Abstract
Recent studies have demonstrated that neurotrophins (NTs) and their Trk tyrosine kinase receptors, thought to be exclusively required for development of the nervous system, are also involved in controlling ovarian development. Here we show that primordial follicle formation is decreased in the absence of nerve growth factor (NGF) or its receptor TrkA, and in the absence of TrkB, the receptor for neurotrophin-4 (NT4) and brain-derived neurotrophic factor (BDNF). This deficiency is not due to premature oocyte loss, because the ovaries of trkA-/- and trkB-/- mice do not show an increased rate of oocyte death antedating the initiation of folliculogenesis. Moreover, exposure of NGF-deficient ovaries to NGF rescues the defect in follicular assembly, if TrkA receptors are present, suggesting that the absence of neurotrophins causes a delay, and not an irretrievable loss, of follicle formation. Both the number of secondary follicles and FSH receptor (FSHR) expression are diminished in trkA and trkB-null ovaries, but not in ovaries lacking the common NT receptor p75NTR. Transient exposure of wild-type (WT) ovaries to NT4 increases FSHR gene expression and enhances the ability of the ovary to respond to FSH with formation of cyclin D2, a cell-cycle protein mediating the proliferative actions of FSH in the ovary. These results indicate that both TrkA and TrkB receptors are necessary for the timely assembly of primordial follicles and for sustaining early follicular development. They also suggest that a mechanism by which TrkB receptors facilitate subsequent follicle development is by inducing the formation of functional FSHR.
Keywords: trkB receptor knockout, trkA receptor knockout, neurotrophins, follicular essambly, FSH receptor
Introduction
Much is known about the hormonal mechanisms controlling ovarian development. More recently, a major focus of attention in the field has been the identification of regulatory pathways that, operating within the ovarian microenvironment, contribute to the acquisition of ovarian reproductive competence. Within this framework, our laboratory has developed the concept that neurotrophins (NTs) and their Trk tyrosine kinase receptors, long thought to be exclusively required for the development of the nervous system, are also involved in the control of ovarian function (reviewed in (Dissen et al. 2004)).
A role for NTs in the control of ovarian maturation was initially suggested by the finding that the developing ovary not only contains four of the known NTs (nerve growth factor, NGF; brain derived neurotrophic factor, BDNF; neurotrophin 3, NT3; and neurotrophin 4/5, NT-4/5 (Ernfors et al. 1990; Hallböök et al. 1991; Berkemeier et al. 1991; Lara et al. 1990; Dissen et al. 1996; Dissen et al. 1995), but also expresses the receptors for each of them (p75NTR and the tyrosine kinase high affinity receptors TrkA, TrkB and TrkC) (Klein et al. 1989; Lamballe et al. 1991; Dissen et al. 1991; Dissen et al. 1996; Abir et al. 2005; Paredes et al. 2004; Dissen et al. 1995). More recent studies have made clear that the NTs and their respective receptors are expressed in feto-neonatal rodent ovaries and fetal human ovaries before the initiation of follicular assembly (Dissen et al. 1995; Spears et al. 2003; Anderson et al. 2002; Anesetti et al. 2001; Abir et al. 2005).
The importance of NGF in early follicular development was made evident by the reduction in the number of primary and secondary follicles, and the decreased expression of FSHR found at the end of the first postnatal week of life in NGF-null mice (Dissen et al. 2001). These findings suggested that NGF not only promotes the early stages of follicle development, but also induces the initial biochemical differentiation of secondary follicles into gonadotropin-responsive structures.
