Abstract
Protein translocation across the cytoplasmic membrane is an essential process in all bacteria. The Sec system, comprising at its core an ATPase, SecA, and a membrane channel, SecYEG, is responsible for the majority of this protein transport. Recently, a second parallel Sec system has been described in a number of Gram-positive species. This accessory Sec system is characterized by the presence of a second copy of the energizing ATPase, SecA2; where it has been studied, SecA2 is responsible for the translocation of a subset of Sec substrates. In common with many pathogenic Gram-positive species, Clostridium difficile possesses two copies of SecA. Here, we describe the first characterization of the C. difficile accessory Sec system and the identification of its major substrates. Using inducible antisense RNA expression and dominant-negative alleles of secA1 and secA2, we demonstrate that export of the S-layer proteins (SLPs) and an additional cell wall protein (CwpV) is dependent on SecA2. Accumulation of the cytoplasmic precursor of the SLPs SlpA and other cell wall proteins was observed in cells expressing dominant-negative secA1 or secA2 alleles, concomitant with a decrease in the levels of mature SLPs in the cell wall. Furthermore, expression of either dominant-negative allele or antisense RNA knockdown of SecA1 or SecA2 dramatically impaired growth, indicating that both Sec systems are essential in C. difficile.
Keywords: Bacteria, Bacterial Genetics, Cell-surface Protein, Protein Translocation, Secretion, Accessory Sec System, Clostridium difficile, S-layer
Introduction
Clostridium difficile is a spore-forming, Gram-positive, anaerobic bacterium and is the major cause of nosocomial antibiotic-associated diarrhea (1). The importance of this pathogen is highlighted by the recent increased incidence of C. difficile infection in the United States and many European countries (2). Research has focused largely on two toxins (3, 4) that cause tissue damage, neutrophil recruitment, and a severe inflammatory response (5). However, other aspects of C. difficile virulence are poorly understood. In particular, little is known about the mechanisms of gastrointestinal colonization, an undoubtedly essential step in pathogenesis. Bacterial pathogens generally secrete a subset of proteins that mediate essential functions during pathogenesis such as adhesion to host tissues and modulation of the host immune response (6). The identity of such proteins and the mechanisms of secretion employed by C. difficile are currently a subject of active research, and several candidate proteins have been identified (see below).
Exterior to a typical Gram-positive cell wall is the C. difficile S-layer, a paracrystalline proteinaceous array that surrounds the bacterium (7). The S-layer is composed of two proteins, the high molecular weight (HMW)2 and low molecular weight (LMW) S-layer proteins (SLPs), derived by post-translational extracellular cleavage of the precursor SlpA (8). Following cleavage, the HMW and LMW SLPs form a high affinity heterodimer, the basic subunit of the assembled S-layer (9). In addition to the S-layer, C. difficile also possesses a large family of noncovalently anchored cell wall proteins (CWPs) that are related to SlpA and are characterized by the presence of three Pfam04122 cell wall-binding motifs (10). Functions have been assigned to a few of these proteins, including Cwp84, the cysteine protease responsible for SlpA cleavage (11–13); CwpV, a phase-variable autoaggregating factor (14, 15); and Cwp66, a putative adhesin (16). However, despite a number of these proteins being implicated in bacterium-host interactions (16–18), their exact roles in pathogenesis have yet to be elucidated. Cell-surface localization of several CWPs has been demonstrated, and antibodies to many CWPs have been found in convalescent sera from C. difficile-infected patients (19, 20), implying that at least some CWPs are expressed and surface-exposed in vivo.
The mechanisms of secretion of the C. difficile S-layer and CWPs are not understood. Translocation of proteins across the cytoplasmic membrane is an essential process in bacteria and is largely mediated by the multisubunit Sec system (21). Alternative secretion systems such as the twin arginine translocation system (22) have been found in some Gram-positive species, but to date, no additional secretion systems have been described in C. difficile. The essential core components of the Sec system are the heteromeric SecYEG membrane channel and the SecA ATPase. Recently, a parallel accessory Sec system has been described in a small number of Gram-positive species (23–27), characterized by the presence of a second copy of SecA, termed SecA2. Some of these species also possess a second copy of SecY (28). Bacteria that possess an accessory Sec system employ it for translocation of a defined subset of proteins, many of which appear to be virulence factors (28). C. difficile strain 630 (29) encodes the core components of the canonical Sec secretion system, including the SecYEG membrane channel and also has a second copy of the SecA ATPase, indicative of an accessory Sec system. Of the organisms possessing a SecA2-only accessory Sec system, Mycobacterium smegmatis is perhaps the most intensively studied (26, 30, 31). In M. smegmatis, SecA1 and SecA2 have non-redundant functions in protein translocation; SecA1 provides essential housekeeping functions, whereas SecA2 is responsible for the secretion of a small number of lipoproteins (30). Furthermore, the M. smegmatis accessory pathway also appears to require SecA1 (31).
Molecular characterization of C. difficile has recently been made possible by the development of basic genetic tools for the manipulation of the organism, including shuttle vectors transferable by conjugation and insertional gene inactivation using group II introns (32, 33). Here, we describe a controlled inducible expression system in C. difficile, which we used to study the functions of secA1 and secA2. Using antisense RNA and protein expression techniques, we demonstrate that C. difficile has two essential Sec systems, a housekeeping system and an accessory system, and we identify SlpA and several CWPs as the major substrates of the accessory system.
EXPERIMENTAL PROCEDURES
Strains, Plasmids, and Growth Conditions
All plasmids used in this study are described in Table 1; oligonucleotides are listed in supplemental Table S1. Escherichia coli strains were propagated in LB broth and on LB agar supplemented with antibiotics as appropriate: chloramphenicol (15 μg/ml), kanamycin (50 μg/ml), or carbenicillin (50 μg/ml). E. coli strain NovaBlue (Merck) was used as a recipient for all cloning procedures, and the donor strain CA434 (HB101 carrying R702) was used for conjugation of plasmids into C. difficile. C. difficile strain 630 (29) was cultured in TY broth without thioglycolate (34), C. difficile defined medium (35), or brain heart infusion broth and on brain heart infusion agar or blood agar supplemented with thiamphenicol (15 μg/ml) where appropriate. Anhydrotetracycline (ATc; 20–500 ng/ml) was used for induction of the Ptet promoter in the C. difficile expression vectors described below.
TABLE 1.
