Abstract
Background:
The role of Mycoplasma pneumoniae (Mp) in the initiation and persistence of asthma remains elusive. Mp community-acquired respiratory distress syndrome toxin (CARDS Tx) is a unique virulence factor that induces an intense lymphocytic response and exacerbates asthma in animal models. We sought to determine the incidence of Mp infection and the presence of CARDS Tx in subjects with refractory asthma (RA).
Methods:
We conducted a prospective observational study in 64 subjects with RA. Respiratory secretions (sputum, nasal lavage, and throat swab) and blood were analyzed for the presence of CARDS Tx and P1 adhesin (P1) DNA by polymerase chain reaction (PCR), and CARDS Tx by antigen capture. Serum IgM and IgG antibodies to CARDS Tx were determined by enzyme-linked immunosorbent assay (ELISA).
Results:
Thirty-three of 64 subjects (52%) tested positive for Mp: 29 of 33 by CARDS Tx vs 10 of 33 by P1 assays. Ten subjects followed longitudinally for up to 633 days tested persistently positive for Mp. There were no significant differences in Mp-specific IgG responses between Mp-positive and Mp-negative groups. Eight of 10 subjects who tested persistently positive failed to mount a substantial IgG response to CARDS Tx, and up to 8 weeks of clarithromycin failed to eradicate Mp in five subjects.
Conclusions:
Subjects with RA may be chronically infected with Mp. PCR for CARDS Tx appears to be the most sensitive method of identifying Mp infection. Despite the persistence of Mp in subjects with RA, some subjects failed to mount an IgG response, and macrolide therapy was insufficient to eradicate Mp.
Asthma is a prevalent and heterogeneous disease that not only has a marked effect on the quality of life of affected patients but also imparts a significant economic burden on society. The term “refractory asthma” (RA) is used for patients with persistent asthma symptoms in whom comorbidities have been treated, triggers addressed, compliance with treatment evaluated, and alternative diagnoses excluded.1 The link between bacterial processes and RA has emerged as various phenotypes of chronic asthma with persistent inflammation have been recognized.2-7 Many studies have implicated Mycoplasma pneumoniae (Mp) in the initiation and persistence of asthma, although the precise role it plays and its pathogenic mechanisms remain elusive.8 However, several limitations exist in studies of Mp in asthma, including the inability to consistently culture this organism, the poor performance of Mp serology in defining active infection, and the variable sensitivities of polymerase chain reaction (PCR) assays in detecting Mp.
Recently, our group identified a 68-kDa protein unique to Mp called the community-acquired respiratory distress syndrome toxin (CARDS Tx). CARDS Tx is a highly immunogenic protein that possesses adenosine diphosphate-ribosyltransferase activity similar to pertussis toxin.9 We have subsequently developed assays to detect CARDS Tx by PCR and CARDS Tx antigen-capture and to detect antibodies directed against CARDS Tx.10 CARDS Tx gene sequences are more sensitive for the detection of Mp than other sequences using PCR amplification, including the P1 adhesin (P1) and the ATPase gene.11-13
We studied 64 subjects with RA who had persistent symptoms despite being under the care of an asthma specialist and receiving optimal asthma therapy. The purpose of this study was to identify the frequency of Mp infection using both CARDS Tx PCR and conventional P1 PCR, to evaluate antibody responses to CARDS Tx and P1 proteins, and to detect CARDS Tx protein concentrations within the airways of these subjects.
Materials and Methods
Study Subjects
We conducted a prospective observational study in adult subjects (aged 18-65 years) with RA defined by persistent symptoms despite step 5 management of the National Asthma Education and Prevention Program guidelines. An additional 91 subjects undergoing diagnostic bronchoscopy for nonmalignant nonasthmatic lung disease and 104 healthy control subjects were evaluated. This study was approved by the institutional review board of the University of Texas Health Science Center at San Antonio (IRB No. 056-5012-271). Samples from nasal lavage and sputum were collected with Copan flocked swabs (Copan Diagnostics; Murrieta, California) and suspended in SP4 broth.14 Serum and respiratory samples were stored at −80°C until analysis.
