Abstract
Wounding initiates a strong and largely jasmonate-dependent remodelling of the transcriptome in the leaf blades of Arabidopsis (Arabidopsis thaliana). How much control do jasmonates exert on wound-induced protein repatterning in leaves? Replicated shotgun proteomic analyses of 2.5-mm-wide leaf strips adjacent to wounds revealed 106 differentially regulated proteins. Many of these gene products have not emerged as being wound regulated in transcriptomic studies. From experiments using the jasmonic acid (JA)-deficient allene oxide synthase mutant we estimated that approximately 95% of wound-stimulated changes in protein levels were deregulated in the absence of JA. The levels of two tonoplast proteins already implicated in defense response regulation, TWO-PORE CHANNEL1 and the calcium-V-ATPase ACA4 increased on wounding, but their transcripts were not wound inducible. The data suggest new roles for jasmonate in controlling the levels of calcium-regulated pumps and transporters, proteins involved in targeted proteolysis, a putative bacterial virulence factor target, a light-dependent catalyst, and a key redox-controlled enzyme in glutathione synthesis. Extending the latter observation we found that wounding increased the proportion of oxidized glutathione in leaves, but only in plants able to synthesize JA. The oxidizing conditions generated through JA signaling near wounds help to define the cellular environment in which proteome remodelling occurs.
Leaves, often having large surface-to-volume ratios and lacking thickened protective barriers, are particularly prone to wounding. However, since damage elicits strong defense responses extending beyond the wound site, leaves are some of the most common and resilient of living structures. These wound responses, first identified as damage-induced defense protein accumulation occurring in physically damaged leaves as well as in distal leaves (Green and Ryan, 1972), are now known to involve extensive transcriptional reprogramming (Reymond et al., 2004). Importantly, even strong wound responses in adult plants are not truly systemic. Instead, they depend in large part on source-sink relationships, as shown for the expression of the gene WOUND-INDUCED3 in poplar (Populus spp.; Davis et al., 1991). In adult-phase Arabidopsis (Arabidopsis thaliana), the wound-induced expression of the genes JASMONATE ZIM DOMAIN10 (JAZ10) and LIPOXYGENASE2 (LOX2) follows intervascular connections termed parastichies (Glauser et al., 2009). While the maximum extent of wound response domains generated after a single severe wound is not yet established fully for the vegetative tissues of any plant, the approximate limits of wound-induced gene expression should, in theory, be predictable if a plant’s vascular architecture is known. In summary, the regulation of transcription (and, potentially, translation) takes place within the first few hours of wounding in reproducible patterns extending away from the wound into discrete distal tissue domains.
In terms of regulation, the wound response of plants involves the action of multiple signal pathways (e.g. Onkokesung et al., 2010; Walley and Dehesh, 2010). One of them, the jasmonate pathway, is based on the synthesis of the small fatty acid-derived regulator jasmonic acid (JA), and is now known to control and coordinate a particularly large number of responses to wounding (Koo and Howe, 2009). JA signaling relies on the production of amino acid conjugates such as jasmonoyl-l-Ile from JA, followed by the perception of jasmonoyl-l-Ile that probably takes place in the nucleus (Browse, 2009; Fonseca et al., 2009; Gfeller et al., 2010). Significantly, the wound-induced accumulation of JA, like jasmonate-regulated transcripts, also occurs in domains closely reflecting interleaf vascular connections (Glauser et al., 2009).
Statistical analysis has revealed that the levels of 67% to 84% of transcripts were controlled via jasmonate signaling upon wounding Arabidopsis leaves (Reymond et al., 2004). This transcriptome study, however, used whole wounded leaves, i.e. samples containing both crushed and intact tissue. Now, to improve the quantitative assessment of the impact of the jasmonate pathway, better spatial resolution is needed and it will be necessary to focus increasingly on discrete, well-defined tissue regions. Several proteomic studies of leaves in response to wounding or herbivory have been conducted (Shen et al., 2003; Giri et al., 2006; Soares et al., 2009; Collins et al., 2010; Thivierge et al., 2010), and a recent study has compared the proteomes of wild-type leaves with those from a jasmonate perception mutant (Shan et al., 2011). Nevertheless, wound proteomic studies comparing the wild-type and jasmonate mutants have not been reported.
In this work we aimed to quantitate the role of jasmonate in early proteome repatterning after wounding. The selection of the tissue area to be studied proved to be challenging since many wound responses in leaves diminish with increasing distance from the site of damage. This has been shown to be the case for two proteinase inhibitor proteins in wounded tomato (Solanum lycopersicum) leaves (Graham et al., 1986). Similarly, this occurs with wound-stimulated JA synthesis in Arabidopsis leaves (Glauser et al., 2009). Furthermore, other studies indicate that the expression of multiple inducible transcripts decrease with distance from a wound (e.g. Farmer et al., 1992; Reymond et al., 2004; Chung et al., 2008). In summary, protein and transcript levels can form sectors or gradients within leaves or intervascular domains and these are not truly systemic responses in adult-phase plants.
A specific goal of our study was to provide new information on the molecular environment in undamaged plant tissues in the vicinity of a wound, coupled to a quantitative estimation of the extent of control exerted by jasmonate signaling in reshaping the proteome in response to tissue damage. Through the analysis of nearly 6,000 proteins we assessed proteome remodelling events that unfold in the 6 h following wounding and observed a larger than expected impact of jasmonate on the formation of the wound proteome. Among the results was the finding that the levels of proteins of glutathione (GSH) synthesis and deployment were affected by wounding. Based on this we tested whether the oxidation status of GSH pools was affected and this has provided new insights into the nature of the cellular environment near a wound.
RESULTS
Experimental Design
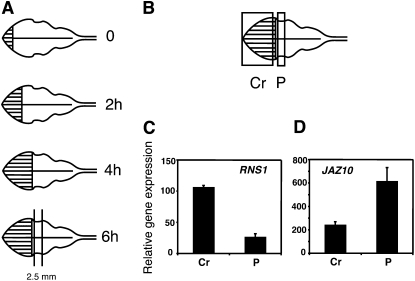
To evolve an experimental design we first examined the possibility that the presence of crushed tissue from the wounding procedure might complicate the interpretation of protein pattern changes. For this, leaves were wounded successively three times (each time damaging approximately 10% of the total leaf area) with forceps at times 0, 2, and 4 h, starting at the apex. Six hours after the first wound, tissue strips of 2.5 ± 0.5 mm width were harvested (Fig. 1, A and B). Quantitative PCR was performed to assess the relative levels of wound-responsive transcripts. From data in Reymond et al. (2000) we used the RIBONUCLEASE1 (RNS1) gene as a crush marker and found that RNS1 transcripts were more highly expressed in crushed tissue than in the proximal strip of undamaged tissue (Fig. 1C). In contrast, the expression of a jasmonate-response reference gene JAZ10 (formerly JAS1; Yan et al., 2007) was higher in intact tissue than in the crushed tissue (Fig. 1D). These results suggested that the inclusion of crushed tissue could, in theory, introduce heterogeneity into proteomic studies. Only intact, undamaged tissue was harvested for subsequent proteomics experiments. We chose a 6 h harvest time since, at the protein level, this is likely to be an early time point and may reduce indirect effects where, for example, an induced protein might alter the level of a second protein. Since many herbivores wound plants in a repetitive manner three successive wounds were inflicted.
