Abstract
T helper cells that produce Interleukin-17 (IL-17) (TH17 cells) are a recently identified CD4+ T-cell subset with characterized pathological roles in autoimmune diseases1–3. The nuclear receptors retinoic acid receptor-related orphan receptors α and γt (RORα and RORγt) have indispensible roles in the development of this cell type4–7. Here we present a first-in-class, high-affinity synthetic ligand, SR1001, specific to both RORα and RORγt that inhibits TH17 cell differentiation and function. SR1001 binds specifically to the ligand binding domains (LBDs) of RORα and RORγt inducing a conformational change within the LBD that encompasses repositioning of helix 12 leading to diminished affinity for coactivators and increased affinity for corepressors resulting in suppression of the receptors transcriptional activity. SR1001 inhibited the development of murine TH17 cells as demonstrated by inhibition of IL-17A gene expression and protein production. Additionally, SR1001 inhibited the expression of cytokines when added to differentiated murine or human TH17 cells. Finally, SR1001 effectively suppressed the clinical severity of autoimmune disease in mice. Thus, our data demonstrates the feasibility of targeting the orphan receptors RORα and RORγt to specifically inhibit TH17 cell differentiation and function and indicates that this novel class of compound has potential utility in the treatment of autoimmune diseases.
TH17 cells are crucial effector cells implicated in the pathology of numerous autoimmune diseases, including multiple sclerosis, rheumatoid arthritis, inflammatory bowel disease, and systemic lupus erythematosis. These cells produce a number of cytokines, including IL-17, which are known to enhance inflammatory processes1–3. The discovery of these cells as critical mediators of autoimmune disorders provides a unique opportunity to develop focused therapeutics that act by inhibiting the function of these cells. An essential role for two nuclear receptors (NRs), RORα and RORγt, has been established in the development of TH17 cells. Both of these NRs are required for the full differentiation of naïve CD4+ T cells into TH17 cells4–7.
Members of the NR superfamily are ligand-dependent transcription factors. A number of drugs utilized in the clinic have been developed that target several NR superfamily members. Therefore, an attractive strategy for the development of novel therapeutics for treatment of TH17-mediated autoimmune disorders is targeting RORα and RORγt with synthetic ligands that inhibit their activity resulting in decreased TH17 cell differentiation and/or function. However, RORs are generally characterized as orphan receptors with no well-characterized ligands, thus it is unclear whether this approach is viable.