Detection of Mp DNA, Protein, and Antibodies
Sputum and nasal lavage samples were homogenized prior to extraction with dithiothreitol. DNA from airways and serum samples was purified using the QIAmp DNA Mini Kit (Qiagen; Valencia, California). Real-time PCR for CARDS Tx (annotated MPN372) and P1 (annotated MPN 141) was performed as described.9,10,12
CARDS Tx protein was detected and quantified using antigen capture enzyme-linked immunosorbent assay (ELISA) methods as described previously.10,12 The detection of antibodies against recombinant CARDS Tx (rCARDS Tx) and recombinant P1 immunodominant carboxy domain (rP1) was performed using ELISA.9,12,15
Immunohistochemistry
Immunohistochemical staining was described previously.10 Briefly, cellular pellets from nasal lavage and sputum were collected, and specimens were fixed with 4% phosphate buffered paraformaldehyde. After paraffin embedding, 4-μm sections were stained with rabbit anti-CARDS Tx polyclonal antibodies and mouse anti-P1 monoclonal antibody (US Biologic; Swampscott, Massachusetts) at 1:1,500 and 1:10 dilutions, respectively, followed by polyclonal anti-rabbit or anti-mouse antibody-horse radish peroxidase, and then developed with diaminobenzidine (Vector Laboratories; Burlington, California) and counterstained with hematoxylin.
Statistical Analysis
All results were expressed as mean ± SD. Data were tabulated in SPSS, version 18.0 (SPSS Inc; Chicago, Illinois), and statistical differences between group means were determined using parametric (two-tailed t test) or nonparametric (Mann-Whitney U test) analyses (significant differences < .05). Differences in categorical data were determined using Pearson χ2 test.
Results
Sixty-four adult subjects with RA were enrolled, with a mean age of 44.8 years (Table 1). Thirty-one subjects were white, 25 Hispanic, and eight black. Forty-three subjects (67%) were women. The predominance of women within the cohort is consistent with other studies, which have shown a female predominance of adult subjects with severe asthma,16 and the ethnic breakdown is proportional to our regional population. Overall, the baseline postbronchodilator FEV1 was 2.35 (73.6% predicted), the mean IgE level was 599 mg/dL, the mean Asthma Control Test score was 17.2, and the mean number of asthma and allergy medications was 7.4. Forty-four subjects (69%) were identified as allergic asthmatics by their referring physician. Comparisons between subjects who were classified as positive or negative for Mp revealed significant differences (P < .05) in ethnic distribution, with higher numbers of whites and fewer Hispanics in the Mp-positive group (Table 1).
Table 1.
—Baseline Clinical Characteristics of Study Population
| Characteristic | Mp Positive | Mp Negative | Total |
| Age, y | 44.1 ± 14.5 | 45.61 ± 12.3 | 44.8 ± 13.4 |
| Female | 51.2 (22) | 48.8 (21) | 67 (43) |
| Ethnicity | |||
| W | 60.6 (20)a | 35.5 (11) | 48.4 (31) |
| H | 24.2 (8) | 54.8 (17) | 39.1 (25) |
| B | 15.2 (5) | 9.7 (3) | 12.5 (8) |
| FEV1b | 2.45 ± 0.91 | 2.24 ± 0.71 | 2.35 ± 0.82 |
| FEV1 % | 75.1 ± 24.8 | 71.9 ± 17.9 | 73.6 ± 21.6 |
| IgE | 700 ± 800 | 511 ± 521 | 599 ± 664 |
| ACT | 17.5 ± 5 | 17 ± 5.6 | 17.2 ± 5.2 |
| Medicationsc | 6.7 ± 2.3a | 8.2 ± 2 | 7.4 ± 2.3 |
Data are presented as mean ± SD except for female sex and ethnicity, which are expressed as % (No.). ACT = Asthma Control Test; B = black; H = Hispanic; Mp = Mycoplasma pneumoniae; W = white.
P < .05 comparing Mp-positive with Mp-negative subjects.
Postbronchodilator FEV1.
Medications includes prescribed medications for asthma, allergic rhinitis, and gastric reflux disease.