Figure 1.
Differential gene expression in crushed and undamaged tissues. A, Design used in large-scale proteomic experiments. B, Detail showing areas of tissue harvested. Cr, Crushed region; P = wound-proximal zone (2.5 ± 0.5 mm width). C, Relative expression (ΔCT) of RNS1 in zones Cr and P. D, Relative expression of JAZ10 in zones Cr and P.
High-Throughput Proteomic Experiments
With linear trap quadrupole ion-trap mass spectrometry (MS/MS) techniques described by Baerenfaller et al. (2008) we performed 240 individual ion-trap MS/MS runs of protein extracts from the 2.5-mm wound-proximal zone and its spatial equivalent in unwounded plants. From these replicated experiments a total of 6,530 proteins were identified based on 216,896 peptide spectrum assignments with two search algorithms at a spectrum false discovery rate of 1%. As proteins are quantified by taking into account solely true tryptic peptides, the number of quantified proteins is 5,936. This led to the identification of 106 wound-regulated proteins (69 induced, 37 repressed) in the wild type (Fig. 2A; Supplemental Table S1). To assess the reliability of the data we used enzymes involved in JA synthesis as landmarks and finding of six of these proteins (LOX3, AOC1, OPR3, ACX1, ACX3, and JAR1) up-regulated in the wounded wild type suggested that we captured many proteins associated with wound responses. Extended analysis revealed Gene Ontology (GO) categories that were up- or down-regulated in wounded wild-type leaves (Fig. 2B; Supplemental Table S2). Next, we compared control and wounded leaves of the allene oxide synthase (aos) mutant and found 114 wound-regulated proteins of which 51 are more abundant in wounded and 63 in control leaves (Supplemental Table S3). The list of proteins that were more abundant in wounded aos leaves only shared four proteins with the list of proteins that are more abundant in wounded wild-type leaves, namely a sulfotransferase family protein, a putative peroxidase, AtEXO70H7, and ALDH6B2. ANNEXIN2 (ANNAT2) protein was repressed both in wild type and aos.
Figure 2.
Summary of results. A, Numbers of differentially regulated proteins from all proteomics experiments. Also indicated are the five wound-induced proteins in common between wild type and aos. B, Overrepresented GO categories for proteins that were more abundant in wounded wild-type leaves (dark gray) and in control (unwounded) wild-type leaves (light gray). C, Overview of protein group regulation by jasmonates in response to wounding based on Supplemental Table S5. Upward arrows indicate that proteins in the category are mostly up-regulated via activity of the jasmonate pathway in response to wounding and downward arrows that they are mostly down-regulated via activity of the jasmonate pathway in response to wounding. Contrasense arrows indicate differential protein regulation controlled by jasmonates.
Remarkably, 95% of the wound-regulated proteins in the wild type were not wound regulated in aos. However 109 proteins (47 induced, 62 repressed; Supplemental Table S3) became wound regulated in aos independently of the JA biosynthesis, with a higher tendency to down-regulation. Major differences between the aos and wild-type wound proteomes were in proteins involved in oxylipin biosynthesis (six induced in wild type; two induced in aos), stress and redox regulation (10 induced, four repressed in wild type; four induced, six repressed in aos), posttranslationnal modification and signaling (seven induced, one repressed in wild type; four induced, eight repressed in aos), transport (six induced, eight repressed in wild type; five induced, 12 repressed in aos), metabolism (12 induced, three repressed in wild type; 12 induced, 15 repressed in aos), and proteolysis (one induced in wild type; five induced, two repressed in aos; Supplemental Tables S1 and S3). To investigate how the aos mutation affects the otherwise healthy plants, the protein levels in resting, unwounded wild-type and aos plants were then compared. The control unwounded wild-type and control aos showed 58 differences with 34 proteins being more abundant in aos (Supplemental Table S4; summarized in Supplemental Fig. S1). Almost all of the differentially regulated proteins in resting leaves were found in lower levels in aos relative to the wild type. At this point we directly compared the ratios of protein induction/repression in the wild type with the analogous ratios for aos (see Supplemental Table S5). This led to the identification of 116 proteins that were differentially regulated in the two genotypes where the levels of 62 proteins were lower in wounded aos than in wounded wild type. The functional classification of the proteins that are more abundant in control wild type as compared to control aos gave JA biosynthetic process as the most overrepresented GO category (Supplemental Table S2). Many proteins not known previously to be jasmonate regulated were observed in this way and the major categories are summarized in Figure 2C. Figure 3 shows annotated proteins that were wound induced in the wild type, and indicates which of these were regulated in a jasmonate-independent manner. Finally, 60% of the wound-regulated proteins we found in wild-type tissues have also been recorded as wound regulated at the transcript level in wild-type plants grown under similar conditions. However, we observed 15 down-regulated and 26 up-regulated proteins that did not show the same pattern of regulation at the transcript level. These proteins are listed in Supplemental Table S6. Using wounding conditions identical to those used for proteomics we assessed the mRNA levels of several of these genes chosen since they represent transport functions. Two proteins that were up-regulated by wounding were chosen: TWO-PORE CHANNEL1 (TPC1) and ACA4. These were not induced at the mRNA level, and both mRNAs were lower in wounded than in unwounded leaves. ADNT1 and ANNAT2 were chosen for transcript abundance analysis since they were both wound repressed at the protein level. ADNT1 transcripts were weakly down-regulated by wounding whereas ANNAT2 transcripts were weakly up-regulated by wounding in the wild type (Fig. 4).
Figure 3.
Protein changes adjacent to the wound in the wild type. Arrows show the 2-fold up- and down-regulation of proteins. Downward arrows mean that wounding leads to decreased protein level. Arabidopsis Gene Identifier codes are given for proteins lacking consistently used acronyms. Quantitative data for this experiment are given in Supplemental Table S1. Black arrows = P value < 0.05; gray arrows = P value 0.05 to 0.1. Proteins indicated in red were regulated in a jasmonate-independent manner. PTM, Proteins potentially involved in posttranslational modification.
Figure 4.
Relative transcript levels for proteins that are significantly regulated upon wounding in the wild type. Leaves were wounded three times with forceps at time 0, 2, and 4 h (10% of the leaf area each time), and the intact 2.5 ± 0.5 mm proximal tissue to the wound was harvested at 6 h. Control leaves were not wounded. The levels of the mRNAs indicated in each section (top section for wild type, bottom section for aos) were measured by PCR relative to the reference gene UBC21. White bars, samples from unwounded leaves; black bars, samples from wounded leaves. Data are from three biologically independent replicates and each value was calculated from the average of three technical replicates. The positive control for transcript induction by wounding was JAZ10, protein levels for this control gene were not investigated.
