Abstract
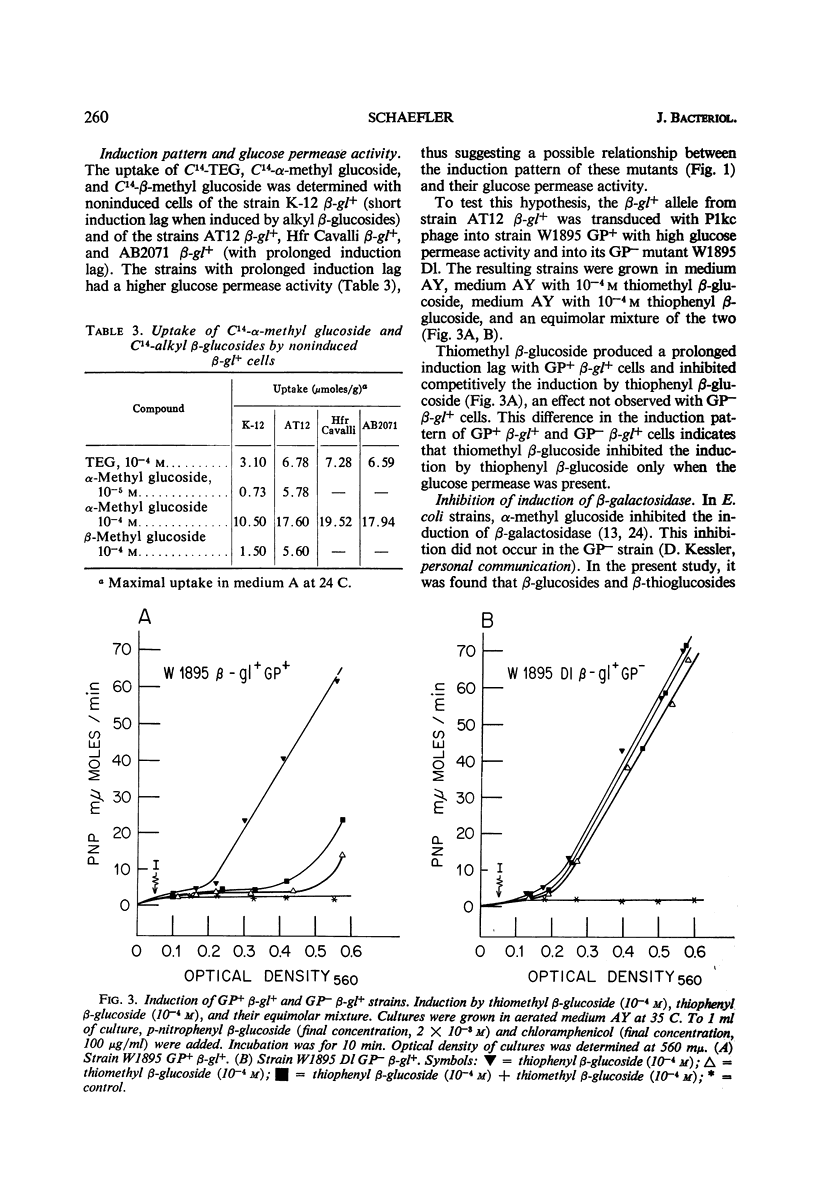
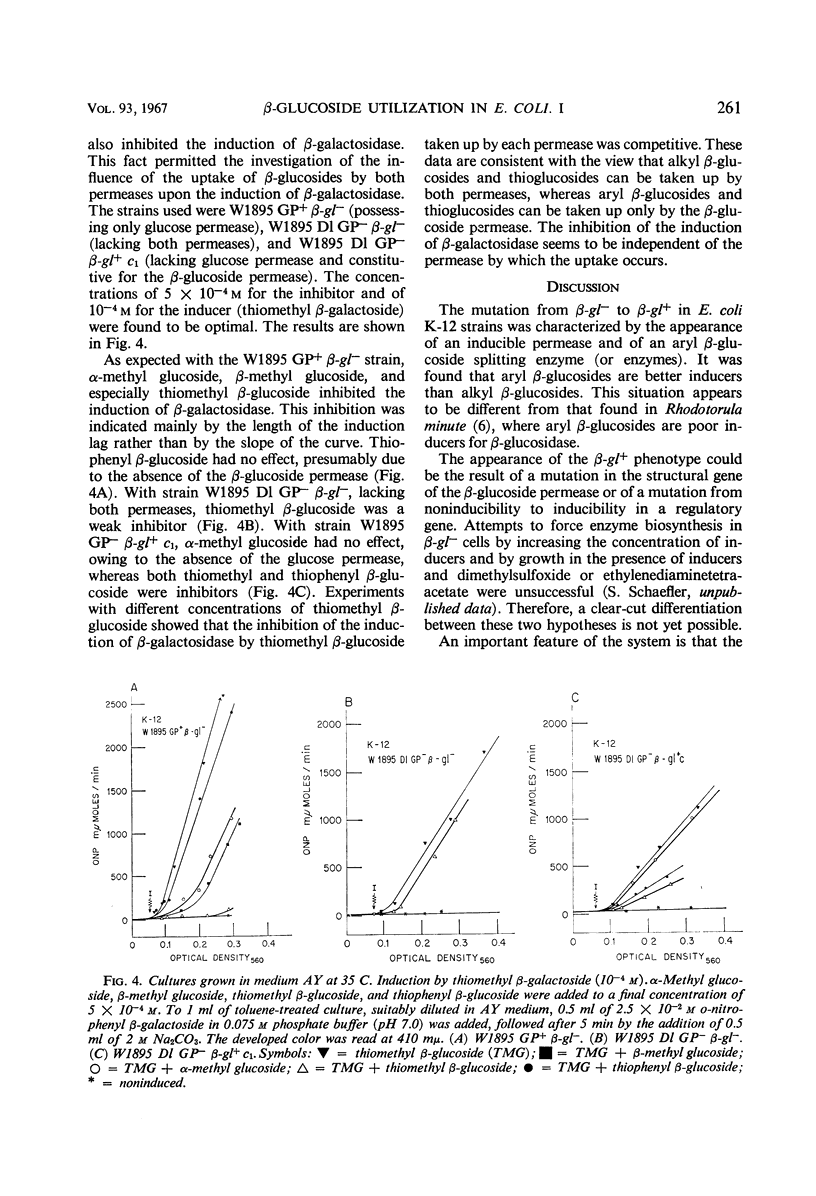
Wild-type Escherichia coli strains (β-gl−) do not split β-glucosides, but inducible mutants (β-gl+) can be isolated which do so. This inducible system consists of a β-glucoside permease and an aryl β-glucoside splitting enzyme. Both can be induced by aryl and alkyl β-glucosides. In β-gl− and noninduced β-gl+ cells, C14-labeled thioethyl β-glucoside (TEG) is taken up by a constitutive permease, apparently identical with a glucose permease (GP). This permease has a high affinity for α-methyl glucoside and a low affinity for aryl β-glucosides. No accumulation of TEG occurs in a β-gl− strain lacking glucose permease (GP−). In induced β-gl+ strains, there appears a second β-glucoside permease with low affinity for α-methyl glucoside and high affinity for aryl β-glucosides. Autoradiography shows that TEG is accumulated by the β-glucoside permease and glucose permease in two different forms (one being identical with TEG, the other probably phosphorylated TEG). In GP+ β-gl+ strains with high GP activity, alkyl β-glucosides induce the enzyme and the β-glucoside permease after a prolonged induction lag, and they competitively inhibit the induction by aryl β-glucosides. The induction lag and competition do not exist in GP− β-gl+ strains. It is assumed that phosphorylated alkyl and thioalkyl β-glucosides inhibit the induction, and that this inhibition is responsible for the induction lag.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- AMES G. F. UPTAKE OF AMINO ACIDS BY SALMONELLA TYPHIMURIUM. Arch Biochem Biophys. 1964 Jan;104:1–18. doi: 10.1016/s0003-9861(64)80028-x. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUERKSEN J. D., HALVORSON H. The specificity of induction of beta-glucosidase in Saccharomyces cerevisiae. Biochim Biophys Acta. 1959 Nov;36:47–55. doi: 10.1016/0006-3002(59)90068-x. [DOI] [PubMed] [Google Scholar]