Thus far, a role for NT3 and its high-affinity receptor TrkC in follicle formation or follicle development has not been revealed (Spears et al. 2003). However, using trkB-null mice, we (Paredes et al. 2004) and others (Spears et al. 2003) recently demonstrated that TrkB signaling is required for oocyte survival and preantral follicular development. Spears and colleagues employed mutant mice lacking the intracellular domain of the receptor and found that these animals have a significantly lower number of oocytes and primordial follicles than WT controls (Spears et al. 2003), suggesting that TrkB signaling is required for germ cell survival before initiation of follicular assembly. Employing mutant mice lacking all TrkB isoforms, we found that the ovaries of these mice (or those lacking both NT-4/5 and BDNF) suffer a stage-selective deficiency in early follicular development that compromises the ability of follicles to grow beyond the primary stage. Proliferation of granulosa cells – required for this transition – and expression of FSH receptors, which reflects the degree of biochemical differentiation of growing follicles, are reduced in these “complete” trkB-null mice. To determine the importance of TrkB receptors for subsequent follicular development, and because trkB-null mice die within the first two weeks of postnatal life, we grafted the ovaries from 4-5-day-old KO animals under the kidney capsule of WT adult female mice and examined the ovaries two weeks later. To our surprise, the trkB- null ovaries failed to sustain follicular development and showed a striking loss of follicular organization, preceded by massive oocyte death. These results indicate that TrkB receptors facilitate the early development of ovarian follicles, and that the receptors become critical for oocyte survival after follicular assembly.
While the importance of TrkB receptors in early ovarian development appears now unquestionable, the general consistency – but also the specific differences – emerging from the aforementioned results (Paredes et al. 2004; Spears et al. 2003) raise an entirely new set of questions. For instance, the size of the primordial follicle population is relatively normal by the end of the first postnatal week of life in both trkA and trkB null mice (Paredes et al. 2004; Dissen et al. 2001), but it is significantly reduced in perinatal TrkB-deficient ovaries (Spears et al. 2003), suggesting that TrkB and/or TrkA receptors may be required for the timely initiation of folliculogenesis. If this is the case, are TrkB and/or TrkA receptors required for oocyte survival before follicular formation? Are TrkA receptors, in addition to TrkB receptors, required for the acquisition of functional FSH receptors? The present study addresses these questions and provides evidence that both TrkA and TrkB receptors contribute not only to the assembly of primordial follicles, but also to the subsequent development of the newly formed follicles, and to the acquisition of FSH receptors by the growing follicles. In addition, these studies show that oocyte death does not increase before the initiation of folliculogenesis in the absence of either TrkA or TrkB receptors, and that ligand-mediated activation of TrkB receptors enhances the capacity of the infantile ovary to respond to FSH with synthesis of cyclin D2, a cell cycle protein underlying the proliferative effects of FSH on granulosa cells (Sicinski et al. 1996).
Materials and Methods
TrkA, trkB, p75 and NGF-null Mice
TrkA+/− (129Sv-C57BL/6) mice (Liebl et al. 2000) were a generous gift of Dr. Lino Tesarollo (National Cancer Institute, Frederick, MD). Ngf+/− (C57BL/6-AB1) mice (Crowley et al. 1994) were kindly provided by Dr. Heike Phillips (University of California at San Francisco, CA) and p75NTR+/− (BALB/c) mice (Lee et al. 1992) were generously provided by Dr. Kuo-Fen Lee (The Salk Institute, CA). TrkB+/− mice (C57BL6-DBA) were generated as previously described (Paredes et al. 2004). The mutant mice were bred to wild-type (WT) animals of the same genetic background, and the null mutant and WT mice used in this study were obtained by crossing F1 heterozygous individuals. The animals were housed under controlled conditions of temperature (23-25 °C) and light (12 h light, 12 h of darkness; lights on from 0700 to 1900 h), and were given ad libitum access to food (LabDiet 5001, PMI Nutrition International Brentwood, St. Louis, MO) and tap water. The use of mice was duly approved by the ONPRC Animal Care and Use Committee, in accordance to the guidelines provided by the NIH Guide for the Care and Use of Laboratory Animals.
Collection of ovarian tissue and genotyping
Ovaries from entire litters were collected at 0 (day of birth), 2 and 4 days after birth, and assigned to different procedures (organ culture, RNA extraction or morphometric analysis) before establishing each genotype. Once this was known, the ovaries were assigned to either a WT or a KO group. No heterozygotes were studied. Genotyping was performed via PCR analysis of tail DNA, employing specifics oligodeoxynucleotide primers that amplify a DNA segment comprising both the targeting vector and the gene sequence specific to each mutant animal (Paredes et al. 2004; Crowley et al. 1994; Lee et al. 1992; Liebl et al. 2000).