GSH Analyses
A number of proteins that were found to be wound inducible participate in GSH synthesis or employ GSH in various reactions. We therefore analyzed the oxidation state of GSH proximal to the wound. A significantly higher proportion of oxidized GSH (GSSG; GSSG/total GSH ratio = 6.24 ± 0.68) was seen in the wounded wild type relative to unwounded wild type (ratio = 3.79 ± 0.35). However, this was not observed in aos leaves where the GSSG/total GSH ratio in unwounded leaves was 4.29 ± 0.21 and this remained essentially constant at 3.76 ± 0.54 after wounding (Fig. 5).
Figure 5.
Jasmonate-dependent GSH oxidation in the wound-proximal zone. Leaves of the wild type and the aos mutant were wounded (W) three times with a forceps at time 0, 2, and 4 h (10% of the leaf area each time), and the intact 2.5 ± 0.5 mm proximal tissue to the wound was harvested at 6 h. The equivalent region was taken from the control (C) leaves that were not wounded. The ratio of oxidized over total GSH (%GSSG/[GSH + GSSG]) was measured. The asterisk indicates a significant difference between the wounded sample and control unwounded wild-type leaves (P value < 0.05, Student’s t test, n = 4–7).
DISCUSSION
Unless very severe, signals emanating from wounds probably only rarely fill the potential boundaries of wound response domains: parastichies in adult-phase Arabidopsis. Instead, wound-generated signals are often restricted to smaller regions. These can take many forms depending on where the leaf is wounded, but for proteomic experiments we used staggered leaf tip wounding in keeping with the fact that many invertebrate herbivores feed at intervals on the same area of leaf and due to the fact that small wounds such as pin pricking do not activate the jasmonate pathway strongly in expanded leaves (Farmer et al., 1992). We chose to harvest tissue at 6 h after the first wound so that secondary effects on the proteome would, in theory, be minimized.
Generalities from Proteome Analysis
Figure 3 summarizes the pattern of differentially regulated proteins in the wounded wild type. The spectrum of these proteins (Supplemental Table S1) differed greatly from those that were wound regulated in aos (Supplemental Table S3). First, it was striking that only five of the wound-regulated proteins in wild-type leaves were still significantly wound regulated in aos. The levels of these proteins may be regulated through signal pathways that are largely JA independent. Second, the large number of protein changes (114) elicited in wounded aos was unexpected. It seems most likely to us that jasmonates otherwise repress the majority of these changes, probably through cross regulation of other, non-jasmonate-based signal pathways. It is also possible that in the absence of jasmonate-regulated increases in GSH oxidation in aos (Fig. 5), the cellular environment near the wound leads to a strong deregulation of many genes. We also compared the protein complement of resting (undamaged) leaves of the wild type and of aos. In apparently healthy leaves the aos mutation impacts the resting chloroplast proteome and, in addition, we noted the deregulation of proteins that were annotated as stress related. In aos a novel set of 47 proteins becomes wound inducible. The simplest explanation of this is that jasmonates normally suppress the accumulation of these proteins in the wild type through modulating the activity of other signal pathways. Indeed, Supplemental Table S5 shows that more proteins that are implicated in signaling (including protein kinases) and posttranslational modification were down-regulated in wounded aos (eight proteins) than in wounded wild type (one protein). To generate results that would be comparable with previous transcriptome studies we compared the ratios of protein induction and repression in the wild type and in aos. This was necessary because of the altered basal levels of proteins that were observed when we compared the two unwounded genotypes. The results of these experiments (Supplemental Table S5) are consistent with results from transcriptome analyses of wild-type plants treated with JA (Sasaki-Sekimoto et al., 2005) and from wounding wild-type plants and jasmonate mutants (Reymond et al., 2004). However, Figure 2C reveals two categories of protein that include members not discussed in detail in microarray studies: endomembrane proteins, and proteins involved in targeted proteolysis. Consistent with the literature showing that genes encoding proteins related to photosynthesis tend to be down-regulated in many biotic stresses (Bilgin et al., 2010), we also found the down-regulation of several photosynthesis-related functions following wounding. It seems likely that the bulk of early proteome remodelling in response to wounding is directed at improving survival and in many cases, protein level changes may be associated indirectly with defense. For example, the down-regulation of the porphyrin-metabolizing enzyme POR C suggests decreased nitrogen flux into porphyrin synthesis. This may ultimately be defense related since nitrogen is needed for glucosinolate and defense protein synthesis.
Wounding Affects Transport Functions
The tonoplast is emerging as a potentially important compartment for the regulation of defense pathways. Three tonoplast transporter proteins, two of which have been implicated previously in the regulation of defense signal pathways, accumulated after wounding. In each case, this required JA synthesis. First is the calcium-regulated TPC1 (At4g03560) protein. The fou2 point mutation in TPC1 increases the open probability of this voltage-gated channel, activating jasmonate synthesis (Bonaventure et al., 2007). Second, genetic disruption of the tonoplast calcium-V-ATPase ACA4 (At2g41560) along with a paralog, ACA11, resulted in the powerful activation of the defense-related salicylic acid pathway (Boursiac et al., 2010). We found that ACA4 protein was wound inducible. The third tonoplast-localized transporter that was wound induced in the wild type was MRP2 (At2g34660). This ABCC-type ABC transporter protein is a known part of the organic anion pump capacity of the vacuole membrane (Frelet-Barrand et al., 2008). Several proteins that might directly regulate water flux (and therefore turgor) were also found to be up-regulated in the wound-proximal region. An example of this was PIP2A (At3g53420), a plasma membrane protein that facilitates the bidirectional flow of water and hydrogen peroxide (Ludewig and Dynowski, 2009).
IMPa-4 (At1g09270), an α-importin, forms part of the nuclear pore-targeting complex responsible for the energy-dependent translocation of proteins into and out of the nucleus. This wound-induced protein is utilized by the pathogen Agrobacterium tumefaciens to efficiently transform plant cells (Bhattacharjee et al., 2008). Another wound-induced protein that has been linked to pathogenesis is PEN3 (PDR8; At1g59870), a plasma membrane-localized ATP binding cassette transporter possibly involved in the export of antimicrobial agents into the apoplast (Stein et al., 2006). PXA1 (At4g39850), a peroxisomal ATP-binding cassette transporter implicated in the transport of fatty acyl CoAs (Nyathi et al., 2010), was up-regulated in resting aos relative to the wild type. Finally, we found that three predicted chloroplast transport proteins were all down-regulated in response to wounding and, in each case, this required JA synthesis. One of these was cpSecY (SCY1; At2g18710), a nuclear-encoded thylakoid translocase component (Dalbey and Kuhn, 2000). To our knowledge this is the first component of a thylakoid protein import machine found to be jasmonate regulated.