Plasmids used in this study
| Plasmid | Relevant details | Ref. or source |
|---|---|---|
| pCBR023 | Pcwp2 driving codon-optimized gusA gene in pUC19 | Ref. 15 |
| pRPF137 | EcoRI site between Pcwp2 and gusA in pCBR023 changed to SacI | This study |
| pMTL960 | E. coli-C. difficile shuttle vector | Nigel Minton |
| pRPF144 | Pcwp2-gusA cassette from pRPF137 subcloned into pMTL960 | This study |
| pRPF150 | gusA gene in pRPF144 replaced with WT secA2 with N-terminal Strep-tag II | This study |
| pRPF151 | gusA gene in pRPF144 replaced with secA2(K106R) with N-terminal Strep-tag II | This study |
| pSTBlue-1 | E. coli cloning vector | Novagen |
| pRMC2 | Tetracycline-inducible vector for S. aureus | Ref. 36 |
| pRPF135 | Ptet from pRMC2 cloned into pSTBlue-1 | This study |
| pRPF139 | Extraneous BamHI and XhoI sites in pRPF135 removed | This study |
| pRPF141 | Extraneous KpnI site in pRPF139 removed | This study |
| pRPF143 | Pcwp2 in pRPF137 replaced with modified Ptet from pRPF141 | This study |
| pRFP146 | Ptet-gusA cassette from pRPF143 cloned into pMTL960 | This study |
| pRPF177 | pRPF146 with slpA transcriptional terminator added after gusA | This study |
| pRPF185 | pRPF177 with fdx transcriptional terminator added after tetR | This study |
| pRPF186 | WT secA2 with N-terminal Strep-tag II cloned into pRPF185 | This study |
| pRPF187 | secA2(K106R) with N-terminal Strep-tag II cloned into pRPF185 | This study |
| pRPF193 | WT secA1 with N-terminal Strep-tag II cloned into pRPF185 | This study |
| pRPF194 | secA1(K106R) with N-terminal Strep-tag II cloned into pRPF185 | This study |
| pRPF195 | Inducible antisense RNA to secA2 | This study |
| pRPF204 | Inducible antisense RNA to secA1 | This study |
Inducible Expression System for C. difficile
A highly optimized Staphylococcus aureus tetracycline-inducible promoter (Ptet) (36) was adapted for use in C. difficile. The entire Ptet promoter was amplified from pRMC2 using oligonucleotides NF1320 and NF1321 and ligated as a blunt-ended fragment into pSTBlue-1, yielding pRPF135. Extraneous BamHI, XhoI, and KpnI sites were removed by sequential rounds of inverse PCR and ligation using NF1322 and NF1323 to generate pRPF139, followed by NF1334 and NF1335 to generate pRPF141.
pCBR023 containing the constitutive Pcwp2 promoter driving a C. difficile codon-optimized gusA gene in a pUC19 backbone (15) was modified by inverse PCR with NF1326 and NF1328 to replace the EcoRI site between the promoter and gusA gene with a SacI site, yielding pRPF137. The Pcwp2 promoter was then excised from pRPF137 and replaced with the modified Ptet promoter on a KpnI-SacI fragment, yielding pRPF143. The Pcwp2-gusA and Ptet-gusA cassettes were subcloned as KpnI-BamHI fragments into the E. coli-C. difficile shuttle vector pMTL960 to produce plasmids pRPF144 and pRPF146, respectively. Two transcriptional terminators were added, one after the gusA gene and a second after the divergent tetR gene. The terminator that follows the slpA gene in C. difficile strain 630 was amplified using NF1437 and NF1438 and cloned as a BamHI-BstXI fragment downstream of the gusA gene in pRPF146, yielding pRPF177. The fdx terminator from Clostridium pasteurianum was reconstituted as two annealed oligonucleotides, NF1469 and NF1470, and cloned downstream of the tetR gene between NheI and KpnI, yielding plasmid pRPF185. This stabilized inducible expression plasmid was used for all further studies.
SecA1 and SecA2 Expression Plasmids
secA2 was amplified from C. difficile strain 630 using oligonucleotides NF1234 and NF1235 and cloned as EcoRI-BamHI into pCBR023. The resulting plasmid was then modified by inverse PCR using NF1260 and NF1261 to introduce an N-terminal Strep-tag II (WSHPQFEKLE) after the initiation methionine and again using NF1327 and NF1328 to change the EcoRI site between Pcwp2 and secA2 to SacI. The resulting Pcwp2-secA2 cassette was subcloned into pMTL960 between KpnI and BamHI, yielding pRPF150. The K106R mutation was introduced by inverse PCR using NF1256 and NF1257, yielding pRPF151. WT secA2 and secA2(K106R) were subsequently subcloned (SacI-BamHI) into pRPF185, generating pRPF186 and pRPF187, respectively. secA1 was amplified from C. difficile strain 630 using oligonucleotides NF1511 and NF1513, and this product was used as a template for a second PCR with NF1512 and NF1513 to add an N-terminal Strep-tag II. The resulting product was cloned as a blunt fragment into pSTBlue-1. The K106R mutation was introduced by inverse PCR using NF1514 and NF1515. WT secA1 and secA1(K106R) were subcloned (SacI-BamHI) into pRPF185, generating pRPF193 and pRPF194, respectively.
Antisense RNA Expression Plasmids
A 178-bp (NF1582 and NF1583) antisense fragment, spanning from the predicted transcription initiation site of secA1, was amplified from C. difficile strain 630 genomic DNA and cloned (SacI-BamHI) into pRPF185, yielding pRPF204. A 600-bp (NF1409 and NF1410) antisense fragment covering the first 300 bp of secA2 and 300 bp upstream of the gene was amplified from C. difficile strain 630 genomic DNA and cloned (SacI-BamHI) into pRPF146. The fdx transcriptional terminator from C. pasteurianum was added downstream of the tetR gene as described above, yielding plasmid pRPF195.
RT-PCR
C. difficile harboring pRPF195 or pRPF204 was grown to an absorbance at 600 nm (A600 nm) of 0.3–0.4, induced with 500 ng/ml ATc, and harvested 90 min later following stabilization with RNAprotect (Qiagen). RNA was purified using the FastRNA Pro Blue kit (BIO 101 Systems), followed by DNase treatment (TURBO DNA-free, Applied Biosystems) and a final cleanup step with the RNeasy MinElute cleanup kit (Qiagen). RNA concentration and purity were assayed using a NanoDrop ND-1000 spectrophotometer. 260/280 nm ratios were all >2.1. To ensure complete removal of DNA, a 16 S rRNA PCR amplification was carried out with 1 μg of RNA. First-strand synthesis was carried out using the RETROscript kit (Applied Biosystems) according to the manufacturer's instructions with random decamers but without heat denaturation of the RNA template. Expression of the individual antisense RNAs was confirmed by semiquantitative PCR using antisense-specific oligonucleotides to secA1a (NF1633 and NF1640) and secA2a (NF1636 and NF1639). Quantitative real-time PCR was carried out with an Applied Biosystems 7300 real-time PCR system using SYBR Green PCR Master Mix (Applied Biosystems) and primers specific for secA1 (NF1634 and NF1635), secA2 (NF1641 and NF1642), and 16 S rRNA (NF1643 and NF1644). A dissociation step was included after each quantitative PCR to confirm the production of a single unique product. The expression of secA1 and secA2 relative to 16 S rRNA was determined according to the method of Pfaffl (37).
