that contain no CHOP element are not up-regulated in UPRmt, suggesting a CHOP element maybe required for mitochondrial stress signaling [51, 52]. However, genes encoding cytosolic proteins with CHOP elements are not induced by the accumulation of unfolded proteins in mitochondria, indicating CHOP element is not sufficient for the regulation of UPRmt [51]. Further analysis of the promoters of UPRmt responsive genes discovered at least two highly conserved and putative regulatory sites on the up- and down-stream of the CHOP element, namely mitochondrial unfolded protein response element 1 (MURE1) and MURE2 [51]. Mutation of each of these elements showed substantial reduction in the UPRmt responsiveness of the promoters, indicating that a highly conserved region containing the MURE1, CHOP and MURE2 sites is critical for the regulation of genes involved in mitochondrial QC. However, the transcriptional factor(s) that binds to this conserved region and regulates UPRmt gene expression is currently unknown.
A two-stage process has been proposed for CHOP-mediated UPRmt. In the first stage, accumulation of unfolded proteins initiates CHOP gene expression via an unidentified signaling pathway. In the second stage, CHOP dimerizes with its partner CAAT enhancer-binding protein β (C/EBPβ), and subsequently binds to promoters of CHOP element-containing UPRmt-responsive genes. The formation of the CHOP/C/EBPβ heterodimer is essential for transcriptional activation of chaperone and protease genes involved in mitochondrial QC [43, 44]. It should be pointed out that CHOP is also up-regulated under the condition of ER stress [54–56]. After dimerization with its partner CAAT enhancer-binding protein α (C/EBPα), the CHOP/C/EBPα dimer binds to the conserved binding site (TGCAAT) within the first intron of bim genes, resulting in bim up-regulation and subsequent initiation of apoptosis [57]. The interaction of CHOP with different partners appears to play distinct roles in mitochondrial and ER stress. However, the mechanisms underlying the specific interaction of CHOP with its distinct partners remain unknown.
In addition to ER and mitochondrial stress, CHOP can also be activated by a range of other cytotoxic stimuli, including amino acid starvation, γ- irradiation or treatment with DNA damaging drugs [58]. These findings raise an interesting question as to how CHOP gene expression is induced under distinct stress conditions. Horibe and Hoogenraad [52] found that the chop promoter containing an mtUPR response activator protein-1 (AP-1) binding element, which lies alongside an ER stress element (ERSE) through which chop transcription is activated in response to UPRer. Since the AP-1 site is bound with c-Jun that is activated by the c-Jun N-terminal kinase (JNK) [59], it is possible that the JNK-AP1 pathway and inflammation are involved in mitochondrial stress. Consistent with this, activation of JNK-dependent transcription factor 2 (ATF2) has been shown to transduce mitochondrial signals to the nucleus [60]. Accumulated evidence implicated that CHOP is involved in ER stress-induced apoptosis [56, 61]. However, it is currently unclear whether mitochondrial stress also induces apoptosis.
Mitochondrial stress not only decreases mitochondrial membrane potential- and electron transport-coupled ATP synthesis, but also elevates steady-state cytosolic Ca2+ levels and promotes the expression of genes involved in Ca2+ transport and storage, as well as activation of the Ca2+-responsive factor calineurin [41, 62, 63]. Mitochondrial stress-induced alteration of Ca2+ homeostasis and nuclear gene expression patterns has also been implicated in tumorigenesis and invasion [42, 64]. mtDNA depletion in lung tumor cells induced by mitochondrial stress enhances the activity of AMP-activated protein kinase (AMPK), hexokinase (HK) and phosphoenolpyruvate carboxykinase (PEPCK) [42], the latter two are important regulators of the glycolysis and gluconeogenesis pathways, respectively. However, whether mitochondrial stress is involved in the onset of the metabolic diseases remains to be established.
