Abstract
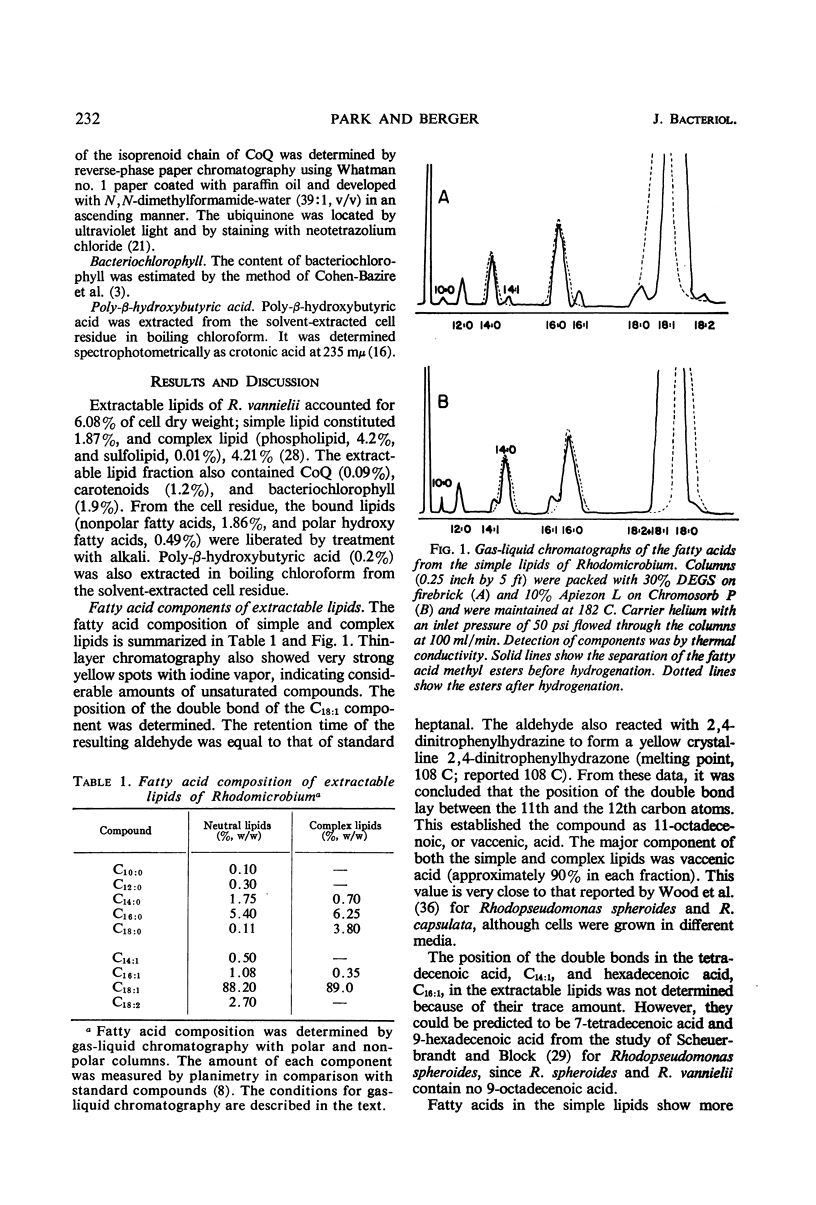
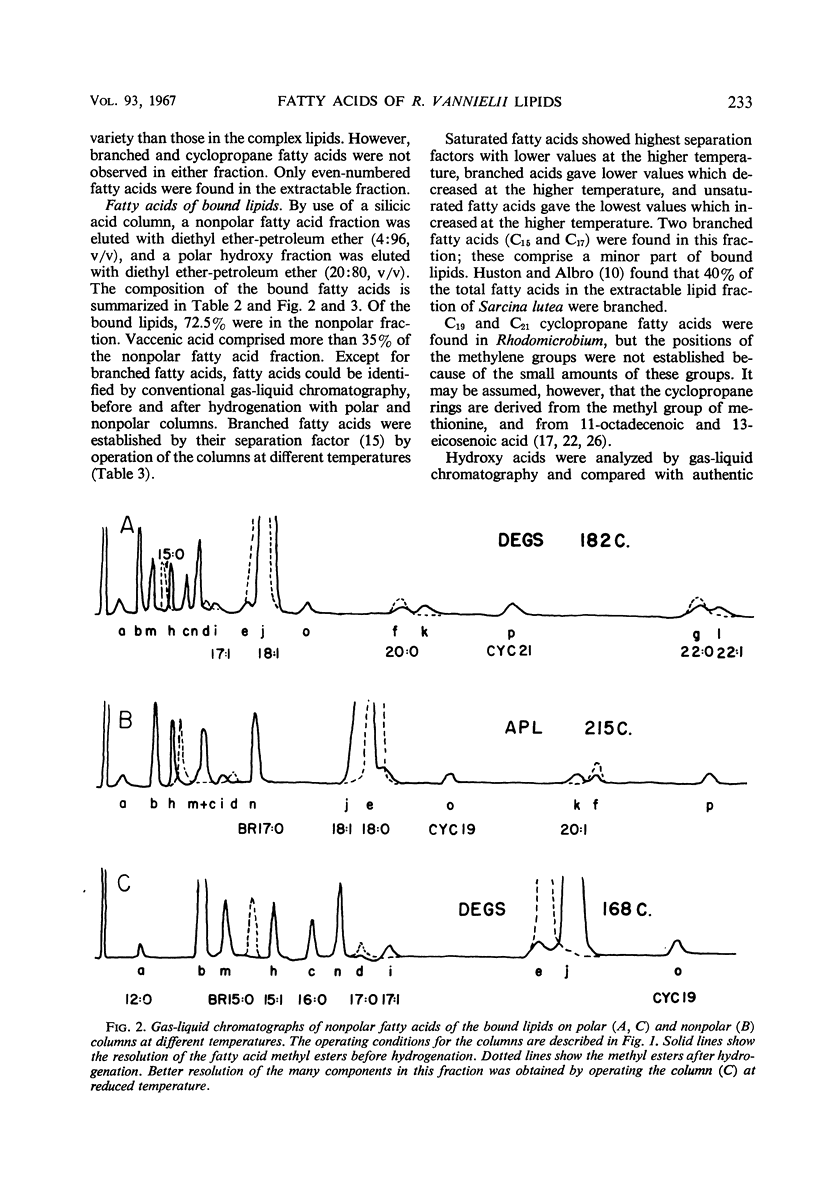
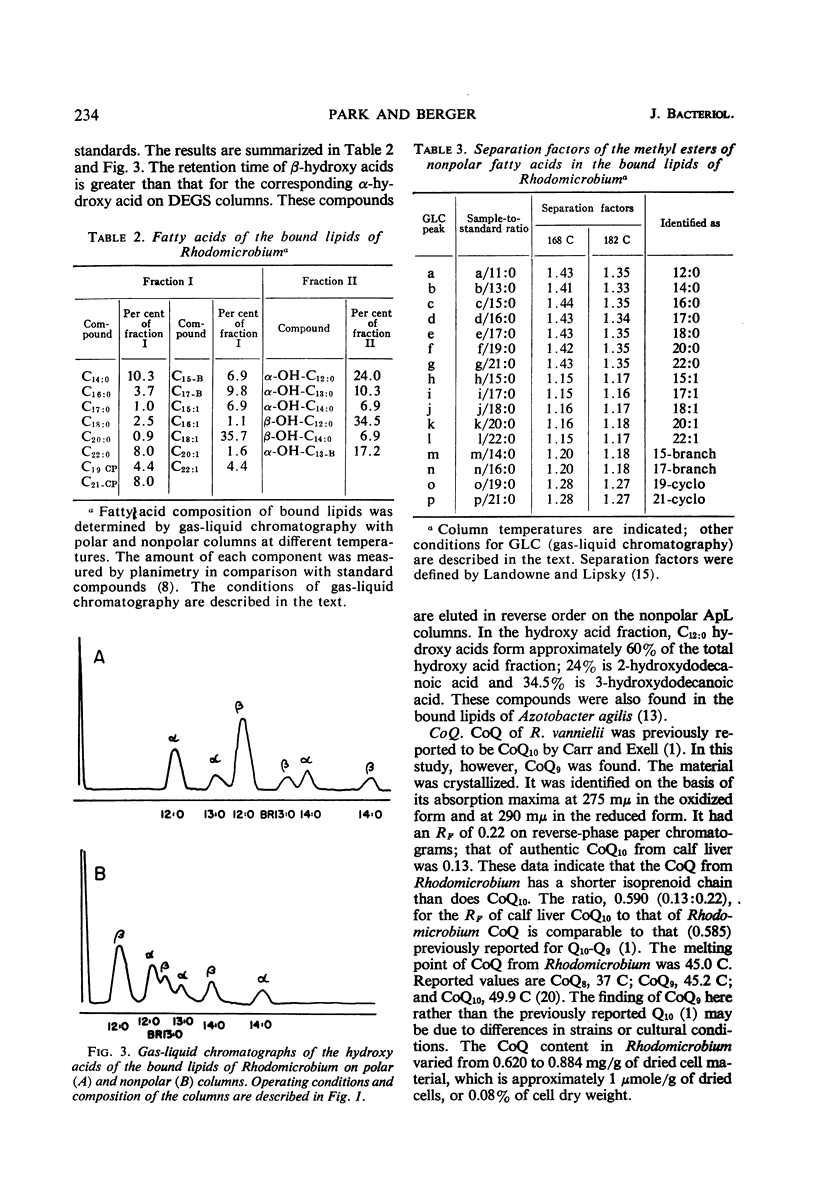
Cells of Rhodomicrobium vannielii grown at 29 C in a lactate-containing medium were extracted at room temperature with organic solvents. The extractable fraction contained the bulk of the simple lipid (1.87% of cell dry weight) and complex lipids (phospholipids, 4.2%; sulfolipid, 0.01%), coenzyme Q (0.09%), and pigments (carotenoids 1.2%; bacteriochlorophyll, 1.9%). The cell residue contained the bound lipids (nonpolar fatty acid fraction, 1.86%; polar hydroxy fatty acids, 0.49%). The residue also contained poly-β-hydroxybutyric acid (0.2%), which was extracted in boiling chloroform. In both the simple and complex lipids, vaccenic acid (11-octadecenoic acid) was the largest single component (approximately 90% in each fraction). The fatty acids of the bound lipid contained 35% vaccenic acid, even- and odd-numbered saturated and unsaturated straight-chain fatty acids, cyclopropane-, branched-, and α- and β-hydroxy fatty acids. The extractable lipids contained only straight-chain saturated and unsaturated even-numbered fatty acids. Nearly 60% of hydroxy fatty acid fraction was α-hydroxydodecanoic acid (24%) and β-hydroxydodecanoic acid (34.5%). Coenzyme Q was crystallized and identified as Q9 on the basis of melting point and chromatographic properties. Q10 had been previously reported.