Cell Lysis, Fractionation, and Protein Analysis
C. difficile is highly resistant to standard chemical and mechanical lysis methods. However, we have observed that C. difficile spontaneously lyses at 37 °C following a single freeze-thaw cycle. This propensity was exploited for the preparation of whole cell lysates and fractionation of C. difficile. For the preparation of whole cell lysates, cultures of C. difficile were harvested by centrifugation at 5000 × g for 10 min at 4 °C, and the pellets were frozen at −20 °C. Bacteria were thawed, resuspended in PBS to an A600 nm of 20, and incubated at 37 °C for 40 min. This method resulted in consistent and reproducible lysis. For analysis by SDS-PAGE, an equal volume of 2× SDS sample buffer (38) was added. For cell fractionation, the frozen bacterial pellets were resuspended in PBS containing 1.4 mg/ml lysozyme and 0.12 μg/ml DNase I to an A600 nm of 20 and incubated at 37 °C for 1 h. Membranes were harvested by centrifugation at 25,000 × g for 10 min at 4 °C. The supernatant (containing the soluble cytoplasmic proteins) was removed and mixed with an equal volume of 2× SDS sample buffer. The harvested membranes were washed twice with 500 μl of PBS, resuspended in PBS, solubilized with 1% SDS to a final equivalent A600 nm of 20, and mixed with an equal volume of 2× SDS sample buffer. SLPs and CWPs were isolated from intact C. difficile cells using low pH glycine as described previously (9, 39). SDS-PAGE and Western immunoblotting were carried out using standard methods and as described previously (9, 39).
β-Glucuronidase Assay
C. difficile strain 630 harboring pRPF185 was grown to an A600 nm of 0.3 in TY broth, induced with ATc (20, 100, or 500 ng/ml) for 210 min, harvested by centrifugation, and frozen. Bacteria harboring the empty vector (pMTL960) or a constitutive gusA expression plasmid (pRPF144) were included as controls. Bacteria were lysed as described above, and the β-glucuronidase activity in the cell lysates was determined as described previously (34).
[35S]Methionine Metabolic Labeling
Overnight TY cultures were subcultured to an A600 nm of 0.05 in complete C. difficile defined medium, grown to an A600 nm of 0.3, and induced with 20 ng/ml ATc. After a 40-min induction, 1 ml of each culture was harvested by centrifugation, resuspended in C. difficile defined medium without methionine, and incubated for a further 30 min to exhaust cellular methionine pools. Cultures were then removed from the anaerobic cabinet to a 37 °C heating block. 100 μCi of EasyTag [35S]methionine (specific activity of 1175 Ci/mmol; PerkinElmer Life Sciences) was added to each culture, and 200-μl samples were removed to fresh tubes containing 50 μl of 1 m NaN3 at defined intervals. 800 μl of unlabeled C. difficile was added to each tube, and S-layer extracts were prepared as described above.
Phase-contrast and Immunofluorescence Microscopy
Overnight C. difficile TY cultures were subcultured into fresh TY broth supplemented with thiamphenicol with and without 500 ng/ml ATc and grown overnight. Bacteria were fixed and immunolabeled using a rat anti-LMW SLP antibody as described previously (15). Immunolabeled bacteria were visualized and photographed using a Nikon Eclipse E600 microscope fitted with a Retiga 2000R Fast 1394 camera.
RESULTS
Construction of an Inducible Promoter System for C. difficile
For many years, the genetic intractability of C. difficile has hampered molecular analysis of this important pathogen; however, recent advances have finally opened the way for fundamental genetic analysis. One crucial tool still missing from the repertoire of C. difficile genetic tools is the ability to control the expression of a plasmid-borne gene. A lac-based inducible promoter, which has been shown to work well in some clostridial species (33) does not appear to be fully functional in C. difficile.3 Tetracycline-inducible promoters have been successfully employed in many Gram-positive species (40, 41). We have taken one such system, optimized for use in S. aureus (36), and adapted it for use in C. difficile. The inducible system consists of a pair of divergent promoters (PtetR and Ptet), each with an overlapping tet operator sequence. PtetR drives the expression of TetR, a transcription factor that negatively regulates both promoters, whereas Ptet is used to drive expression of the gene of interest. Addition of tetracycline relieves TetR repression and results in increased expression of both the gene of interest and TetR. The resulting negative feedback ensures tight regulation and dose-dependent induction.
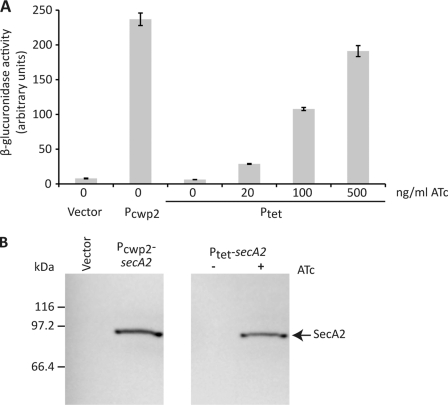
The tet promoter system from pRMC2 (36) was modified as described under “Experimental Procedures” and transferred into the E. coli-C. difficile shuttle vector pMTL960. The gusA gene, encoding the β-glucuronidase enzyme, was cloned downstream of the Ptet promoter, and β-glucuronidase activity used as a readout of inducible gene expression in C. difficile. Although C. difficile strain 630 carries tetM and is resistant to tetracycline, there is a lag between addition of the antibiotic and complete expression of resistance. To avoid any effects on growth and to allow use of the inducible system in tetracycline-sensitive strains of C. difficile, the non-antibiotic analog ATc was used for induction of the system. In the absence of induction, there was no detectable β-glucuronidase activity, demonstrating the extremely tight repression of Ptet by TetR (Fig. 1A). Induction with ATc was exquisitely dose-dependent with a linear induction profile. Furthermore, induction with 500 ng/ml ATc resulted in levels of β-glucuronidase activity similar to those seen with the constitutive Pcwp2 promoter (15).
FIGURE 1.
Inducible protein expression in C. difficile. A, β-glucuronidase activity of mid-logarithmic phase cultures of C. difficile strain 630 harboring the empty E. coli-C. difficile shuttle vector (pMTL960), the constitutive gusA expression plasmid (pRPF144, Pcwp2), or the inducible gusA expression vector (pRPF185, Ptet). Cultures of C. difficile pRPF185 were induced with the indicated concentrations of ATc for 210 min. Shown are the averages of triplicate measurements, with S.D. displayed as error bars. B, constitutive and inducible expression of SecA2. Expression of SecA2 in mid-logarithmic phase cultures of C. difficile strain 630 harboring pMTL960 (Vector), pRPF150 (Pcwp2-secA2), or pRPF186 (Ptet-secA2) with (+) and without (−) ATc induction (500 ng/ml, 1 h) was assayed by SDS-PAGE and Western immunoblotting using an anti-Strep-tag II antibody. The position of Strep-tagged SecA2 is indicated.