The changes of protein expressions and disruption of the mitochondrial stress response signaling by environmental factors, such as nutrition, are likely to induce mitochondrial function changes associated with metabolic dysregulation. Studies have found that the expression levels of HSP60, an important marker of mitochondrial stress, are significantly suppressed in rats under high fat diet (HFD) conditions, accompanied by reduced expression levels of cytochrome c and peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), decreased mtDNA copy numbers and impaired glucose tolerance [65]. Similar results are also found in adipose tissue of ob/ob [66] and db/db [27] mice. On the other hand, treatment of ob/ob mice with rosiglitazone, an insulin sensitizer, reverses these effects and induces the expression levels of HSP60 and HSP70 [66]. A preliminary epidemiological study demonstrates that lower blood HSP60 level is associated with increased risk of type 2 diabetes [67]. Exercise training with dietary counseling increases mitochondrial HSP60 and glucose-regulated protein 75 (GRP75) expression in skeletal muscle in middle-aged subjects with impaired glucose tolerance [68], suggesting a protective role of HSP60, or possibly the UPRmt in glucose metabolism and antioxidative capacity. In addition to metabolic dysregulation, mitochondrial stress and/or impairment in the UPRmt pathway such as reducing the expression levels or activities of mitochondrial chaperones has also been implicated in neurological diseases and sporadic diseases of aging [32, 69–71].
5. Cross-talk between mitochondria and ER during stress
Mitochondria and ER form a delicate network connection that is fundamental for the maintenance of cellular homeostasis and survival. In addition to a structural connection [72], there is a direct transfer of lipids between the ER and mitochondria [73]. Furthermore, there is dynamic exchange of Ca2+ ions between these two organelles, which regulates processes such as ER chaperone-assisted folding of newly synthesized proteins, regulation of mitochondria-localized dehydrogenases involved in ATP-producing Krebs cycle, and the activation of Ca2+-dependent enzymes that execute cell death programs [74].
While ER and mitochondria are subjected to distinct regulation in response to stress, these two organelles are connected at multiple levels during the stress response. First, the regulation of certain nuclear genes encoding mitochondrial proteins appears to be influenced by ER stress. In cultured rat astrocytes, hypoxia-induced ER stress triggers the expression of mitochondrial ATP-dependent proteases Yme1 and Lon, leading to the transmission of cell stress from ER to mitochondria [75]. This ER-mitochondria connection is dependent on the ER-resident protein kinase PERK since ER stress-induced Lon or GRP75/mtHSP70 expression is inhibited in PERK−/− cells [75, 76]. In Drosophila Schneider cells, Lon regulates mitochondrial transcription by stabilizing the ratio of mitochondrial transcription factor A (TFAM): mtDNA via selective degradation of TFAM [77]. ER stress may also regulate mitochondrial function by reducing the steady-state levels of nuclear encoded subunits of OXPHOS complexes and mtDNA numbers via inducing mitochondrial-resident proteases [75].
Protein translocation between ER and mitochondria provide another mechanism by which ER and mitochondria communicate during stress. One of such transporting proteins is GRP78, also known as BiP (immunoglobulin heavy-chain-binding protein). As a Ca2+-binding molecular chaperone in the ER, GRP78 controls UPRer signaling by binding to three ER membrane-associated proteins PERK, IRE1, and ATF6 [78] and suppresses stress-induced apoptosis [79, 80]. Inducing ER stress by treatments with calcium ionophore A23187 or ER Ca2+-ATPase inhibitor thapsigargin leads to GRP78 retargeted to mitochondria [81]. This mitochondrial relocalization of GRP78 might be due to its ability to modulate Ca2+ buffering store with sigma-1 receptor (Sig-1R), the later is an important ER chaperone that regulates Ca2+ signaling between ER and mitochondria and undergoes chronic ER stress-induced mitochondrial translocation [82]. The dissociation of Sig-1R from GRP78/BiP upon ER Ca2+ depletion or ligand stimulation leads to a prolonged Ca2+ signaling into mitochondria. Increased Sig-1R expression in cells counteracts with ER stress response, whereas decreased expression levels of this protein associates with apoptosis [82]. These ER chaperones are important not only for sensing ER Ca2+ concentrations and regulating ER-mitochondrial inter-organelle Ca2+ signaling and cell survival, but are also critical for correlating UPR signaling between these two organelles.
