Abstract
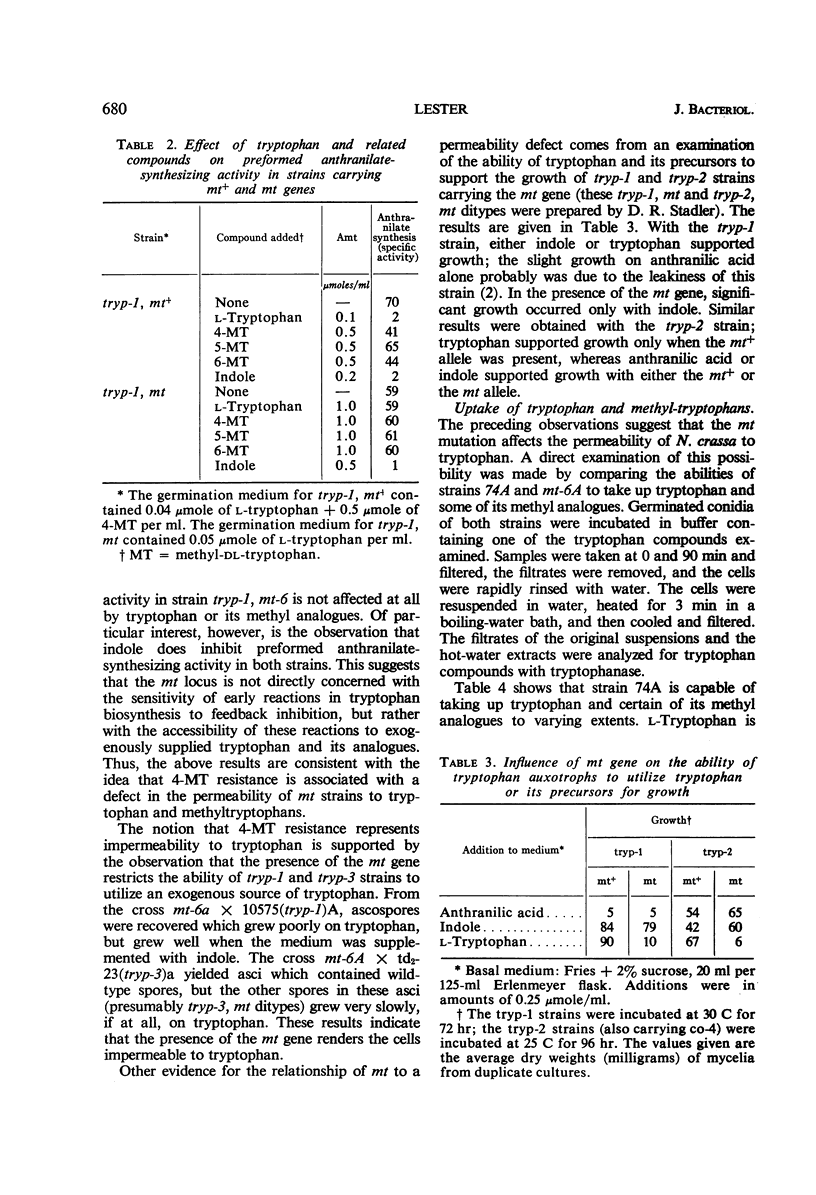
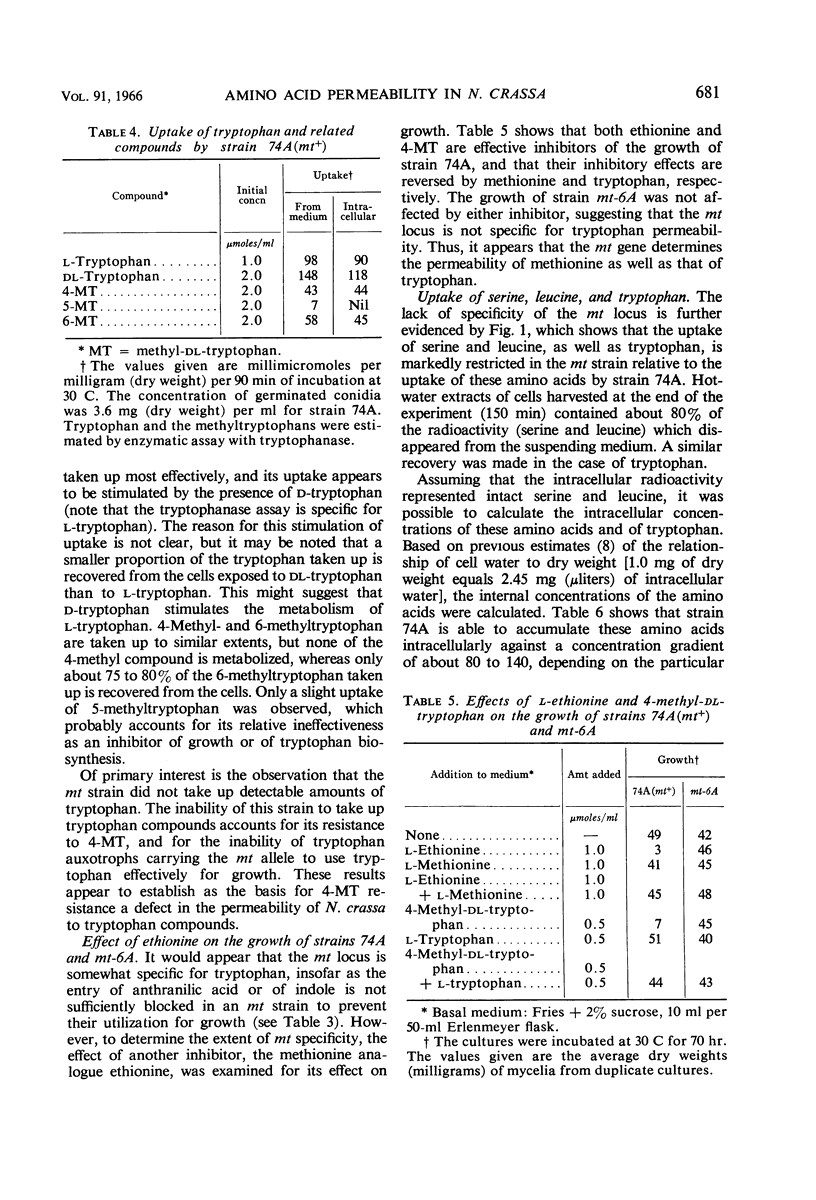
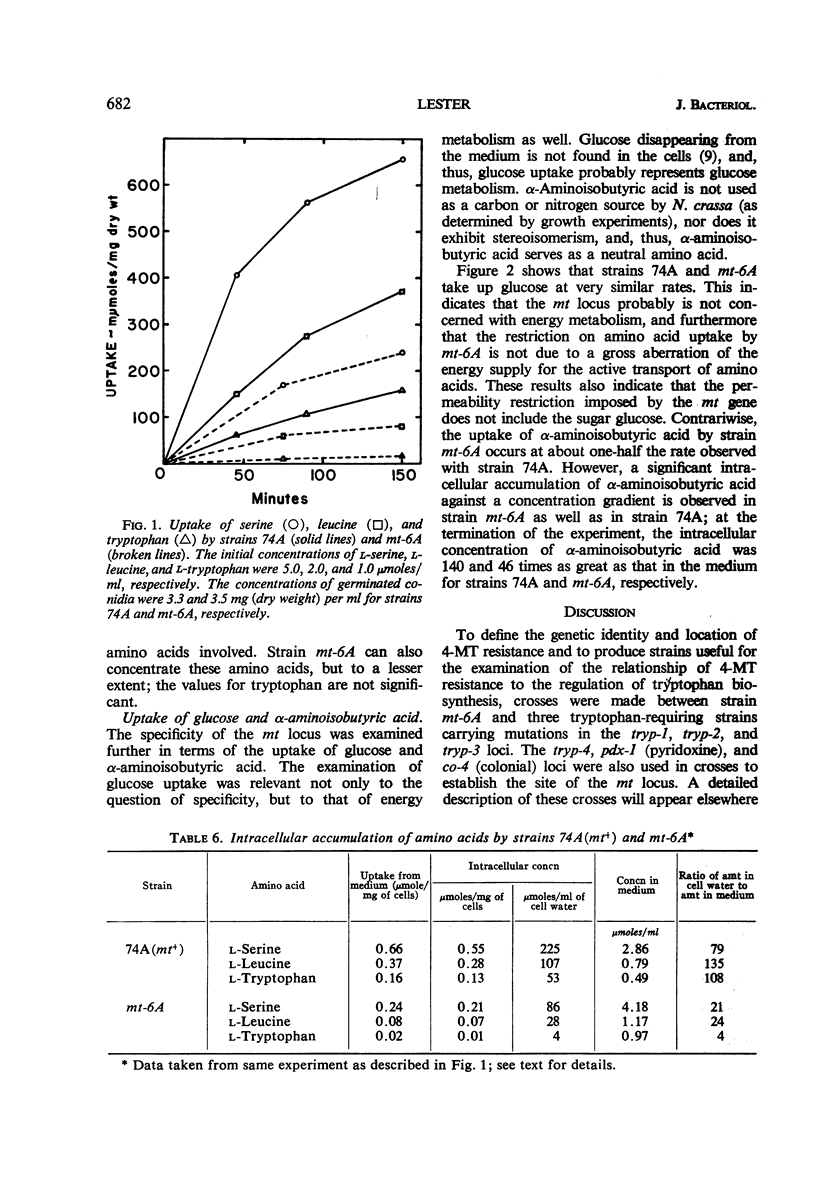
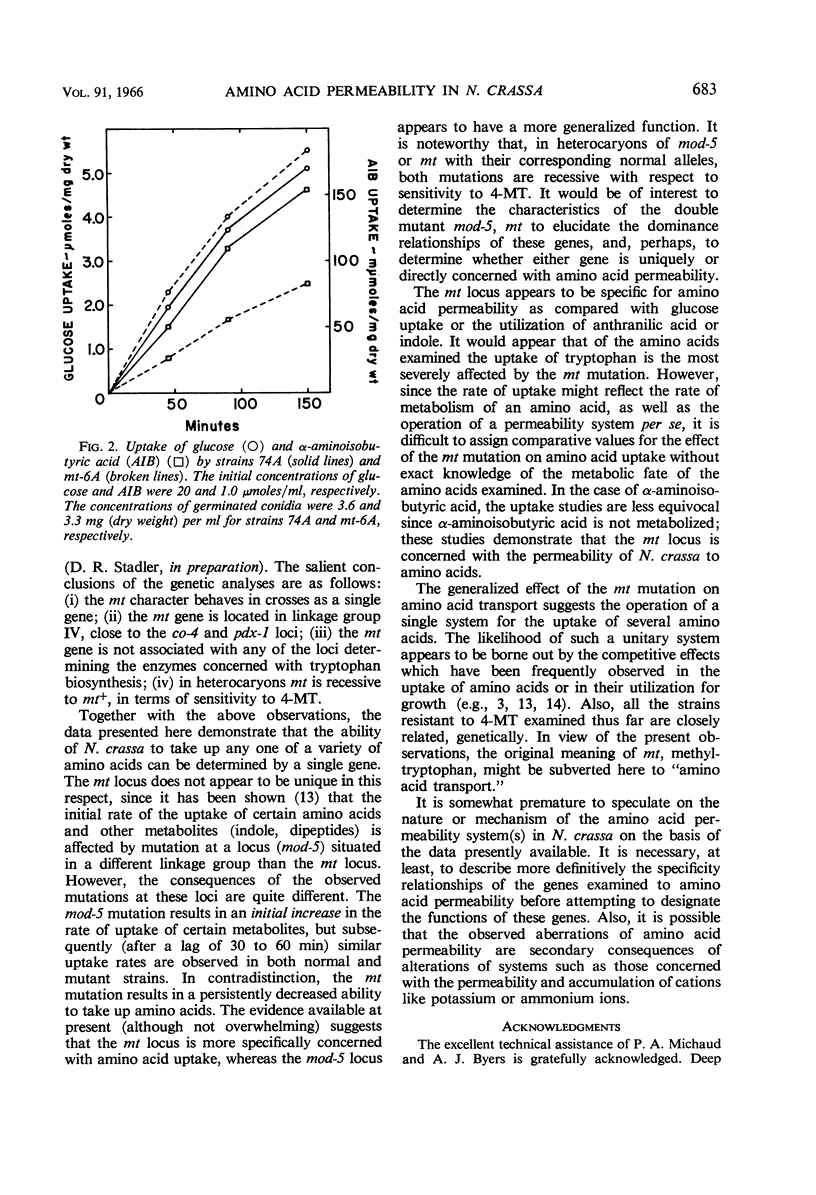
Lester, Gabriel (Reed College, Portland, Ore.). Genetic control of amino acid permeability in Neurospora crassa. J. Bacteriol. 91:677–684. 1966.—Strains of Neurospora crassa resistant to 4-methyltryptophan (4-MT) were isolated from populations of conidia exposed to ultraviolet light. In genetic crosses, 4-MT resistance behaved as a single-gene difference. Resistance to 4-MT could not be attributed to a relaxation of control of the formation or the activity of the enzymes of tryptophan biosynthesis. Growth studies involving tryptophan auxotrophs carrying the aberrant mt gene and uptake studies with normal and 4-MT-resistant strains showed that 4-MT resistance could be attributed to an inability of 4-MT-resistant strains to take up tryptophan and its methyl analogues. The mt gene is not specific for tryptophan; strains resistant to 4-MT are also resistant to ethionine, and they have a markedly reduced ability to take up serine, leucine, and α-aminoisobutyric acid. No difference was observed