C. difficile Possesses an Accessory Sec System
In species that possess both canonical and accessory Sec systems, there are several consistent characteristics that distinguish the respective SecA proteins (reviewed in Ref. 28). For example, the housekeeping SecA1 protein has a higher molecular weight than the accessory protein, SecA2. This size difference is due largely to the absence, in SecA2, of the C-terminal linker; the C-terminal linker has been implicated in interactions with both lipid and SecB (42). SecA1 also shares higher sequence identity with the well characterized SecA proteins of E. coli and Bacillus subtilis. Sequenced strains of C. difficile all possess two copies of SecA, putatively annotated as SecA1 and SecA2 (29, 43). SecA1 (CD0143 in strain 630) displays 47 and 51% amino acid sequence identity to the E. coli and B. subtilis SecA proteins, respectively, compared with 41 and 47% for SecA2 (CD2792) (supplemental Fig. S1). Furthermore, SecA2 is smaller than SecA1 (781 and 891 residues, respectively) and lacks the C-terminal linker. Based on these characteristics, it is likely that CD0143 is the housekeeping SecA1 protein and that the second protein, SecA2, may form the core of an accessory Sec system.
SecA2 is encoded within a genomic locus containing at least 12 surface-localized gene products, including the S-layer precursor SlpA, the S-layer protease Cwp84, and the adhesin Cwp66 (supplemental Fig. S2). Given the genomic localization of secA2, it is tempting to speculate that SecA2 may play a role in the export of the S-layer precursor SlpA and perhaps other surface proteins. This would not be without precedent, as in Listeria monocytogenes, one of the substrates of the accessory Sec system, p60, is encoded by a gene adjacent to, but divergent from, secA2 (24).
Conservative Walker Box Mutations in secA1 and secA2 Are Dominant-negative and Lethal in C. difficile
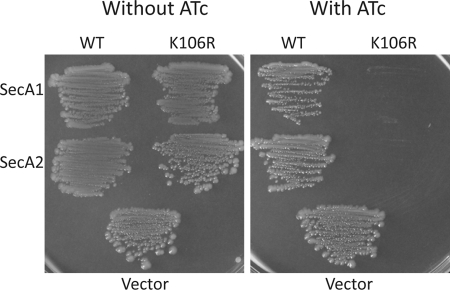
Despite repeated attempts, we were unable to create insertion-inactivated mutants of secA2, suggestive of an essential function. However, we took advantage of a previous observation that a conservative Lys-to-Arg substitution in the Walker box of SecA, which negates binding to ATP, confers a dominant-negative phenotype in both E. coli and M. smegmatis (31, 44). In M. smegmatis, overexpression of dominant-negative SecA2(K129R) gave the same phenotype as a secA2 knock-out (31). Lys-129 corresponds to Lys-106 in both SecA1 and SecA2 of C. difficile (supplemental Fig. S3). E. coli-C. difficile shuttle plasmids were constructed with the Strep-tagged wild-type (pRPF150) and K106R (pRPF151) alleles of secA2 under the control of the constitutive cwp2 promoter (Pcwp2) (15). The plasmid carrying the wild-type secA2 allele was transferred into C. difficile strain 630, which carries wild-type secA1 and secA2 in its genome, from an E. coli conjugal donor strain with high efficiency, and Strep-tagged SecA2 was found to be well expressed (Fig. 1B). However, despite repeated attempts, the secA2(K106R) allele could not be conjugated into C. difficile, suggesting that secA2(K106R) was lethal in C. difficile. Both the wild-type and K106R alleles of secA2 were subcloned into the tetracycline-inducible expression vector pRPF185, yielding pRPF186 and pRPF187, respectively. Expression of SecA2 from pRPF186 was found to be tightly repressed, and induction with 500 ng/ml ATc resulted in high level protein expression, albeit slightly less than seen with the constitutive Pcwp2 promoter (Fig. 1B). In contrast with the constitutive secA2(K106R) plasmid, pRPF187 (Ptet-secA2(K106R)) was easily conjugated into C. difficile. To test if expression of SecA2(K106R) was indeed lethal, bacteria were grown on brain heart infusion agar plates with and without ATc. Expression of SecA2(K106R) resulted in a severe growth defect, whereas expression of the wild-type protein had no apparent effect on C. difficile growth (Fig. 2). Plasmids encoding inducible wild-type SecA1 and a K106R derivative were also constructed (pRPF193 and pRPF194, respectively) and conjugated into C. difficile strain 630. Expression of SecA1(K106R) resulted in a growth defect similar to that seen with SecA2(K106R). However, in contrast with SecA2, expression of wild-type SecA1 also resulted in a partial growth defect. Interestingly, this growth defect was far greater on blood agar (supplemental Fig. S4).
FIGURE 2.
Growth phenotypes resulting from SecA1 and SecA2 expression in C. difficile. C. difficile harboring pRPF193 (WT SecA1), pRPF194 (SecA1(K106R)), pRPF186 (WT SecA2), pRPF187 (SecA2(K106R)), or pMTL960 (Vector) was streaked on brain heart infusion agar plates with and without ATc (500 ng/ml). Plates were incubated anaerobically at 37 °C for 48 h and then photographed.
Expression of SecA1(K106R) or SecA2(K106R) Blocks Translocation of SlpA
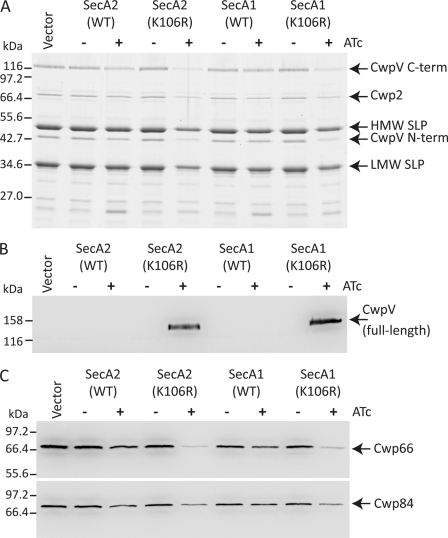
To examine the effect of expression of the SecA proteins on the surface protein composition of C. difficile, cells were grown to early exponential phase and induced with ATc for 4 h, and surface proteins were isolated following standard methods. Bacteria overexpressing either SecA1(K106R) or SecA2(K106R) had considerably lower levels of SLPs (SecA1(K106R), 68% of control levels; SecA2(K106R), 48%) and associated CWPs (Fig. 3, A and B). SlpA is cleaved post-translocation by the cell wall cysteine protease Cwp84, yielding the LMW and HMW SLP subunits (11). We therefore reasoned that inhibition of SlpA translocation should result in the accumulation of the full-length protein in the cell cytoplasm. To determine whether SlpA was indeed accumulating in cells expressing SecA1(K106R) or SecA2(K106R), bacteria carrying the appropriate plasmid were induced with ATc as before, and whole cell lysates were analyzed for the presence of full-length SlpA (Fig. 3, C and D). Expression of SecA2(K106R) or SecA1(K106R) resulted in significant accumulation of SlpA in the cell cytoplasm, although the effect was greater with SecA2(K106R) than with SecA1(K106R). This observation was in complete agreement with the reduction in the amount of the SLPs observed on the cell surface. Expression of wild-type SecA1 also resulted in a small but clear accumulation of SlpA in the cell cytoplasm. In contrast, expression of SecA2 had no effect on the amount of SlpA detected.