While numerous studies have demonstrated a close biochemical and physiological connection between ER and mitochondria, an interesting question remains to be answered is the cause-and-effect relationship between ER and mitochondrial stress. Increased cytosolic Ca2+ concentration caused by ER Ca2+ mobilization leads to overload of mitochondrial Ca2+, which in turn causes the release of cytochrome c from the intermembrane space of mitochondria into the cytosol [83, 84]. While these data suggest that ER dysfunction may have a causative effect on mitochondrial stress, other studies suggest mitochondrial dysfunction may have a contributing role in ER stress. Given the requirement for ATP in ER chaperone function and activation, the initiation and continuation of the UPRer process are likely to be influenced by the cellular energy status and thus mitochondrial function. Consistent with this, a recent study showed that RNAi-mediated suppression of adenylate kinase 2 (AK2), a mitochondrial enzyme that regulates adenine nucleotide inter-conversion within the intermembrane space, decreased UPRer-associated gene expression in 3T3-L1 cells, probably owing to impaired mitochondrial energy metabolism and biogenesis [85]. Therefore, it is conceivable that mitochondrial dysfunction, such as insufficient ATP supply from mitochondria, will have a significant impact on ER function such as protein folding, modification and assembly, leading to UPRer. In support of this, reducing mitochondrial function by chemicals has been shown to increase the levels of ER stress markers such as eIF2α phosphorylations and XBP-1 splicing [86]. Furthermore, in parallel to mitochondrial dysfunction as demonstrated by outer mitochondrial membrane translocation of Bax/Bak, increased cytochrome c release, loss of membrane potential, and caspase-9 activation, there is an increased ER stress-induced apoptosis [87]. In addition, inhibition of mitochondrial permeability and deletion of APAF1 or Bax/Bak strongly decrease ER stress-induced cell death [87], further highlighting the intimate connection between ER and mitochondria.
ER-associated protein degradation (ERAD), which specifically target unfolded and misfolded proteins from the ER to the cytosol for ubiquitin-proteasome-mediated degradation, is a well-established mechanism for ER QC [56, 78, 88, 89]. A recent study suggests there is a similar mechanism in mitochondria. Upon mitochondrial stress, Vms1, a VCP/Cdc48-associated mitochondrial stress-responsive protein, is translocated from the cytosol to mitochondria [90]. This translocation triggers Vms1-dependent mitochondrial localization of Cdc48, a component of the ubiquitin/proteasome system with a well-defined role in ERAD. Loss-of-function analysis shows that Vms1 is critical for ubiquitin-dependent mitochondrial protein degradation, mitochondrial respiratory function and cell viability [90]. Thus, by recruiting components in the ERAD pathway to the stressed mitochondria, a mitochondria-associated protein degradation (MAD) pathway is initiated to remove damaged mitochondrial components, thus preventing mitochondria from stress-induced damage [91].
Finally, intracellular levels of Ca2+ appear to play an important role in the functional link between mitochondria and ER under stress. ER is the major place for Ca2+ storage in cells. ER stress has been shown to trigger Ca2+ release from the ER, which could be subsequently taken up by mitochondria [72]. The efficiency of this process depends on the proximity between ER and mitochondria, which is facilitated by the dynamin-like GTPase mitofusin 2 [92]. Ca2+ may play a dual role in mediating ER stress-induced cellular events. First, the increase of cytosolic Ca2+ concentration triggers autophagy through Ca2+/calmodulin-dependent protein kinase kinase-β (CaMKKβ)-mediated activation of AMPK pathway, leading to the inhibition of mammalian target of rapamycin signaling complex 1 (mTORC1) [93]. In addition, prolonged ER stress conditions can cause a slow but sustained increase of free Ca2+ in the mitochondrial matrix, which may induce the pro-apoptotic mitochondrial membrane permeabilzation [87]. Taken together, these results suggest another mechanism underlying the interplay between ER and mitochondria during stress.