Subjects were considered positive if Mp was detectable by PCR (CARDS Tx or P1) or by CARDS Tx antigen capture on their initial study visit. Based on this analysis, 31 subjects were negative, and 33 (52%) were positive for Mp. Of the Mp-positive group, 29 of 33 subjects (88%) tested positive for CARDS Tx by PCR, 10 of 33 (30%) tested positive for P1 by PCR, and 52% tested positive by CARDS Tx antigen capture. Only one subject was positive by P1 alone. P1-negative specimens that were PCR positive to CARDS Tx were submitted to an outside reference laboratory (ARUP; Salt Lake City, Utah), which substantiated our P1 results. By comparison, 15 of 91 subjects (16.5%) with nonasthmatic lung disease were positive for CARDS Tx by PCR, and four of 91 (4.4%) were positive by CARDS Tx antigen capture (P < .05). During the peak respiratory season, 104 healthy subjects had airway specimens tested for CARDS Tx by antigen capture, and only three of 104 (2.9%) were positive.
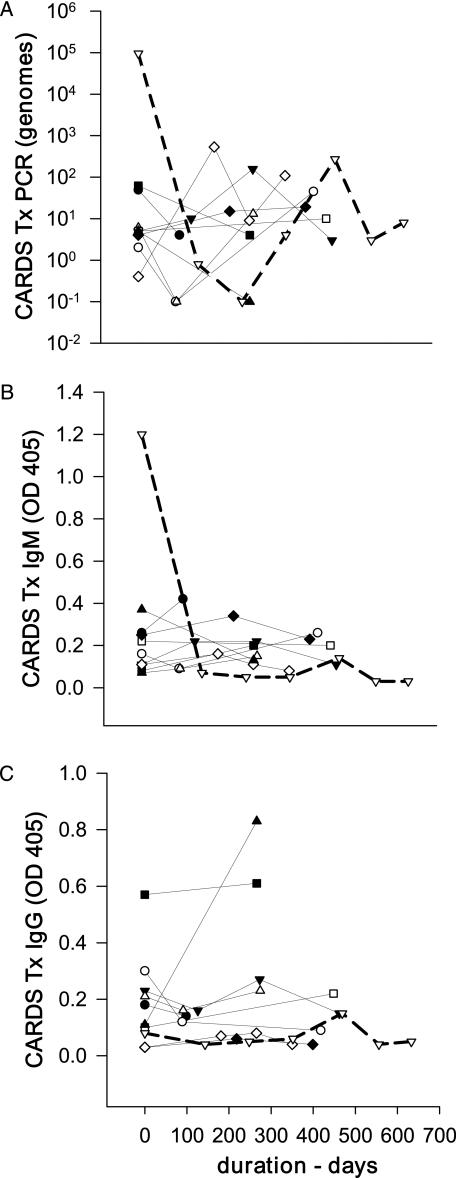
The majority of positive subjects were analyzed only during the initial visit. However, 10 of the 33 Mp-positive subjects were followed longitudinally and analyzed at multiple visits. This subgroup exhibited persistent detection of Mp DNA (CARDS Tx or P1) and/or CARDS Tx by antigen capture for > 90 days (average duration, 324 days; range, 91-633 days) (Fig 1). Within this “persistent” group, all 10 were PCR positive for CARDS Tx, one of 10 was PCR positive for P1, six of 10 were positive by CARDS Tx antigen capture on their initial visit, and all 10 were positive for CARDS Tx antigen capture during the extended period of observation.
Figure 1.
CARDS Tx PCR levels and serologic responses in 10 subjects with persistent Mycoplasma pneumoniae infection. A, PCR. B, CARDS Tx IgM. C, IgG titers. Each line represents an individual case. The index case is shown in bold dashed lines. CARDS Tx genome levels graphed at 10−1 genomes represent samples that were below the detectable limits for the assay. CARDS Tx = community-acquired respiratory distress syndrome toxin; OD = optical density; PCR = polymerase chain reaction.
