Abstract
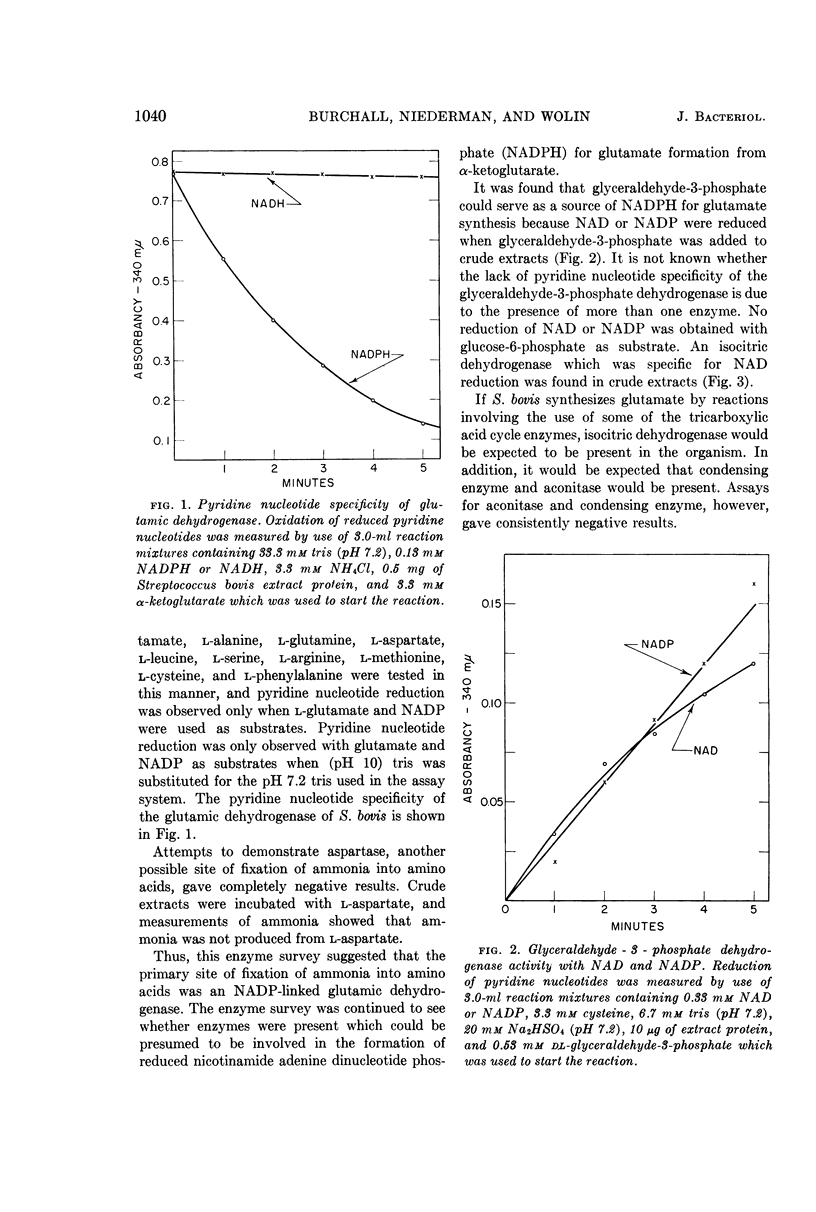
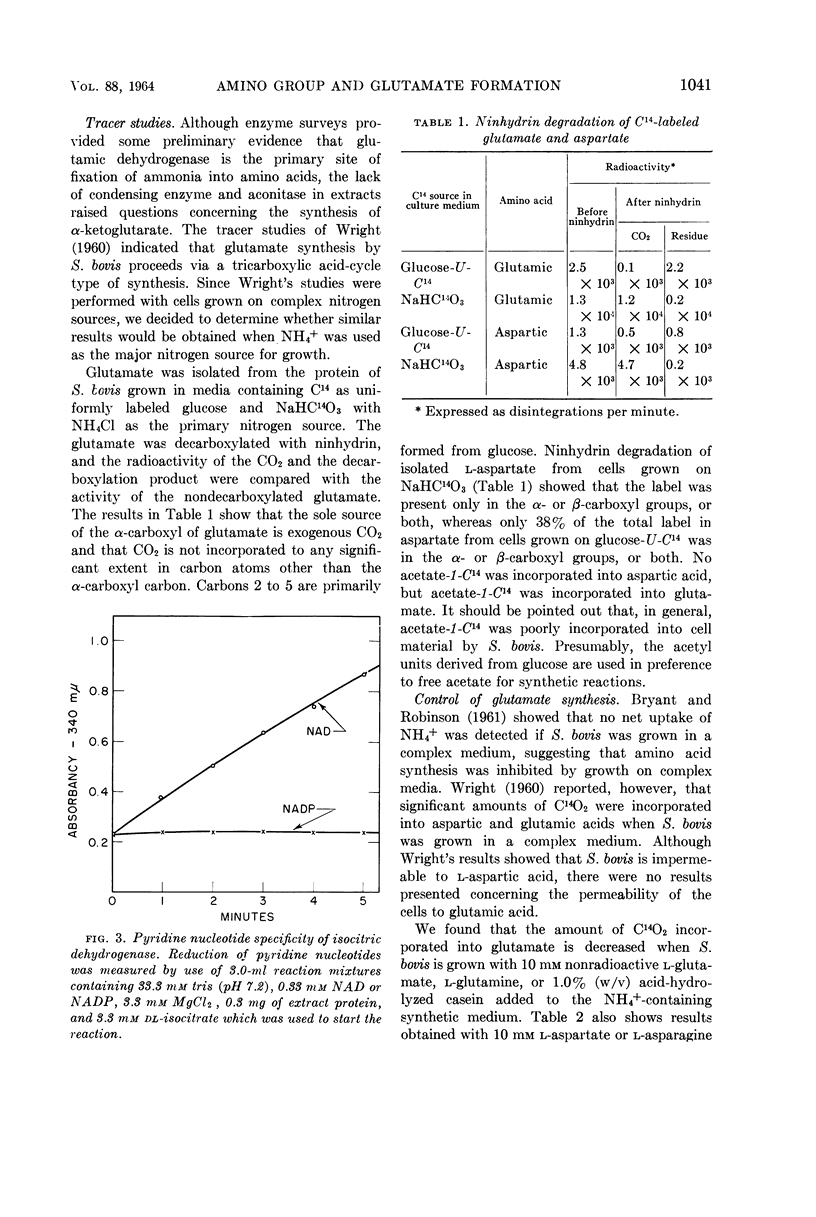
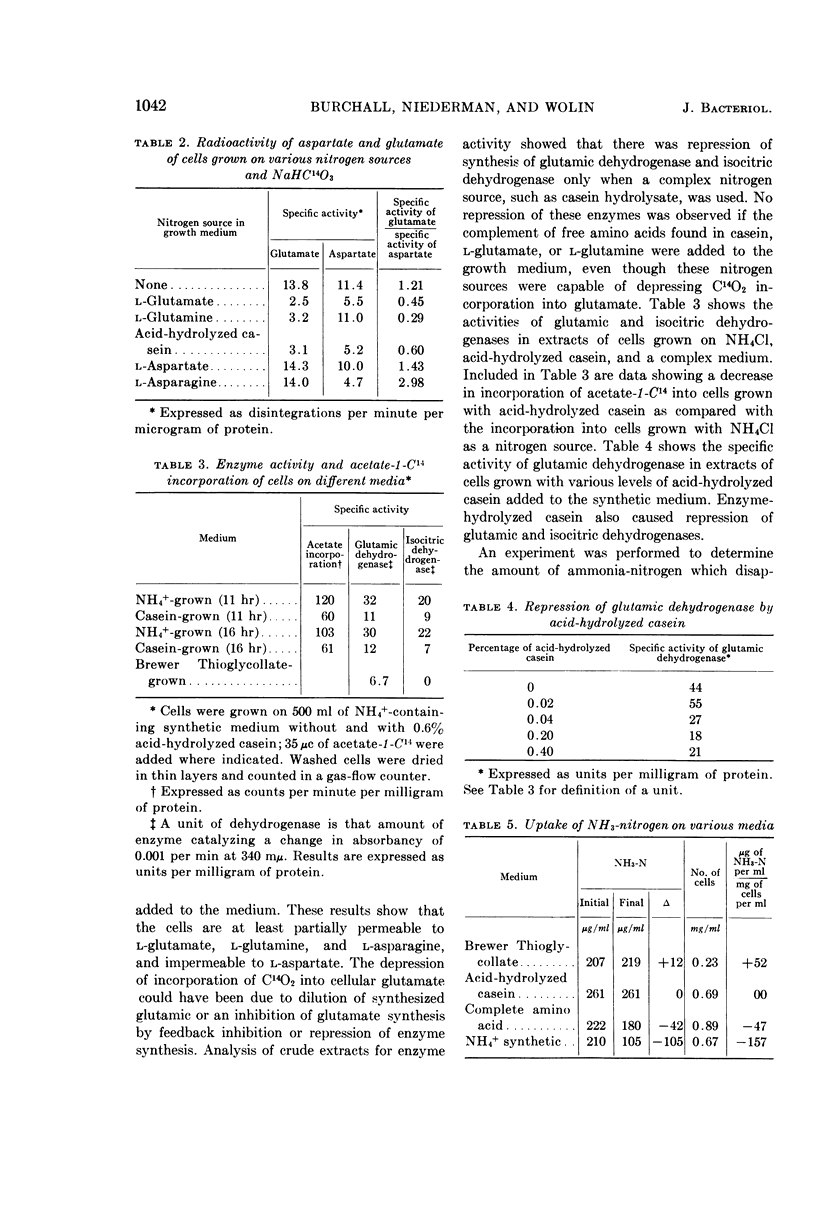
Burchall, J. J. (University of Illinois, Urbana), R. A. Niederman, and M. J. Wolin. Amino group formation and glutamate synthesis in Streptococcus bovis. J. Bacteriol. 88:1038–1044. 1964.—Extracts of Streptococcus bovis grown on NH4+ as a nitrogen source contain a nicotinamide adenine dinucleotide phosphate (NADP)-linked glutamic dehydrogenase and are devoid of alanine dehydrogenase, other amino acid dehydrohygenases, and aspartase. A potential source of reduced nicotinamide adenine dinucleotide phosphate for glutamate synthesis is a NADP and nicotinamide adenine dinucleotide (NAD)-linked glyceraldehyde-3-phosphate dehydrogenase present in the extracts. Experiments with C14-labeled glucose and NaHCO3 indicate that the glutamate carbon skeleton is synthesized by a tricarboxylic acid pathway. The