Abstract
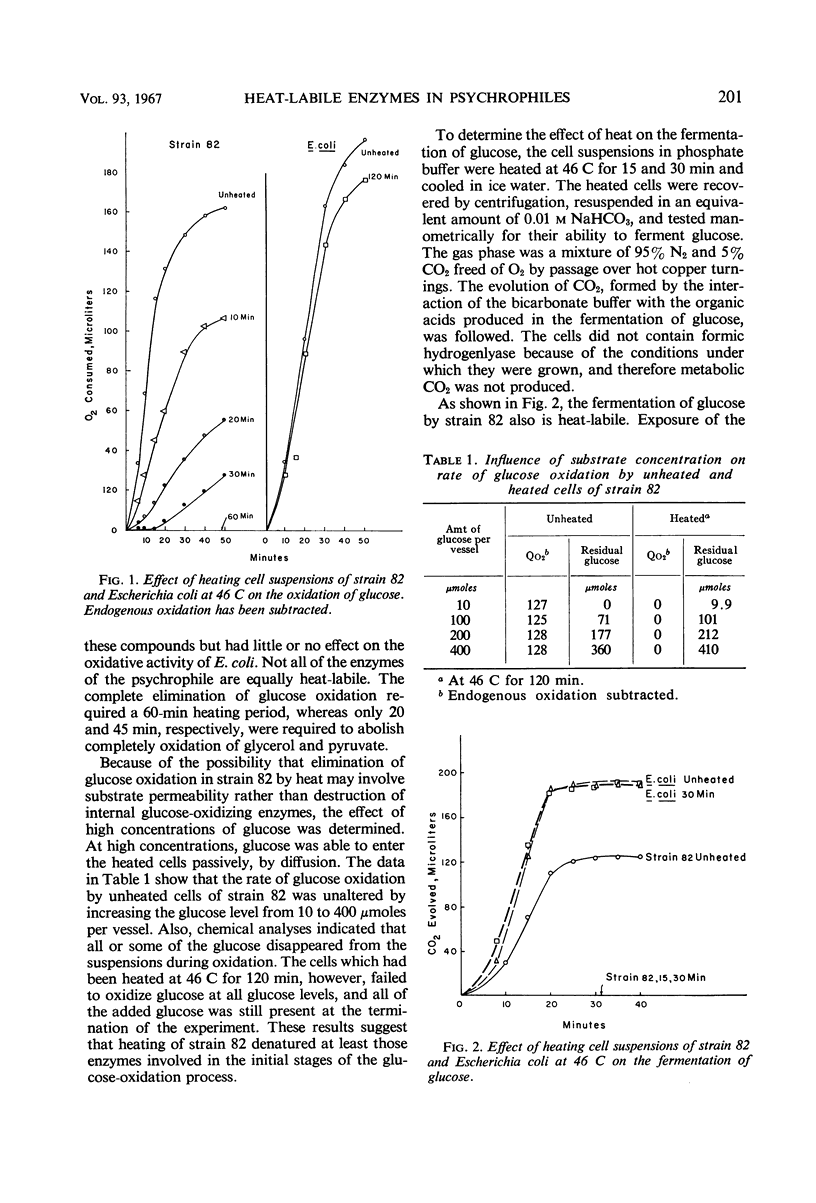
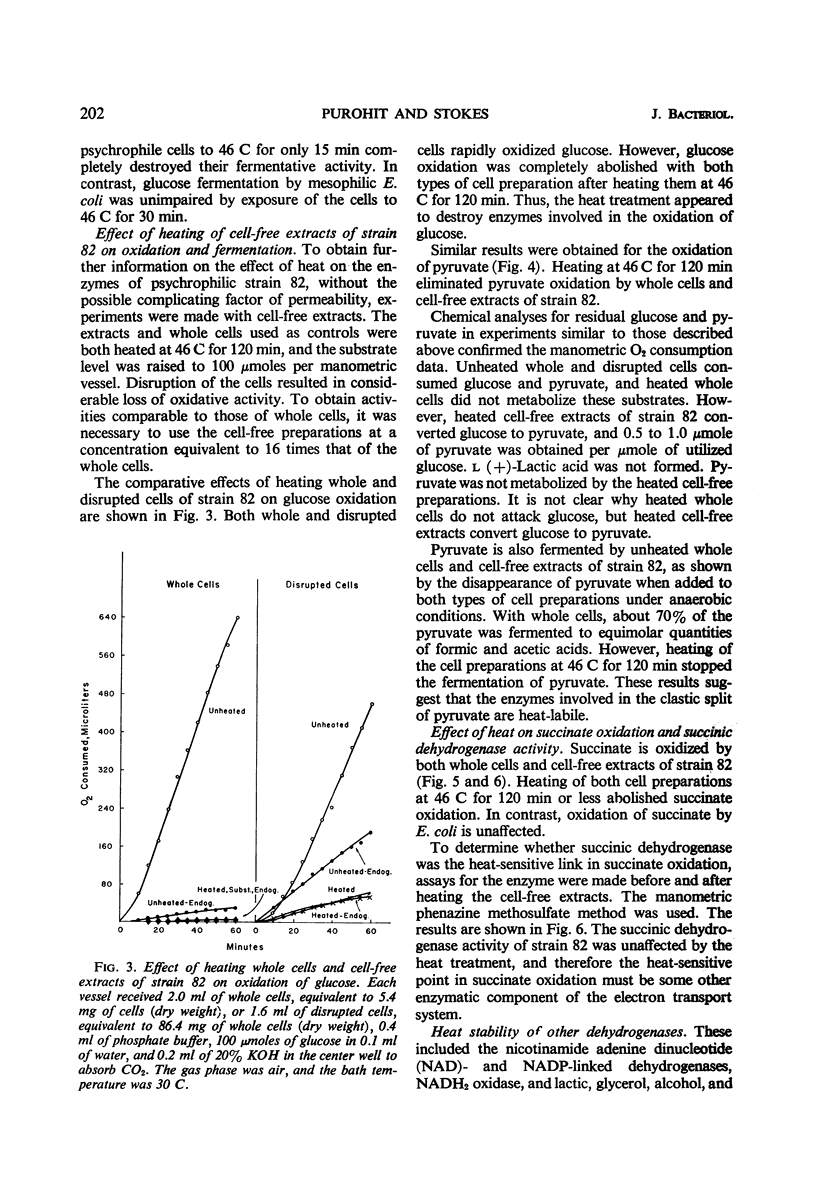
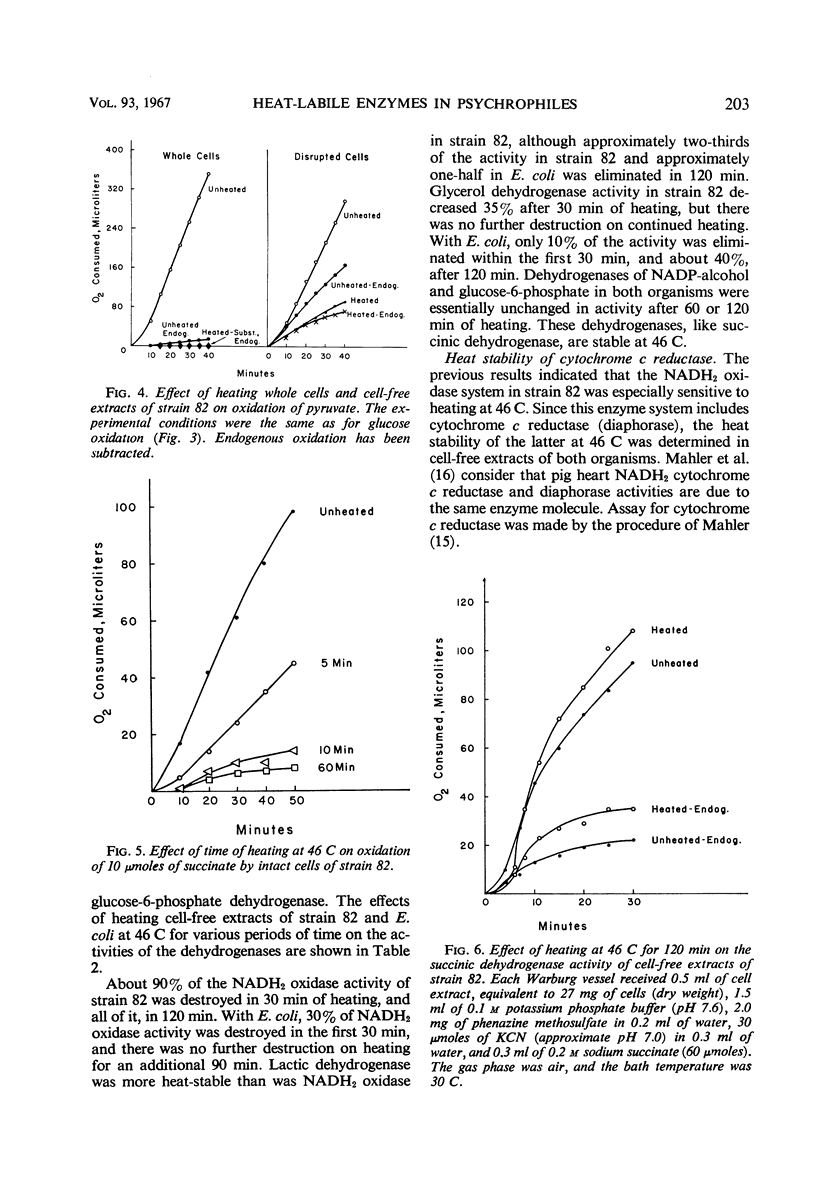
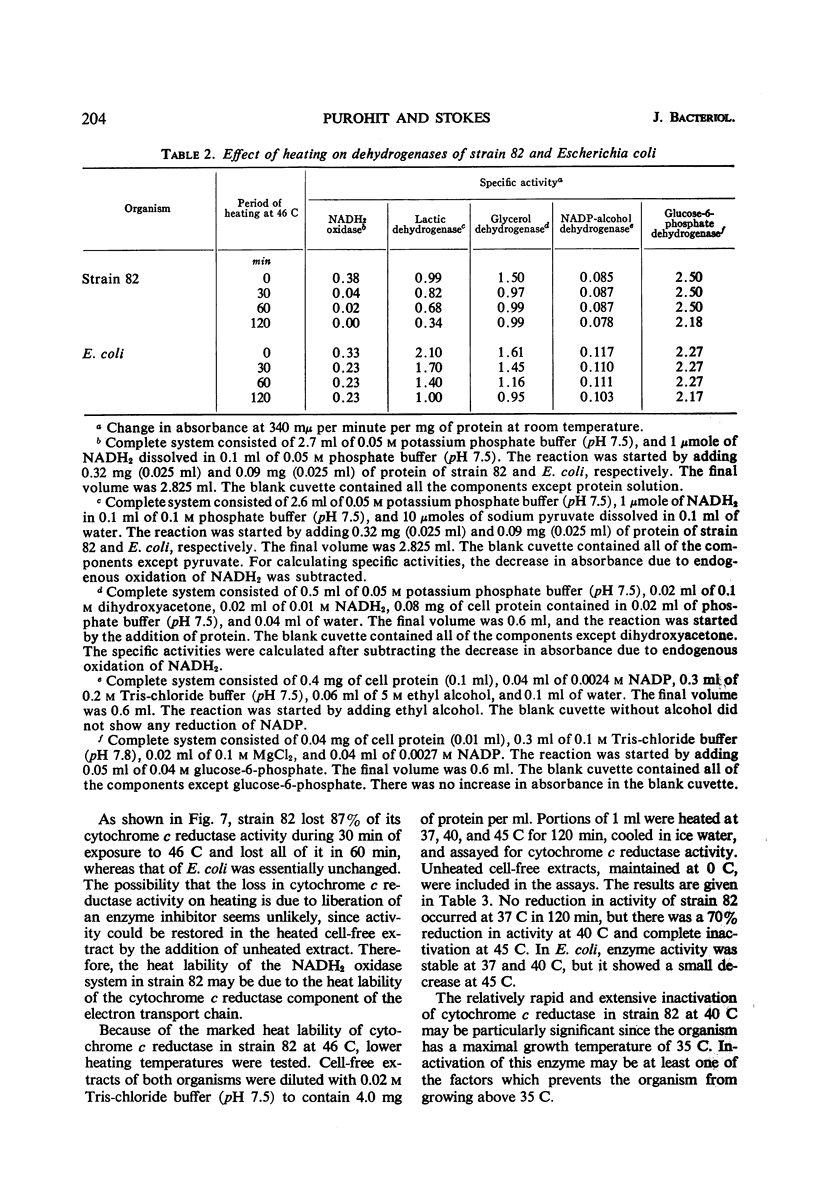
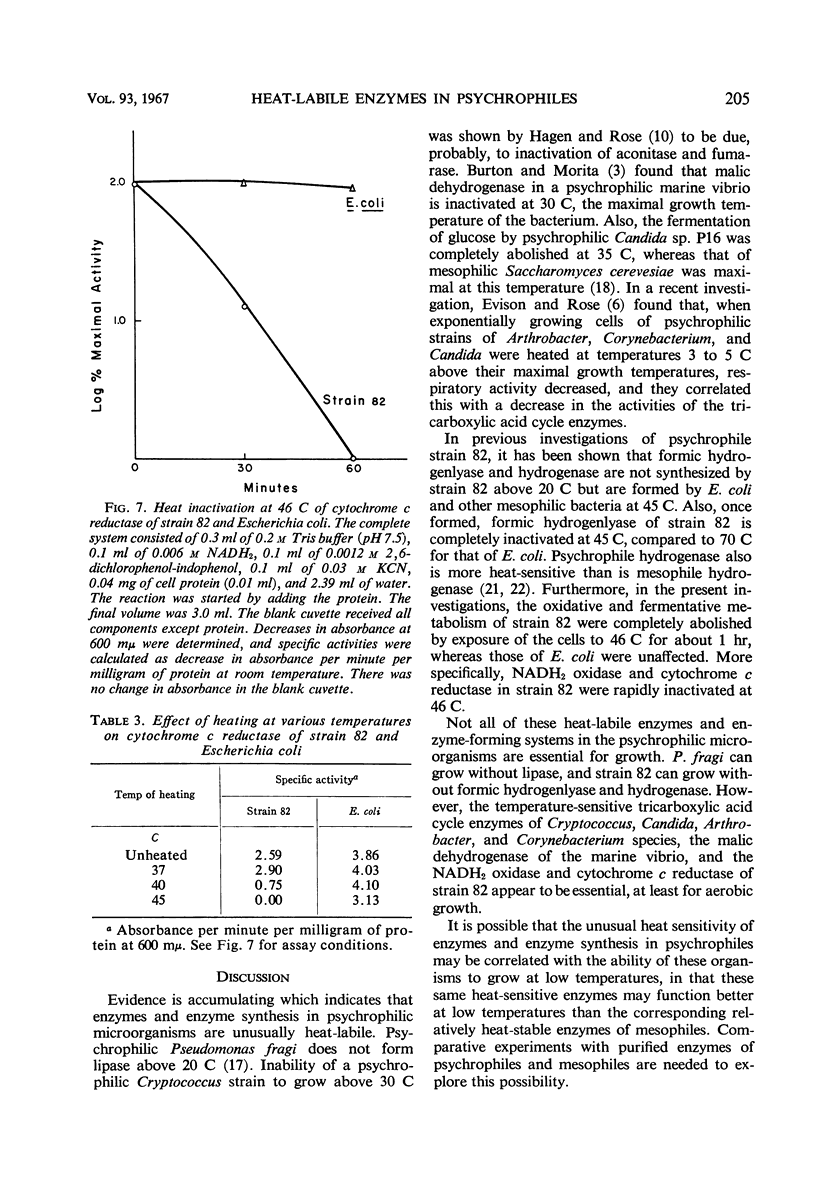
The oxidative and fermentative activities of a psychrophilic bacterium (strain 82), whose maximal growth temperature is 35 C, were completely destroyed by exposure of the cells to 46 C for about 1 hr, whereas those of mesophilic Escherichia coli were unaffected. Similar results were obtained with cell-free extracts. In attempts to determine some of the specific enzymes inactivated in strain 82 by exposure to 46 C, it was found that reduced