Abstract
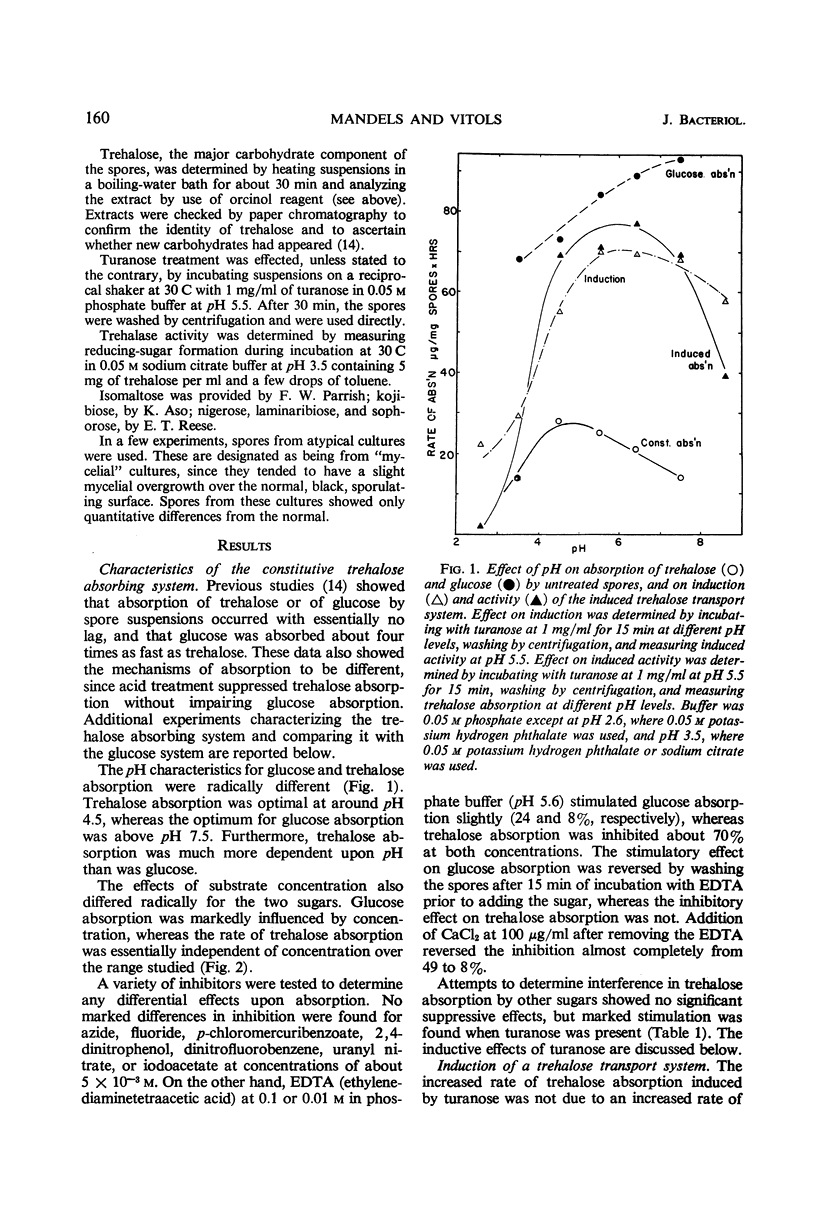
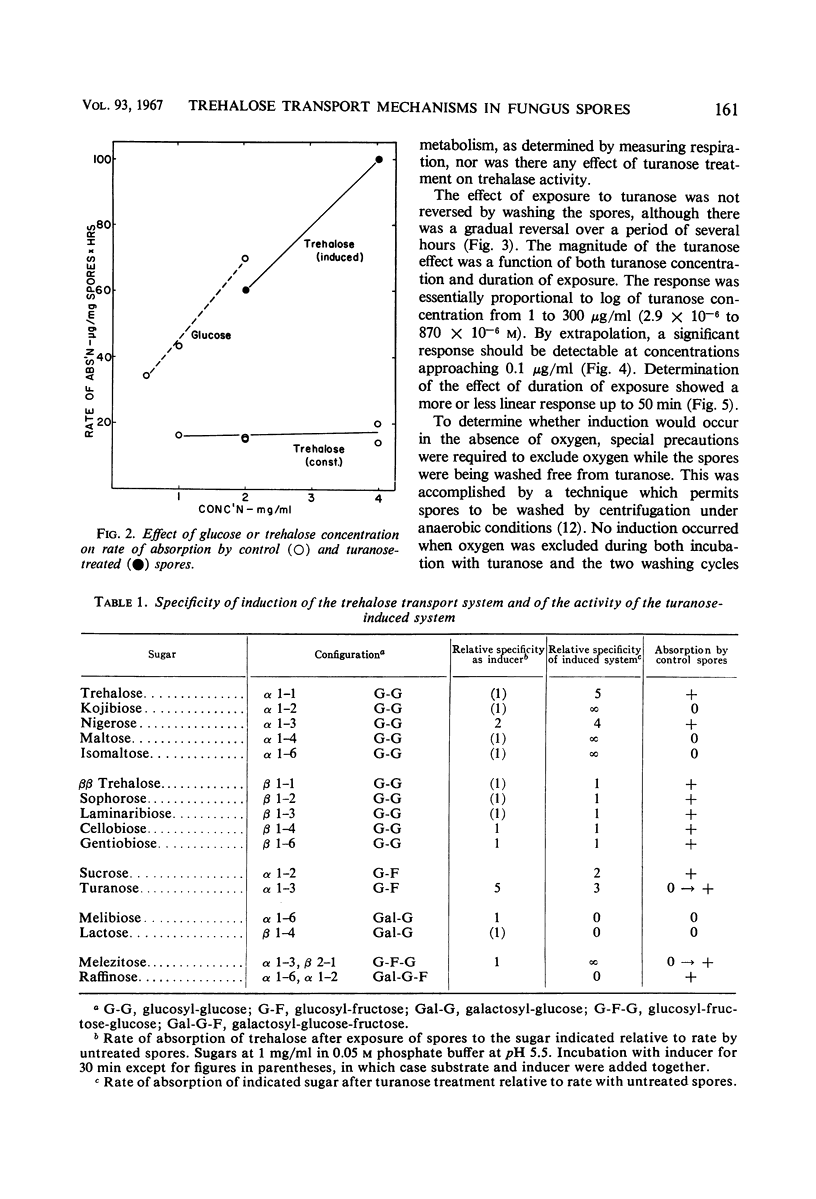
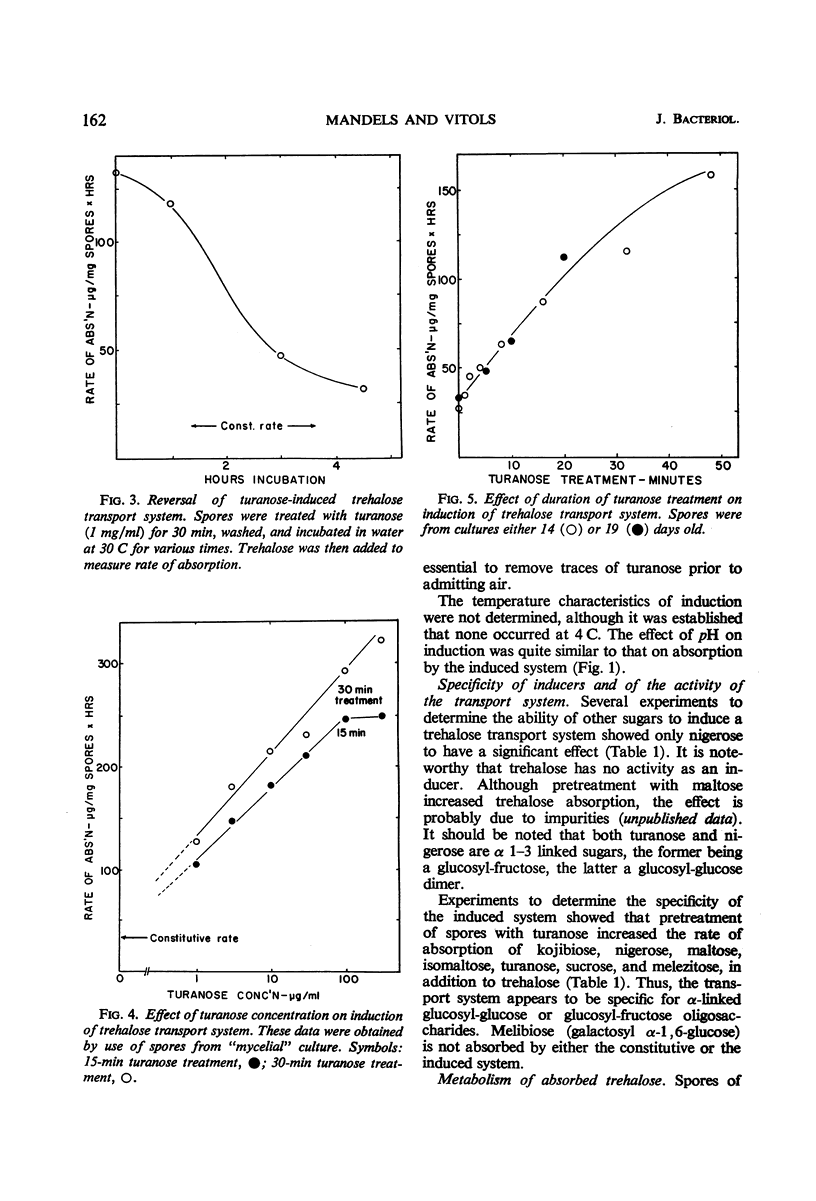
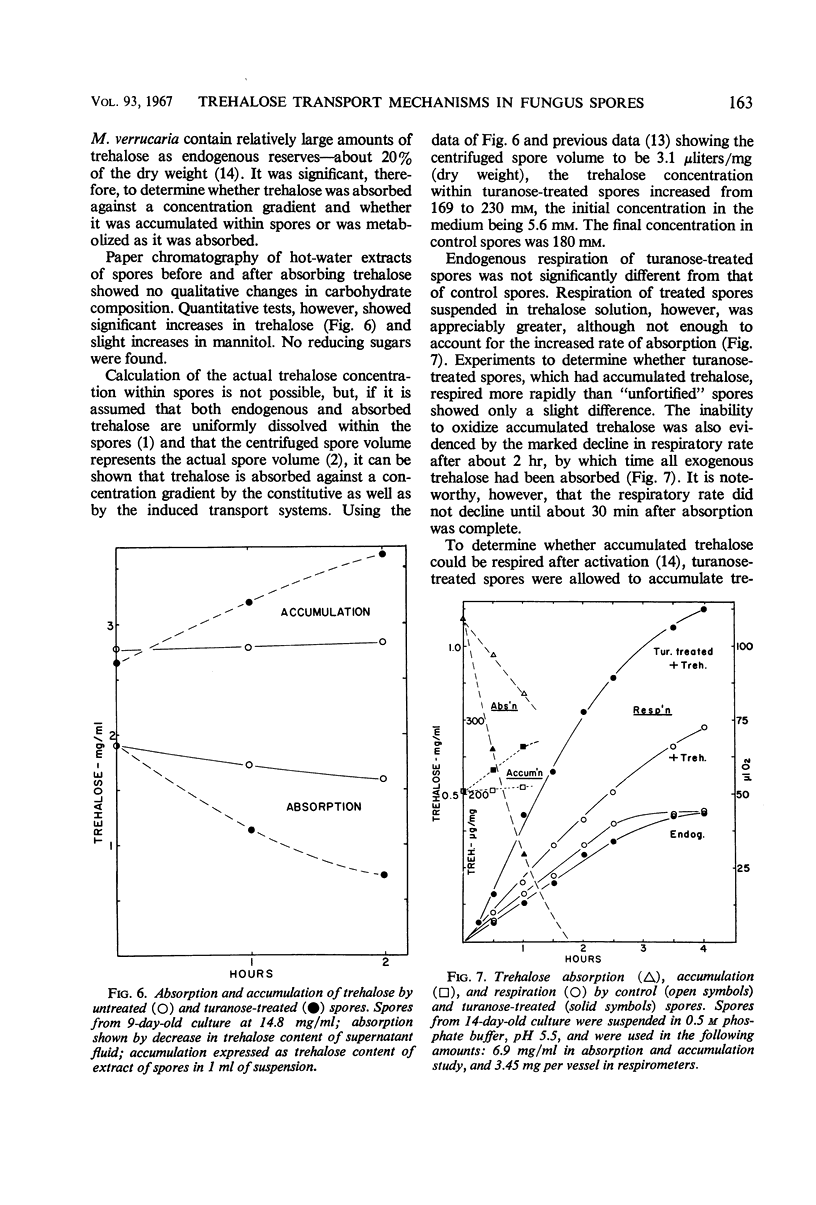
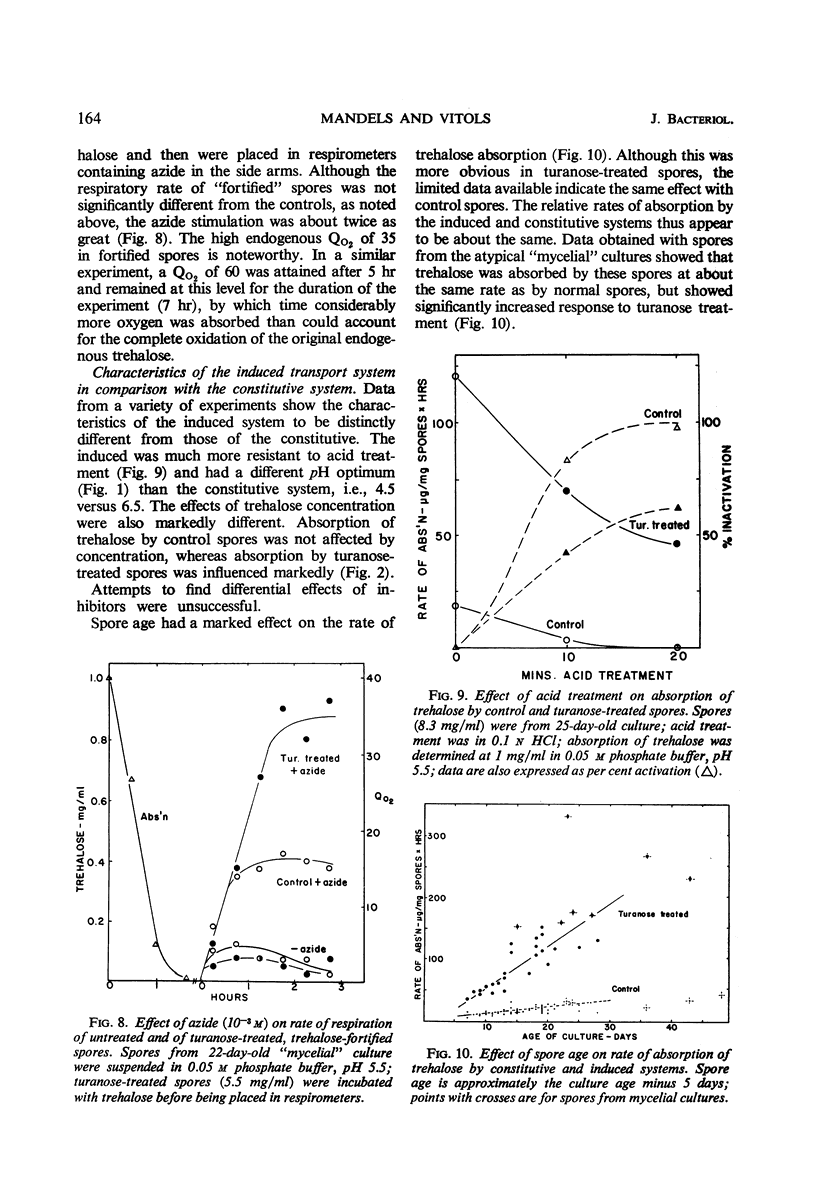
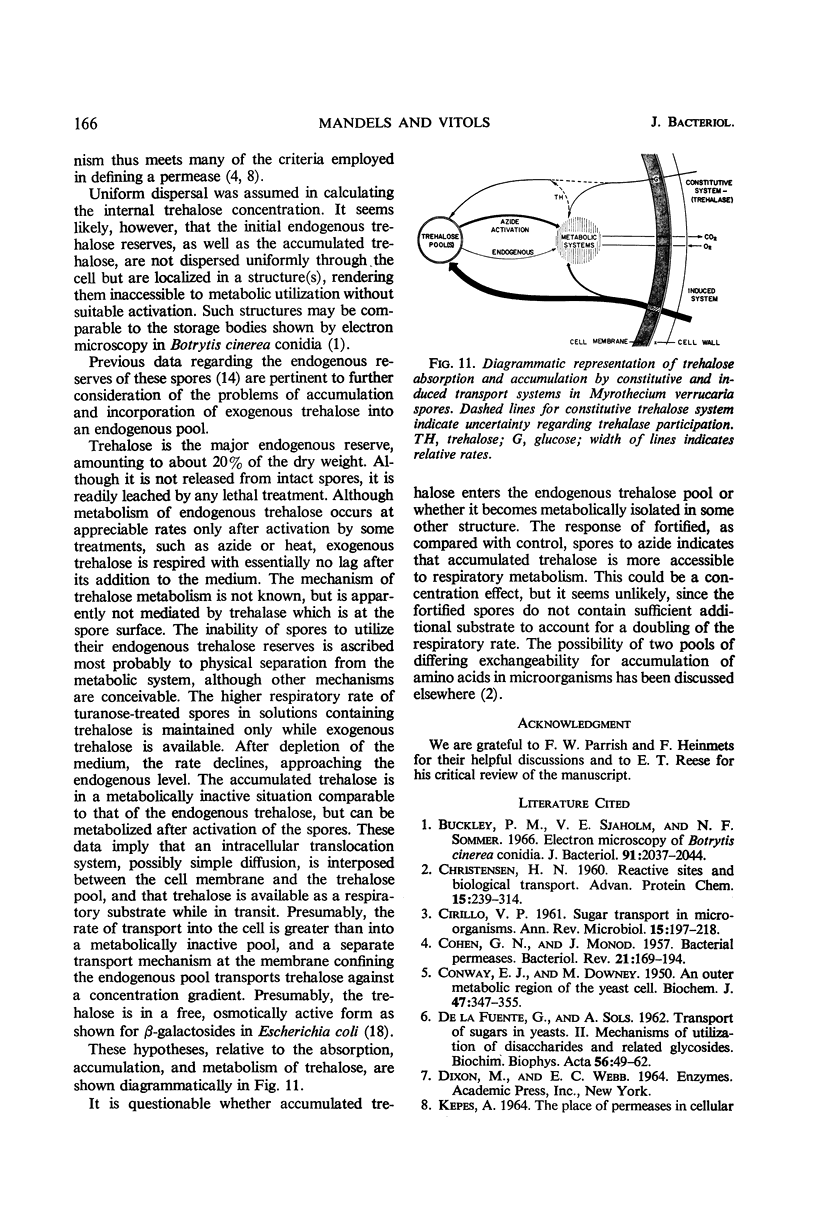
Trehalose is absorbed by two distinct systems—one constitutive, the other induced by turanose and to a lesser extent by nigerose but not by trehalose. The constitutive system is apparently mediated by a surface trehalase; the induced system has the characteristics of a permease. The specificity of the induced system is apparently limited to the α glucosyl-glucose or glucosyl-fructose linkage, because absorption of kojibiose, nigerose, maltose, isomaltose, turanose, sucrose, and