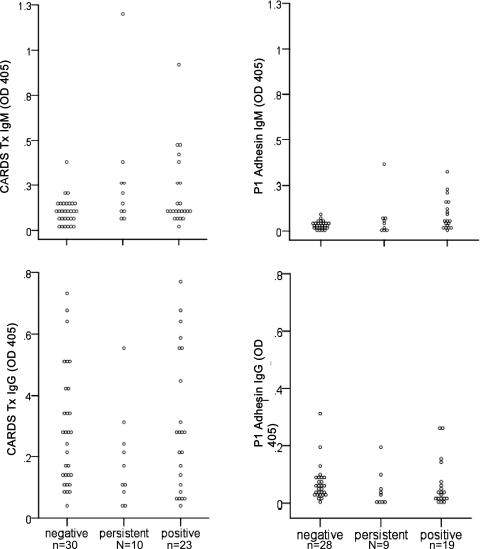
We measured serologic responses to rCARDS Tx and rP1 in most subjects (Fig 2, Table 2). The 10 subjects (labeled “persistent” in Fig 2 and Table 2) who persistently tested positive were analyzed separately to determine them from the remaining subjects, who were analyzed at only a single visit (designated as “negative” and “positive”). IgM antibodies directed against rCARDS Tx were significantly elevated (P < .01) in both the Mp-positive and persistent groups compared with Mp-negative subjects at the initial visit. However, no significant differences were detected in IgG responses between the groups. Similarly, IgM antibodies directed against rP1 were significantly elevated (P < .05) in the persistent and positive groups compared with the negative group at the initial visits, but there were no differences in IgG responses. When peak IgM and IgG responses were tabulated for the persistent group, who were the only subjects with multiple samples taken, there was a significant increase in IgM levels to rCARDS Tx and rP1 (P < .001) compared with the negative group, but no significant differences in the IgG responses. Two subjects in the persistent group were evaluated for immune deficiency and demonstrated normal total immunoglobulins and normal antibody titers to tetanus and diphtheria.
Figure 2.
Serologic responses to CARDS Tx and P1 adhesin. Serum IgG and IgM directed against CARDS Tx and P1 adhesin were analyzed by enzyme-linked immunosorbent assay at the initial visit for all subjects. Data are expressed as OD 405. See Figure 1 legend for expansion of abbreviations.
Table 2.
—Mp IgM and IgG Antibody Titers
| Immunoglobulin Assay | Negative | Persistent | Positive |
| CARDS Tx IgM | 0.103 ± 0.076 | 0.284 ± 0.335 | 0.207 ± 0.207 |
| CARDS Tx IgM peak | … | 0.353 ± 0.311 | … |
| CARDS Tx IgG | 0.284 ± 0.192 | 0.184 ± 0.161 | 0.3 ± 0.227 |
| CARDS Tx IgG peak | … | 0.294 ± 0.242 | … |
| P1 IgM | 0.033 ± 0.02 | 0.068 ± 0.115 | 0.093 ± 0.087 |
| P1 IgM peak | … | 0.089 ± 0.103 | … |
| P1 IgG | 0.069 ± 0.062 | 0.046 ± 0.063 | 0.065 ± 0.081 |
| P1 IgG peak | … | 0.069 ± 0.061 | … |
Data are presented as mean ± SD. CARDS Tx = community-acquired respiratory distress syndrome toxin; P1 = P1 adhesin. See Table 1 for expansion of the other abbreviation.
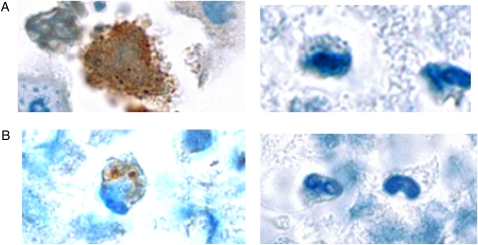
The subjects’ physicians, who were notified in the event of persistent Mp infection, subsequently determined antibiotic therapy. Five subjects in the persistent infection group received up to 8 weeks of clarithromycin but had no discernible improvement in their symptoms, rescue medication, Asthma Control Test scores, or spirometric values. All five subjects continued to have persistent detection of Mp in respiratory secretions after completion of antibiotic therapy. One of those subjects (shown in bold, dashed lines in Fig 1) was followed for 633 days. On initial enrollment, the subject had high levels of anti-rCARDS Tx IgM (1.2 OD405), high levels of Mp detected by PCR to CARDS Tx (95,133 genomes/5 μL nasal lavage) and P1 (39,887 genomes/5 μL), and high levels of CARDS Tx antigen (2.3 pg/12.5 μL nasal lavage or sputum), but no substantial increase in IgG antibody to rCARDS Tx or rP1 (Fig 1). After completion of 8 weeks of clarithromycin, Mp was still detectable by CARDS Tx PCR, and immunohistochemical staining of sputum for CARDS Tx and P1 demonstrated surface-associated and intracytoplasmic Mp (Fig 3). The immunostaining was similar to that observed in airway epithelium from Mp-infected mice.17
Figure 3.
