Abstract
Telomeres containing vertebrate-type DNA repeats can be stably maintained in Saccharomyces cerevisiae cells. We show here that telomerase is required for growth of yeast cells containing these vertebrate-type telomeres. When present at the chromosome termini, these heterologous repeats elicit a DNA damage response and a certain deprotection of telomeres. The data also show that these phenotypes are due only to the terminal localization of the vertebrate repeats because if they are sandwiched between native yeast repeats, no phenotype is observed. Indeed and quite surprisingly, in this latter situation, telomeres are of virtually normal lengths, despite the presence of up to 50% of heterologous repeats. Furthermore, the presence of the distal vertebrate-type repeats can cause increased problems of the replication fork. These results show that in budding yeast the integrity of the 3′ overhang is required for proper termination of telomere replication as well as protection.
Keywords: Chromosomes, DNA Synthesis, DNA-Protein Interaction, Telomeres, Yeast Genetics, Chromosome Capping, Telomerase
Introduction
Specialized ribonucleoprotein complexes known as telomeres protect the natural ends of eukaryotic chromosomes. In the budding yeast Saccharomyces cerevisiae, telomeres are composed of an ∼300-bp double-stranded (ds)4 tract of irregular repeats, abbreviated TG1–3/C1–3A, and terminate with a 12- to 14-nt single-stranded (ss) 3′ overhang made of the TG1–3 motifs (1–3). In contrast, vertebrate telomeres contain a ds tract containing thousands of T2AG3/C3TA2 repeats and terminate with a ss 3′ overhang made of T2AG3 motifs (2, 4, 5).
The complete replication of linear eukaryotic chromosomes requires the de novo addition of telomeric repeats to chromosome ends by telomerase, a specialized reverse transcriptase (for reviews, see Refs. 6–9). The core telomerase components are a reverse-transcriptase catalytic subunit and a RNA moiety. In yeast, these components are known as Est2p and TLC1, respectively (8). The telomerase RNA always contains a short region that is complementary to the G-rich strand of telomeric repeats, and that region is used as a template for repeat addition (7, 9).
Telomerase invalidation leads to gradual telomere shortening and ultimately causes cells to stop dividing (7, 9, 10). Nevertheless, cellular backup mechanisms can maintain telomeres in the absence of telomerase and ultimately preserve genome integrity. In this mode of telomere maintenance, which is known as alternative lengthening of telomeres in human cells, and survivor mode in Saccharomyces cerevisiae, telomeric DNA is maintained via homologous recombination-based mechanisms (11, 12).
By differentiating chromosomal ends from internal ds breaks, telomeres also prevent chromosomal ends from eliciting DNA damage checkpoint activation and protect telomeres from inappropriate DNA repair activity. Binding of the multiprotein complex shelterin is essential for both protecting and maintaining the length of mammalian telomeres (13, 14). Rap1p, one of the several proteins associated with similar functions at budding yeast telomeres, binds directly to ds telomeric repeats and functions to regulate telomere length homeostasis and to prevent telomere-telomere fusions (7, 9, 15–17).
In S. cerevisiae, the terminal telomeric repeat tracts can be changed from the budding yeast-specific sequences to vertebrate-type repeats by changing the RNA template region of TLC1 to a C3TA2-rich sequence (18). Analyses of S. cerevisiae strains containing such C3TA2-rich telomeres have shown that these telomeres are devoid of Rap1p but are bound by Del ScTbf1p, which appears to be able to maintain telomeres in the absence of Rap1p (19–21). However, there is also evidence that these vertebrate-type repeats are subject to high turnover, the cells are subjected to cellular stress, and that in the absence of the DNA damage checkpoint kinases Tel1p and Mec1p, telomeres are prone to fusing (22, 23). It therefore remained unclear how these new chimeric telomeric protein-DNA complexes ensure chromosome protection and the maintenance of telomeric repeat tracts.
Our results shown here demonstrate that yeast strains with distal vertebrate telomeric repeats absolutely depend on the telomerase enzyme for growth, suggesting that such chromosome ends require very frequent telomerase elongation events. Furthermore, the presence of significant 3′ vertebrate repeat overhangs at chromosomal ends activates the DNA damage response. Quite surprisingly, composite telomeres, on which as much as 146 bp of vertebrate telomeric repeats are sandwiched between native yeast repeats, remained at a length indistinguishable of endogenous wild-type yeast telomeres and did not cause any cellular phenotypes. We propose that yeast-specific telomere proximal and distal repeats may allow some structural arrangement that can determine overall telomere length, even in the presence of intervening non-yeast telomeric sequences.
EXPERIMENTAL PROCEDURES
Strains, Media, and Plasmids
All yeast strains and plasmids used in this study are summarized in supplemental Tables S1 and S2, respectively. Yeasts were manipulated according to standard methods (24). Plasmids and deletion cassettes were transformed according to Ref. 25.
Strain Constructions
Deletions of indicated genes were generated by a one-step PCR gene replacement technique where the complete ORF was replaced by a kanMX4 or natMX4 resistance cassette (26). Doxycycline-regulatable strains were generated by a one-step PCR substitution of the original promoter by a kanMX4-tTA-ADH1t-tetO2 promoter amplified from pCM224 (27). All created strains were validated for correct gene disruption or promoter substitution by appropriate phenotypic analysis and genomic locus arrangement by PCR.