FIGURE 3.
Expression of SecA1(K106R) and SecA2(K106R) causes a severe S-layer translocation defect. A, mid-log cultures of C. difficile strain 630 harboring pMTL960 (Vector), pRPF186 (WT SecA2), pRPF187 (SecA2(K106R)), pRPF193 (WT SecA1), or pRPF194 (SecA1(K106R)) were induced with ATc (500 ng/ml) for 4 h, and surface proteins were isolated using low pH glycine (39). Surface proteins were separated on 12% SDS-polyacrylamide gels and Coomassie Blue-stained. B, densitometry analysis of the gel shown in A. Densitometry was performed independently on the LMW and HMW SLPs; the average densitometry values with S.D. are shown. C and D, Western immunoblot analysis of the SlpA translocation defect in cultures expressing wild-type and dominant-negative SecA1 and SecA2. Cultures of C. difficile strain 630 harboring pRPF186 (WT SecA2), pRPF187 (SecA2(K106R)), pRPF193 (WT SecA1), or pRPF194 (SecA1(K106R)) were induced for 3 h with the indicated concentrations of ATc. Whole cell lysates were separated on 10% SDS-polyacrylamide gels and probed with anti-Strep-tag II or anti-LMW SLP antibody. E, [35S]methionine labeling of de novo synthesized C. difficile surface proteins. Cultures of C. difficile strain 630 carrying pMTL960, pRPF187 (SecA2(K106R)), pRPF186 (WT SecA2), pRPF194 (SecA1(K106R)), and pRPF193 (WT SecA1) were 35S-labeled as described under “Experimental Procedures.” Surface proteins were isolated using low pH glycine and visualized by SDS-PAGE, followed by autoradiography.
Pulse Labeling Reveals a Substantial Defect in S-layer Translocation upon Overexpression of Dominant-negative SecA1 or SecA2
To determine the degree of the S-layer translocation defect, cells were induced with ATc for 40 min, washed and resuspended in a defined medium lacking methionine, incubated to exhaust cellular methionine pools, and pulse-labeled with [35S]methionine. The surface protein repertoire synthesized during the [35S]methionine pulse was then analyzed by SDS-PAGE and autoradiography (Fig. 3E). Expression of either dominant-negative SecA protein, SecA1(K106R) or SecA2(K106R), resulted in a substantial blockade of translocation of the SLPs and all detectable CWPs. There was also a clear but less severe defect in surface protein translocation upon overexpression of wild-type SecA1, but not SecA2.
Translocation of Other CWPs Is Affected by SecA1(K106R) and SecA2(K106R) Expression
As SlpA translocation was dramatically inhibited by expression of SecA1(K106R) and SecA2(K106R), we sought to determine whether other members of the cell-surface protein family were also affected in a similar manner. Unlike SlpA, most C. difficile CWPs are not modified post-translocation, making it difficult to distinguish the cytoplasmic and surface forms by size alone. However, there is one additional surface protein (CwpV) that undergoes a cleavage event analogous to SlpA, resulting in two fragments localized to the cell wall (15). Expression of CwpV is phase-variable (15), but we have recently described a C. difficile strain (630ΔrecV ON) with a mutation in the gene encoding the recombinase (RecV) responsible for CwpV phase variation, rendering CwpV expression constitutive (14). Using this constitutive CwpV strain, we sought to analyze the effect of SecA1(K106R) and SecA2(K106R) expression on the translocation of CwpV. Expression of SecA1(K106R) or SecA2(K106R) resulted in a severe defect in the surface localization of CwpV, and analogous to SlpA, there was a concomitant accumulation of full-length CwpV in the cell cytoplasm (Fig. 4, A and B). There were also reductions in the amount of several other CWPs on the cell surface, including Cwp2, Cwp66, and Cwp84 (Fig. 4, A and C). However, as these proteins are not modified post-translocation, it was impossible to demonstrate accumulation of these proteins in the cell cytoplasm.
FIGURE 4.
Expression of SecA1(K106R) and SecA2(K106R) impairs the translocation of several CWPs. A, C. difficile strain 630ΔrecV ON (14) harboring pMTL960 (Vector), pRPF186 (WT SecA2), pRPF187 (SecA2(K106R)), pRPF193 (WT SecA1), or pRPF194 (SecA1(K106R)) was induced with ATc (500 ng/ml) for 4 h. Surface proteins were isolated using low pH glycine (39) and analyzed by SDS-PAGE. B, whole cell lysates of the same cultures were also prepared and analyzed for the presence of full-length CwpV using an antibody raised against the 41.2-kDa N-terminal fragment of the protein. C, the surface protein samples described above were further analyzed using antibodies recognizing two additional CWPs (Cwp66 and Cwp84). The positions of the two CwpV subunits (C-term and N-term), Cwp2, HMW and LMW SLPs, and full-length CwpV, Cwp66, and Cwp84 are indicated.
Knockdown of SecA2, but Not SecA1, Disrupts SlpA and CwpV Translocation
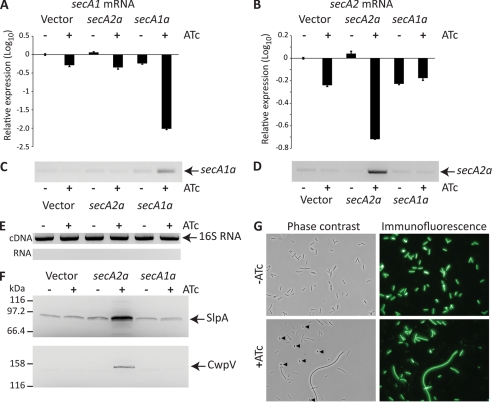
The experiments described above used plasmids overexpressing secA1 or secA2 alleles in the context of wild-type levels of both proteins, driven from chromosomally encoded genes. To investigate the effects of down-regulation of expression of chromosomally encoded secA1 and secA2, antisense fragments to the secA1 and secA2 genes were cloned separately under the control of the inducible Ptet promoter. Both antisense fragments were designed such that the 5′-end of the target mRNA, including the ribosome-binding site, would be sequestered in the resulting RNA duplex. Expression of the antisense RNAs and sequestration of the target mRNA were confirmed using semiquantitative and quantitative RT-PCR (Fig. 5, A–D). RNA samples were not heated prior to first-strand cDNA synthesis to preserve the mRNA/antisense RNA duplex. As an RNA duplex is refractory to cDNA synthesis, the cDNA synthesized is representative of the available (and translatable) mRNA. When secA1a was expressed, there was an almost complete sequestration of the secA1 mRNA but no effect on the amount of available secA2 mRNA, demonstrating the target specificity of the antisense RNA (Fig. 5A). Expression of secA2a resulted in a >70% decrease in the amount of available secA2 mRNA but had no effect on the levels of secA1 mRNA (Fig. 5B).
