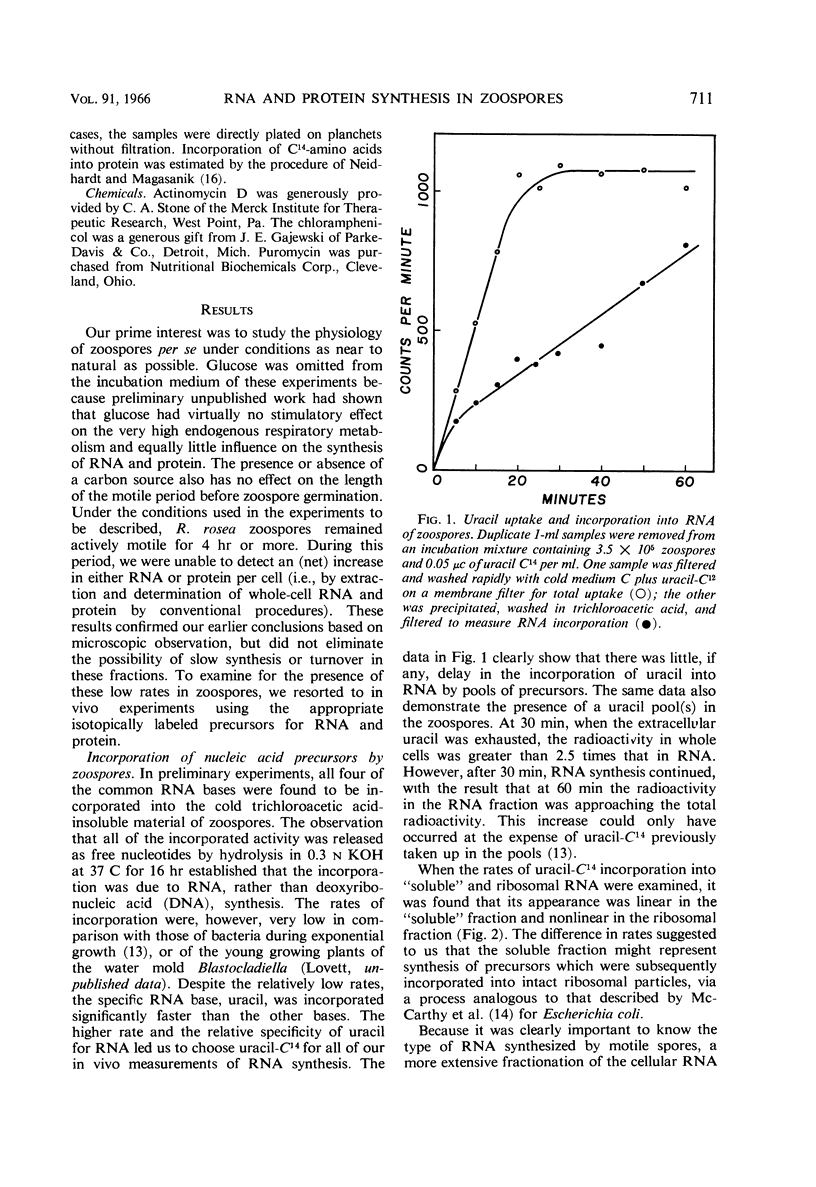
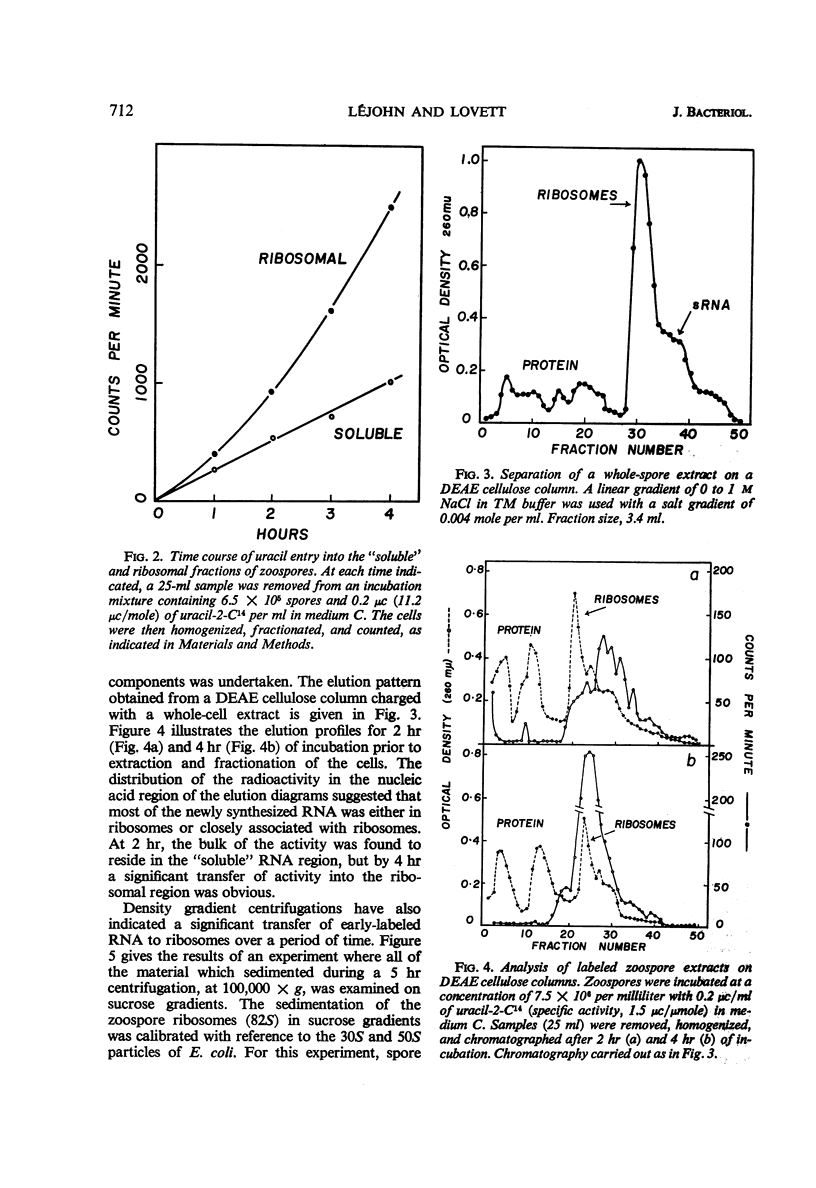
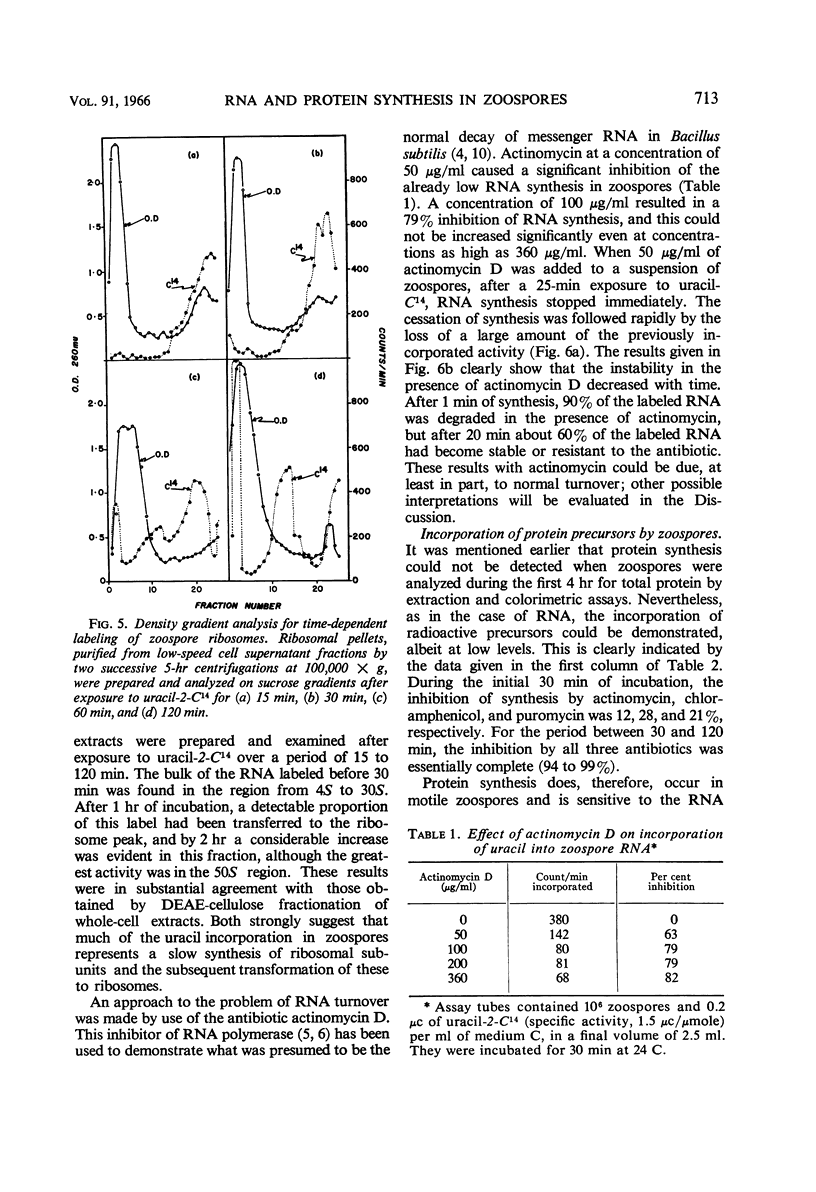
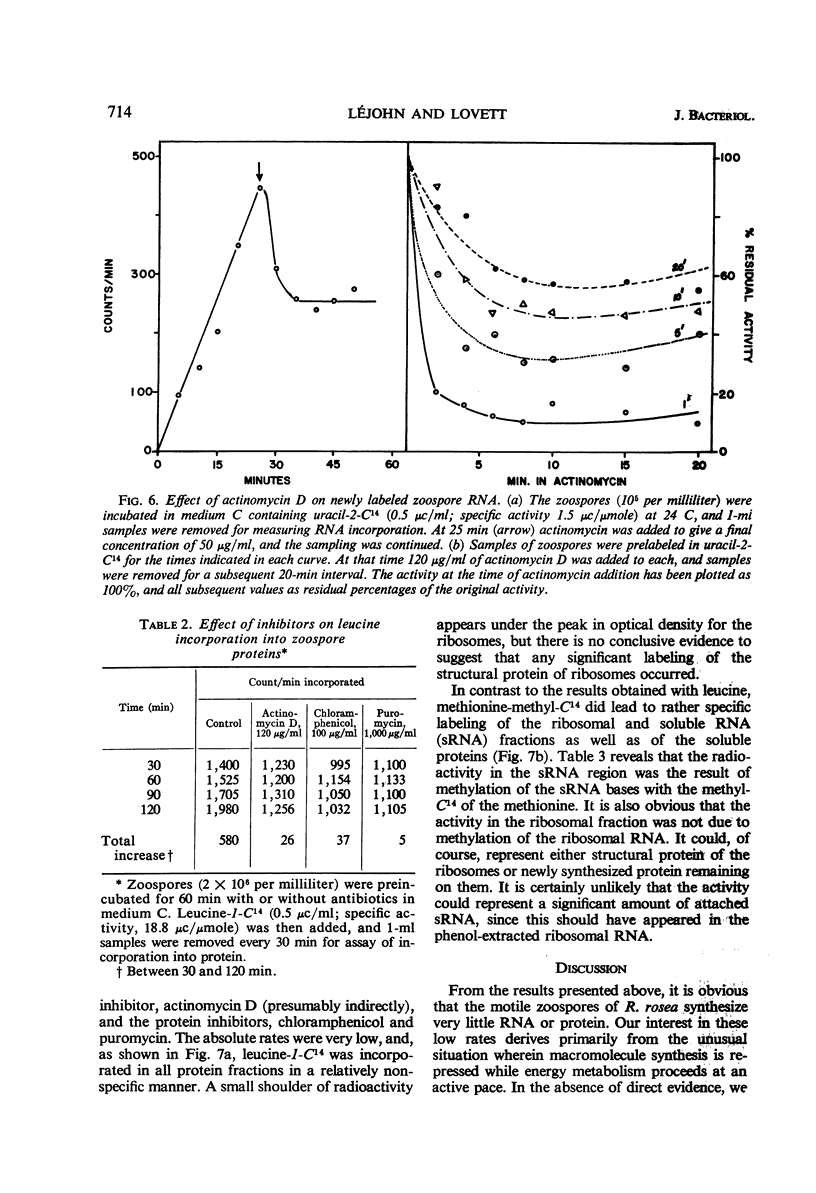
Abstract
LéJohn, Herbert B. (Purdue University, Lafayette, Ind.), and James S. Lovett. Ribonucleic acid and protein synthesis in Rhizophlyctis rosea zoospores. J. Bacteriol. 91:709–717. 1966.—The uniflagellate zoospores of Rhizophlyctis rosea display active motility and a high endogenous respiratory metabolism, but neither growth nor net ribonucleic acid (RNA) or protein synthesis can be measured by ordinary procedures. Nevertheless, synthesis can be detected with isotopic precursors. Uracil-C14 is incorporated slowly into both the soluble and ribosomal RNA. Analysis of zoospore extracts (on diethylaminoethyl cellulose columns or sucrose gradients) after various periods of labeling suggested that most of the uracil incorporation represents slow synthesis of ribosomal precursor RNA and, ultimately, ribosomes. Actinomycin D caused an 80% inhibition of uracil incorporation. The most rapidly labeled RNA was susceptible to extensive degradation in cells treated with actinomycin, but the percentage of stable