melezitose, in addition to that of trehalose, was increased. Absorption of β-linked or of galactose-containing disaccharides was not increased. The constitutive and induced trehalose-absorbing systems differ in their activity, specificity, lability to acid treatment, effects of substrate concentration, and pH optima. Both systems require oxygen, and no marked differential effects of inhibitors were observed. The activity of the induced system is proportional to log turanose concentration (from about 1 to 300 μg/ml), and is an approximate linear function of time of exposure (from about 1 to 50 min). Accumulation of trehalose occurred against a concentration gradient in both systems but particularly in the induced. No leakage was observed. The activity of the induced system declined slowly upon removal of the inducer. Accumulated trehalose is metabolized after activation by azide as are the endogenous trehalose reserves. The accumulated trehalose appears to enter the endogenous trehalose pool found in these spores, although some data suggest it may be more accessible. Respiratory data indicate that absorbed trehalose is available for metabolism while in transit from the external membrane to the internal pool.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buckley P. M., Sjaholm V. E., Sommer N. F. Electron microscopy of Botrytis cinerea conidia. J Bacteriol. 1966 May;91(5):2037–2044. doi: 10.1128/jb.91.5.2037-2044.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTENSEN H. N. Reactive sites and biological transport. Adv Protein Chem. 1960;15:239–314. doi: 10.1016/s0065-3233(08)60310-1. [DOI] [PubMed] [Google Scholar]
- COHEN G. N., MONOD J. Bacterial permeases. Bacteriol Rev. 1957 Sep;21(3):169–194. doi: 10.1128/br.21.3.169-194.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONWAY E. J., DOWNEY M. An outer metabolic region of the yeast cell. Biochem J. 1950 Sep;47(3):347–355. doi: 10.1042/bj0470347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE LA FUENTE G., SOLS A. Transport of sugars in yeasts. II. Mechanisms of utilization of disaccharides and related glycosides. Biochim Biophys Acta. 1962 Jan 1;56:49–62. doi: 10.1016/0006-3002(62)90526-7. [DOI] [PubMed] [Google Scholar]