Viability Assays
Yeast strains were grown in the appropriate media until they reached exponential phase (1 × 107 cells/ml). Cell concentrations were measured at A660 nm. A volume of an equal number of cells for each strain was serially diluted in steps of 10-fold and spotted on the indicated plate media.
Protein Extracts and Western Blotting
Yeast cells were grown to exponential phase, and total protein extracts were prepared by a TCA precipitation procedure as described in Ref. 28. Proteins were resolved on a 8% acrylamide/Bis-acrylamide (30:0.39) SDS-PAGE gel, transferred to a membrane, and Rad53p forms were detected with an anti-Rad53 rabbit antibody (29) (generous gift of J. Heierhorst, St. Vincent's Institute of Medical Research, Melbourne, Australia), followed by secondary detection with horseradish peroxidase-conjugated donkey anti-rabbit antibody (GE Healthcare).
Telomere PCR and Terminal Restriction Fragment Analyses
Amplification and cloning of the unique V-R ADE2 telomere from ScV500Sc and ScV1000Sc strains was performed as described previously (23). Amplification of telomere-telomere (T-T) fusions from ScV2000 and ScV2500 strains was performed as described in Ref. 16, with slight modifications. HindIII-X2 (cgcAAGCTTTGTGGTGGTGGGATTAGAGTGGTAG) and EcoRI-Y'2 (cgGAATTCTTAGGGCTATGTAGAAGTGCTG) primers were used for T-T PCR, and products were cloned in pUC19. As a loading control for each sample, ARO1+ (TGACTGGTACTACCGTAACGGTTC) and ARO1- (GAATACCATCTGGTAATTCTGTAGTTTTGAC) primers were used to amplify a 371 bp fragment corresponding to the ORF of the non-telomeric gene ARO1 (30). All plasmids were sequenced at the Centre Hôpitalier Universitaire Laval Research Center, Quebec City, Canada.
32P-radiolabeled probes were used to detect single-stranded and double-stranded telomeric DNA containing either vertebrate- or yeast-specific repeats in XhoI-digested genomic DNAs resolved on agarose gels, essentially as described previously (23, 31).
Cell Cycle Analysis by FACS
DNA content from asynchronously and exponentially growing cultures at 30 °C was measured as described previously (32).
RESULTS
Telomerase Loss in Yeast with Vertebrate-type Telomeres Causes Immediate Growth Arrest
We replaced the WT allele of the gene encoding the RNA moiety (TLC1) of the core telomerase of S. cerevisiae with the tlc1h allele, which encodes a vertebrate-type telomeric repeat sequence. This mutation causes a replacement of the distal yeast-specific telomeric repeat sequence (TG1–3)n with (T2AG3)n (18). The S. cerevisiae strains that rely on tlc1h for telomerase activity are designated “ScV yeast,” and the yeast-type and mixed-type telomeric repeats are hereafter designated “Sc” and “ScV” or “ScVSc,” respectively.
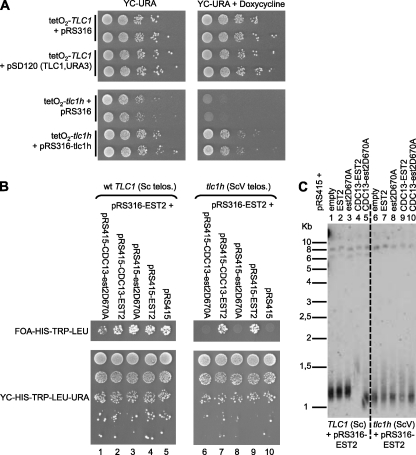
As expected (33), doxycycline-induced repression of the WT TLC1 gene in otherwise non-modified strains is tolerated for at least 50 generations without detectable major loss of viability (Fig. 1A, upper panels). In contrast, the repression of tlc1h in the ScV yeast cells led to an immediate growth arrest (Fig. 1A, lower panels). Consistent with this result, the ScV yeast cells required the catalytic activity of Est2p for growth (Fig. 1B) and were sensitive to Pif1p overexpression, which is known to inhibit telomere elongation by telomerase (supplemental Fig. S1, A–C and Ref. 34).
FIGURE 1.