RNA increased with the time of incorporation before addition of the antibiotic. Neither the effects of actinomycin nor the results of chase experiments have established unequivocally the existence of turnover or the presence of a short-lived “messenger” fraction in motile spores. Both leucine and methionine were slowly incorporated into a spectrum of cellular proteins. The methyl group of C14-methylmethionine also served as a methyl donor for the methylation of soluble RNA but not of ribosomal RNA. The observations that some of the newly synthesized RNA and protein occur in the intact 82S ribosomes and that actinomycin inhibits the low level of protein synthesis provide some indirect evidence for a very low rate of “messenger” synthesis and turnover in zoospores.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Britten R. J., Roberts R. B. High-Resolution Density Gradient Sedimentation Analysis. Science. 1960 Jan 1;131(3392):32–33. doi: 10.1126/science.131.3392.32. [DOI] [PubMed] [Google Scholar]
- CANTINO E. C., LOVETT J. S. NON-FILAMENTOUS AQUATIC FUNGI: MODEL SYSTEMS FOR BIOCHEMICAL STUDIES OF MORPHOLOGICAL DIFFERENTIATION. Adv Morphog. 1964;4:33–93. doi: 10.1016/b978-1-4831-9950-4.50005-9. [DOI] [PubMed] [Google Scholar]