6. Conclusion remarks: Is mitochondrial stress a bridge between mitochondrial dysfunction and insulin resistance?
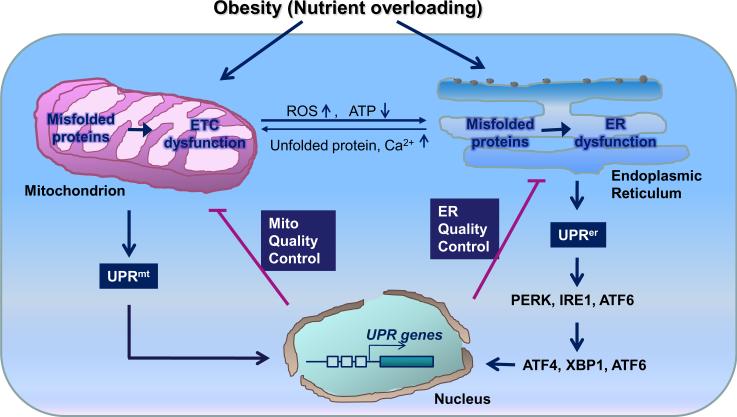
Mitochondria are vital for cell function and survival, and it is not surprising that the integrity of this organelle is safeguarded by various QC mechanisms such as UPRmt and MAD. Like UPRer, UPRmt senses perturbations of protein homeostasis in mitochondria and, in turn, activates genes that encoding QC proteins such as mitochondrial chaperons and proteases, leading to enhanced mitochondrial protein-handling capacity and protein homeostasis [40, 45–47]. The ultimate outcome of this protective response to stress is to reestablish normal function of the organelles and restore cellular homeostasis [94] (Fig. 1).
Fig. 1.
Great progresses have been made recently on our understanding of the role of mitochondrial stress in mitochondrial dysfunction and metabolic diseases such as obesity, insulin resistance, and type 2 diabetes. A growing list of studies have demonstrated the importance of the UPRmt, QC and MAD machineries in the maintenance of mitochondrial integrity and homeostasis suggesting a functional link between mitochondria and ER under physiological and stress conditions. However, a number of important questions remain to be answered. For example, is mitochondrial dysfunction a contributing factor or a consequence of metabolic diseases? In addition, what are the players involved in UPRmt and MAD? Finally, how mitochondria and ER are functionally linked underlying physiological and pathophysiological conditions such as ER and mitochondrial stress? Answers to these questions should yield new information on the potential mechanisms underlying obesity-induced insulin resistance and provide insight to the development of therapeutic treatment of metabolic diseases such as insulin resistance and type 2 diabetes.
Abbreviations
- AMPK
AMP-activated protein kinase
- CaMKKβ
Ca2+/calmodulin-dependent protein kinase kinase-β
- C/EBPβ
CAAT enhancer-binding protein β
- CHOP
C/EBP homologous protein
- ER
endoplasmic reticulum
- ERAD
ER-associated protein degradation
- ERSE
ER stress element
- ETC
electron transport chain
- GRP75
glucose-regulated protein 75
- HFD
high fat diet
- HSPs
heat-shock proteins
- JNK
c-Jun N-terminal kinase
- MAD
mitochondria-associated protein degradation
- mtHSP60
mitochondrial HSP60
- mtHsp70
mitochondrial HSP70
- mTORC1
mammalian target of rapamycin signaling complex 1
- MURE1
Mitochondrial Unfolded Protein Response Element 1
- MURE2
Mitochondrial Unfolded Protein Response Element 2
- NAFLD
nonalcoholic fatty liver disease
- NDUFB2
NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 2
- OXPHOS
oxidative phosphorylation
- PEPCK
phosphoenolpyruvate carboxykinase
- PGC-1α
peroxisome proliferator-activated receptor-γ coactivator-1α
- QC
quality control
- ROS
Reactive oxygen species
- TGs
triglycerides
- UPRer
ER-specific unfolded protein response
- UPRmt
mitochondrial unfolded protein response
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Pollex RL, Hegele RA. Nat Clin Pract Cardiovasc Med. 2006;3:482–489. doi: 10.1038/ncpcardio0638. [DOI] [PubMed] [Google Scholar]
- [2].Hinney A, Hebebrand J. Obes Facts. 2008;1:35–42. doi: 10.1159/000113935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hebebrand J, Hinney A. Child Adolesc Psychiatr Clin N Am. 2009;18:83–94. doi: 10.1016/j.chc.2008.07.006. [DOI] [PubMed] [Google Scholar]