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- Carr N. G., Exell G. Ubiquinone concentrations in athiorhodaceae grown under various environmental conditions. Biochem J. 1965 Sep;96(3):688–692. doi: 10.1042/bj0960688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUDOROFF M., STANIER R. Y. Role of poly-beta-hydroxybutyric acid in the assimilation of organic carbon by bacteria. Nature. 1959 May 23;183(4673):1440–1442. doi: 10.1038/1831440a0. [DOI] [PubMed] [Google Scholar]
- Duchow E., Douglas H. C. RHODOMICROBIUM VANNIELII, A NEW PHOTOHETEROTROPHIC BACTERIUM. J Bacteriol. 1949 Oct;58(4):409–416. doi: 10.1128/jb.58.4.409-416.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- HOFMANN K., LUCAS R. A., SAX S. M. The chemical nature of the fatty acids of Lactobacillus arabinosus. J Biol Chem. 1952 Apr;195(2):473–485. [PubMed] [Google Scholar]
- HORNING E. C., AHRENS E. H., Jr, LIPSKY S. R., MATTSON F. H., MEAD J. F., TURNER D. A., GOLDWATER W. H. QUANTITATIVE ANALYSIS OF FATTY ACIDS BY GAS-LIQUID CHROMATOGRAPHY. J Lipid Res. 1964 Jan;5:20–27. [PubMed] [Google Scholar]
- HUSTON C. K., ALBRO P. W. LIPIDS OF SARCINA LUTEA. I. FATTY ACID COMPOSITION OF THE EXTRACTABLE LIPIDS. J Bacteriol. 1964 Aug;88:425–432. doi: 10.1128/jb.88.2.425-432.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENSEN A., AASMUNDRUD O., EIMHJELLEN K. E. CHLOROPHYLLS OF PHOTOSYNTHETIC BACTERIA. Biochim Biophys Acta. 1964 Nov 29;88:466–479. doi: 10.1016/0926-6577(64)90089-0. [DOI] [PubMed] [Google Scholar]