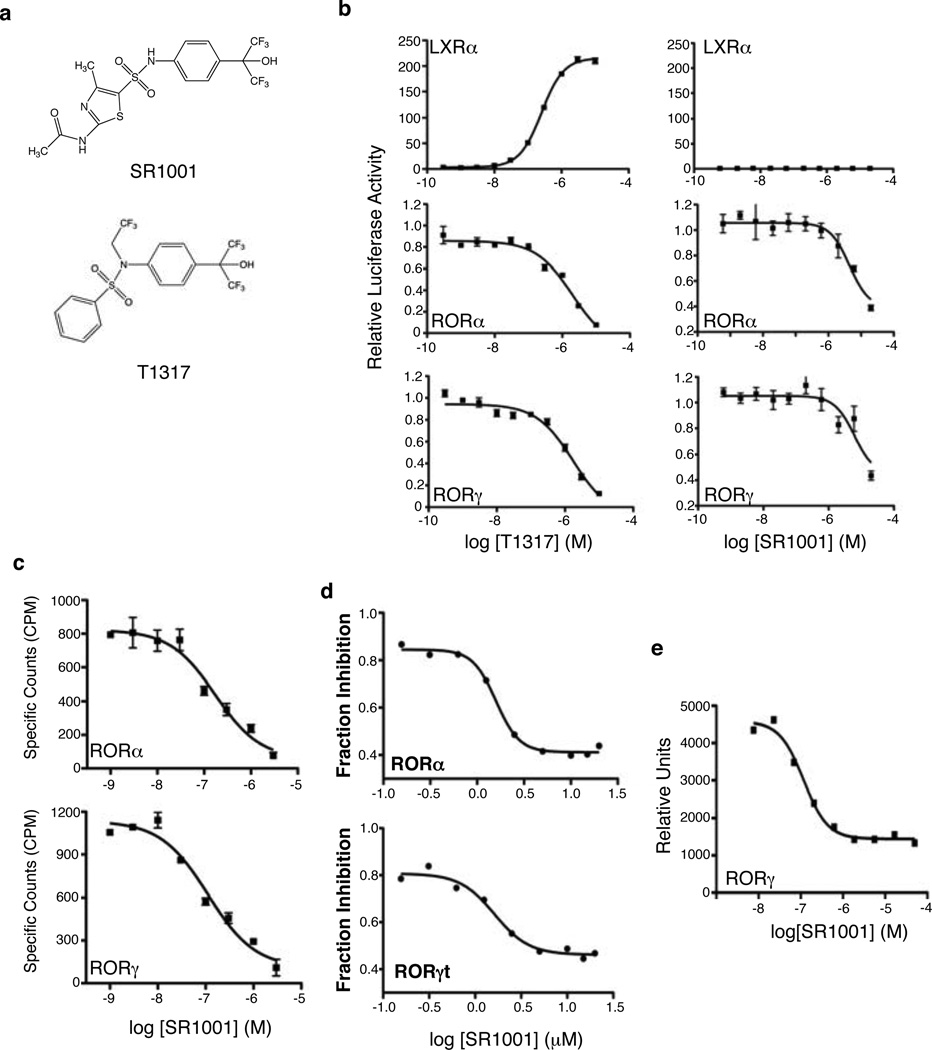
We recently characterized the benzenesulfonamide liver X receptor (LXR) agonist T0901317 (T1317) as a promiscuous ligand that modulates the activity of several NRs including RORα and RORγ8. While T1317 is a very potent and efficacious agonist of LXR, it also acts as an inverse agonist of RORα and RORγ by suppressing their basal transcriptional activity8. Using the T1317 scaffold as a lead compound we developed a derivative, SR1001 (Fig. 1a and Supplementary Fig. 1) that was devoid of all LXR activity yet retained its ability to suppress the activity of RORα and RORγ. We found that SR1001 repressed both GAL4-RORα and GAL4-RORγ transcriptional activity in a dose dependent manner (Fig. 1b), but demonstrated no effect on LXRα activity (Fig. 1b). We assessed the specificity of SR1001 in a panel of all 48 human nuclear receptors in a cell-based cotransfection assay 8 and did not observe activity on receptors other than RORα or RORγ (data not shown). We examined the direct binding of SR1001 to RORα and RORγ using competitive radioligand binding assays. SR1001 dose dependently displaced [3H]25-hydroxycholesterol binding to RORα and RORγ (Ki = 172 and 111 nM, respectively) (Fig. 1c) but demonstrated no binding to RORβ (data not shown).
Figure 1. SR1001 is a selective RORα and RORγ inverse agonist.
a, Structure of SR1001 and T0901317 (T1317). b, GAL4-LXRα, GAL4-RORα, and GAL4-RORγ cotransfection assays in HEK293 cells comparing T1317 to SR1001 (n=8). c, Competition radioligand binding assays illustrating the direct binding of SR1001 to the LBD of RORα and RORγ relative to [3H]25-hydroxycholesterol (n=4). d, SR1001 dose-dependently inhibits an Il17 promoter-driven luciferase construct in the presence of RORα or RORγt in HEK293 cells. Results are normalized to vehicle (DMSO) control (n=4). e, AlphaScreen assay indicating SR1001 dose-dependently inhibits the recruitment of a TRAP220 NR box 2 peptide to the LBD of RORγ (n=3). Error bars denote sem.
We examined whether SR1001 would affect RORα- and RORγ-dependent regulation of an Il17 promoter-driven luciferase reporter9. HEK293 cells were transfected with the Il17 reporter and either full-length RORα or RORγ and treated with SR1001 or vehicle. As illustrated in Fig. 1d, SR1001 dose-dependently suppressed the Il17 promoter driven activity by each of the receptors. Since SR1001 bound RORα and RORγ, resulting in suppression of each receptors’ transcriptional activity, we expected that SR1001 would inhibit coactivator binding to the receptors. SR1001 reduced the interaction of a coactivator TRAP220 NR box 2 peptide with RORγ in a dose dependent manner (Fig. 1e) (IC50 value ~117 nM). Collectively, these data demonstrate that SR1001 function as an inverse agonist ligand of RORα/RORγ.