Loss of telomerase activity causes immediate death of yeast cells with terminal vertebrate telomeric repeats. A, strains in which either the WT or the tlc1h allele of TLC1 are controlled by the tetO promoter (respectively tetO2-TLC1 or tetO2-tlc1h) and that also contained a centromeric plasmid, as indicated, were plated on selective media on which the tetO-controlled gene was expressed (left panels) or repressed (right panels). Note that the alleles on the plasmid are not controlled by tetO. B, cells with deletions of both EST2 and TLC1 (est2::HIS3 tlc1::KanMX4) contained either a WT TLC1 (left panels) or the allele templating vertebrate repeats (tlc1h) on a plasmid (right panels). For viability, all strains initially also contained a WT EST2 gene on a pRS316(URA3) plasmid as positive controls (bottom panels). Finally, strains contained pRS415(LEU2) plasmids harboring the EST2 alleles as indicated. Exponentially growing cultures were diluted by steps of 10-fold, and an equal number of cells were spotted onto plates. Cells on the top plates only harbor the EST2 alleles on the pRS415 plasmid as indicated on top (rightmost panel; lanes 5 and 10, negative control, empty pRS415; lanes 4 and 9, positive control with pRS415-EST2wt). Pictures were taken after incubating the plates for 3 days at 30 °C. Note that for the plates showing cells after pRS316-EST2 loss, only the row with the highest cell number is shown (top panels). C, genomic DNA was extracted from the above strains cultivated in synthetic complete drop-out media, digested with XhoI, and analyzed by Southern blotting using a telomere Y'-specific probe. All strains do harbor the WT allele of EST2 (as indicated with the pRS316-EST2 plasmid) and also express a second allele on the pRS315 plasmid as indicated on top of the lanes. Lanes 1–5, DNA derived from strains that express the WT allele of TLC1. Note the telomere elongation with the Cdc13-EST2 fusion protein (compare lanes 1 and 4). Lanes 6–10, DNA derived from strains that express the tlc1h allele. Note a slight telomere elongation with the Cdc13-EST2 fusion protein (compare lanes 6 and 9).
The observation that ScV yeast cells required continuous expression of telomerase for viability might be explained by the fact that the ScV telomeres were, on average, shorter than the WT telomeres. Thus, the ScV telomeres might rapidly become too short or otherwise dysfunctional after the loss of telomerase. However, the expression of a fused protein Cdc13-Est2p, which slightly increases the mean length of ScV telomeres (Fig. 1C and supplemental Fig. S2), did not allow the ScV cells to bypass their immediate requirement for telomerase (supplemental Fig. S2A). Consistent with the presence of irreversible telomere damage in these cells, ScV cells lacking RAD9 also lost viability without delay (data not shown).
Telomerase Loss Is Tolerated if Vertebrate-type Repeats Are Located Internally
The above results led us to examine whether the presence of Sc-type termini would ameliorate the exquisite sensitivity of ScV yeast to the loss of telomerase. Toward this end, after the cells were grown for 500 or 1000 generations with the tlc1h allele, the tlc1h allele was exchanged again with a WT TLC1 gene carried on a plasmid (creating strains designated ScV500Sc and ScV1000Sc, respectively). Sequencing of the resulting telomeres confirmed that yeast-specific sequences were incorporated distal to the preexisting vertebrate-type repeats (supplemental Fig. S3). In all cases examined, the telomeres retained a significant number of vertebrate-type repeats, so that T2AG3 repeats were sandwiched between TG1–3 repeats (supplemental Fig. S3, D and E). Importantly, the tracts of vertebrate-type telomeric repeats in ScVSc telomeres and in ScV telomeres were in most cases comparable in length (about 75 to 145 nt, see supplemental Fig. S3 and Ref. 23).
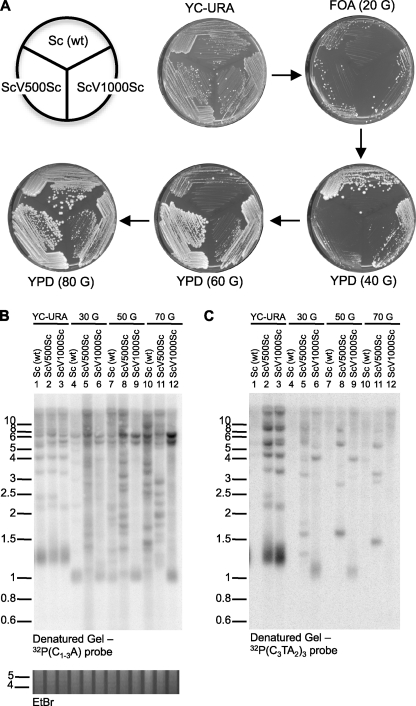
Like cells with WT Sc telomeres, cells with ScV500Sc or ScV1000Sc telomeres were initially able to form colonies on a plate after the loss of telomerase (Fig. 2A, plate FOA (20 G)). However, although the WT cells continued to be able to form normal colonies for at least one more passage, the colonies resulting from subsequent passages of the cells initially containing ScVSc telomeres were heterogeneous in size, and many were very small (Fig. 2A, plate YPD(40 G)). This latter pattern is reminiscent of that seen in senescing WT cultures after telomerase loss (10, 35, 36) (Fig. 2A, plate YPD(60 G)).
FIGURE 2.