Chloroplast and Light-Related Functions
Treatment of excised barley (Hordeum vulgare) leaf segments with exogenous jasmonate is known to impair the translation of mRNAs for Rubisco. In the case of the plastid-encoded large subunit, jasmonate treatment resulted in the production of abnormally long transcripts (Reinbothe et al., 1993). We did not observe changes in Rubisco protein levels in the 6 h time frame of our experiments, although we did observe down-regulation of a putative Rubisco activase (At1g73110; Fig. 3), a protein that functions to expulse inhibitory sugar phosphates from the active site of Rubisco. Giri et al. (2006) found a strong reduction in the levels of Rubisco activase isoforms after wounding tobacco (Nicotiana tabacum) leaves and treating them with caterpillar oral secretions. Additionally, Shan et al. (2011) also found that Rubisco activase in Arabidopsis functions to repress senescence and is jasmonate repressible. Our study also revealed that PSI SUBUNIT P (PSAP, At2g46820) was strongly down-regulated in the resting leaves of aos with respect to the wild type, meaning that both the dark and light reactions of photosynthesis are wound and jasmonate regulated at the protein level. A number of other proteins involved in various aspects of photobiology were wound regulated (Supplemental Tables S1 and S5). Interestingly, POR C (At1g03630), one of the rare enzymes requiring light for full catalytic activity (Frick et al., 2003), was down-regulated following wounding.
Defense Functions
LOX2 (At3g45140) levels increased significantly in the wounded wild type compared to wounded aos but is not indicated in Figure 3 since its induction fell just below the 2-fold threshold we set. LOX2 functions in the synthesis of arabidopsides and the increased growth of a generalist herbivore relative to growth on the wild type was observed on lox2-1 plants (Glauser et al., 2009). The function of LOX3 in leaves is unknown; its level of induction after wounding exceeded the 2-fold threshold. The wound induction of HopW1-1 INTERACTING PROTEIN1 (At1g80600), a potential target of the Pseudomonas virulence factor HopW1 (Lee et al., 2008), was found to be deregulated in wounded aos relative to the wounded wild type. Additionally, the levels of two jacalin lectins also increased in a JA-dependent manner after wounding (Supplemental Table S1 and S5) as did β-glucosidase BGL1 (At1g52400), a hydrolase known to hydrolyze Glc-conjugated abscisic acid to release the active hormone (Lee et al., 2006).
Strong Impact on Proteins of Vesicular Transport, Cell Wall, and Cytoskeleton
Levels of dynamin-like protein 6 (ADL6; At1g10290) and V-Snare 11 (VTI11; At5g39510), both of which are annotated as being potentially involved in vesicle trafficking from Golgi to vacuoles, were altered by wounding. These candidates could now be employed as fusion proteins to probe endomembrane dynamics in the wound-proximal zone. Vesicle secretion (exocytosis) may modify the apoplast in response to damage and Soares et al. (2009) found that apoplastic glucanases, chitinases, and peroxidases accumulated in response to leaf wounding in Medicago truncatulata. We observed wound-regulated proteins involved in pectin methylation, glycosyl bond formation or hydrolysis, and peroxidation, indicating the possible catalytic and structural remodelling of the cell wall. Finally, our experiments revealed increased levels of either TUBULIN2 or 3 in the wound-proximal zone of the wild type but not in this zone in aos. Hamant et al. (2008) observed cell-autonomous microtubule realignment around wound sites and this peaked between 1.5 and 5.5 h after wounding, i.e. within the general time frame of our study.
Jasmonate-Dependent Remodelling of the Protein Synthesis and Degradation Machinery
We observed that INITIATION FACTOR3 (At5g39510) was down-regulated in leaves in response to wounding and five putative ribosomal proteins (both prokaryotic and eukaryotic) and one ribosome-associated protein were also down-regulated in the wounded wild type. It is known that a ribosome-inactivating protein, JIP60, is induced upon jasmonate treatment in barley (Reinbothe et al., 1994). While JIP60 activity was not itself affected by jasmonate treatment, its substrates (ribosomes) themselves were probably modified in response to jasmonate treatment. Our results are also consistent with ribosome remodelling under control of the jasmonate pathway near the wound. Additionally, the comparison of resting wild-type and aos leaves also suggests that some of the protein synthesis machinery in resting leaves may be modeled in part due to the activity of the jasmonate pathway. For example, two 40S ribosomal proteins, RPS3aA (At3g04840) and RPS24B (At5g28060) were down-regulated in the resting leaves of aos relative to the wild type and a putative eukaryotic cytosolic elongation factor (At1g57720) was up-regulated at the protein level in resting aos relative to wild type. Another interesting aspect was the indication that wounding, via activity of the jasmonate pathway, may regulate components of ubiquitin-dependent proteolysis. One such example is proteasome-associated 200, PA200 (At3g13330), a regulatory subunit of the 26S proteosome (Book et al., 2010) that we now find to be up-regulated by wounding. A second example, SKP1 (At1g75950), emerged from comparison of the proteomes of wounded wild-type and aos plants.
Translational and Posttranslational Events
Some of the proteins identified in Supplemental Table S6 as being wound induced at the protein level but not or less so at the transcript level have appeared in genetic screens related to jasmonates, as was the case with the ion channel TPC1. A nearly 4-fold increase in TPC1 protein level was observed in wounded wild-type leaves whereas levels of TPC1 transcript were reported to be relatively stable during development and were not strongly wound regulated under conditions different to those used herein (Bonaventure et al., 2007). We examined the behavior of mRNAs for TPC1 and for several other calcium binding/regulated proteins as well as a putative nucleotide transporter (ADNT1) in the same wounding conditions used for proteomics. Of the four gene products tested three, TPC1, ACA4, and ANNAT2, are likely to be regulated posttranscriptionally in the wild type since the levels of their RNA and protein products behave oppositely on wounding. Only one of the genes tested (ADNT1) behaved similarly at both the protein and RNA levels although it was much more strongly repressed at the protein level than at the transcript level. It seems, then, that transcription and translation may not be well correlated for these proteins. A number of polypeptides we found to be JA regulated are known to be themselves involved in posttranslational modification, potentially modifiying the activities of proteins rather than their levels. An example of this is TIP1 (At5g20350), a thio-acyl transferase (Hemsley et al., 2005). Other wound-regulated proteins are themselves known to be subject to in vivo posttranslational modification e.g. GCL (RML1/PAD2; Hicks et al., 2007) in which oxidation of the disulfide bond Cys-186-Cys-406 due to oxidative stress increases enzyme activity. In summary, our lists provide candidates for proteins for which levels might be regulated posttranscriptionally by jasmonate and for enzyme activities that might depend on the cellular redox conditions.