- [4].Hamilton MT, Hamilton DG, Zderic TW. Diabetes. 2007;56:2655–2667. doi: 10.2337/db07-0882. [DOI] [PubMed] [Google Scholar]
- [5].Ritz P, Berrut G. Diabetes Metab. 2005;31(Spec No 2):5S–67S. doi: 10.1016/s1262-3636(05)73654-5. [DOI] [PubMed] [Google Scholar]
- [6].Wallace DC. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Frisard M, Ravussin E. Endocrine. 2006;29:27–32. doi: 10.1385/ENDO:29:1:27. [DOI] [PubMed] [Google Scholar]
- [8].Kim JA, Wei Y, Sowers JR. Circ Res. 2008;102:401–414. doi: 10.1161/CIRCRESAHA.107.165472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lesnefsky EJ, Moghaddas S, Tandler B, Kerner J, Hoppel CL. J Mol Cell Cardiol. 2001;33:1065–1089. doi: 10.1006/jmcc.2001.1378. [DOI] [PubMed] [Google Scholar]
- [10].Henze K, Martin W. Nature. 2003;426:127–128. doi: 10.1038/426127a. [DOI] [PubMed] [Google Scholar]
- [11].McBride HM, Neuspiel M, Wasiak S. Curr Biol. 2006;16:R551–R560. doi: 10.1016/j.cub.2006.06.054. [DOI] [PubMed] [Google Scholar]
- [12].Kelley DE, He J, Menshikova EV, Ritov VB. Diabetes. 2002;51:2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- [13].Wiederkehr A, Wollheim CB. Endocrinology. 2006;147:2643–2649. doi: 10.1210/en.2006-0057. [DOI] [PubMed] [Google Scholar]
- [14].Hojlund K, Mogensen M, Sahlin K, Beck-Nielsen H. Endocrinol Metab Clin North Am. 2008;37:713–731. doi: 10.1016/j.ecl.2008.06.006. [DOI] [PubMed] [Google Scholar]
- [15].Ashrafian H, Frenneaux MP, Opie LH. Circulation. 2007;116:434–448. doi: 10.1161/CIRCULATIONAHA.107.702795. [DOI] [PubMed] [Google Scholar]
- [16].Abdul-Ghani MA, DeFronzo RA. Curr Diab Rep. 2008;8:173–178. doi: 10.1007/s11892-008-0030-1. [DOI] [PubMed] [Google Scholar]
- [17].De Pauw A, Tejerina S, Raes M, Keijer J, Arnould T. Am J Pathol. 2009;175:927–939. doi: 10.2353/ajpath.2009.081155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, Nair KS. Proc Natl Acad Sci U S A. 2005;102:5618–5623. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dumas JF, Simard G, Flamment M, Ducluzeau PH, Ritz P. Diabetes Metab. 2009;35:159–167. doi: 10.1016/j.diabet.2009.02.002. [DOI] [PubMed] [Google Scholar]
- [20].Pagel-Langenickel I, Bao J, Pang L, Sack MN. Endocr Rev. 2010;31:25–51. doi: 10.1210/er.2009-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fromenty B, Pessayre D. Pharmacol Ther. 1995;67:101–154. doi: 10.1016/0163-7258(95)00012-6. [DOI] [PubMed] [Google Scholar]
- [22].Perez-Carreras M, Del HP, Martin MA, Rubio JC, Martin A, Castellano G, Colina F, Arenas J, Solis-Herruzo JA. Hepatology. 2003;38:999–1007. doi: 10.1053/jhep.2003.50398. [DOI] [PubMed] [Google Scholar]
- [23].Pessayre D, Fromenty B. J Hepatol. 2005;42:928–940. doi: 10.1016/j.jhep.2005.03.004. [DOI] [PubMed] [Google Scholar]
- [24].Mitchell C, Robin MA, Mayeuf A, Mahrouf-Yorgov M, Mansouri A, Hamard M, Couton D, Fromenty B, Gilgenkrantz H. Am J Pathol. 2009;175:1929–1937. doi: 10.2353/ajpath.2009.090332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rossmeisl M, Syrovy I, Baumruk F, Flachs P, Janovska P, Kopecky J. Faseb J. 2000;14:1793–1800. doi: 10.1096/fj.99-0965com. [DOI] [PubMed] [Google Scholar]
- [26].Lowell BB, Shulman GI. Science. 2005;307:384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- [27].Rong JX, Qiu Y, Hansen MK, Zhu L, Zhang V, Xie M, Okamoto Y, Mattie MD, Higashiyama H, Asano S, Strum JC, Ryan TE. Diabetes. 2007;56:1751–1760. doi: 10.2337/db06-1135. [DOI] [PubMed] [Google Scholar]