Culture of ovaries
To determine the effect of NGF on primordial follicle formation, ovaries from 0-day-old Ngf −/− and trkA−/− mice were dissected under a stereomicroscope using aseptic conditions, placed on sterile lens paper and cultured on metal grids in a 24-well plate at the interface of air/culture medium, under an atmosphere of 60% O2-35% N2-5% CO2, as described (Romero et al. 2002; Paredes et al. 2004). One ovary from each animal was cultured in presence of NGF (100 ng/ml) for 4 days; the contralateral ovary served as an untreated control. At the end of this period, the ovaries were collected, fixed in Kahle’s fixative (Hirshfield & DeSanti 1995), embedded in paraffin, serially sectioned at 6 μm and stained for morphometric analysis, as reported earlier (Dissen et al. 2001).
To determine the effect of TrkB activation on FSHR mRNA levels, and on cyclin D2 abundance, ovaries from 4-day-old WT mice were dissected and cultured as outlined above. When used for measurement of FSHR mRNA, the ovaries were cultured for 8 h in presence or absence of NT4 (100 ng/ml), then frozen in dry ice, and stored at −85 °C until RNA extraction. Cultures employed for western blot analysis of cyclin D2 protein levels were subjected to the same experimental protocol we used earlier to document an effect of NGF on the formation of functional FSHR (Romero et al. 2002). In brief, the ovaries were treated with NT4 for 8 h or left untreated. At the end of this period, the medium was replaced with fresh medium alone or medium containing FSH (500 ng/ml), in the absence of NT4. Twenty-four h later the ovaries were collected, frozen on dry ice, and stored at −85 °C until protein extraction.
RNA extraction and Real-time PCR
Total RNA was isolated from cultured ovaries treated with NT4 using Tri-Reagent (Molecular Research Center, Inc, Cincinnati, OH) as previously described (Paredes et al. 2004). FSHR mRNA was detected by real-time PCR using a procedure previously described (Romero et al. 2002) with some modifications. After reverse transcribing 200 ng of total RNA, aliquots of each reaction (10 ng cDNA/μl) were diluted 1:10 before using 2 μl for real-time PCR. Each sample was run in triplicate along with a relative and an absolute standard curve. Relative standard curves, generated by serially diluting one sample 1:10 to 1:10,000 times, served to estimate the content of 18S ribosomal RNA of each sample. The primers used to detect 18s ribosomal RNA were purchased as a kit (TaqMan Ribosomal RNA Control Reagents Kit, Perkin Elmer Applied Biosystems, Foster City, CA). Absolute standard curves were constructed by using serial dilutions (1:10) of sense FSHR RNA (2 ag-2 ng, see below). The threshold cycle number (CT) from each sample was referred to this curve to estimate the corresponding RNA content, and each RNA value was then normalized for procedural losses using the 18S ribosomal RNA values estimated from the relative standard curve. CT was the fractional cycle number at which the fluorescence accumulated to a level 10 times greater than 1 SD from basal values. The FSHR and 18s ribosomal RNA primers employed were those already reported (Romero et al. 2002). These primers and the fluorescent probes for real-time PCR were selected with the assistance of the program, Primer Express (Perkin Elmer Applied Biosystems). Real-time PCR reactions were performed in a total volume of 10 μl, each reaction containing 2 μl of the diluted reverse transcribed sample or 2 μl of sense FSHR mRNA standard, 5 μl TaqMan Universal PCR Master Mix (PE Applied Biosystems), 250 nM of each gene specific- and ribosomal fluorescent probes, 300 nM of each gene specific primer, and 10 nM of each ribosomal primer.