Yeast cells with internal vertebrate telomeric repeats are able to lose telomerase but senesce very rapidly. A, all yeast strains harbored a deletion of the TLC1 gene in the genome (tlc1Δ::LEU2) and contained the indicated type telomeric repeats. The strains initially also contained the WT TLC1 gene on a URA3 plasmid (YC-URA). Strains were then replated onto a FOA plate that only allows growth of cells that had lost the URA3/TLC1 plasmid (FOA 20 G). Next, individual colonies from the FOA plate were consecutively restreaked onto rich plates YPD (40 G), YPD (60 G), and YPD (80 G). B, colonies from the strains in A were picked from the FOA and YPD plates, grown in liquid cultures, and XhoI-digested genomic DNA was analyzed by Southern blotting using a 32P(C1–3A) probe so as to detect total yeast telomeric DNA. DNA size markers are indicated on the left, and the estimated total number of generations grown is indicated on top. C, the gel shown in B was rehybridized to a vertebrate telomere-repeat specific probe. Note that the DNAs in lanes 1, 4, 7, and 10 from the Sc (WT) strain are not expected to hybridize to this probe. The EtBr-stained gel slice below B is shown as control for equal DNA loading.
Cultured cells after each growth period were examined for the structure of their telomeres (Fig. 2, B and C). About half of all telomeres in yeast lab strains contain a conserved subtelomeric element called Y' that harbors an XhoI site about 950 bp from the transition to the terminal repeat DNA (31). On Southern blots of XhoI-digested DNA probed with telomere-specific probes, the terminal restriction fragments of these telomeres are about 1.0 to 1.4 kb in size, depending on the length of the terminal repeats. The other half of the telomeres harbor a less conserved X element, and the terminal restriction fragments of those telomeres will be of various sizes above 2 kb (31). Cells initially containing WT Sc telomeres exhibited gradual telomere shortening (Fig. 2B, lanes 1 and 4), whereas the telomeres of both ScVSc telomere-containing cells were clearly rearranged after 30 generations (Fig. 2B, lanes 5 and 6), despite the fact that they started out with telomeres of about the same length as those of WT cells (Fig. 2B, compare lanes 1–3). This new arrangement was similar to that seen in survivors type I for the ScV500Sc telomere strain (Fig. 2, B and C, lanes 6, 9, and 12) and in survivors type II for the ScV1000Sc telomere strain (Fig. 2, B and C, lanes 5, 8, and 11) (see Refs. 37, 38). These data suggest that the majority of the distal yeast repeats were lost during initial cell growth and that as soon as the vertebrate-type repeats were exposed at the ends, the cells stopped growing, just as cells harboring ScV telomeres did (Fig. 1). Thus, in the absence of telomerase, the vertebrate-type repeats appear to represent an immediate threat to telomere stability when they constitute the 3′ terminus but not when they are located internally as a dsDNA sequence. However and remarkably, despite the presence of up to 24 T2AG3 repeats (about 145 bp) in these telomeres, the mean lengths of the ScV500Sc and ScV1000Sc telomeres are indistinguishable from those of telomeres containing yeast repeats only, i.e. WT telomeres (Fig. 2B, lanes 1–3) (see also Fig. 3 and supplemental Fig. S3F).
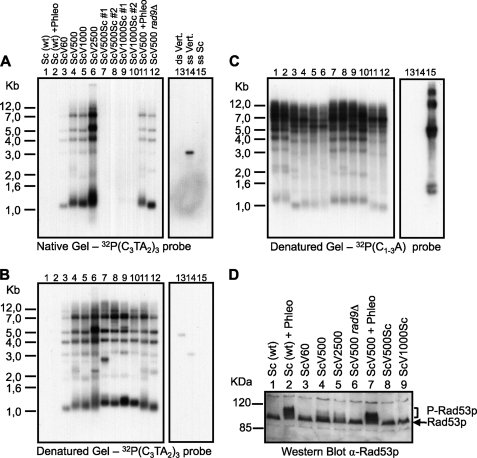
FIGURE 3.
Telomeres with internally located vertebrate repeats are not sensed as DNA damage. A–C, genomic DNAs were isolated from cells with wild-type telomeric repeats (Sc, lanes 1 and 2). Cells that have distal vertebrate repeats (ScV(n), lanes 3, 4, 5, 6, 11 and 12; where n indicates the number of generations the cells had grown in the presence of the tlc1h allele) or cells that had divided n times in the presence of tlc1h and then were converted back to a wild-type TLC1 allele (ScV(n)Sc, lanes 7, 8, 9 and 10). + Phleo indicates that the cells had been incubated with the radiomimetic phleomycin before analysis, and rad9Δ indicates that the corresponding strain lacked the RAD9 gene. XhoI-digested DNA fragments were resolved on 0.75% agarose gels and the DNA was hybridized first in non-denaturing conditions with a 32P(C3TA2)3 probe to detect the vertebrate repeat-specific telomeric overhangs (A). The gel was then denatured and hybridized to the same probe to detect total vertebrate-specific telomeric DNA (B). In parallel, the same samples of XhoI-digested DNA as in A were also subjected to regular Southern analysis using a 32P(C1–3A) probe to detect total yeast telomeric DNA (C). Lanes 13-15 in each panel show control DNAs harboring vertebrate telomeric repeats in double- and single-stranded forms (ds Vert., ss Vert.) or yeast telomeric repeats in single-stranded form (ss Sc). D, Western blot analysis of total protein extracts derived from wild-type cells (lanes 1 and 2). Shown are cells with distal vertebrate repeats (lanes 3 to 7) and cells with previously distal vertebrate repeats reconverted to wild-type repeats (lanes 8 and 9). The blot was probed with an anti-Rad53p antibody. + Phleo and rad9Δ are as in A–C. Molecular weight markers are indicated on the left.