- KANESHIRO T., MARR A. G. Hydroxy fatty acids of Azotobacter agilis. Biochim Biophys Acta. 1963 Jun 18;70:271–277. doi: 10.1016/0006-3002(63)90751-0. [DOI] [PubMed] [Google Scholar]
- LANDOWNE R. A., LIPSKY S. R. A simple method for distinguishing between unsaturated and branched fatty acid isomers by gas chromatography. Biochim Biophys Acta. 1961 Mar 4;47:589–592. doi: 10.1016/0006-3002(61)90555-8. [DOI] [PubMed] [Google Scholar]
- LAW J. H., SLEPECKY R. A. Assay of poly-beta-hydroxybutyric acid. J Bacteriol. 1961 Jul;82:33–36. doi: 10.1128/jb.82.1.33-36.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESTER R. L., CRANE F. L. The natural occurrence of coenzyme Q and related compounds. J Biol Chem. 1959 Aug;234(8):2169–2175. [PubMed] [Google Scholar]
- LESTER R. L., HATEFI Y., WIDMER C., CRANE F. L. Studies on the electron transport system. XX. Chemical and physical properties of the coenzyme Q family of compounds. Biochim Biophys Acta. 1959 May;33(1):169–185. doi: 10.1016/0006-3002(59)90511-6. [DOI] [PubMed] [Google Scholar]
- LESTER R. L., RAMASARMA T. Chromatography of the coenzyme Q family of compounds on silicone-impregnated paper. J Biol Chem. 1959 Mar;234(3):672–676. [PubMed] [Google Scholar]
- LIU T. Y., HOFMANN K. Cyclopropane ring blosynthesis. Biochemistry. 1962 Jan;1:189–191. doi: 10.1021/bi00907a028. [DOI] [PubMed] [Google Scholar]
- MORRISON W. R., SMITH L. M. PREPARATION OF FATTY ACID METHYL ESTERS AND DIMETHYLACETALS FROM LIPIDS WITH BORON FLUORIDE--METHANOL. J Lipid Res. 1964 Oct;5:600–608. [PubMed] [Google Scholar]