- KOCH A. L. THE ROLE OF PERMEASE IN TRANSPORT. Biochim Biophys Acta. 1964 Jan 27;79:177–200. doi: 10.1016/0926-6577(64)90050-6. [DOI] [PubMed] [Google Scholar]
- MANDELS G. R. An apparatus for centrifugation and washing of particulate matter in a controlled atmosphere. Science. 1953 Aug 7;118(3058):158–159. doi: 10.1126/science.118.3058.158. [DOI] [PubMed] [Google Scholar]
- MANDELS G. R., DARBY R. T. A rapid cell volume assay for fungitoxicity using fungus spores. J Bacteriol. 1953 Jan;65(1):16–26. doi: 10.1128/jb.65.1.16-26.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandels G. R., Vitols R., Parrish F. W. Trehalose as an endogenous reserve in spores of the fungus Myrothecium verrucaria. J Bacteriol. 1965 Dec;90(6):1589–1598. doi: 10.1128/jb.90.6.1589-1598.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimington C. Seromucoid and the bound carbohydrate of the serum proteins. Biochem J. 1940 Jun;34(6):931–940. doi: 10.1042/bj0340931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SISTROM W. R. On the physical state of the intracellularly accumulates substrates of beta-galactoside-permease in Escherichia coli. Biochim Biophys Acta. 1958 Sep;29(3):579–587. doi: 10.1016/0006-3002(58)90015-5. [DOI] [PubMed] [Google Scholar]
- WILBRANDT W. Transport through biological membranes. Annu Rev Physiol. 1963;25:601–630. doi: 10.1146/annurev.ph.25.030163.003125. [DOI] [PubMed] [Google Scholar]