FIGURE 5.
Inducible antisense RNA knockdown of SecA1 and SecA2 expression. A–E, RNA was isolated from C. difficile strain 630 carrying pMTL960 (Vector), pRPF195 (secA2a), or pRPF204 (secA1a) grown with (+) and without (−) ATc (500 ng/ml). The available secA1 (A) and secA2 (B) mRNAs were quantified relative to 16 S rRNA by quantitative RT-PCR. Displayed are the relative expression values (log10); each is the average of triplicate reactions. Error bars indicate S.D. RT-PCR using antisense RNA-specific oligonucleotides was used to confirm expression of secA1a (C) and secA2a (D). Purified RNA and cDNA samples were subjected to PCR using 16 S rRNA-specific oligonucleotides to confirm RNA purity and cDNA synthesis (E). F, C. difficile strain 630 carrying pMTL960, pRPF195 (secA2a), or pRPF204 (secA1a) was grown overnight with (+) and without (−) ATc (500 ng/ml). Whole cell lysates were prepared, and full-length SlpA and CwpV were detected using antibodies raised against the LMW SLP and CwpV N-terminal fragment, respectively. G, morphological phenotypes of SecA1 knockdown. Shown are representative phase-contrast and immunofluorescence images of C. difficile strain 630 carrying pRPF204 (Ptet-secA1a) grown overnight with and without ATc (500 ng/ml). Spores present in the induced culture are indicated (arrowheads), and cell debris is clearly visible in the corresponding immunofluorescence image (lower right panel).
Expression of the secA1 antisense RNA (secA1a) led to a rapid cessation of growth; after induction, growth stopped within two to three generations. When examined microscopically, there was evidence of severe stress, including defective septation, increased sporulation, and cell lysis (Fig. 5G). Induction of the secA2 antisense RNA (secA2a) also resulted in impaired growth, but the effect was far less severe than with secA1a (growth stopped eight generations after induction), and there was no visible change in cell morphology when examined microscopically (data not shown). To ascertain what effect the knockdown of SecA1 and SecA2 had on surface protein translocation, we examined cells for the accumulation of full-length SlpA and CwpV in the cell cytoplasm (Fig. 5F). Knockdown of SecA2 expression resulted in significant accumulation of both CwpV and SlpA in the cell, demonstrating an absolute requirement for SecA2 for the translocation of both proteins. However, despite the highly efficient sequestration of secA1 mRNA and the extreme stress phenotype induced by secA1a expression, there was no apparent effect on the translocation of either SlpA or CwpV.
Cellular Distribution of SecA1 and SecA2
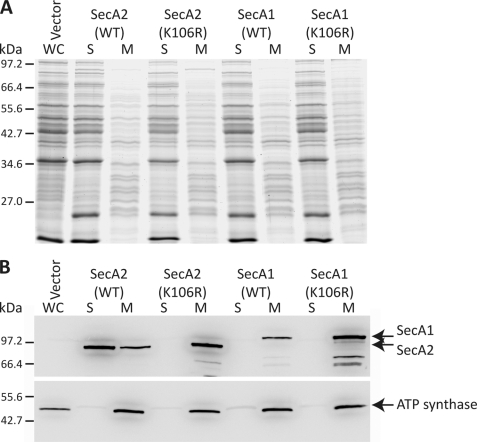
It has been previously reported that M. smegmatis SecA1 and SecA2 differ in their subcellular localization; SecA1 was found equally in cytoplasmic and membrane fractions, whereas SecA2 was predominantly cytoplasmic (31). Furthermore, the SecA2(K129R) mutation, analogous to the SecA2(K106R) mutation described in this study, relocalized SecA2 predominantly to the membrane. We sought to determine whether C. difficile SecA1 and SecA2 were localized in a similar manner. Wild-type SecA1 and SecA2 differed dramatically in their subcellular localization (Fig. 6); SecA2 was found predominantly in the soluble fraction with only a small amount of protein detected at the membrane, whereas SecA1 was detected only at the membrane. This is in stark contrast to M. smegmatis, where SecA1 was found to be localized equally between the membrane and cytoplasm (31). However, as was observed in M. smegmatis, the dominant-negative K106R mutation in SecA2 switched the localization of the protein entirely to the membrane fraction.
FIGURE 6.
Subcellular localization of SecA1 and SecA2. Mid-log cultures of C. difficile strain 630 harboring pMTL960 (Vector), pRPF186 (WT SecA2), pRPF187 (SecA2(K106R)), pRPF193 (WT SecA1), or pRPF194 (SecA1(K106R)) were induced with ATc (500 ng/ml) for 90 min. A, Coomassie Blue-stained SDS-polyacrylamide gel showing whole cell lysate (WC), soluble (S), and membrane (M) protein fractions. B, the location of SecA1 and SecA2 was detected using anti-Strep-tag II antibody. The integral membrane protein ATP synthase was used as a control for cell fractionation.
DISCUSSION
In synthesizing and maintaining a complete cell envelope, all living cells face a similar physical problem: how to transport largely polar proteins across the non-polar membrane? Bacteria have evolved many complex proteinaceous systems to facilitate this transport, but the majority of translocated proteins in bacteria utilize the multisubunit Sec translocase (21). The Sec systems of the model organisms E. coli and B. subtilis have been studied in remarkable detail, but there are still many research questions remaining to be answered. In the last decade, a second accessory Sec system has been described in several Gram-positive species (24–27, 45). In this study, we have characterized the accessory Sec system of the nosocomial pathogen C. difficile and identified its major substrates.
A conservative amino acid substitution in the SecA2 Walker box, which prevents binding of ATP, has previously been shown to confer a dominant-negative phenotype in M. smegmatis (31). Indeed, expression of dominant-negative SecA2 resulted in the same phenotype as a secA2 knock-out. We have shown that an equivalent mutation (K106R) in C. difficile SecA2 is also dominant-negative. However, unlike in mycobacteria, the dominant-negative SecA2(K106R) mutant was found to be lethal in C. difficile, suggesting an essential role for SecA2. Consistent with this, we were unable to construct a secA2 knock-out despite repeated attempts. Previous to these observations, SecA2 has been found to be essential only in one species where an accessory Sec system has been described, Corynebacterium glutamicum (45). In the absence of a secA2 knock-out, we resorted to using an inducible antisense RNA to knockdown SecA2 expression. Although the knockdown was only partial, as judged by quantitative PCR, there was a significant growth defect. Taken together, these data support the hypothesis that SecA2 performs an essential function in C. difficile. We also confirmed the essentiality of SecA1 in C. difficile by constructing an analogous dominant-negative allele (secA1(K106R)) and knocking down expression with antisense RNA. This result was as predicted due to a requirement for the canonical Sec system for translocation of many essential proteins into and across the cytoplasmic membrane (46). As SecA2 is also essential in C. difficile, it is likely that one or more of its translocated substrates are essential. It is clear that SecA2 is necessary for the secretion of a subset of proteins and does not rely on SecA1 for this function.