- FAN D. P., HIGA A., LEVINTHAL C. MESSENGER RNA DECAY AND PROTECTION. J Mol Biol. 1964 Feb;8:210–222. doi: 10.1016/s0022-2836(64)80130-3. [DOI] [PubMed] [Google Scholar]
- GOLDBERG I. H., RABINOWITZ M., REICH E. Basis of actinomycin action. I. DNA binding and inhibition of RNA-polymerase synthetic reactions by actinomycin. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2094–2101. doi: 10.1073/pnas.48.12.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HURWITZ J., FURTH J. J., MALAMY M., ALEXANDER M. The role of deoxyribonucleic acid in ribonucleic acid synthesis. III. The inhibition of the enzymatic synthesis of ribonucleic acid and deoxyribonucleic acid by actinomycin D and proflavin. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1222–1230. doi: 10.1073/pnas.48.7.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ S., COMB D. G. A NEW METHOD FOR THE DETERMINATION OF THE BASE COMPOSITION OF RIBONUCLEIC ACID. J Biol Chem. 1963 Sep;238:3065–3067. [PubMed] [Google Scholar]
- KENNELL D. PERSISTENCE OF MESSENGER RNA ACTIVITY IN BACILLUS MEGATERIUM TREATED WITH ACTINOMYCIN. J Mol Biol. 1964 Sep;9:789–800. doi: 10.1016/s0022-2836(64)80185-6. [DOI] [PubMed] [Google Scholar]