nicotinamide adenine dinucleotide oxidase was completely inactivated at 46 C in 2 hr. Also, cytochrome c reductase was completely destroyed at 46 C in 1 hr and was 70% destroyed at 40 C in 2 hr. The heat lability of the latter may determine the maximal growth temperature of the organism. In addition, the results indicated that the enzymes of strain 82 involved in the clastic split of pyruvate to formate and acetate are inactivated by exposure to 46 C and that the lactic and glycerol dehydrogenases are more heat-labile than those in E. coli. Succinic, nicotinamide adenine dinucleotide phosphate-alcohol, and glucose-6-phosphate dehydrogenases, however, in both strain 82 and E. coli, were essentially unaffected by exposure to 46 C for 2 hr.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON S. D., MORITA R. Y. DENATURATION AND RENATURATION OF MALIC DEHYDROGENASE IN A CELL-FREE EXTRACT FROM A MARINE PSYCHROPHILE. J Bacteriol. 1963 Nov;86:1019–1024. doi: 10.1128/jb.86.5.1019-1024.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGEN P. O., ROSE A. H. Studies on the biochemical basis of the low maximum temperature in a psychrophilic cryptococcus. J Gen Microbiol. 1962 Jan;27:89–99. doi: 10.1099/00221287-27-1-89. [DOI] [PubMed] [Google Scholar]