To construct the sense FSHR RNA standard curve used to quantify FSHR mRNA abundance in the ovary samples, we used a 346 bp cDNA generated by RT-PCR of total RNA derived from adult mouse ovaries. The primers used were a 5′ forward primer (5′-GCCCTGGCCTTTGTGGTCATCTGT-3′) corresponding to nucleotides 1675-1698 in mouse FSHR mRNA (NM_013523) and a 3′ reverse primer (5′-AAATCTGGGCTTGCACCTCATAAC-3′) complementary to nucleotides 1997-2020. To amplify this fragment we used a PCR amplification protocol consisting of 33 cycles of denaturing at 94 °C (30 s), annealing at 57 °C (60 s) and extension at 72 °C (60 s). The resulting PCR product was cloned into the pGEM-T vector (Promega Corp., Madison, WI), and sequenced from both ends to verify its identity.
Morphometric analysis
Ovaries from 2 and 4-day-old mice, and 0-day-old ovaries maintained for 4 days in organ culture were fixed in Kahle’s fixative, embedded in paraffin, serially sectioned at 6 μm, stained with Weigert’s iron hematoxylin and counterstained with picric acid-methyl blue, as reported (Dissen et al. 2001; Paredes et al. 2004; Romero et al. 2002). Every other section was imaged as described (Paredes et al. 2004) and the degree of follicle development was morphometrically analyzed counting only follicles in which the nucleus of the oocyte was visible (Paredes et al. 2004). The total number of follicles per ovary was then calculated by first dividing the total number of follicles counted per ovary by the number of sections examined and then multiplying this number by the total number of sections made from each ovary. Because each ovary yielded about 140 sections, we counted about 50 sections per ovary. All counts were performed without previous knowledge of the animal’s genotype. The follicles were classified in different developmental stages according to well-established criteria (Peters 1969) that we have previously used (Dissen et al. 2001; Paredes et al. 2004; Romero et al. 2002). Briefly, primordial follicles are the initial result of follicular assembly; they contain an oocyte surrounded by a single layer of flattened pregranulosa cells (Peters 1969). Primordial follicles become primary follicles (type 3a) (Peters 1969) by a process that results in the differentiation of the flattened granulosa cells into a cuboidal morphology (Hirshfield 1991; Peters 1969). Granulosa cell proliferation and oocyte growth begin at this point resulting in the formation of larger (type 3b) primary follicles first, and secondary follicles with two (type 4) or more layers of granulosa cells (type 5 and larger) subsequently.
Assessment of Apoptosis
To determine if the decrease in the number of primordial follicles observed in trkA−/− and trkB−/− mice on postnatal days 2 and 4 is due to death of oocytes before they become organized into follicular structures, we used ovaries from 0-day-old mice. The ovaries were immersed in Zamboni’s fixative overnight at 4 °C and processed as described (Paredes et al. 2004), before preparing 14 μm cryostat sections. Six-eight randomly selected sections derived from 3 mice per group were then subjected to combined immunohistofluorescence-TUNEL. Oocytes were identified using a rat monoclonal antibody (Mab KMC8, 5 μg/ml, BD Pharmigen, San Diego, CA) that recognizes mouse CD9, a member of the tetraspanin family of membrane proteins expressed in the plasma membrane of oocytes (Zhu et al. 2002). CD9 is also present in several cell types of the immune system (BD Pharmigen, product specifications). After an overnight incubation at 4 °C with the CD9 antibody, the immunoreaction was developed by incubating the sections for 1 h at room temperature with Alexas 594 donkey anti-rat gamma globulin (1:500). Following extensive rinsing, the sections were subjected to the TUNEL reaction, which was performed following the manufacturer’s instructions. To detect apoptotic ovarian cells by TUNEL we used an in situ cell death detection kit coupled with fluorescein detection (Roche Diagnostics Co, Basel, Switzerland). Positive controls for the TUNEL reaction included sections from the ovary of a 28-day-old mouse (to detect apoptotic granulosa cells) and sections from day 0 ovaries treated with DNase I (Ambion, Austin, TX; 10 U/ml for 20 min at room temperature).