between strains carrying either mt allele in their ability to take up glucose; also, the uptake of anthranilic acid or of indole was not sufficiently impaired by the aberrant mt gene to prevent these tryptophan precursors from satisfying the nutritional requirement of certain tryptophan auxotrophs. The role of the mt gene in determining the permeability of N. crassa to amino acids is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROCKMAN H. E. EFFECTS OF AMINO ACIDS ON THE UTILIZATION OF TRYPTOPHAN AND INDOLE FOR GROWTH BY A MUTANT OF NEUROSPORA CRASSA. J Gen Microbiol. 1964 Jan;34:31–41. doi: 10.1099/00221287-34-1-31. [DOI] [PubMed] [Google Scholar]
- Bonner D. M., Yanofsky C., Partridge C. W. Incomplete Genetic Blocks in Biochemical Mutants of Neurospora. Proc Natl Acad Sci U S A. 1952 Jan;38(1):25–34. doi: 10.1073/pnas.38.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN G., JACOB F. Sur la répression de la synthèse des enzymes intervenant dans la formation du tryptophane chez Escherichia coll. C R Hebd Seances Acad Sci. 1959 Jun 15;248(24):3490–3492. [PubMed] [Google Scholar]
- LESTER G., AZZENA D., HECHTER O. Permeability and metabolism of lactose in Neurospora crassa. J Bacteriol. 1962 Aug;84:217–227. doi: 10.1128/jb.84.2.217-227.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESTER G. Regulation of early reactions in the biosynthesis of tryptophan in Neurospora crassa. J Bacteriol. 1963 Feb;85:468–475. doi: 10.1128/jb.85.2.468-475.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESTER G. Repression and inhibition of indole-synthesizing activity in Neurospora crassa. J Bacteriol. 1961 Aug;82:215–223. doi: 10.1128/jb.82.2.215-223.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESTER G., STONE D., HECHTER O. The effects of deoxycorticosterone and other steroids on Neurospora crassa. Arch Biochem Biophys. 1958 May;75(1):196–214. doi: 10.1016/0003-9861(58)90410-7. [DOI] [PubMed] [Google Scholar]
- LESTER G. Some aspects of tryptophan synthetase formation in Neurospora crassa. J Bacteriol. 1961 Jun;81:964–973. doi: 10.1128/jb.81.6.964-973.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESTER G., YANOFSKY C. Influence of 3-methylanthranilic and anthranilic acids on the formation of tryptophan synthetase in Escherichia coli. J Bacteriol. 1961 Jan;81:81–90. doi: 10.1128/jb.81.1.81-90.