To identify the substrates of the C. difficile accessory Sec system, we used a novel inducible expression system to express dominant-negative SecA1(K106R) and SecA2(K106R) proteins and antisense RNAs. We then examined the surface protein complement to identify those proteins whose surface display was SecA2-dependent. Expression of either dominant-negative SecA resulted in a severe defect in the translocation of the S-layer precursor SlpA. However, the defect was considerably more severe when SecA2(K106R) was expressed. In M. smegmatis, it was observed that translocation of SecA2 substrates also required SecA1 (31), demonstrating a degree of interdependence between the two systems. To determine whether a similar interdependence existed between the C. difficile canonical and accessory Sec systems, we employed antisense RNA to independently knockdown the expression of either SecA1 or SecA2. SecA2 knockdown resulted in the same SlpA translocation defect seen with SecA2(K106R) expression. However, despite efficient knockdown of SecA1 and the resultant severe growth phenotypes, there was no effect on the translocation of SlpA. These data suggest that, unlike in M. smegmatis, the C. difficile accessory Sec system is not dependent on canonical SecA. Furthermore, it is clear that transport of the C. difficile S-layer precursor across the membrane is dependent on the accessory Sec system. As C. difficile appears to possess only the canonical SecYEG translocase, SecA1- and SecA2-dependent translocation presumably utilizes the same membrane channels. As dominant-negative SecA1 is predicted to non-productively interact with the SecYEG translocase (31), overexpression of this SecA1 mutant would likely block SecA2 from accessing the translocase. This would explain the SlpA translocation defect seen upon SecA1(K106R) expression.
Interestingly, overexpression of wild-type SecA1 also resulted in a partial SlpA translocation defect, but expression of wild-type SecA2 had no effect. We also observed that, when overexpressed, SecA1 accumulated at the membrane. Therefore, it is possible that the artificial excess of SecA1 reduces the access of SecA2 to the SecYEG translocase and thus prevents efficient translocation of SlpA. We examined the amount of SecA2 at the membrane in cells with and without overexpressed SecA1, and although we did observe a slight but reproducible decrease in the amount of SecA2 at the membrane with a concomitant increase in the cytoplasm, the difference was too small to be conclusive (data not shown). However, it is possible that even a small decrease in the amount of SecA2 accessing SecYEG could result in the partial SlpA defect observed upon SecA1 overexpression.
SlpA is a member of a large family of C. difficile CWPs (10) related by a common putative cell-anchoring mechanism. The C. difficile strain used in this study, 630, encodes a further 28 of these proteins in addition to SlpA. We have demonstrated that at least one of these proteins, CwpV, is also a SecA2 substrate. The translocation of three more CWPs, Cwp2, Cwp66, and Cwp84, also appear to be affected by the expression of dominant-negative SecA1 or SecA2 in a manner similar to SlpA. It is possible that SecA2-dependent translocation is a feature of the entire CWP family, but confirmation of this will require additional study.
The experiments described here required the development of a number of new genetic tools and techniques for C. difficile. The inducible promoter system described in this study adds another powerful genetic tool to our repertoire and will undoubtedly prove useful to the wider clostridial community. The Ptet promoter is very tightly repressed and displays exquisite dose-dependent induction with tetracycline or the non-antibiotic analog ATc. Here, we used this promoter system for the high level expression of several proteins and also for inducible antisense RNA production. This allowed us to study the function of a pair of essential genes, which would previously have been impossible given the current lack of tools to construct conditional mutants in C. difficile. The combination of inducible antisense RNA with the ability to test knockdown specificity and efficiency using quantitative PCR should be widely applicable to the study of essential genes in clostridia.
We have demonstrated that the C. difficile accessory Sec system in required for the secretion of at least two substrates, the major C. difficile surface proteins SlpA and CwpV. SlpA is the most highly expressed protein in C. difficile, accounting for ∼10–15% of total cellular protein. CwpV is an autoaggregating adhesin and, although expressed in a phase-variable manner (15), is the dominant CWP in phase ON cells and accounts for 13% of the S-layer (14). Although it is possible to knock-out many of the genes encoding CWPs, including cwpV (15), we have found it impossible to generate an slpA knock-out strain. The essential requirement for secA2 likely reflects a requirement for the S-layer in C. difficile. S-layers are ubiquitous structures in nature, found on many Gram-positive and Gram-negative bacteria and most archaea (47). The clostridia are an evolutionarily ancient lineage of bacteria (48). It will be fascinating to see if other species possessing S-layers also have a dedicated system for their secretion.
Supplementary Material
Acknowledgments
We thank Christopher Thompson for assistance with quantitative PCR and Catherine Reynolds, Lucia de la Riva, and Marcin Dembek for helpful discussions, critical reading, and technical assistance.
This work was supported by Medical Research Council Grant G0800170.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4 and Table S1.
R. P. Fagan and N. F. Fairweather, unpublished data.
- HMW
- high molecular weight
- LMW
- low molecular weight
- SLP
- S-layer protein
- CWP
- cell wall protein
- ATc
- anhydrotetracycline.