A T2AG3 3′ Overhang Triggers a DNA Damage Response in S. cerevisiae
Because there is evidence that the DNA damage response (DDR) is activated in ScV yeast cells as shown by Rad53p phosphorylation assays (22), we next examined whether the DDR was associated with the presence of the T2AG3 3′ overhangs or was a consequence of other problems occurring during replication of vertebrate telomeric repeats in yeast.
As noted previously, the 3′ overhang made of vertebrate repeats gradually lengthened as the number of generations in the presence of the tlc1h allele increased (Fig. 3, A and B) (see also Refs.18, 23). However, significant Rad53p phosphorylation was not discernible before 60 generations of growth but was detectable after 500 generations of growth, coincident with a slight delay in the G2/M transition in such cultures (Fig. 3D, compare lanes 3 and 4, and supplemental Fig. S4). The level of Rad53 phosphorylation was relatively low compared with that triggered by the DNA-damaging agent phleomycin. In the absence of the checkpoint mediator Rad9p, Rad53p phosphorylation was lost, and the G2/M delay was abolished (Fig. 3D, lane 6 and supplemental Fig. S4). Upon reverting the T2AG3 3′ overhang to the normal yeast TG1–3 type (Fig. 3A, lanes 7–10), Rad53p phosphorylation and the G2/M delay are abolished (Fig. 3D, lanes 8 and 9 and supplemental Fig. S4).
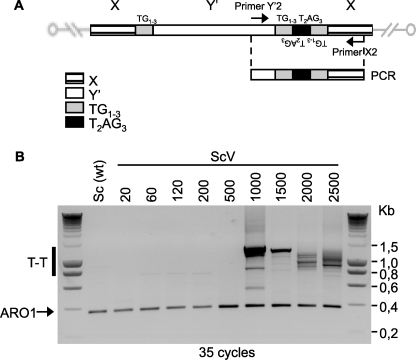
These data are thus consistent with the interpretation that terminal vertebrate-type telomeric repeats are deficient in providing normal capping function. Using a PCR-based assay specifically developed for detecting telomere fusions in yeast (16), we indeed detected telomere-telomere fusions in cultures of ScV yeast cells that had grown for more than 500 generations (Fig. 4). Interestingly, sequencing the fusion PCR products revealed human-type repeats at the fusion points (see examples in supplemental Fig. S5), indicating that at least in these cases, a failure of telomere capping allowed fusions directly onto the existing distal human-type repeats.
FIGURE 4.
Fusions of telomeres with terminal vertebrate telomeric repeats. A, schematic representation of a telomere-telomere fusion (T-T) between a Y' and X telomere in yeast cells with distal vertebrate repeats (ScV) and positioning of primers. The Y'2 and X2 primers are, respectively, 120 and 340 bp away from the first TG1–3 repeats, and amplification of a fragment where two telomeres are engaged in a fusion will generate a product of 460 bp (plus telomeric repeats, if present). B, PCR detection of telomere-telomere fusions (T-T) in Sc and ScV telomere yeast strains after the indicated numbers of generations of outgrowth. ARO1 indicates a 380-bp internal control fragment. DNA size standards are indicated on the right, and the black bar (T-T) indicates the area on the gel where telomere-telomere fusions should migrate.
We conclude that telomeres ending with vertebrate-type T2AG3 repeats are partially uncapped, leading to an activation of DDR and telomere fusions. Thus, telomerase appears to be required to maintain a level of capping compatible with growth.
Terminal T2AG3 Repeats Lead to Replication Damage
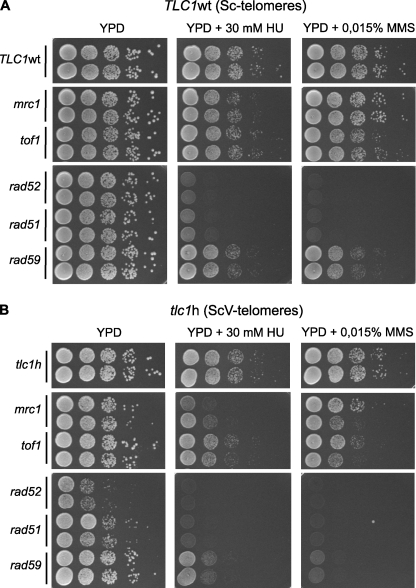
To be able to tolerate telomerase loss and maintain telomeric repeats, WT S. cerevisiae cells depend on Rad52 and Mms1, two proteins involved in the repair of stalled replication forks (36, 37). Indeed, deleting the RAD52 gene from ScV cells significantly decreased their ability to grow (Fig. 5). Moreover, when replication stress was induced by hydroxyurea treatment, the ScV cells were more sensitive than the WT cells to a loss of MRC1 and to a slightly lesser extent TOF1, two proteins required for normal fork progression (39, Fig. 5). Furthermore, as revealed by two-dimensional agarose gel electrophoresis, replication forks in ScV cells appeared to arrest near the telomeric repeats and signals for Holliday junctions, and replication bubbles were increased (supplemental Fig. S6). Therefore, yeast strains harboring vertebrate-type termini are impaired in the replication of their telomeres with heterologous repeats.