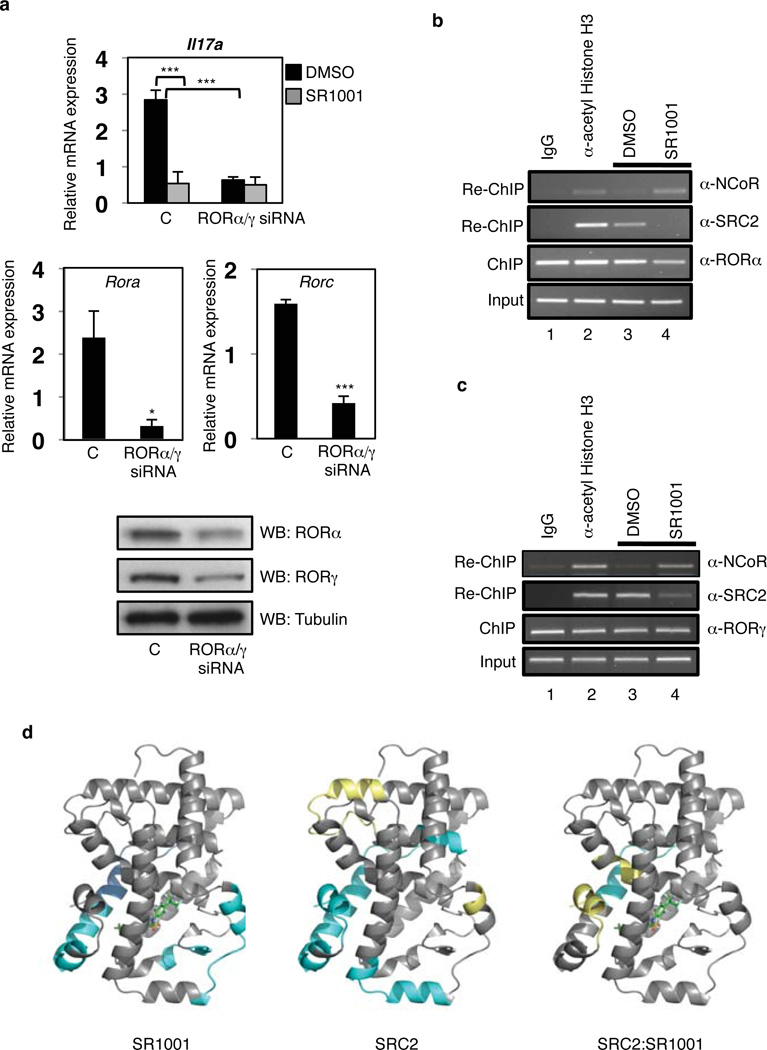
Next, we determined whether SR1001 affected endogenous Il17a gene expression. The EL4 murine tumor cell line constitutively expresses RORα (Rora), RORγt (Rorc), and IL-17A (Il17a)10. EL4 cells were treated with either control siRNA or a mixture of RORα/γ siRNA followed by treatment with either vehicle or SR1001. Reduction in the expression of RORα and RORγt significantly reduced the expression of IL-17A mRNA as measured by quantitative PCR (Fig. 2a). More importantly, treatment of cells with SR1001 suppressed Il17a mRNA expression whereas treatment of RORα/γ depleted cells displayed a significantly blunted response indicating that SR1001 suppression of Il17a mRNA expression is RORα/RORγ dependent (Fig. 2a). Furthermore, SR1001 suppressed the expression of the RORα and RORγ target gene G6Pase in HepG2 cells, a human hepatocellular carcinoma cell line, providing further proof that the effect of SR1001 is mediated by RORα and RORγ (Supplementary Fig. 2)11, 12.