Sulfur Metabolism, GSH, Redox-Related Functions, and the Redox Environment Near a Wound
Consistent with the known jasmonate inducibility of many genes of sulfur (S) assimilation (Sasaki-Sekimoto et al., 2005) the levels of several proteins involved in S metabolism were strongly affected in the wound-proximal zone: ATP SULFURYLASE PRECURSOR2 (At1g19920) and ADENOSINE 5′-PHOSPHOSULFATE kinase1 (APK1; At2g14750) are two examples. APK1 has been implicated previously in the control of oxophytodienoic acid, sulpho-hydroxyjasmonate, and glucosinolate levels (Mugford et al., 2009). A wound-inducible phosphoadenosine phosphosulfate reductase family protein (At5g03430) we found may merit attention since a genetic screen has implicated phosphoadenosine phosphosulfate or a closely related molecule in the control of resting JA levels (Rodríguez et al., 2010). Figure 3 shows that many proteins involved in stress and redox are wound inducible in a number of cellular compartments including the mitochondrion, an organelle seldom implicated in wound responses. Two putative respiratory chain components are down-regulated in a JA-dependent manner in this organelle and this might affect cellular energetics or the production of reactive oxygen species.
These considerations and the fact that GCL (RML1/PAD2; At4g23100), the major regulatory enzyme of GSH synthesis, was wound inducible prompted us to assess GSH oxidation status and we found that the ratio of GSSG over total GSH increased in the wounded wild type, but not in wounded aos (Fig. 5). Therefore JA production actively signals the generation of a relatively oxidizing cellular environment near the wound border and this might potentiate the activities of many proteins. The jasmonate-controlled redox environment in wounded tissue may help to directly or indirectly regulate the activities of many genes and proteins including jasmonate-regulated genes such as JAZ10, and also some of the redox-sensitive proteins seen in our proteomics experiments.
Proteomics Summary: An Estimated 95% of Protein Repatterning in a Zone Near the Wound Is Jasmonate Regulated
Many processes that result from wounding are known to be heavily JA dependent and among these are transcriptome remodelling. Previous estimates of transcript accumulation after wounding in Arabidopsis leaves are within the 1 to 5 h time frame (Reymond et al., 2004). Within this interval 67% to 84% of transcriptome remodelling in leaves was controlled by jasmonate signaling. The new results show that, after a series of wounds inflicted at 2 to 6 h prior to harvest, 95% of the differentially expressed proteins recovered in a strip of healthy tissue 0.5 to 3.0 mm from a wound were regulated in a jasmonate-dependent manner. This percentage should be taken only as a rough estimate since (1) the proportion of low-abundance proteins (not accessible to our analyses) regulated by the jasmonate pathway remains unknown and (2) the results depend on the statistical criteria we used. This excluded many proteins up-regulated or down-regulated by <2-fold after wounding.
MATERIALS AND METHODS
Plant Material
Arabidopsis (Arabidopsis thaliana) Heynl. wild-type (Columbia-0) and aos mutant plants (Park et al., 2002; Mène-Saffrané et al., 2009) were soil grown under long-day conditions (14-h light, 100 μE m−2 s−1) for 4.5 to 5 weeks. The wounding and tissue harvest strategy used is detailed in Figure 1. Five expanded leaves (leaves 8–12) per plant were successively wounded (starting at the leaf tip, each time 10% of the leaf surface was wounded) at 0, 2, and 4 h with metal forceps. The 2.5 ± 0.5 mm of tissue neighboring the wound were harvested, excluding a border of approximately 0.5 mm of unwounded tissue directly abutting the wound. Eight plants were utilized for each proteomic sample and each data set was from three biologically independent replicates.
Real-Time PCR Quantitation
Total RNA (1 μg; purified according to Oñate-Sánchez and Vicente-Carbajosa, 2008) was copied to cDNA with the M-MLV reverse transcriptase RNAse H(-) (Promega) according to the manufacturer’s instructions. The quantitative PCR reaction mixture contained 0.2 mm dNTPs, 2.5 mm MgCl2, 0.5× SYBR Green I (Invitrogen), 30 nm 6-carboxy-X-rhodamine, 0.5 units of GoTaq polymerase (Promega), and 0.25 μm of each primer in a total volume of 20 μL. The primers used were for RNS1 (At2g02990): 5′-GGAGCTCTAACCAAAGCCGGGATT-3′ and 5′-AGAACCATCTCTGTTACACTCAACCC-3′. For JAZ10 (At5g13220): 5′-ATCCCGATTTCTCCGGTCCA-3′ and 5′-ACTTTCTCCTTGCGATGGGAAGA-5′. For TPC1 (At4g03560): 5′-GGC AAG TAT GGA TGG AGA GC-3′ and 5′-GGC AAC AAC CAA ATTCAACA-3′: ACA4 (At2g41560), TTTGCCAGGTGTTCAATGAG-3′ and 5′-TACCGTCACAGTCATTACCC-3′: ADNT1 (At4g01100), 5′-GCACAGCTTCACTTGAATACAC-3′ and 5′-CGATGGTACAACCTTCACTG-3′:ANNAT2 (At5g65020) 5′-CGCCATTAACAAGAACTTGAAGG-3′ and 5′-GATTGATAGACGAAGAACCTTCTC-3′. Primers used for the UBC21 reference gene (At5g25760; Glauser et al., 2009) were 5′-CAGTCTGTGTGTAGAGCTATCATAGCAT-3′ and 5′-AGAAGATTCCCTGAGTCGCAGTT-3′. The PCR program consisted of an initial denaturation step at 95°C for 2 min followed by 40 cycles of 10 s at 95°C, 30 s at 60°C, and 30 s at 72°C. Real-time PCR was performed using an Mx3000P real-time PCR system (Stratagene). Data were analyzed essentially as described by Livak and Schmittgen (2001). The UBC21 reference gene was amplified similarly in unwounded and wounded leaves (ΔCT = 0.59).
Protein Sample Preparation
Frozen leaf strips (50 mg) were powdered with a 5-mm stainless steel bead (Qiagen) in a TissueLyser II (Qiagen). Proteins were solubilized in 200 μL buffer A (40 mm Tris-HCl pH 8.0, 2× complete protease inhibitor cocktail [Roche Diagnostics], 5 mm MgCl2) and centrifuged at 16,100g for 10 min at 4°C. The supernatant was ultracentrifuged at 100,000g for 45 min at 4°C and the pellet was washed twice with equal amounts of buffer A and subsequently centrifuged under the same conditions prior to solubilization in 200 μL buffer B (40 mm Tris base, 4% SDS, 2× complete protease inhibitor cocktail [Roche Diagnostics]) by slow vortexing for 15 min. The resuspended pellet was centrifuged at 16,100g for 10 min at 25°C. The resulting supernatant was ultracentrifuged at 100,000g for 45 min at 25°C. The supernatants from the two centrifugation steps were combined. Protein concentration of the soluble fractions was determined with a Bradford assay. About 250 μg of each protein fraction were subjected to SDS-PAGE. After electrophoresis, the gels were cut into five equal parts and each gel slice was diced into small pieces. Gel pieces were destained in 50% (v/v) methanol containing 100 mm ammonium bicarbonate, then washed with water and stored at −20°C prior to in-gel tryptic digest according to Shevchenko et al. (1996). After tryptic digest, the peptides were purified using Sep-Pak reverse-phase cartridges (Waters).