FIGURE 5.
Distal vertebrate repeats on telomeres cause sensitivity to replication stress. A, yeast cells with the WT allele of TLC1 (TLC1 wt, Sc telomeres) or B, cells with the allele templating distal vertebrate telomeric repeats (tlc1h, ScV telomeres) were engineered to also contain deletions of the genes indicated on the left. Cultures were pregrown in rich media to exponential growth phase, and 10-fold serial dilutions of equal number of cells were plated on YPD, YPD + 30 mm hydroxyurea, and YPD + 0.015% methyl methanesulfonate media. Plates were incubated at 30 °C for 2 days (TLC1 wt, A) or 3 days (tlc1h, B) before photographs were taken.
DISCUSSION
Budding yeast cells can be quite tolerant in terms of their requirements for the precise sequence of the telomeric repeats capping their chromosomes. Indeed, even telomeres composed entirely of vertebrate-type repeats are kept stable during mitotic growth (18, 20, 21). Remarkably, on these telomeres, the hallmark telomere binding protein Rap1p appears to be replaced with Tbf1p, and there is some evidence to show that this latter protein can maintain a telomere length regulatory mechanism (19, 20). However, it remained unclear how this mechanism could ensure telomere capping and stability in this situation.
The results shown here demonstrate that when cells harbor hybrid yeast-vertebrate telomeres, the enzyme telomerase becomes absolutely essential for continued cell division. Further, cells behaved the same whether a catalytic dead allele of Est2p is expressed or Est2p is lost altogether, showing that it is the activity of telomerase that is required (Fig. 1). One interpretation of these data would be that in ScV yeast cells, there always are a number of critically short telomeres that would cause growth arrest immediately after telomerase loss. However, several considerations lead us to suggest otherwise. First, in yku80Δ or tel1Δ cells containing telomeres that are as short as or even shorter than those described above, the transient loss of telomerase is tolerated. yku80Δtlc1Δ cells can grow for at least a few generations to generate microcolonies on plates, and tel1Δtlc1Δ cells can even form colonies (40, 41). Second, forced expression of a Cdc13-Est2p product, although only slightly elongating telomeres and formally not excluding the presence of a few critically short telomeres, also does not allow growth (Fig. 1, B and C). Third, yeast cells with ScVSc telomeres do allow telomerase loss, but cells stop growing after only about 20–30 generations of outgrowth (Fig. 2). This latter observation is consistent with the observation that during those first 20–30 generations, the distal most yeast repeats are progressively lost, and as soon as the vertebrate-type repeats become terminal, telomeres are destabilized. Taken together, these results therefore suggest that 3′ ends consisting of vertebrate-type T2AG3 repeats uncap yeast chromosome ends even in the presence of internal yeast-type repeats. As a consequence, telomeres with terminal vertebrate repeats may be subject to much more dynamic turnover than WT telomeres and therefore require telomerase-mediated extension at a very frequent rate. Furthermore, such ends will eventually elicit a chronic low-level DDR response and be subject to telomere-telomere fusions (Figs. 3 and 4). Of note, these fusions do occur in the presence of active Mec1p and Tel1p kinases. Therefore, the telomere-telomere fusions observed in late-generation ScV cells are not completely preventable by these kinases, as was reported previously (22). Presently, we ignore the reason for these experimental differences, but strain backgrounds and differing technical protocols could cause different outcomes. Nevertheless, the telomere-telomere fusions observed in the presence of the Tel1p/Mec1p kinases could also explain the increased gross chromosomal instability observed in late ScV yeast cells (22).
Quite remarkably, if located internally, as much as half of the repeats on yeast telomeres can be of the vertebrate-type without causing any telomere length changes or other perceptible phenotypes. Previous results showed that telomeric vertebrate-type repeats in yeast are bound by Tbf1p, but no Rap1p or Rif2p associations are detectable (21). Furthermore, the homeostasis of overall telomere length has been proposed to be regulated by a mechanism that somehow accounts for the actual number of Rap1p and, in particular, Rif-proteins present at telomeres (17), but evidence for Rap1p-independent telomere length control mechanisms exists (20). The near-WT length of mixed ScVSc-telomeres that contain about 50% fewer yeast repeats than an average WT Sc-telomere suggests that Rap1p binding at the subtelomere-telomere transition and at the distal-most repeats is crucial for allowing normal telomere homeostasis. It remains unclear whether in the situation of ScVSc telomeres, the vertebrate-type repeats, via the binding of Tbf1p, do actively contribute to overall telomere length regulation. Tbf1p has been shown to be able to contribute to telomere length regulation (19) and to promote telomere elongation of short telomeres, at least in the absence of Tel1p (42). However, it is also possible that the ∼75- to 145-bp vertebrate repeats are too short to separate the proximal repeats from the distal repeat tracts to allow the establishment of a new and distally located independent telomere (43). Yet, one previous report showed that 138 bp without a Rap1p binding site is sufficient to separate two tracts of Rap1p binding sequence to separate those domains (43).