Western blots
Proteins and RNA derived from the experiments described in the subsection “Culture of Ovaries” were extracted simultaneously, as recommended (Tolosa et al. 2007; Morse et al. 2006) using a nucleic acid column extraction kit (RNeasy minikit, Qiagen Inc, Valencia, CA). Briefly, pools of four ovaries (i.e., from two animals) were homogenized using an ultra-Turrax homogeneizer in 300 μl of a guanidine hydrochloride-containing buffer (RLT buffer), provided with the kit supplemented with 10% β-mercaptoethanol. The RNA was then extracted using the RNeasy minicolumn, following the manufacturer’s instructions. The flow-through containing the proteins was collected at every step and pooled. The proteins were precipitated overnight at −20 °C, the precipitates were collected by centrifugation at 10,000 × g (15 min at 4 °C), and the pellets were washed three times with 100% cold ethanol (each wash for 30 min at −20 °C), before collecting the proteins by centrifugation at 10,000 × g for 30 min at 4 °C. Thereafter, the pellets were air-dried and each sample was resuspended in 40-50 μl of Laemli sample buffer (187 mM Tris-base, 9% SDS, 15% Glycerol, and 15% β-mercaptoethanol, pH 6.8), boiled 5 min and fractionated in a 4-20% precast SDS-PAGE gel (Invitrogen, Carlsbad, CA). Due to the small amount of tissue per sample, the protein concentration of each sample was not determined before gel loading. After electrophoresis at 130V for 2 h, the proteins were transferred for 1.5 h at 4 °C onto polyvinylidene difluoride membranes (Millipore, Billerica, MA). The membranes were blocked in 5% non-fat milk for 1 h. A rat monoclonal antibody raised against recombinant cyclin D2 (34B1-3; Santa Cruz Biotechnology, Santa Cruz, CA) was used at a 1:1,000 dilution (overnight at 4 °C) followed by a goat anti-rat HRP antibody (1 h at room temperature, 1:25,000 sc-2006, Santa Cruz Biotechnology). The signal was developed by enhanced chemiluminescence using the Super Signal West Dura Extended Duration Substrate (Pierce Biotechnology Inc, Thermo Fisher Scientific Inc, Rockford, IL). For quantitation purposes, the membrane was washed several times in Tris-buffered saline 0.5% Tween-20 before an overnight exposure (at 4 °C) to a mouse monoclonal antibody against GAPDH (Abcam Inc, Cambridge, MA; 1:10,000 dilution), followed by an anti-mouse HRP antibody (Invitrogen; 1 h at room temperature, 1:50,000 dilution). To develop the signal an enhanced chemiluminescence substrate (Western Lighting, Perkin Elmer) was used.
Statistical Analysis
Before specific analyses were performed, the data was subjected to a normality test. Data passing this test were then analyzed using the 2-tailed Student’s t test to assess differences between 2 groups of animals or independent observations, or a one-way ANOVA followed by the Student-Neuman-Keuls multiple comparison test for unequal replications, when comparing several groups. When the data failed the normality test, they were analyzed using an ANOVA test on ranks followed by the Kruskal-Wallis One Way Analysis of Variance on Ranks test (SigmaStat, Systat Software Inc. v3.11, San Jose, CA). A p value <0.05 was considered statistically significant.