- ENGLESBERG E., WATSON J. A., HOFFEE P. A. The glucose effect and the relationship between glucose permease, acid phosphatase, and glucose resistance. Cold Spring Harb Symp Quant Biol. 1961;26:261–276. doi: 10.1101/sqb.1961.026.01.033. [DOI] [PubMed] [Google Scholar]
- HAGIHIRA H., WILSON T. H., LIN E. C. STUDIES ON THE GLUCOSE-TRANSPORT SYSTEM IN ESCHERICHIA COLI WITH ALPHA-METHYLGLUCOSIDE AS SUBSTRATE. Biochim Biophys Acta. 1963 Nov 15;78:505–515. doi: 10.1016/0006-3002(63)90912-0. [DOI] [PubMed] [Google Scholar]
- HELFERICH B., SIEPMANN R. [o-Nitrophenol-alpha- and-beta-D-glycosides. Use of beta-D-glycosides in the synthesis of alpha-D-glycosides]. Hoppe Seylers Z Physiol Chem. 1962 Sep 6;329:105–108. doi: 10.1515/bchm2.1962.329.1.105. [DOI] [PubMed] [Google Scholar]
- HOFFEE P., ENGLESBERG E., LAMY F. THE GLUCOSE PERMEASE SYSTEM IN BACTERIA. Biochim Biophys Acta. 1964 Mar 30;79:337–350. [PubMed] [Google Scholar]
- KESSLER D. P., RICKENBERG H. V. A NEW METHOD FOR THE SELECTION OF MUTANTS OF ESCHERICHIA COLI FORMING BETA-GALACTOSIDASE CONSTITUTIVELY. Biochim Biophys Acta. 1964 Sep 4;90:609–610. doi: 10.1016/0304-4165(64)90241-7. [DOI] [PubMed] [Google Scholar]
- MONOD J., PAPPENHEIMER A. M., Jr, COHEN-BAZIRE G. La cinétique de la biosynthèse de la beta-galactosidase chez E. coli considérée comme fonction de la croissance. Biochim Biophys Acta. 1952 Dec;9(6):648–660. doi: 10.1016/0006-3002(52)90227-8. [DOI] [PubMed] [Google Scholar]