Figure 2. SR1001 modulates the expression of ROR target genes by decreasing coactivator recruitment.
a, Il17a, Rora, and Rorc mRNA expression in EL4 cells treated with control (C), or mouse RORα/γ siRNA, vehicle (DMSO), or SR1001 (10µM, 24 hours) (n=3). Protein expression of RORα and RORγ is shown. *P<0.05; ***P<0.005. b, ChIP-reCHIP assay in EL4 cells illustrating that SR1001 reduces b, RORα- and c, RORγ-dependent recruitment of SRC-2 and promotes recruitment of NCoR to the Il17 promoter. d, Illustration of the HDX kinetics of peptic peptides derived from the RORγ LBD.. Cyan indicates an increase in protection to exchange; yellow represents a decrease in protection to exchange. Error bars denote sem.
We hypothesized that SR1001 would inhibit binding of the coactivator SRC2 to either RORα or RORγ when these receptors are occupying the Il17 promoter. We performed a sequential chromatin immunoprecipitation assay (ChIP-reChIP) assessing the relative amount of SRC2 associated with either RORα or RORγ resident at the Il17 promoter in EL4 cells. SR1001 suppressd the ability of SRC2 to bind to RORα and RORγ at the Il17 promoter and increased the recruitment of the corepressor NCoR (Fig. 2b, lanes 3 and 4 and Fig. 2c, lanes 3 and 4). Thus, SR1001 suppresses Il17a expression by directly inhibiting coactivator binding and promoting the recruitment of corepressors to RORα and RORγ.
To understand how ligand mediates the transcriptional activation of RORγ, we performed comprehensive differential hydrogen-deuterium exchange mass spectrometry (HDX) analysis of the RORγ LBD in the presence and absence of SR1001. This approach provides a measure of the localized ligand-induced perturbation in the conformational ensemble of the receptor. HDX kinetics of peptic peptides derived from the RORγ LBD were measured and the average difference in percentage of incorporated deuterium between apo RORγ LBD and SR1001 bound RORγ LBD are presented in Supplementary Figure 3. A negative value represents an increase in protection to exchange (more stable, less dynamic) in that region of the LBD when bound to ligand as compared to apo whereas a positive value represents a decrease in protection to exchange (less stable, more dynamic). HDX kinetics are sensitive to hydrogen bond networks and perturbations in these networks upon ligand binding can be determined using differential HDX. The differential HDX induced by SR1001 binding to RORγ correlates with the co-crystal structure of RORγ complexed with the sterol ligand, 25-hydroxycholesterol 25-OHC (PDB:3LOL)13 (Supplementary Fig. 4). In the RORγ/25-OHC structure, the C25 hydroxyl tail is oriented towards helix 11 (H11) and the A ring toward H1/H2. As can be inferred from PDB:3LOL, the hydroxyl group at the C1 position of 25-OHC is hydrogen bonded to Qln-286 (H1) and the 25-hydroxyl is hydrogen bonded to His-479 (H11). The regions within the RORγ LBD that show increased protection to exchange upon binding of SR1001 include portions of H1 and H11. To highlight this, the HDX data in Supplementary Fig.3 is represented graphically by overlay onto PDB:3LOL with SR1001 docked (Fig. 2d). Consistent with the differential HDX data, docking of SR1001 to PDB:3LOL suggests a similar binding mode for SR1001 to RORγ (Supplementary Fig. 4).
In order to examine the role of SR1001 in modulation of this interaction between SRC2 and RORγ, we performed differential HDX on RORγ LBD in the presence and absence of the receptor interaction domain (RID) of SRC2 (Fig. 2d & Supplementary Fig.3), which contains three NR boxes (~18kDa). Several regions of the LBD demonstrate reduced HDX kinetics in the presence of SRC2 RID, indicating an interaction between the two proteins. One region stabilized is H12, containing the AF2 domain of the receptor, which has been shown to be important for NR interaction with coactivators. Furthermore, differential HDX analysis of the RORγ:SRC2 complex in the presence and absence of SR1001 clearly demonstrates that ligand disrupts the receptors interaction with SRC2 RID (Fig. 2d). These data provide strong mechanistic insight into how inverse agonists such as SR1001 repress transcriptional output of RORγ target genes.