- [28].Calvo S, Jain M, Xie X, Sheth SA, Chang B, Goldberger OA, Spinazzola A, Zeviani M, Carr SA, Mootha VK. Nat Genet. 2006;38:576–582. doi: 10.1038/ng1776. [DOI] [PubMed] [Google Scholar]
- [29].Neupert W, Herrmann JM. Annu Rev Biochem. 2007;76:723–749. doi: 10.1146/annurev.biochem.76.052705.163409. [DOI] [PubMed] [Google Scholar]
- [30].Bukau B, Horwich AL. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- [31].Horwich AL, Weber-Ban EU, Finley D. Proc Natl Acad Sci U S A. 1999;96:11033–11040. doi: 10.1073/pnas.96.20.11033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hartl FU, Hayer-Hartl M. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- [33].Benedetti C, Haynes CM, Yang Y, Harding HP, Ron D. Genetics. 2006;174:229–239. doi: 10.1534/genetics.106.061580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tatsuta T. J Biochem. 2009;146:455–461. doi: 10.1093/jb/mvp122. [DOI] [PubMed] [Google Scholar]
- [35].Ngo JK, Davies KJ. Ann N Y Acad Sci. 2007;1119:78–87. doi: 10.1196/annals.1404.015. [DOI] [PubMed] [Google Scholar]
- [36].Bota DA, Ngo JK, Davies KJ. Free Radic Biol Med. 2005;38:665–677. doi: 10.1016/j.freeradbiomed.2004.11.017. [DOI] [PubMed] [Google Scholar]
- [37].Ondrovicova G, Liu T, Singh K, Tian B, Li H, Gakh O, Perecko D, Janata J, Granot Z, Orly J, Kutejova E, Suzuki CK. J Biol Chem. 2005;280:25103–25110. doi: 10.1074/jbc.M502796200. [DOI] [PubMed] [Google Scholar]
- [38].Kang SG, Dimitrova MN, Ortega J, Ginsburg A, Maurizi MR. J Biol Chem. 2005;280:35424–35432. doi: 10.1074/jbc.M507240200. [DOI] [PubMed] [Google Scholar]
- [39].Flynn JM, Neher SB, Kim YI, Sauer RT, Baker TA. Mol Cell. 2003;11:671–683. doi: 10.1016/s1097-2765(03)00060-1. [DOI] [PubMed] [Google Scholar]
- [40].Haynes CM, Petrova K, Benedetti C, Yang Y, Ron D. Dev Cell. 2007;13:467–480. doi: 10.1016/j.devcel.2007.07.016. [DOI] [PubMed] [Google Scholar]
- [41].Biswas G, Adebanjo OA, Freedman BD, Anandatheerthavarada HK, Vijayasarathy C, Zaidi M, Kotlikoff M, Avadhani NG. Embo J. 1999;18:522–533. doi: 10.1093/emboj/18.3.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Amuthan G, Biswas G, Ananadatheerthavarada HK, Vijayasarathy C, Shephard HM, Avadhani NG. Oncogene. 2002;21:7839–7849. doi: 10.1038/sj.onc.1205983. [DOI] [PubMed] [Google Scholar]
- [43].Zhao Q, Wang J, Levichkin IV, Stasinopoulos S, Ryan MT, Hoogenraad NJ. Embo J. 2002;21:4411–4419. doi: 10.1093/emboj/cdf445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ryan MT, Hoogenraad NJ. Annu Rev Biochem. 2007;76:701–722. doi: 10.1146/annurev.biochem.76.052305.091720. [DOI] [PubMed] [Google Scholar]
- [45].Yoneda T, Benedetti C, Urano F, Clark SG, Harding HP, Ron D. J Cell Sci. 2004;117:4055–4066. doi: 10.1242/jcs.01275. [DOI] [PubMed] [Google Scholar]
- [46].Haynes CM, Ron D. J Cell Sci. 2010;123:3849–3855. doi: 10.1242/jcs.075119. [DOI] [PubMed] [Google Scholar]
- [47].Broadley SA, Hartl FU. Trends Cell Biol. 2008;18:1–4. doi: 10.1016/j.tcb.2007.11.003. [DOI] [PubMed] [Google Scholar]
- [48].Gottesman S. Annu Rev Cell Dev Biol. 2003;19:565–587. doi: 10.1146/annurev.cellbio.19.110701.153228. [DOI] [PubMed] [Google Scholar]
- [49].Young L, Leonhard K, Tatsuta T, Trowsdale J, Langer T. Science. 2001;291:2135–2138. doi: 10.1126/science.1056957. [DOI] [PubMed] [Google Scholar]