MS
MS was performed on a linear trap quadrupole ion trap (Thermo Finnigan) coupled with an Eksigent AS-SX-1100 NanoLC 1D-HPLC system (Eksigent Technologies). Peptide mixtures were loaded onto laboratory-made capillary columns (75 μm i.d., 8-cm length, packed with Magic C18 AQ beads, 5 μm, 100 Å [Micromass BioResources]) at a flow rate of 500 nL/min in 3% buffer B in buffer A (buffer A: 0.2% formic acid in water, buffer B: 0.2% formic acid and 99.8% acetonitrile). Peptides were eluted from the column with increasing acetonitrile concentration in the mobile phase (3%–10% in buffer B for 0–1 min, 10%–35% in buffer B for 1–71 min, 35%–80% in buffer B for 71–75 min, 80% in buffer B for 75–85 min, 80%–3% in buffer B for 85–88 min, 3% in buffer B for 88–102 min). Peptide ions were detected in a survey scan from 400 to 2,000 amu followed by three data-dependent MS/MS scans (isolation width 3 amu, relative collision energy 35%, dynamic exclusion enabled, repeat count 1, repeat duration 30 s, followed by peak exclusion for 4 min). Each sample was injected and measured twice.
Interpretation of MS/MS Spectra, Data Filtering, and Export to PRIDE
Peptide spectrum assignment and data filtering have been described (Baerenfaller et al., 2008, 2011). In brief, these consisted of searching the MS/MS spectra (1) with TurboSEQUEST v.27 and PeptideProphet (Keller et al., 2002) using the Trans-Proteomic Pipeline (TPP v.3.2.1_SQUALL) against the TAIR8 protein database with concatenated decoy database (download on May 13, 2008) supplemented with contaminants (65,908 entries), and (2) with PepSplice (Roos et al., 2007) both against the protein database with an extended search space and the genome database. The search parameters for PepSplice searches were: requirement for tryptic ends, one missed cleavage allowed, mass tolerance = ±3 D. The search parameters for the PepSplice standard search were: requirement for tryptic ends, maximum penalty = 0.61, mass tolerance = +5/−1 D. The search parameters in the extended search additionally allowed a whole-genome hit, one nontryptic end, or one variable modification. Posttranslational modifications included in the searches with both algorithms were C iodoacetamide derivative as static and M oxidation as variable modifications. In addition, PepSplice searches in the extended search space included the variable modifications N-terminal acetyl, C without iodoacetamide, C S-Methyl, D iodoacetamide derivative, D Phospho, H iodoacetamide derivative, H Phospho, K Acetyl/Carboxy/Trimethyl, K Hypusine, S Phospho, T Phospho, W Oxidation, and Y Phospho/Sulfo that do not overlap with the error tolerance values. For PeptideProphet search results, the cutoff was set to a minimum probability of 0.9, for PepSplice the false discovery rate was adjusted to <0.01, assessed for each search space separately. All peptide spectrum assignments above the determined threshold, except those of known contaminants, were filtered for ambiguity. Peptides matching to several proteins were excluded from further analyses. This does not apply to different splice variants of the same protein or to different loci sharing exactly the same sequence. All remaining spectrum assignments were entered into the pep2pro database (Baerenfaller et al., 2011). After database upload, the following spectrum assignments were flagged and not taken into consideration for further analyses: (1) spectrum assignments to decoy database peptides, and (2) spectra for which PeptideProphet and PepSplice assign a different peptide to the same spectrum (including different posttranslational modifications and differently charged peptides). The spectrum false discovery rate was calculated by dividing the number of decoy database spectrum assignments by the number of spectrum assignments in the final dataset. PRIDE 2.1 XML files were created from the final fully integrated dataset and exported to the PRIDE database (Vizcaíno et al., 2010: accessions 13,327–13,334). The dataset is also available at www.pep2pro.ethz.ch in assembly Arabidopsis thaliana leaf wound proteome.
Protein Quantification, Statistics, and GO Classification
The normalized spectrum count (factor) for the proteins is determined by calculating the expected contribution of each individual protein to the sample’s total peptide pool as described in Baerenfaller et al. (2008). For normalization, the number of measured spectra of tryptic peptides and the sum of the theoretical tryptic peptides of the identified proteins have to be calculated for each sample. For quantification, only true tryptic peptides are taken into account, which are peptides with tryptic ends not containing missed cleavage sites. Analyses were performed in three biologically independent replicates. Proteins were considered to be differentially regulated if they fulfilled the following criteria: (1) the P value of the two-tailed paired Student’s t test between the normalized factors expressing the abundance of the protein had to be below 0.1; (2) the fold induction or repression ratio had to be ≥2 or ≤0.5; (3) there had to be at least 10 spectra per protein; (4) the spectral counts and normalized factors had to show the same pattern of induction or repression in the three independent replicates. Assignment of protein functions was based on the The Arabidopsis Information Resource GO categories from the aspect biological process (download ATH_GO_GOSLIM_20080510.txt; Berardini et al., 2004). The assignment was performed in R (http://www.r-project.org) using the elim method from the topGO package (Alexa et al., 2006). Fisher’s exact test was used for assessing the GO term significance.
Comparison of Protein and Transcript Levels
Wound-induced protein levels were compared with transcriptome data in Yan et al. (2007); ArrayExpress accession E-ATMX-9. In this analysis only proteins and transcripts from genes covered in both types of analysis were considered. Wound-induced proteins were considered likely to be transcriptionally regulated if the average of the log-transformed ratio (log2) of transcript level between unwounded and wounded leaves was below −0.59 or above 0.58 in the microarray dataset, corresponding to a 1.5-fold change in transcript levels.
GSH Redox State
GSH redox state in 50-mg fresh weight samples from the 2.5-mm region adjacent to the wound was determined with an enzyme-cycling assay (Griffith, 1980).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Predicted cellular locations of proteins differentially regulated in wild type and aos.
Supplemental Table S1. Wound-regulated proteins in wild type 6 h after wounding.
Supplemental Table S2. P values for wound-regulated proteins.
Supplemental Table S3. Wound-regulated proteins in aos 6 h after wounding.
Supplemental Table S4. Differentially regulated proteins in unwounded aos and wild-type leaves.
Supplemental Table S5. Differentially regulated proteins in wounded aos and wild-type leaves.
Supplemental Table S6. Potentially posttranscriptionally wound-regulated proteins.
Acknowledgments
We thank Patrice Waridel and Manfredo Quadroni (Protein Analysis Facility, University of Lausanne) and for technical support, Iván-Felipe Acosta (University of Lausanne) for discussion and advice on PCRs, and the Functional Genomics Center Zurich for infrastructure and technical support.