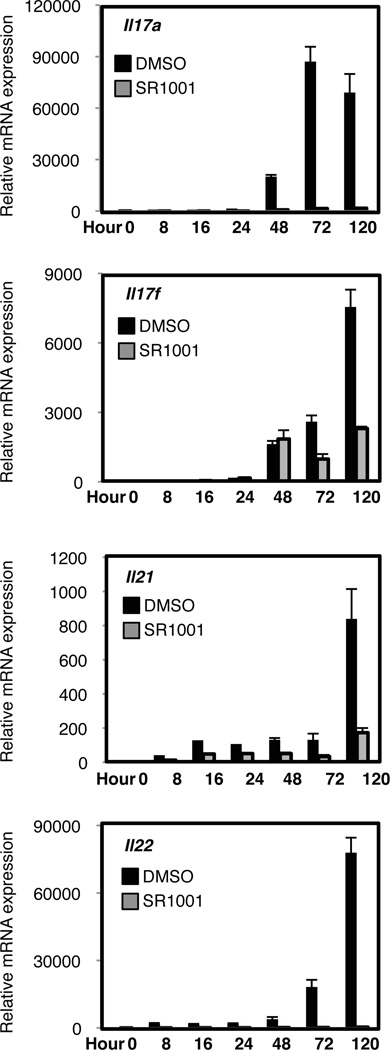
Since RORα and RORγt activity is required for optimal TH17 cell development4, we explored whether SR1001 would inhibit TH17 cell differentiation. Splenocytes were cultured under TH17 polarizing conditions (TGF-β and IL-6) with SR1001 or vehicle control for 5 days. The combination of TGF-β and IL-6 increased the mRNA expression of Il17a, Il17f, Il21, and Il22, in vehicle treated cells whereas SR1001 treated cells failed to significantly upregulate these cytokines (Fig. 3). Propidium iodide staining indicated that SR1001 was not toxic and did not induce cell death (Supplementary Fig. 5). TH17 cells and inducible T regulatory cells (iTreg) are both dependent on TGFβ for their differentiation. We evaluated whether expression of the Treg-specific transcription factor Foxp3, was affected by SR1001 treatment. Similar to vehicle control, Foxp3 mRNA expression was unaffected by SR1001 treatment suggesting that inhibition of TH17 cell differentiation by SR1001 did not drive the cells into an iTreg phenotype (Supplementary Fig. 6)9. Furthermore, suppression of TH17 cell development with SR1001 treatment did not drive the splenocyte cultures into any of the other T helper lineages, TH1 or TH2, as indicated by the decrease in Tbx21 (T-bet) and Gata3 mRNA expression, respectively (Supplementary Fig. 6).
Figure 3. SR1001 inhibits the expression of cytokines expressed by TH17 cells.
Il17a, Il17f, Il21, and Il22 mRNA expression in splenocytes differentiated under TH17 polarizing conditions in the presence of vehicle (DMSO) or SR1001 (5µM) for 5 days. mRNA expression levels are normalized to Gapdh (n=3). Error bars denote sem.
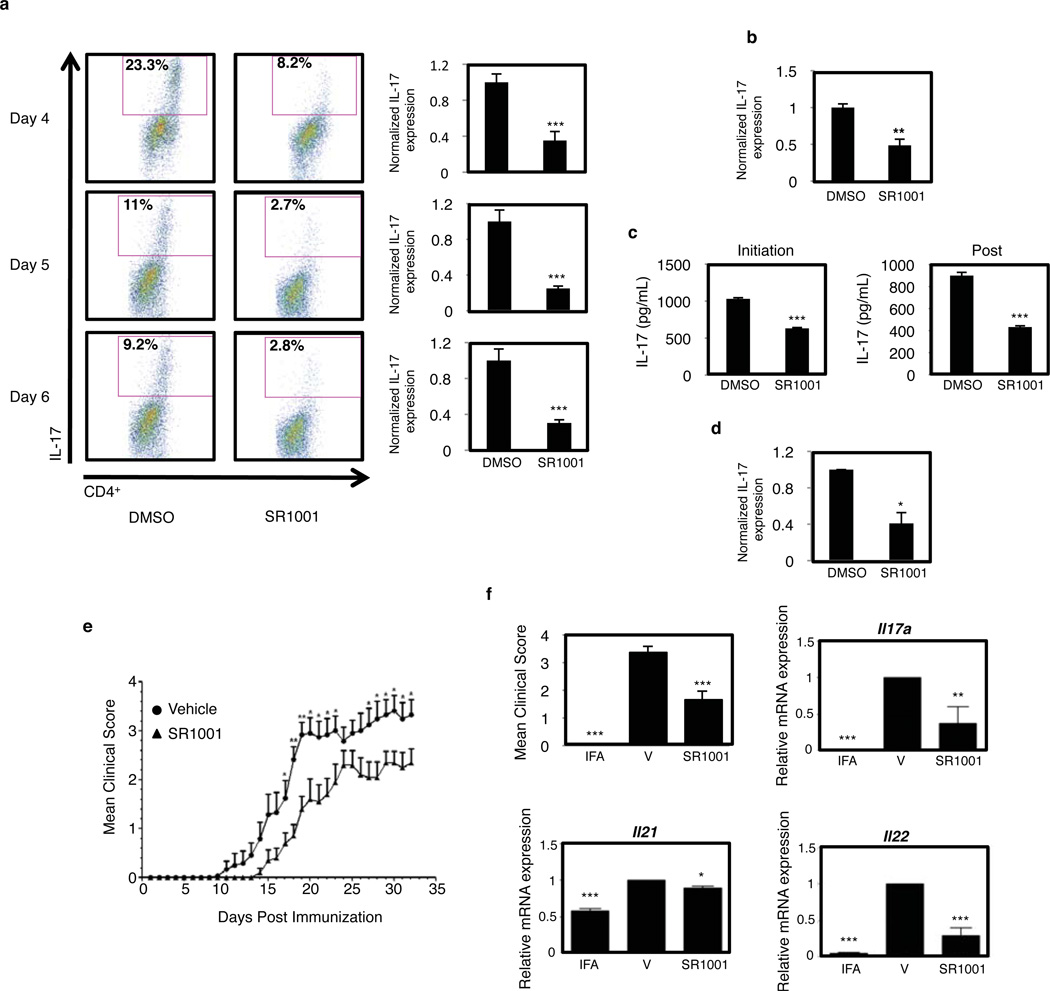