Sputum sample. A, Stained with rabbit anti-CARDS TX polyclonal antibodies (left) or control rabbit antibody (right) (original magnification × 60). B, Stained with mouse anti-P1 monoclonal antibody (left) or control mouse antibody (right) (original magnification × 60). Both techniques demonstrated mycoplasmas associated with mononuclear cells in the sputum of a subject (shown in bold dashed lines in Figure 1) who had been treated with 8 weeks of macrolide therapy.
Discussion
The results of this study demonstrate that Mp is often present in the airways of patients with RA and can be detected by CARDS Tx assays described earlier. In this cohort of patients with refractory asthma, 52% of subjects tested positive for Mp by PCR or antigen capture, which was significantly more frequent than in subjects with nonasthmatic lung disease.
Only 30% of Mp-positive subjects were detected by the conventionally used PCR assay to P1. If assays using CARDS Tx had not been used in this study, then 70% of subjects potentially infected with Mp may have been misclassified as Mp negative. This finding has significant implications for recently published studies, in which macrolide or ketolide therapy was based on less sensitive assays.18,19 In the study of telithromycin in acute exacerbations of asthma, 61% of the 278 subjects met at least one criterion for atypical infection; however, only three subjects were PCR positive for Mp or Chlamydophila pneumoniae.18 Sutherland et al19 reported on the multicenter Mycoplasma in Asthma study, which used a nested PCR to detect P1 and Mp-specific 16S ribosomal RNA sequences, and identified 12/92 Mp-positive subjects with RA. Although there have been no direct comparisons between this nested PCR assay and the CARDS Tx PCR assay described here, a recent analysis by the US Centers for Disease Control and Prevention evaluated three real-time PCR assays for the detection of Mp in an outbreak of community-acquired pneumonia.20 Their data showed that real-time PCR assays targeting the CARDS Tx gene were the most sensitive in identifying positive specimens and recommended that the assay be used as the initial screening marker in respiratory clinical specimens. Of note, one subject in our study was P1 positive and CARDS Tx negative on initial PCR screening. This patient converted to negative on subsequent follow-up visits and never demonstrated CARDS Tx by antigen capture assay. This illustrates the difficulty in distinguishing true-positive from false-positive results when there is no clearly defined gold standard for the diagnosis of Mp infection.
Serologic assays for Mp have demonstrated variable clinical usefulness in the diagnosis of mycoplasma infection.21 Prior studies have shown that CARDS Tx is highly immunogenic and results in dramatic seroconversion in pneumonia patients.9 In the current study, anti-rCARDS Tx antibody titers were higher than anti-rP1 antibody titers. However, there were no differences in IgG to either rCARDS Tx or rP1 between those subjects who were Mp positive and those who were Mp negative. This finding held up even among subjects who were persistently positive for Mp as well as subjects among whom CARDS Tx was identified by antigen capture. IgM antibodies directed against rCARDS Tx or rP1 were significantly elevated in both the Mp-positive and persistent groups compared with Mp-negative subjects. However, the overall IgM responses still failed to rise to the conventional standard of a three- to fourfold increase in titer. Two subjects in the persistently positive group had robust IgM responses to rCARDS Tx but did not exhibit substantial increases in IgG antibodies. This lack of antibody response to Mp has been reported by others. In a study by Petitjean et al22 comparing four commercial Mp ELISAs in hospitalized subjects with upper or lower respiratory tract infections, 22% to 48% of children and 17% to 25% of adults had negative serology despite positive PCR results to P1. In another study, Atkinson et al23 identified 7/9 (78%) pediatric subjects with asthma who had negative IgG titers despite positive PCR results to Mp 16S ribosomal RNA. In the setting of Mp community-acquired pneumonia, up to 47% of subjects who were culture positive, PCR positive, or both, remained seronegative.24 One might speculate that the low levels of Mp infecting the airway in RA could explain the poor immune responses noted in our subjects; alternatively, these asthmatic subjects may have been unable to mount an appropriate immune response, resulting in a chronic airway infection.