References
- Alexa A, Rahnenführer J, Lengauer T. (2006) Improved scoring of functional groups from gene expression data by decorrelating GO graph structure. Bioinformatics 22: 1600–1607 [DOI] [PubMed] [Google Scholar]
- Baerenfaller K, Grossmann J, Grobei MA, Hull R, Hirsch-Hoffmann M, Yalovsky S, Zimmermann P, Grossniklaus U, Gruissem W, Baginsky S. (2008) Genome-scale proteomics reveals Arabidopsis thaliana gene models and proteome dynamics. Science 320: 938–941 [DOI] [PubMed] [Google Scholar]
- Baerenfaller K, Hirsch-Hoffmann M, Svozil J, Hull R, Russenberger D, Bischof S, Lu Q, Gruissem W, Baginsky S. (2011) pep2pro: a new tool for comprehensive proteome data analysis to reveal information about organ-specific proteomes in Arabidopsis thaliana. Integr Biol 3: 225–237 [DOI] [PubMed] [Google Scholar]
- Berardini TZ, Mundodi S, Reiser L, Huala E, Garcia-Hernandez M, Zhang P, Mueller LA, Yoon J, Doyle A, Lander G, et al. (2004) Functional annotation of the Arabidopsis genome using controlled vocabularies. Plant Physiol 135: 745–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee S, Lee LY, Oltmanns H, Cao HB, Veena, Cuperus J, Gelvin SB. (2008) IMPa-4, an Arabidopsis importin alpha isoform, is preferentially involved in agrobacterium-mediated plant transformation. Plant Cell 20: 2661–2680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilgin DD, Zavala JA, Zhu J, Clough SJ, Ort DR, DeLucia EH. (2010) Biotic stress globally downregulates photosynthesis genes. Plant Cell Environ 33: 1597–1613 [DOI] [PubMed] [Google Scholar]
- Bonaventure G, Gfeller A, Proebsting WM, Hörtensteiner S, Chételat A, Martinoia E, Farmer EE. (2007) A gain-of-function allele of TPC1 activates oxylipin biogenesis after leaf wounding in Arabidopsis. Plant J 49: 889–898 [DOI] [PubMed] [Google Scholar]
- Book AJ, Gladman NP, Lee SS, Scalf M, Smith LM, Vierstra RD. (2010) Affinity purification of the Arabidopsis 26 S proteasome reveals a diverse array of plant proteolytic complexes. J Biol Chem 285: 25554–25569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boursiac Y, Lee SM, Romanowsky S, Blank R, Sladek C, Chung WS, Harper JF. (2010) Disruption of the vacuolar calcium-ATPases in Arabidopsis results in the activation of a salicylic acid-dependent programmed cell death pathway. Plant Physiol 154: 1158–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browse J. (2009) Jasmonate passes muster: a receptor and targets for the defense hormone. Annu Rev Plant Biol 60: 183–205 [DOI] [PubMed] [Google Scholar]
- Chung HS, Koo AJK, Gao XL, Jayanty S, Thines B, Jones AD, Howe GA. (2008) Regulation and function of Arabidopsis JASMONATE ZIM-domain genes in response to wounding and herbivory. Plant Physiol 146: 952–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins RM, Afzal M, Ward DA, Prescott MC, Sait SM, Rees HH, Tomsett AB. (2010) Differential proteomic analysis of Arabidopsis thaliana genotypes exhibiting resistance or susceptibility to the insect herbivore, Plutella xylostella. PLoS ONE 5: e10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalbey RE, Kuhn A. (2000) Evolutionarily related insertion pathways of bacterial, mitochondrial, and thylakoid membrane proteins. Annu Rev Cell Dev Biol 16: 51–87 [DOI] [PubMed] [Google Scholar]
- Davis JM, Gordon MP, Smit BA. (1991) Assimilate movement dictates remote sites of wound-induced gene expression in poplar leaves. Proc Natl Acad Sci USA 88: 2393–2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer EE, Johnson RR, Ryan CA. (1992) Regulation of expression of proteinase inhibitor genes by methyl jasmonate and jasmonic acid. Plant Physiol 98: 995–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca S, Chini A, Hamberg M, Adie B, Porzel A, Kramell R, Miersch O, Wasternack C, Solano R. (2009) (+)-7-Iso-jasmonoyl-L-isoleucine is the endogenous bioactive jasmonate. Nat Chem Biol 5: 344–350 [DOI] [PubMed] [Google Scholar]
- Frelet-Barrand A, Kolukisaoglu HU, Plaza S, Rüffer M, Azevedo L, Hörtensteiner S, Marinova K, Weder B, Schulz B, Klein M. (2008) Comparative mutant analysis of Arabidopsis ABCC-type ABC transporters: AtMRP2 contributes to detoxification, vacuolar organic anion transport and chlorophyll degradation. Plant Cell Physiol 49: 557–569 [DOI] [PubMed] [Google Scholar]
- Frick G, Su QX, Apel K, Armstrong GA. (2003) An Arabidopsis porB porC double mutant lacking light-dependent NADPH:protochlorophyllide oxidoreductases B and C is highly chlorophyll-deficient and developmentally arrested. Plant J 35: 141–153 [DOI] [PubMed] [Google Scholar]
- Gfeller A, Liechti R, Farmer EE. (2010) Arabidopsis jasmonate signaling pathway. Sci Signal 3: cm4. [DOI] [PubMed] [Google Scholar]
- Giri AP, Wünsche H, Mitra S, Zavala JA, Muck A, Svatos A, Baldwin IT. (2006) Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. VII. Changes in the plant’s proteome. Plant Physiol 142: 1621–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauser G, Dubugnon L, Mousavi SA, Rudaz S, Wolfender JL, Farmer EE. (2009) Velocity estimates for signal propagation leading to systemic jasmonic acid accumulation in wounded Arabidopsis. J Biol Chem 284: 34506–34513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JS, Hall G, Pearce G, Ryan CA. (1986) Regulation of synthesis of proteinase inhibitors I and II mRNAs in leaves of wounded tomato plants. Planta 169: 399–405 [DOI] [PubMed] [Google Scholar]
- Green TR, Ryan CA. (1972) Wound-induced proteinase inhibitor in plant leaves: a possible defense mechanism against insects. Science 175: 776–777 [DOI] [PubMed] [Google Scholar]
- Griffith OW. (1980) Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem 106: 207–212 [DOI] [PubMed] [Google Scholar]
- Hamant O, Heisler MG, Jönsson H, Krupinski P, Uyttewaal M, Bokov P, Corson F, Sahlin P, Boudaoud A, Meyerowitz EM, et al. (2008) Developmental patterning by mechanical signals in Arabidopsis. Science 322: 1650–1655 [DOI] [PubMed] [Google Scholar]
- Hemsley PA, Kemp AC, Grierson CS. (2005) The TIP GROWTH DEFECTIVE1 S-acyl transferase regulates plant cell growth in Arabidopsis. Plant Cell 17: 2554–2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks LM, Cahoon RE, Bonner ER, Rivard RS, Sheffield J, Jez JM. (2007) Thiol-based regulation of redox-active glutamate-cysteine ligase from Arabidopsis thaliana. Plant Cell 19: 2653–2661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A, Nesvizhskii AI, Kolker E, Aebersold R. (2002) Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem 74: 5383–5392 [DOI] [PubMed] [Google Scholar]