Finally, we explored whether SR1001 would inhibit IL-17 protein production and secretion. Splenocytes were cultured under TH17 polarizing conditions and analyzed for IL-17 expression by intracellular flow cytometry. Treatment with SR1001 inhibited the expression of IL-17 from CD4+ T cells at Day 4, 5, and 6 (Fig. 4a). Similar to splenocyte cultures, intracellular flow cytometry demonstrated that SR1001 significantly repressed IL-17 expression in purified differentiated murine CD4+ T cells (CD4+CD25− CD62LhiCD44lo) (Fig. 4b). Next we assessed the effect of SR1001 on IL-17 secretion from splenocyte cultures by ELISA. Treatment with SR1001 inhibited IL-17 secretion over a three-day time course, when SR1001 was added at either the initiation of TH17 cell differentiation (initiation) or 48 hours post initiation of differentiation (post) (Fig. 4c). SR1001 was also effective at inhibiting intracellular IL-17 expression in human peripheral blood mononuclear cells (hPBMCs) (Fig. 4d). Finally, we examined the effects of SR1001 on other T helper cell lineages. Differentiation of TH1, TH2, and iTreg cells was unaffected by SR1001 treatment as similar amounts of IFNγ, IL-4, or Foxp3, respectively, were expressed compared to vehicle controls, indicating that SR1001 specifically targets TH17 cells (Supplementary Fig. 7).
Figure 4. SR1001 inhibits TH17 cell development and IL-17A secretion.
a, IL-17 expression in splenocytes cultured under TH17 polarizing conditions with vehicle control (DMSO) or SR1001 (5µM). Graphs represent the average percentage of IL-17A expressing cells normalized to vehicle control (n=3). b, IL-17 expression in differentiated purified naïve murine CD4+ T cells normalized to vehicle control (n=3). c, IL-17A secretion from splenocytes cultured under TH17 polarizing conditions with SR1001 (5µM) for 3 days (n=3). d, Intracellular IL-17A expression in hPBMCs cultured for 24 hours with vehicle or SR1001 (5µM) (n=3). e, Treatment with SR1001 suppresses the clinical severity of EAE: vehicle (●, n=12) or SR1001 (25 mg kg−1) (▲, n=10). f, Il17a, Il21, and Il22 mRNA expression from spinal cords of sham-operated (IFA), vehicle control (V), or drug treated (SR1001) mice. (n=4). Error bars denote sem. ***P <0.001; **P <0.01; and *P <0.05.