We demonstrated previously that rCARDS Tx is a potent inducer of pulmonary inflammation in mice and baboons.25 rCARDS Tx-mediated inflammatory responses were characterized by the rapid expression of cytokines and chemokines with the concurrent development of an intense lymphocytic inflammatory response. Additionally, significant increases in both airway obstruction and airway hyperreactivity correlated with the degree of peribronchiolar and perivascular airway inflammation.25
Although this study was not designed with a therapeutic arm, the primary treating physicians were notified when Mp was identified within the airway of their patient and of persistent infection. Five subjects with persistent infection were treated with up to 8 weeks of clarithromycin therapy. We were unable to detect clinical improvement or eradication of Mp or CARDS Tx using this form of therapy. Whether prolonged antimicrobial therapy would impact the clinical course or eliminate the organism from the airway remains speculative.
There were several limitations to this study. Ideally, all subjects would have been followed longitudinally and would have included a population of healthy individuals and asthmatic subjects with various levels of severity. Without this data, we are unable to determine if low genome values might be associated with colonization. Another limitation is the difficulty in determining the precise timing of when the enrolled subjects initially became infected with Mp. In fact, none of the subjects reported either an acute respiratory illness or pneumonia during the 6-month period prior to enrollment in the study. We chose our definition of persistent infection based on an epidemiologic study of an acute outbreak of Mp infection, in which the median duration of carriage was 7 weeks and 70% of the subjects had no detectable Mp after 3 months.20 Thus, according to this definition, 10 of our subjects remained persistently positive for Mp, and no immediate differences were noted in their clinical or immunologic parameters. Therefore, we cannot speculate as to whether there were unique characteristics, immunologic or otherwise, in the subjects with persistent infection. However, the fact that the inflammatory CARDS Tx protein was persistently identified in the airways of these subjects suggests that Mp and the accompanying pathophysiology of infection and intoxication may have played a pathogenic role in the refractory nature of their disease.
Conclusions
The results of this study confirm that Mp commonly exists within the airways of patients with RA and can be detected optimally with assays targeting CARDS Tx DNA and protein. The fact that 45% (29 of 64) of the RA subjects in this study tested positive by PCR to CARDS Tx, whereas only 16% (10 of 64) tested positive by PCR to P1, further demonstrates the superiority of CARDS Tx assays when screening for Mp. Our failure to demonstrate a distinguishing IgG response to Mp rCARDS Tx and rP1in this group of severe asthmatics is relevant and raises the possibility that some patients may be unable to mount a robust immune response to Mp. The role of Mp in both acute and chronic asthma and other respiratory and extrapulmonary diseases may need to be reassessed because many of the prior studies in the literature have relied on serologic diagnosis and less sensitive PCR assays in its detection.
Acknowledgments
Author contributions:
Dr Peters: contributed to study design, data collection and analysis, and obtaining funding and was primary author of the manuscript.
Dr Singh: contributed to the oversight of acquisition and analysis of data and preparation of the manuscript.
Dr Brooks: contributed to study design, immunologic assays, data analysis, coauthorship, and review of the manuscript.
Dr Diaz: contributed to patient clinical data and review of the manuscript.
Dr Kannan: contributed to design of study, data acquisition and analysis, and preparation of the manuscript.
Dr Coalson: contributed to study design, core pathology, data analysis, and review of the manuscript.
Dr J. G. Baseman: contributed to statistical analysis of the data and preparation of the manuscript.
Ms Cagle: contributed to data acquisition and analysis and preparation of the manuscript.
Dr J. B. Baseman: contributed to study study, obtaining funding, data collection and analysis, and preparation of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: No funding source had any role in the design or conduct of the study or in collection, management, analysis, interpretation of the data, or preparation of the manuscript.