The partial telomere uncapping described above is accompanied by increases in problems for telomere replication by the conventional machinery (Fig. 5 and supplemental Fig. S6), which suggests a tight and specific coupling between the 3′ overhang and replication termination at chromosome ends. We propose that in ScV yeast cells, this coupling is impaired, leading to aberrant replication termination, deprotection of chromosome ends, and DDR activation. A previous report showed that abolishing Tbf1p binding near telomeric repeat sequences did not affect replication fork stalling, suggesting that Tbf1p association with the block of vertebrate repeat DNA should not be a particular problem (44). However, juxtaposition and accumulation of a significant number of Tbf1p proteins may create a particular structure that is difficult to displace. Consistent with our fork collapse proposal, the ScV yeast cells are sensitive to loss of the ability to perform homologous recombination (Fig. 5), whereas a previous report did not observe this (21). We ignore the precise methodology used by those authors and therefore cannot make direct comparisons between the two. Thus, the precise mechanism by which telomerase prevents this replication-coupled and terminus-specific telomere injury remains to be determined.
Recent evidence obtained in a variety of systems, including budding yeast, fission yeast, and mammalian cells, strongly suggests that the passage of the replication fork through telomeric repeats is a challenge and requires the presence of telomere repeat binding proteins and specialized helicases (45–48). It has been proposed that these characteristics have important ramifications for human chromosome stability during DNA replication and for integration of mobile DNA elements (46). Being able to engineer hybrid yeast-vertebrate type telomeres in a genetically tractable system should allow us to further detail those evolutionary important mechanisms.
Supplementary Material
Acknowledgments
We thank J. Heierhorst for the Rad53 antibody and V. Lundblad and V. Zakian for plasmids.
This work was supported Canadian Institutes of Health Research (CIHR) Grant MOP-110982) (to R. J. W.) and by grants from the Association de la Recherche contre le Cancer (ARC), from the Institut National du Cancer (Program TELOFUN), from the Agence Nationale de la Recherche (ANR) (Program TELOREP and INNATELO), and from the European Community (TELOMARKER Health Grant F2-2007-200950) (to E. G.). This work was also supported by the Lavoisier Grant from the French Ministry of Foreign Affairs (to A. B.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S6, Tables S1 and S2, and references.
- ds
- double-stranded
- ss
- single-stranded
- T-T
- telomere-telomere
- DDR
- DNA damage response
- FOA
- 5-fluoro-orotic acid
- YPD
- yeast extract peptone D-glucose.
REFERENCES
- 1. Shampay J., Szostak J. W., Blackburn E. H. (1984) Nature 310, 154–157 [DOI] [PubMed] [Google Scholar]
- 2. LeBel C., Wellinger R. J. (2005) J. Cell Sci. 118, 2785–2788 [DOI] [PubMed] [Google Scholar]
- 3. Larrivée M., LeBel C., Wellinger R. J. (2004) Genes Dev. 18, 1391–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moyzis R. K., Buckingham J. M., Cram L. S., Dani M., Deaven L. L., Jones M. D., Meyne J., Ratliff R. L., Wu J. R. (1988) Proc. Natl. Acad. Sci. U.S.A. 85, 6622–6626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McElligott R., Wellinger R. J. (1997) EMBO J. 16, 3705–3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Greider C. W., Blackburn E. H. (1987) Cell 51, 887–898 [DOI] [PubMed] [Google Scholar]
- 7. Gilson E., Géli V. (2007) Nat. Rev. Mol. Cell Biol. 8, 825–838 [DOI] [PubMed] [Google Scholar]
- 8. Hug N., Lingner J. (2006) Chromosoma 115, 413–425 [DOI] [PubMed] [Google Scholar]
- 9. Shore D., Bianchi A. (2009) EMBO J. 28, 2309–2322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lundblad V., Szostak J. W. (1989) Cell 57, 633–643 [DOI] [PubMed] [Google Scholar]
- 11. Lundblad V. (2002) Oncogene 21, 522–531 [DOI] [PubMed] [Google Scholar]
- 12. Cesare A. J., Reddel R. R. (2010) Nat. Rev. Genet. 11, 319–330 [DOI] [PubMed] [Google Scholar]
- 13. Palm W., de Lange T. (2008) Annu. Rev. Genet. 42, 301–334 [DOI] [PubMed] [Google Scholar]
- 14. Giraud-Panis M. J., Pisano S., Poulet A., Le Du M. H., Gilson E. (2010) FEBS Lett. 584, 3785–3799 [DOI] [PubMed] [Google Scholar]
- 15. Conrad M. N., Wright J. H., Wolf A. J., Zakian V. A. (1990) Cell 63, 739–750 [DOI] [PubMed] [Google Scholar]
- 16. Pardo B., Marcand S. (2005) EMBO J. 24, 3117–3127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marcand S., Gilson E., Shore D. (1997) Science 275, 986–990 [DOI] [PubMed] [Google Scholar]