synthesis of the carbon skeleton of glutamate is repressed when glutamate or casein hydrolysate supplement the NH4+-containing growth medium. Repression of glutamic dehydrogenase and a NAD-linked isocitric dehydrogenase occurs only when complex nitrogen sources, but not when free amino acids, are added to the growth medium.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNSTEIN I. A. Synthesis of ribose by the chick. J Biol Chem. 1953 Nov;205(1):317–329. [PubMed] [Google Scholar]
- Bryant M. P., Robinson I. M. Studies on the Nitrogen Requirements of Some Ruminal Cellulolytic Bacteria. Appl Microbiol. 1961 Mar;9(2):96–103. doi: 10.1128/am.9.2.96-103.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALPERN Y. S., UMBARGER H. E. Conversion of ammonia to amino groups in Escherichia coli. J Bacteriol. 1960 Sep;80:285–288. doi: 10.1128/jb.80.3.285-288.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HONG M. M., SHEN S. C., BRAUNSTEIN A. E. Distribution of L-alanine dehydrogenase and L-glutamate dehydrogenase in Bacilli. Biochim Biophys Acta. 1959 Nov;36:288–289. doi: 10.1016/0006-3002(59)90111-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- OPPERMANN R. A., NYSTROM R. F., NELSON W. O., BROWN R. E. Use of tertiary alkyl primary C12-C14 amines for the assay of radiocarbon-labeled carbon dioxide by liquid scintillation counting. Int J Appl Radiat Isot. 1959 Nov;7:38–42. doi: 10.1016/0020-708x(59)90278-9. [DOI] [PubMed] [Google Scholar]
- WOLIN M. J., MANNING G. B., NELSON W. O. Ammonium salts as a sole source of nitrogen for the growth of Streptococcus bovis. J Bacteriol. 1959 Jul;78(1):147–147. doi: 10.1128/jb.78.1.147-147.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WRIGHT D. E. The metabolism of carbon dioxide by Streptococcus bovis. J Gen Microbiol. 1960 Jun;22:713–725. doi: 10.1099/00221287-22-3-713. [DOI] [PubMed] [Google Scholar]