- O'LEARY W. M. Studies of the utilization of C14-labeled octadecenoic acids by Lactobacillus arabinosus. J Bacteriol. 1959 Mar;77(3):367–373. doi: 10.1128/jb.77.3.367-373.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLSON R. E., DIALAMIEH G. H., BENTLEY R., SPRINGER C. M., RAMSEY V. G. STUDIES ON COENZYME Q. PATTERN OF LABELING IN COENZYME Q9 AFTER ADMINISTRATION OF ISOTOPIC ACETATE AND AROMATIC AMINO ACIDS TO RATS. J Biol Chem. 1965 Jan;240:514–523. [PubMed] [Google Scholar]
- Park C. E., Berger L. R. Complex lipids of Rhodomicrobium vannielii. J Bacteriol. 1967 Jan;93(1):221–229. doi: 10.1128/jb.93.1.221-229.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skipski V. P., Smolowe A. F., Sullivan R. C., Barclay M. Separation of lipid classes by thin-layer chromatography. Biochim Biophys Acta. 1965 Oct 4;106(2):386–396. doi: 10.1016/0005-2760(65)90047-0. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Doudoroff M., Kunisawa R., Contopoulou R. THE ROLE OF ORGANIC SUBSTRATES IN BACTERIAL PHOTOSYNTHESIS. Proc Natl Acad Sci U S A. 1959 Aug;45(8):1246–1260. doi: 10.1073/pnas.45.8.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood B. J., Nichols B. W., James A. T. The lipids and fatty acid metabolism of photosynthetic bacteria. Biochim Biophys Acta. 1965 Oct 4;106(2):261–273. doi: 10.1016/0005-2760(65)90034-2. [DOI] [PubMed] [Google Scholar]