- [50].Martinus RD, Garth GP, Webster TL, Cartwright P, Naylor DJ, Hoj PB, Hoogenraad NJ. Eur J Biochem. 1996;240:98–103. doi: 10.1111/j.1432-1033.1996.0098h.x. [DOI] [PubMed] [Google Scholar]
- [51].Aldridge JE, Horibe T, Hoogenraad NJ. PLoS One. 2007;2:e874. doi: 10.1371/journal.pone.0000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Horibe T, Hoogenraad NJ. PLoS One. 2007;2:e835. doi: 10.1371/journal.pone.0000835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ryan MT, Herd SM, Sberna G, Samuel MM, Hoogenraad NJ, Hoj PB. Gene. 1997;196:9–17. doi: 10.1016/s0378-1119(97)00111-x. [DOI] [PubMed] [Google Scholar]
- [54].Ron D, Habener JF. Genes Dev. 1992;6:439–453. doi: 10.1101/gad.6.3.439. [DOI] [PubMed] [Google Scholar]
- [55].Fawcett TW, Eastman HB, Martindale JL, Holbrook NJ. J Biol Chem. 1996;271:14285–14289. doi: 10.1074/jbc.271.24.14285. [DOI] [PubMed] [Google Scholar]
- [56].Ron D, Walter P. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- [57].Puthalakath H, O'Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, Gotoh T, Akira S, Bouillet P, Strasser A. Cell. 2007;129:1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- [58].Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D. Genes Dev. 2004;18:3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Weiss C, Schneider S, Wagner EF, Zhang X, Seto E, Bohmann D. Embo J. 2003;22:3686–3695. doi: 10.1093/emboj/cdg364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Butow RA, Avadhani NG. Mol Cell. 2004;14:1–15. doi: 10.1016/s1097-2765(04)00179-0. [DOI] [PubMed] [Google Scholar]
- [61].Spear E, Ng DT. Traffic. 2001;2:515–523. doi: 10.1034/j.1600-0854.2001.20801.x. [DOI] [PubMed] [Google Scholar]
- [62].Goffart S, Wiesner RJ. Exp Physiol. 2003;88:33–40. doi: 10.1113/eph8802500. [DOI] [PubMed] [Google Scholar]
- [63].Kelly DP, Scarpulla RC. Genes Dev. 2004;18:357–368. doi: 10.1101/gad.1177604. [DOI] [PubMed] [Google Scholar]
- [64].Li CH, Cheng YW, Liao PL, Yang YT, Kang JJ. Toxicol Sci. 2010;116:140–150. doi: 10.1093/toxsci/kfq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Sutherland LN, Bomhof MR, Capozzi LC, Basaraba SA, Wright DC. J Physiol. 2009;587:1607–1617. doi: 10.1113/jphysiol.2008.165464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Wilson-Fritch L, Nicoloro S, Chouinard M, Lazar MA, Chui PC, Leszyk J, Straubhaar J, Czech MP, Corvera S. J Clin Invest. 2004;114:1281–1289. doi: 10.1172/JCI21752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Imatoh T, Sugie T, Miyazaki M, Tanihara S, Baba M, Momose Y, Uryu Y, Une H. Diabetes Res Clin Pract. 2009;85:208–212. doi: 10.1016/j.diabres.2009.06.004. [DOI] [PubMed] [Google Scholar]
- [68].Venojarvi M, Aunola S, Puhke R, Marniemi J, Hamalainen H, Halonen JP, Lindstrom J, Rastas M, Hallsten K, Nuutila P, Hanninen O, Atalay M. BMC Endocr Disord. 2008;8:3. doi: 10.1186/1472-6823-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Lindholm E, Klannemark M, Agardh E, Groop L, Agardh CD. J Diabetes Complications. 2004;18:103–107. doi: 10.1016/S1056-8727(03)00019-9. [DOI] [PubMed] [Google Scholar]
- [70].Beal MF. Ann Neurol. 2005;58:495–505. doi: 10.1002/ana.20624. [DOI] [PubMed] [Google Scholar]