Given that RORα and RORγt are required for development of TH17-mediated autoimmune diseases4,5 and SR1001 inhibits the activity of both of these receptors leading to suppressed TH17 cell development in vitro, we evaluated the effects of SR1001 treatment in an animal model of multiple sclerosis, experimental autoimmune encephalomyelitis (EAE), a well characterized model of TS17 cell-mediated autoimmune disease14, 15. After myelin oligodendrocyte glycoprotein (MOG35–55) immunization at day 0, mice were treated with 25 mg kg−1 of SR1001 b.i.d. i.p. for the duration of the study. As shown in Figure 4e, SR1001 treatment delayed the onset and clinical severity of EAE. Further analysis of spinal cords from mice harvested at day 18 post-immunization revealed that SR1001 repressed Il17a mRNA expression by ~60%, as well as reduced Il21, and Il22 mRNA expression (Fig. 4f). Intracellular cytokine analysis of splenocytes indicated a significant reduction in IL-17 expression and reduced total CD4+ T cells with no effect on CD8+ T cells. mRNA expression of IL-4 and IFNγ was unaffected in both spleen and spinal cords (Supplementary Fig. 8). These data are consistent with our interpretation that SR1001 suppresses EAE through its effects on TH17 cell function in vivo. Further optimization of SR1001 may yield compounds with greater activity.
While RORα and RORγt expression and activity are essential for full TH17 cell development, it is important to note that RORα and RORγ have roles outside of the immune system and are critical regulators of hepatic metabolism. Administration of SR1001 to C57BL/6 mice suppressed the expression of hepatic ROR target genes, Cyp7b1, Rev-erbα, and Serpine 1 (Pai-1) suggesting that this class of compound may have metabolic effects; however, we noted no obvious toxicity in animals treated with SR1001 (Supplementary Fig. 9)16–18.
In summary, we describe a novel, first-in-class, highly selective drug targeting the orphan NRs RORα and RORγ that effectively suppresses TH17 cell differentiation and cytokine expression and reduces the severity of disease in an animal model of multiple sclerosis. Our data indicates that the targeting of TH17 cells, by blocking RORα/γ function with a synthetic ligand, is a tractable approach for potential therapeutic intervention. Current treatments for TH17-mediated autoimmune diseases, including multiple sclerosis, utilize agents that are general immunosuppressant’s and thus the side effect profile is significant. Clearly, our data demonstrates that by targeting RORα and RORγ one can specifically inhibit TH17 cells without affecting other T helper cell lineages thereby providing a more focused therapy that will not be a general immunosuppressant. Therefore, SR1001 and derivatives of this compound may represent a novel class of superior drugs to not only treat TH17-mediated autoimmune disorders, but ROR-mediated metabolic disorders as well.
Methods Summary
Synthesis of SR1001 (N-(5-(N-(4-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)phenyl)sulfamoyl)-4-methylthiazol-2-yl)acetamide). A solution of 2-(4-aminophenyl)-1,1,1,3,3,3-hexafluoropropan-2-ol (0.88 g, 3.4 mmol), 2-acetamido-4-methylthiazole-5-sulfonyl chloride (0.79 g, 3.1 mmol) in acetone (15 mL) and 2,6-lutidine (0.73 mL, 6.2 mmol) was warmed to 60°C for 18 h. The reaction was judged complete by analytical HPLC (starting materials consumed).
Supplementary Material
Acknowledgements
This work was supported by NIH grants to T.P.B (DK080201, DK089984 and MH092769) and P.R.G (GM084041) and a grant from the National Multiple Sclerosis Society grant to P.D.D. (RG389A2/1). Additionally, the efforts of P.R.G. and W.R.R. were supported by the NIH Molecular Library Screening Center Network (MLSCN) grant U54MH074404 (Hugh Rosen, Principal Investigator).