Abbreviations
- CARDS Tx
community-acquired respiratory distress syndrome toxin
- ELISA
enzyme-linked immunosorbent assay
- Mp
Mycoplasma pneumoniae
- P1
P1 adhesin
- PCR
polymerase chain reaction
- RA
refractory asthma
- rCARDS Tx
recombinant community-acquired respiratory distress syndrome toxin
- rP1
recombinant P1 adhesin immunodominant carboxy domain
Footnotes
Funding/Support: This study was supported by the National Institutes of Health [Grant U19A1070412-01] and The Kleberg Foundation to J. B. B.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Bel EH, Sousa A, Fleming L, et al. Diagnosis and definition of severe refractory asthma: an international consensus statement from the Innovative Medicine Initiative (IMI) [published online ahead of print November 23, 2010] Thorax. doi: 10.1136/thx.2010.153643. doi:10.1136/thx.2010.138552. [DOI] [PubMed] [Google Scholar]
- 2.Emre U, Roblin PM, Gelling M, et al. The association of Chlamydia pneumoniae infection and reactive airway disease in children. Arch Pediatr Adolesc Med. 1994;148(7):727–732. doi: 10.1001/archpedi.1994.02170070065013. [DOI] [PubMed] [Google Scholar]
- 3.Hahn DL, McDonald R. Can acute Chlamydia pneumoniae respiratory tract infection initiate chronic asthma? Ann Allergy Asthma Immunol. 1998;81(4):339–344. doi: 10.1016/S1081-1206(10)63126-2. [DOI] [PubMed] [Google Scholar]
- 4.Hahn DL, Dodge RW, Golubjatnikov R. Association of Chlamydia pneumoniae (strain TWAR) infection with wheezing, asthmatic bronchitis, and adult-onset asthma. JAMA. 1991;266(2):225–230. [PubMed] [Google Scholar]
- 5.Gil JC, Cedillo RL, Mayagoitia BG, Paz MD. Isolation of Mycoplasma pneumoniae from asthmatic patients. Ann Allergy. 1993;70(1):23–25. [PubMed] [Google Scholar]
- 6.Yano T, Ichikawa Y, Komatu S, Arai S, Oizumi K. Association of Mycoplasma pneumoniae antigen with initial onset of bronchial asthma. Am J Respir Crit Care Med. 1994;149(5):1348–1353. doi: 10.1164/ajrccm.149.5.8173777. [DOI] [PubMed] [Google Scholar]
- 7.Kraft M, Cassell GH, Henson JE, et al. Detection of Mycoplasma pneumoniae in the airways of adults with chronic asthma. Am J Respir Crit Care Med. 1998;158(3):998–1001. doi: 10.1164/ajrccm.158.3.9711092. [DOI] [PubMed] [Google Scholar]
- 8.Leaver R, Weinberg EG. Is Mycoplasma pneumoniae a precipitating factor in acute severe asthma in children? S Afr Med J. 1985;68(2):78–79. [PubMed] [Google Scholar]
- 9.Kannan TR, Baseman JB. ADP-ribosylating and vacuolating cytotoxin of Mycoplasma pneumoniae represents unique virulence determinant among bacterial pathogens. Proc Natl Acad Sci U S A. 2006;103(17):6724–6729. doi: 10.1073/pnas.0510644103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kannan TR, Musatovova O, Balasubramanian S, et al. Mycoplasma pneumoniae community acquired respiratory distress syndrome toxin expression reveals growth phase and infection-dependent regulation. Mol Microbiol. 2010;76(5):1127–1141. doi: 10.1111/j.1365-2958.2010.07092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winchell JM, Thurman KA, Mitchell SL, Thacker WL, Fields BS. Evaluation of three real-time PCR assays for detection of Mycoplasma pneumoniae in an outbreak investigation. J Clin Microbiol. 2008;46(9):3116–3118. doi: 10.1128/JCM.00440-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muir MT, Cohn SM, Louden C, et al. Novel toxin assays implicate Mycoplasma pneumoniae in prolonged ventilator course and hypoxemia. Chest. 2011;139(2):305–310. doi: 10.1378/chest.10-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Techasaensiri C, Tagliabue C, Cagle M, et al. Variation in colonization, ADP-ribosylating and vacuolating cytotoxin, and pulmonary disease severity among Mycoplasma pneumoniae strains. Am J Respir Crit Care Med. 2010;182(6):797–804. doi: 10.1164/rccm.201001-0080OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dallo SF, Kannan TR, Blaylock MW, Baseman JB. Elongation factor Tu and E1 beta subunit of pyruvate dehydrogenase complex act as fibronectin binding proteins in Mycoplasma pneumoniae. Mol Microbiol. 2002;46(4):1041–1051. doi: 10.1046/j.1365-2958.2002.03207.x. [DOI] [PubMed] [Google Scholar]