- Koo AJ, Howe GA. (2009) The wound hormone jasmonate. Phytochemistry 70: 1571–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Piao HL, Kim HY, Choi SM, Jiang F, Hartung W, Hwang I, Kwak JM, Lee IJ, Hwang I. (2006) Activation of glucosidase via stress-induced polymerization rapidly increases active pools of abscisic acid. Cell 126: 1109–1120 [DOI] [PubMed] [Google Scholar]
- Lee MW, Jelenska J, Greenberg JT. (2008) Arabidopsis proteins important for modulating defense responses to Pseudomonas syringae that secrete HopW1-1. Plant J 54: 452–465 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Ludewig U, Dynowski M. (2009) Plant aquaporin selectivity: where transport assays, computer simulations and physiology meet. Cell Mol Life Sci 66: 3161–3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mène-Saffrané L, Dubugnon L, Chételat A, Stolz S, Gouhier-Darimont C, Farmer EE. (2009) Nonenzymatic oxidation of trienoic fatty acids contributes to reactive oxygen species management in Arabidopsis. J Biol Chem 284: 1702–1708 [DOI] [PubMed] [Google Scholar]
- Mugford SG, Yoshimoto N, Reichelt M, Wirtz M, Hill L, Mugford ST, Nakazato Y, Noji M, Takahashi H, Kramell R, et al. (2009) Disruption of adenosine-5′-phosphosulfate kinase in Arabidopsis reduces levels of sulfated secondary metabolites. Plant Cell 21: 910–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyathi Y, De Marcos Lousa C, van Roermund CW, Wanders RJ, Johnson B, Baldwin SA, Theodoulou FL, Baker A. (2010) The Arabidopsis peroxisomal ABC transporter, comatose, complements the Saccharomyces cerevisiae pxa1 pxa2Delta mutant for metabolism of long-chain fatty acids and exhibits fatty acyl-CoA-stimulated ATPase activity. J Biol Chem 285: 29892–29902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oñate-Sánchez L, Vicente-Carbajosa J. (2008) DNA-free RNA isolation protocols for Arabidopsis thaliana, including seeds and siliques. BMC Res Notes 1: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onkokesung N, Gális I, von Dahl CC, Matsuoka K, Saluz HP, Baldwin IT. (2010) Jasmonic acid and ethylene modulate local responses to wounding and simulated herbivory in Nicotiana attenuata leaves. Plant Physiol 153: 785–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Halitschke R, Kim HB, Baldwin IT, Feldmann KA, Feyereisen R. (2002) A knock-out mutation in allene oxide synthase results in male sterility and defective wound signal transduction in Arabidopsis due to a block in jasmonic acid biosynthesis. Plant J 31: 1–12 [DOI] [PubMed] [Google Scholar]
- Reinbothe S, Reinbothe C, Heintzen C, Seidenbecher C, Parthier B. (1993) A methyl jasmonate-induced shift in the length of the 5′ untranslated region impairs translation of the plastid rbcL transcript in barley. EMBO J 12: 1505–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinbothe S, Reinbothe C, Lehmann J, Becker W, Apel K, Parthier B. (1994) JIP60, a methyl jasmonate-induced ribosome-inactivating protein involved in plant stress reactions. Proc Natl Acad Sci USA 91: 7012–7016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond P, Bodenhausen N, Van Poecke RMP, Krishnamurthy V, Dicke M, Farmer EE. (2004) A conserved transcript pattern in response to a specialist and a generalist herbivore. Plant Cell 16: 3132–3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond P, Weber H, Damond M, Farmer EE. (2000) Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell 12: 707–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez VM, Chételat A, Majcherczyk P, Farmer EE. (2010) Chloroplastic phosphoadenosine phosphosulfate metabolism regulates basal levels of the prohormone jasmonic acid in Arabidopsis leaves. Plant Physiol 152: 1335–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos FF, Jacob R, Grossmann J, Fischer B, Buhmann JM, Gruissem W, Baginsky S, Widmayer P. (2007) PepSplice: cache-efficient search algorithms for comprehensive identification of tandem mass spectra. Bioinformatics 23: 3016–3023 [DOI] [PubMed] [Google Scholar]
- Sasaki-Sekimoto Y, Taki N, Obayashi T, Aono M, Matsumoto F, Sakurai N, Suzuki H, Hirai MY, Noji M, Saito K, et al. (2005) Coordinated activation of metabolic pathways for antioxidants and defence compounds by jasmonates and their roles in stress tolerance in Arabidopsis. Plant J 44: 653–668 [DOI] [PubMed] [Google Scholar]
- Shan X, Wang J, Chua L, Jiang D, Peng W, Xie D. (2011) The role of Arabidopsis Rubisco activase in jasmonate-induced leaf senescence. Plant Physiol 155: 751–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Jing Y, Kuang T. (2003) Proteomics approach to identify wound-response related proteins from rice leaf sheath. Proteomics 3: 527–535 [DOI] [PubMed] [Google Scholar]
- Shevchenko A, Jensen ON, Podtelejnikov AV, Sagliocco F, Wilm M, Vorm O, Mortensen P, Shevchenko A, Boucherie H, Mann M. (1996) Linking genome and proteome by mass spectrometry: large-scale identification of yeast proteins from two dimensional gels. Proc Natl Acad Sci USA 93: 14440–14445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares NC, Francisco R, Vielba JM, Ricardo CP, Jackson PA. (2009) Associating wound-related changes in the apoplast proteome of Medicago with early steps in the ROS signal-transduction pathway. J Proteome Res 8: 2298–2309 [DOI] [PubMed] [Google Scholar]
- Stein M, Dittgen J, Sánchez-Rodríguez C, Hou BH, Molina A, Schulze-Lefert P, Lipka V, Somerville S. (2006) Arabidopsis PEN3/PDR8, an ATP binding cassette transporter, contributes to nonhost resistance to inappropriate pathogens that enter by direct penetration. Plant Cell 18: 731–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thivierge K, Prado A, Driscoll BT, Bonneil E, Thibault P, Bede JC. (2010) Caterpillar- and salivary-specific modification of plant proteins. J Proteome Res 9: 5887–5895 [DOI] [PubMed] [Google Scholar]
- Vizcaíno JA, Côté R, Reisinger F, Barsnes H, Foster JM, Rameseder J, Hermjakob H, Martens L. (2010) The Proteomics Identifications database: 2010 update. Nucleic Acids Res (Database issue) 38: D736–D742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walley JW, Dehesh K. (2010) Molecular mechanisms regulating rapid stress signaling networks in Arabidopsis. J Integr Plant Biol 52: 354–359 [DOI] [PubMed] [Google Scholar]
- Yan Y, Stolz S, Chételat A, Reymond P, Pagni M, Dubugnon L, Farmer EE. (2007) A downstream mediator in the growth repression limb of the jasmonate pathway. Plant Cell 19: 2470–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]