Footnotes
Author Contributions P.R.G and T.P.B. conceived the project. L.A.S., P.R.G., and T.P.B. planned the project. Medicinal chemistry was planned and performed by P.N., T.M.K. and W.R.R. Biochemical and cell based assays were performed by L.A.S., N.S., Y.W., J.L., and M.A.I. Molecular modeling was performed by D.V and S.C.C. The EAE model was designed and performed by J.X., G.W. and P.D.D. HDX studies were performed by J.L.L. The manuscript was written by L.A.S. and T.P.B.
Competing Financial Interests The authors declare no competing financial interests.
References
- 1.McGeachy MJ, Cua DJ. Th17 cell differentiation: The long and winding road. Immunity. 2008;28:445–453. doi: 10.1016/j.immuni.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Littman DR, Rudensky AY. Th17 and Regulatory T Cells in Mediating and Restraining Inflammation. Cell. 140:845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 4.Yang XXO, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ivanov II, et al. The orphan nuclear receptor ROR gamma t directs the differentiation program of proinflammatory IL-17(+) T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 6.Ivanov II, Zhou L, Littman DR. Transcriptional regulation of Th17 cell differentiation. Semin. Immunol. 2007;19:409–417. doi: 10.1016/j.smim.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manel N, Unutmaz D, Littman DR. The differentiation of human T-H-17 cells requires transforming growth factor-beta and induction of the nuclear receptor ROR gamma t. Nat. Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar N, et al. The benzenesulfonamide T0901317 is a novel ROR{alpha}/{gamma} Inverse Agonist. Molecular Pharmacology. 2010;77:228–236. doi: 10.1124/mol.109.060905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang FP, Meng GX, Strober W. Interactions among the transcription factors Runx1, ROR gamma t and Foxp3 regulate the differentiation of interleukin 17-producing T cells. Nat. Immunol. 2008;9:1297–1306. doi: 10.1038/ni.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ichiyama K, et al. Foxp3 inhibits ROR gamma t-mediated IL-17A mRNA transcription through direct interaction with ROR gamma t. Journal of Biological Chemistry. 2008;283:17003–17008. doi: 10.1074/jbc.M801286200. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, et al. Modulation of retinoic acid receptor-related orphan receptor alpha and gamma activity by 7-oxygenated sterol ligands. J Biol Chem. 285:5013–5025. doi: 10.1074/jbc.M109.080614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chopra AR, et al. Absence of the SRC-2 coactivator results in a glycogenopathy resembling Von Gierke's disease. Science. 2008;322:1395–1399. doi: 10.1126/science.1164847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin LH, et al. Structural Basis for Hydroxycholesterols as Natural Ligands of Orphan Nuclear Receptor ROR gamma. Molecular Endocrinology. 2010;24:923–929. doi: 10.1210/me.2009-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu J, Wagoner G, Douglas JC, Drew PD. Liver X receptor agonist regulation of Th17 lympocyte function in autoimmunity. J Leukoc Biol. 2009;86:401–409. doi: 10.1189/jlb.1008600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu JH, Racke MK, Drew PD. Peroxisome proliferator-activated receptor-alpha agonist fenofibrate regulates IL-12 family cytokine expression in the CNS: relevance to multiple sclerosis. Journal of Neurochemistry. 2007;103:1801–1810. doi: 10.1111/j.1471-4159.2007.04875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delerive P, Chin WW, Suen CS. Identification of Reverb(alpha) as a novel ROR(alpha) target gene. J Biol Chem. 2002;277:35013–35018. doi: 10.1074/jbc.M202979200. [DOI] [PubMed] [Google Scholar]
- 17.Wada T, et al. Identification of oxysterol 7alpha-hydroxylase (Cyp7b1) as a novel retinoid-related orphan receptor alpha (RORalpha) (NR1F1) target gene and a functional cross-talk between RORalpha and liver X receptor (NR1H3) Mol Pharmacol. 2008;73:891–899. doi: 10.1124/mol.107.040741. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Yin L, Lazar MA. The orphan nuclear receptor Rev-erb alpha regulates circadian expression of plasminogen activator inhibitor type 1. J Biol Chem. 2006;281:33842–33848. doi: 10.1074/jbc.M607873200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.