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATCHETT W. H., DEMOSS J. A. Factors affecting increased production of tryptophan synthetase by a TD mutant of Neurospora crassa. J Bacteriol. 1962 Jun;83:1294–1300. doi: 10.1128/jb.83.6.1294-1300.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOYED H. S. False feedback inhibition: inhibition of tryptophan biosynthesis by 5-methyltryptophan. J Biol Chem. 1960 Apr;235:1098–1102. [PubMed] [Google Scholar]
- STLAWRENCE P., MALING B. D., ALTWERGER L., RACHMELER M. MUTATIONAL ALTERATION OF PERMEABILITY IN NEUROSPORA: EFFECTS ON GROWTH AND THE UPTAKE OF CERTAIN AMINO ACIDS AND RELATED COMPOUNDS. Genetics. 1964 Dec;50:1383–1402. doi: 10.1093/genetics/50.6.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soboren J., Nyc J. F. AMINO ACID INTERACTIONS IN NEUROSPORA CRASSA. J Bacteriol. 1961 Jul;82(1):20–25. doi: 10.1128/jb.82.1.20-25.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRUDINGER P. A., COHEN G. N. The effect of 4-methyltryptophan on growth and enzyme systems of Escherichia coli. Biochem J. 1956 Mar;62(3):488–491. doi: 10.1042/bj0620488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]