- [71].Durieux J, Wolff S, Dillin A. Cell. 2011;144:79–91. doi: 10.1016/j.cell.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Giorgi C, De Stefani D, Bononi A, Rizzuto R, Pinton P. Int J Biochem Cell Biol. 2009;41:1817–1827. doi: 10.1016/j.biocel.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Beller M, Thiel K, Thul PJ, Jackle H. Febs Lett. 2010;584:2176–2182. doi: 10.1016/j.febslet.2010.03.022. [DOI] [PubMed] [Google Scholar]
- [74].Franzini-Armstrong C. Physiology (Bethesda) 2007;22:261–268. doi: 10.1152/physiol.00017.2007. [DOI] [PubMed] [Google Scholar]
- [75].Hori O, Ichinoda F, Tamatani T, Yamaguchi A, Sato N, Ozawa K, Kitao Y, Miyazaki M, Harding HP, Ron D, Tohyama M, M SD, Ogawa S. J Cell Biol. 2002;157:1151–1160. doi: 10.1083/jcb.200108103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Harding HP, Zhang Y, Ron D. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- [77].Matsushima Y, Goto Y, Kaguni LS. Proc Natl Acad Sci U S A. 2010;107:18410–18415. doi: 10.1073/pnas.1008924107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Yoshida H. FEBS J. 2007;274:630–658. doi: 10.1111/j.1742-4658.2007.05639.x. [DOI] [PubMed] [Google Scholar]
- [79].Lievremont JP, Rizzuto R, Hendershot L, Meldolesi J. J Biol Chem. 1997;272:30873–30879. doi: 10.1074/jbc.272.49.30873. [DOI] [PubMed] [Google Scholar]
- [80].Reddy RK, Mao C, Baumeister P, Austin RC, Kaufman RJ, Lee AS. J Biol Chem. 2003;278:20915–20924. doi: 10.1074/jbc.M212328200. [DOI] [PubMed] [Google Scholar]
- [81].Sun FC, Wei S, Li CW, Chang YS, Chao CC, Lai YK. Biochem J. 2006;396:31–39. doi: 10.1042/BJ20051916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Hayashi T, Su TP. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- [83].Strasser A, O'Connor L, Dixit VM. Annu Rev Biochem. 2000;69:217–245. doi: 10.1146/annurev.biochem.69.1.217. [DOI] [PubMed] [Google Scholar]
- [84].Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R. Oncogene. 2008;27:6407–6418. doi: 10.1038/onc.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Burkart A, Shi X, Chouinard M, Corvera S. J Biol Chem. 2011;286:4081–4089. doi: 10.1074/jbc.M110.134106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Koh EH, Park JY, Park HS, Jeon MJ, Ryu JW, Kim M, Kim SY, Kim MS, Kim SW, Park IS, Youn JH, Lee KU. Diabetes. 2007;56:2973–2981. doi: 10.2337/db07-0510. [DOI] [PubMed] [Google Scholar]
- [87].Deniaud A, Sharaf EDO, Maillier E, Poncet D, Kroemer G, Lemaire C, Brenner C. Oncogene. 2008;27:285–299. doi: 10.1038/sj.onc.1210638. [DOI] [PubMed] [Google Scholar]
- [88].Romisch K. Annu Rev Cell Dev Biol. 2005;21:435–456. doi: 10.1146/annurev.cellbio.21.012704.133250. [DOI] [PubMed] [Google Scholar]
- [89].Malhotra JD, Kaufman RJ. Semin Cell Dev Biol. 2007;18:716–731. doi: 10.1016/j.semcdb.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Heo JM, Livnat-Levanon N, Taylor EB, Jones KT, Dephoure N, Ring J, Xie J, Brodsky JL, Madeo F, Gygi SP, Ashrafi K, Glickman MH, Rutter J. Mol Cell. 2010;40:465–480. doi: 10.1016/j.molcel.2010.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Chatenay-Lapointe M, Shadel GS. Cell Metab. 2010;12:559–560. doi: 10.1016/j.cmet.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].de Brito OM, Scorrano L. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- [93].Hoyer-Hansen M, Jaattela M. Autophagy. 2007;3:381–383. doi: 10.4161/auto.4240. [DOI] [PubMed] [Google Scholar]
- [94].Kirstein-Miles J, Morimoto RI. Cell Metab. 2010;11:177–178. doi: 10.1016/j.cmet.2010.02.011. [DOI] [PubMed] [Google Scholar]