REFERENCES
- 1. Kuipers E. J., Surawicz C. M. (2008) Lancet 371, 1486–1488 [DOI] [PubMed] [Google Scholar]
- 2. Freeman J., Bauer M. P., Baines S. D., Corver J., Fawley W. N., Goorhuis B., Kuijper E. J., Wilcox M. H. (2010) Clin. Microbiol. Rev. 23, 529–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kuehne S. A., Cartman S. T., Heap J. T., Kelly M. L., Cockayne A., Minton N. P. (2010) Nature 467, 711–713 [DOI] [PubMed] [Google Scholar]
- 4. Lyras D., O'Connor J. R., Howarth P. M., Sambol S. P., Carter G. P., Phumoonna T., Poon R., Adams V., Vedantam G., Johnson S., Gerding D. N., Rood J. I. (2009) Nature 458, 1176–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Voth D. E., Ballard J. D. (2005) Clin. Microbiol. Rev. 18, 247–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Finlay B. B., Falkow S. (1997) Microbiol. Mol. Biol. Rev. 61, 136–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kawata T., Takeoka A., Takumi K., Masuda K. (1984) FEMS Microbiol. Lett. 24, 323–328 [Google Scholar]
- 8. Calabi E., Ward S., Wren B., Paxton T., Panico M., Morris H., Dell A., Dougan G., Fairweather N. (2001) Mol. Microbiol. 40, 1187–1199 [DOI] [PubMed] [Google Scholar]
- 9. Fagan R. P., Albesa-Jové D., Qazi O., Svergun D. I., Brown K. A., Fairweather N. F. (2009) Mol. Microbiol. 71, 1308–1322 [DOI] [PubMed] [Google Scholar]
- 10. Fagan R. P., Janoir C., Collignon A., Mastrantonio P., Poxton I. R., Fairweather N. F. (2011) J. Med. Microbiol. 10.1099/jmm.0.028472-0 [DOI] [PubMed] [Google Scholar]
- 11. Dang T. H., de la Riva L., Fagan R. P., Storck E. M., Heal W. P., Janoir C., Fairweather N. F., Tate E. W. (2010) ACS Chem. Biol. 5, 279–285 [DOI] [PubMed] [Google Scholar]
- 12. Kirby J. M., Ahern H., Roberts A. K., Kumar V., Freeman Z., Acharya K. R., Shone C. C. (2009) J. Biol. Chem. 284, 34666–34673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de la Riva L., Willing S., Tate E. W., Fairweather N. F. (2011) J. Bacteriol. 193, 3276–3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reynolds C. B., Emerson J. E., de la Riva L., Fagan R. P., Fairweather N. F. (2011) PLoS Pathog. 7, e1002024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Emerson J. E., Reynolds C. B., Fagan R. P., Shaw H. A., Goulding D., Fairweather N. F. (2009) Mol. Microbiol. 74, 541–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Waligora A. J., Hennequin C., Mullany P., Bourlioux P., Collignon A., Karjalainen T. (2001) Infect. Immun. 69, 2144–2153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Calabi E., Calabi F., Phillips A. D., Fairweather N. F. (2002) Infect. Immun. 70, 5770–5778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Janoir C., Péchiné S., Grosdidier C., Collignon A. (2007) J. Bacteriol. 189, 7174–7180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Péchiné S., Gleizes A., Janoir C., Gorges-Kergot R., Barc M. C., Delmée M., Collignon A. (2005) J. Med. Microbiol. 54, 193–196 [DOI] [PubMed] [Google Scholar]
- 20. Wright A., Drudy D., Kyne L., Brown K., Fairweather N. F. (2008) J. Med. Microbiol. 57, 750–756 [DOI] [PubMed] [Google Scholar]
- 21. Driessen A. J., Nouwen N. (2008) Annu. Rev. Biochem. 77, 643–667 [DOI] [PubMed] [Google Scholar]
- 22. Coulthurst S. J., Palmer T. (2008) Mol. Microbiol. 69, 1331–1335 [DOI] [PubMed] [Google Scholar]
- 23. Chen Q., Wu H., Fives-Taylor P. M. (2004) Mol. Microbiol. 53, 843–856 [DOI] [PubMed] [Google Scholar]
- 24. Lenz L. L., Portnoy D. A. (2002) Mol. Microbiol. 45, 1043–1056 [DOI] [PubMed] [Google Scholar]
- 25. Bensing B. A., Sullam P. M. (2002) Mol. Microbiol. 44, 1081–1094 [DOI] [PubMed] [Google Scholar]
- 26. Braunstein M., Brown A. M., Kurtz S., Jacobs W. R., Jr. (2001) J. Bacteriol. 183, 6979–6990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Siboo I. R., Chaffin D. O., Rubens C. E., Sullam P. M. (2008) J. Bacteriol. 190, 6188–6196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rigel N. W., Braunstein M. (2008) Mol. Microbiol. 69, 291–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sebaihia M., Wren B. W., Mullany P., Fairweather N. F., Minton N., Stabler R., Thomson N. R., Roberts A. P., Cerdeño-Tárraga A. M., Wang H., Holden M. T., Wright A., Churcher C., Quail M. A., Baker S., Bason N., Brooks K., Chillingworth T., Cronin A., Davis P., Dowd L., Fraser A., Feltwell T., Hance Z., Holroyd S., Jagels K., Moule S., Mungall K., Price C., Rabbinowitsch E., Sharp S., Simmonds M., Stevens K., Unwin L., Whithead S., Dupuy B., Dougan G., Barrell B., Parkhill J. (2006) Nat. Genet. 38, 779–786 [DOI] [PubMed] [Google Scholar]
- 30. Gibbons H. S., Wolschendorf F., Abshire M., Niederweis M., Braunstein M. (2007) J. Bacteriol. 189, 5090–5100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rigel N. W., Gibbons H. S., McCann J. R., McDonough J. A., Kurtz S., Braunstein M. (2009) J. Biol. Chem. 284, 9927–9936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Purdy D., O'Keeffe T. A., Elmore M., Herbert M., McLeod A., Bokori-Brown M., Ostrowski A., Minton N. P. (2002) Mol. Microbiol. 46, 439–452 [DOI] [PubMed] [Google Scholar]
- 33. Heap J. T., Pennington O. J., Cartman S. T., Carter G. P., Minton N. P. (2007) J. Microbiol. Methods 70, 452–464 [DOI] [PubMed] [Google Scholar]
- 34. Dupuy B., Sonenshein A. L. (1998) Mol. Microbiol. 27, 107–120 [DOI] [PubMed] [Google Scholar]
- 35. Karasawa T., Ikoma S., Yamakawa K., Nakamura S. (1995) Microbiology 141, 371–375 [DOI] [PubMed] [Google Scholar]
- 36. Corrigan R. M., Foster T. J. (2009) Plasmid 61, 126–129 [DOI] [PubMed] [Google Scholar]
- 37. Pfaffl M. W. (2001) Nucleic Acids Res. 29, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Laemmli U. K. (1970) Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 39. Fagan R., Fairweather N. (2010) Methods Mol. Biol. 646, 117–134 [DOI] [PubMed] [Google Scholar]
- 40. Geissendörfer M., Hillen W. (1990) Appl. Microbiol. Biotechnol. 33, 657–663 [DOI] [PubMed] [Google Scholar]
- 41. Bateman B. T., Donegan N. P., Jarry T. M., Palma M., Cheung A. L. (2001) Infect. Immun. 69, 7851–7857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Breukink E., Nouwen N., van Raalte A., Mizushima S., Tommassen J., de Kruijff B. (1995) J. Biol. Chem. 270, 7902–7907 [DOI] [PubMed] [Google Scholar]
- 43. Stabler R. A., He M., Dawson L., Martin M., Valiente E., Corton C., Lawley T. D., Sebaihia M., Quail M. A., Rose G., Gerding D. N., Gibert M., Popoff M. R., Parkhill J., Dougan G., Wren B. W. (2009) Genome Biol. 10, R102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mitchell C., Oliver D. (1993) Mol. Microbiol. 10, 483–497 [DOI] [PubMed] [Google Scholar]
- 45. Caspers M., Freudl R. (2008) Arch. Microbiol. 189, 605–610 [DOI] [PubMed] [Google Scholar]
- 46. Economou A. (2001) Expert Opin. Ther. Targets 5, 141–153 [DOI] [PubMed] [Google Scholar]
- 47. Sára M., Sleytr U. B. (2000) J. Bacteriol. 182, 859–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sheridan P. P., Freeman K. H., Brenchley J. E. (2003) Geomicrobiol. J. 20, 1–14 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.