- MCCARTHY B. J., BOLTON E. T. INTERACTION OF COMPLEMENTARY RNA AND DNA. J Mol Biol. 1964 Feb;8:184–200. doi: 10.1016/s0022-2836(64)80128-5. [DOI] [PubMed] [Google Scholar]
- McCarthy B. J., Britten R. J., Roberts R. B. The Synthesis of Ribosomes in E. coli: III. Synthesis of Ribosomal RNA. Biophys J. 1962 Jan;2(1):57–82. doi: 10.1016/s0006-3495(62)86841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy B. J., Britten R. J. The Synthesis of Ribosomes in E. coli: I. The Incorporation of C-Uracil into the Metabolic Pool and RNA. Biophys J. 1962 Jan;2(1):35–47. doi: 10.1016/s0006-3495(62)86839-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKADA D., ANDERSON I. A., MAGASANIK B. FATE OF THE RIBOSOMAL RNA PRODUCED BY A "RELAXED" MUTANT OF ESCHERICHIA COLI. J Mol Biol. 1964 Aug;9:472–488. doi: 10.1016/s0022-2836(64)80220-5. [DOI] [PubMed] [Google Scholar]
- NEIDHARDT F. C., MAGASANIK B. Studies on the role of ribonucleic acid in the growth of bacteria. Biochim Biophys Acta. 1960 Jul 29;42:99–116. doi: 10.1016/0006-3002(60)90757-5. [DOI] [PubMed] [Google Scholar]