- MUELLER-HILL B., RICKENBERG H. V., WALLENFELS K. SPECIFICITY OF THE INDUCTION OF THE ENZYMES OF THE LAC OPERON IN ESCHERICHIA COLI. J Mol Biol. 1964 Nov;10:303–318. doi: 10.1016/s0022-2836(64)80049-8. [DOI] [PubMed] [Google Scholar]
- PARDEE A. B. An inducible mechanism for accumulation of melibiose in Escherichia coli. J Bacteriol. 1957 Mar;73(3):376–385. doi: 10.1128/jb.73.3.376-385.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAFLER S., BENES S. Recherches sur des souches d'Escherichia freundii à antigène Vi et leur position dans le groupe des Escherichia freundii. Ann Inst Pasteur (Paris) 1959 Feb;96(2):231–237. [PubMed] [Google Scholar]
- SCHAFLER S., MINTZER L. Acquisition of lactose-fermenting properties by salmonellae. I. Interrelationship between the fermentation of cellobiose and lactose. J Bacteriol. 1959 Aug;78:159–163. doi: 10.1128/jb.78.2.159-163.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAFLER S., MINTZER L., SCHAFLER C. Acquisition of lactose fermenting properties by salmonellae. II. Role of the medium. J Bacteriol. 1960 Feb;79:203–212. doi: 10.1128/jb.79.2.203-212.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefler S., Maas W. K. Inducible system for the utilization of beta-glucosides in Escherichia coli. II. Description of mutant types and genetic analysis. J Bacteriol. 1967 Jan;93(1):264–272. doi: 10.1128/jb.93.1.264-272.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VEIBEL S., JENSEN K. A., KLAJN E. [beta-Glucosidase from mussels (Mytilus edulis L.)]. Biochem Z. 1963;337:146–155. [PubMed] [Google Scholar]
- WAINWRIGHT S. D. The role of alpha-methyl glucoside as an inhibitor of induced enzyme biosynthesis (enzymatic adaptation). Arch Biochem Biophys. 1953 Dec;47(2):445–454. doi: 10.1016/0003-9861(53)90481-0. [DOI] [PubMed] [Google Scholar]