- 18. Henning K. A., Moskowitz N., Ashlock M. A., Liu P. P. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 5667–5671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Berthiau A. S., Yankulov K., Bah A., Revardel E., Luciano P., Wellinger R. J., Géli V., Gilson E. (2006) EMBO J. 25, 846–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brevet V., Berthiau A. S., Civitelli L., Donini P., Schramke V., Géli V., Ascenzioni F., Gilson E. (2003) EMBO J. 22, 1697–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alexander M. K., Zakian V. A. (2003) EMBO J. 22, 1688–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. di Domenico E. G., Auriche C., Viscardi V., Longhese M. P., Gilson E., Ascenzioni F. (2009) DNA Repair 8, 209–218 [DOI] [PubMed] [Google Scholar]
- 23. Bah A., Bachand F., Clair E., Autexier C., Wellinger R. J. (2004) Nucleic Acids Res. 32, 1917–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rose M., Winston F., Hieter P. (1990) Methods in Yeast Genetics: A Laboratory Course Manual, pp. 1–200, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York [Google Scholar]
- 25. Gietz R. D., Schiestl R. H. (2007) Nat. Protoc. 2, 31–34 [DOI] [PubMed] [Google Scholar]
- 26. Brachmann C. B., Davies A., Cost G. J., Caputo E., Li J., Hieter P., Boeke J. D. (1998) Yeast 14, 115–132 [DOI] [PubMed] [Google Scholar]
- 27. Bellí G., Garí E., Aldea M., Herrero E. (1998) Yeast 14, 1127–1138 [DOI] [PubMed] [Google Scholar]
- 28. Paciotti V., Clerici M., Lucchini G., Longhese M. P. (2000) Genes Dev. 14, 2046–2059 [PMC free article] [PubMed] [Google Scholar]
- 29. Pike B. L., Yongkiettrakul S., Tsai M. D., Heierhorst J. (2003) J. Biol. Chem. 278, 30421–30424 [DOI] [PubMed] [Google Scholar]
- 30. Fisher T. S., Taggart A. K., Zakian V. A. (2004) Nat. Struct. Mol. Biol. 11, 1198–1205 [DOI] [PubMed] [Google Scholar]
- 31. LeBel C., Larrivée M., Bah A., Laterreur N., Lvesque N., Wellinger R. J. (2006) Methods Mol. Biol. 313, 265–316 [DOI] [PubMed] [Google Scholar]
- 32. Vodenicharov M. D., Laterreur N., Wellinger R. J. (2010) EMBO J. 29, 3007–3019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Khadaroo B., Teixeira M. T., Luciano P., Eckert-Boulet N., Germann S. M., Simon M. N., Gallina I., Abdallah P., Gilson E., Géli V., Lisby M. (2009) Nat. Cell Biol. 11, 980–987 [DOI] [PubMed] [Google Scholar]
- 34. Vega L. R., Phillips J. A., Thornton B. R., Benanti J. A., Onigbanjo M. T., Toczyski D. P., Zakian V. A. (2007) PLoS Genet. 3, e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lingner J., Hughes T. R., Shevchenko A., Mann M., Lundblad V., Cech T. R. (1997) Science 276, 561–567 [DOI] [PubMed] [Google Scholar]
- 36. Abdallah P., Luciano P., Runge K. W., Lisby M., Géli V., Gilson E., Teixeira M. T. (2009) Nat. Cell Biol. 11, 988–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lundblad V., Blackburn E. H. (1993) Cell 73, 347–360 [DOI] [PubMed] [Google Scholar]
- 38. Teng S. C., Zakian V. A. (1999) Mol. Cell Biol. 19, 8083–8093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tourrière H., Versini G., Cordón-Preciado V., Alabert C., Pasero P. (2005) Mol. Cell 19, 699–706 [DOI] [PubMed] [Google Scholar]
- 40. Gravel S., Larrivée M., Labrecque P., Wellinger R. J. (1998) Science 280, 741–744 [DOI] [PubMed] [Google Scholar]
- 41. Ritchie K. B., Mallory J. C., Petes T. D. (1999) Mol. Cell Biol. 19, 6065–6075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Arnerić M., Lingner J. (2007) EMBO Rep. 8, 1080–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ray A., Runge K. W. (1999) Mol. Cell Biol. 19, 31–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Makovets S., Herskowitz I., Blackburn E. H. (2004) Mol. Cell. Biol. 24, 4019–4031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Paeschke K., McDonald K. R., Zakian V. A. (2010) FEBS Lett. 584, 3760–3772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sfeir A., Kosiyatrakul S. T., Hockemeyer D., MacRae S. L., Karlseder J., Schildkraut C. L., de Lange T. (2009) Cell 138, 90–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dehé P. M., Cooper J. P. (2010) FEBS Lett. 584, 3725–3733 [DOI] [PubMed] [Google Scholar]
- 48. Ye J., Lenain C., Bauwens S., Rizzo A., Saint-Léger A., Poulet A., Benarroch D., Magdinier F., Morere J., Amiard S., Verhoeyen E., Britton S., Calsou P., Salles B., Bizard A., Nadal M., Salvati E., Sabatier L., Wu Y., Biroccio A., Londoño-Vallejo A., Giraud-Panis M. J., Gilson E. (2010) Cell 142, 230–242 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.