- Ingraham J. L., Stokes J. L. PSYCHROPHILIC BACTERIA. Bacteriol Rev. 1959 Sep;23(3):97–108. doi: 10.1128/br.23.3.97-108.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAHLER H. R., SARKAR N. K., VERNON L. P. Studies on diphosphopyridine nucleotide-cytochrome c reductase. II. Purification and properties. J Biol Chem. 1952 Dec;199(2):585–597. [PubMed] [Google Scholar]
- SINCLAIR N. A., STOKES J. L. OBLIGATELY PSYCHROPHILIC YEASTS FROM THE POLAR REGIONS. Can J Microbiol. 1965 Apr;11:259–269. doi: 10.1139/m65-032. [DOI] [PubMed] [Google Scholar]
- SINGER T. P., KEARNEY E. B. Determination of succinic dehydrogenase activity. Methods Biochem Anal. 1957;4:307–333. doi: 10.1002/9780470110201.ch9. [DOI] [PubMed] [Google Scholar]
- Stokes J. L. FERMENTATION OF GLUCOSE BY SUSPENSIONS OF ESCHERICHIA COLI. J Bacteriol. 1949 Feb;57(2):147–158. doi: 10.1128/jb.57.2.147-158.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UPADHYAY J., STOKES J. L. TEMPERATURE-SENSITIVE HYDROGENASE AND HYDROGENASE SYNTHESIS IN A PSYCHROPHILIC BACTERIUM. J Bacteriol. 1963 Nov;86:992–998. doi: 10.1128/jb.86.5.992-998.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UPADHYAY J., STOKES R. L. Temperature-sensitive formic hydrogenlyase in a psychrophilic bacterium. J Bacteriol. 1963 Jan;85:177–185. doi: 10.1128/jb.85.1.177-185.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]