- 15.Dallo SF, Su CJ, Horton JR, Baseman JB. Identification of P1 gene domain containing epitope(s) mediating Mycoplasma pneumoniae cytoadherence. J Exp Med. 1988;167(2):718–723. doi: 10.1084/jem.167.2.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.European Network for Understanding Mechanisms of Severe Asthma The ENFUMOSA cross-sectional European multicentre study of the clinical phenotype of chronic severe asthma. Eur Respir J. 2003;22(3):470–477. doi: 10.1183/09031936.03.00261903. [DOI] [PubMed] [Google Scholar]
- 17.Kannan TR, Coalson JJ, Cagle M, et al. Synthesis and distribution of CARDS toxin during Mycoplasma pneumoniae infection in a murine model. J Infect Dis. doi: 10.1093/infdis/jir557. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnston SL, Blasi F, Black PN, Martin RJ, Farrell DJ, Nieman RB. TELICAST Investigators The effect of telithromycin in acute exacerbations of asthma. N Engl J Med. 2006;354(15):1589–1600. doi: 10.1056/NEJMoa044080. [DOI] [PubMed] [Google Scholar]
- 19.Sutherland ER, King TS, Icitovic N, et al. National Heart, Lung and Blood Institute’s Asthma Clinical Research Network A trial of clarithromycin for the treatment of suboptimally controlled asthma. J Allergy Clin Immunol. 2010;126(4):747–753. doi: 10.1016/j.jaci.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nilsson AC, Björkman P, Persson K. Polymerase chain reaction is superior to serology for the diagnosis of acute Mycoplasma pneumoniae infection and reveals a high rate of persistent infection. BMC Microbiol. 2008;8:93. doi: 10.1186/1471-2180-8-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marmion BP, Williamson J, Worswick DA, Kok TW, Harris RJ. Experience with newer techniques for the laboratory detection of Mycoplasma pneumoniae infection: Adelaide, 1978-1992. Clin Infect Dis. 1993;17(suppl 1):S90–S99. doi: 10.1093/clinids/17.supplement_1.s90. [DOI] [PubMed] [Google Scholar]
- 22.Petitjean J, Vabret A, Gouarin S, Freymuth F. Evaluation of four commercial immunoglobulin G (IgG)- and IgM-specific enzyme immunoassays for diagnosis of Mycoplasma pneumoniae infections. J Clin Microbiol. 2002;40(1):165–171. doi: 10.1128/JCM.40.1.165-171.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atkinson TP, Duffy LB, Pendley D, Dai Y, Cassell GH. Deficient immune response to Mycoplasma pneumoniae in childhood asthma. Allergy Asthma Proc. 2009;30(2):158–165. doi: 10.2500/aap.2009.30.3207. [DOI] [PubMed] [Google Scholar]
- 24.Block S, Hedrick J, Hammerschlag MR, Cassell GH, Craft JC. Mycoplasma pneumoniae and Chlamydia pneumoniae in pediatric community-acquired pneumonia: comparative efficacy and safety of clarithromycin vs. erythromycin ethylsuccinate. Pediatr Infect Dis J. 1995;14(6):471–477. doi: 10.1097/00006454-199506000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Hardy RD, Coalson JJ, Peters J, et al. Analysis of pulmonary inflammation and function in the mouse and baboon after exposure to Mycoplasma pneumoniae CARDS toxin. PLoS ONE. 2009;4(10):e7562. doi: 10.1371/journal.pone.0007562. [DOI] [PMC free article] [PubMed] [Google Scholar]