Results
Absence of neurotrophin signaling delays follicle assembly
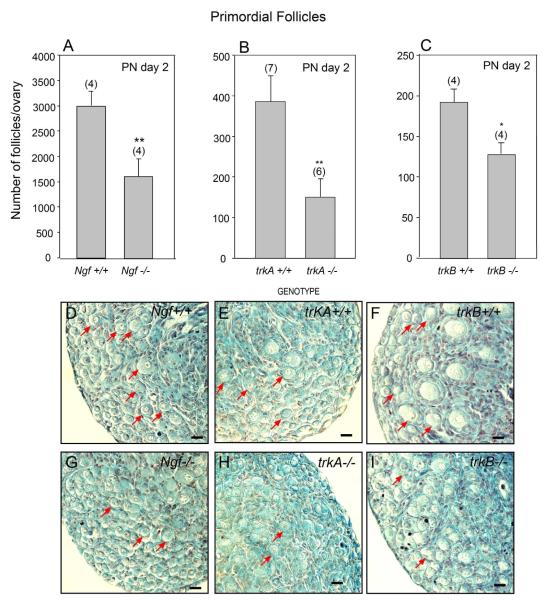
It was previously shown that 7-day-old ovaries from Ngf −/− mice and from mice lacking all isoforms of the TrkB receptor have a deficiency of early follicle development, but a relatively normal complement of primordial follicles (Romero et al. 2002; Paredes et al. 2004). To determine if this apparent normalcy represents a true lack of an NT role in facilitating follicular assembly or, instead, reflects a delay of follicular formation, we examined the ovaries of 2-day-old mice carrying null mutations of the genes encoding NGF and its high-affinity TrkA receptor, in addition to mice lacking all TrkB receptor isoforms. In all cases, and despite of the strain-related differences in the absolute number of follicles present at this early age, the number of primordial follicles was significantly lower in the mutant ovaries than in the ovaries of their respective WT littermates (Fig. 1 A-C). In each case, a clear immaturity of the ovary was apparent, with differences in the size of the primordial follicle population more clearly manifested in ovaries lacking NGF or its TrkA receptor (Fig. 1 D,E and F, G) than in trkB-deficient ovaries (Fig. 1 H, I). The KO ovaries not only have a reduced number of primordial follicles (examples denoted by red arrows), but also appear to exhibit a greater number of non-encapsulated oocytes than WT ovaries (Fig. 1 E, G and I vs. D, F and H).
Figure 1.
Formation of primordial follicles is reduced in the ovaries of mice lacking the Ngf, trkA or the trkB genes, as assessed by morphometric analysis of 2-day-old ovaries. (A) Ngf+/+ and Ngf −/− mice. (B) trkA+/+ and trkA−/− mice. (C) trkB+/+ and trkB−/− mice. (D) Histological aspect of an Ngf+/+ ovary showing an abundance of primordial follicles (examples denoted by red arrows), and the presence of primary follicles. (E) Similar aspect of a trkA+/+ ovary. (F) A trkB+/+ ovary showing that follicular development is more advanced in this strain at this time of postnatal life, with several primary follicles being apparent. (G) An Ngf −/− ovary showing a paucity of primordial follicles and many “naked” oocytes. (H) A trkA−/− ovary also showing a deficiency of primordial follicles, and an accumulation of naked oocytes. (I) Similar aspect of a trkB−/− ovary. Numbers in parentheses above bars are number of animals per group, and vertical lines are SEM. * = p<0.05 and ** = p<0.02 vs. WT control groups. Scale bars = 20 μm.
The neonatal reduction in primordial follicles of trkA and trkB-null ovaries is not due to perinatal death of oocytes
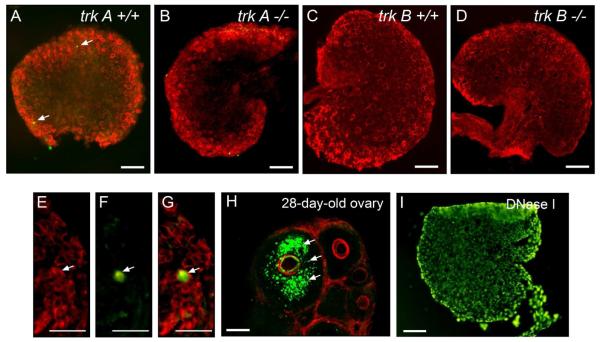
Because the absence of TrkA and TrkB-mediated signaling may result in oocyte death similar to that reported in mutant mice lacking the intracellular domain of the TrkB receptor (Spears et al. 2003), we used a TUNEL assay to examine the ovaries of trkA−/− and trkB−/− mice on the day of birth, i.e. preceding the initiation of folliculogenesis, for signs of apoptosis. Very few, if any, apoptotic cells (green color) were detected (Fig. 2 A-D, arrows), including apoptotic oocytes, which were rarely observed (Fig. 2 A-D; higher magnification images in panels E-G). Neither trkA −/− nor trkB−/− ovaries had a higher incidence of apoptotic oocytes than WT controls (Fig. 2A-D). This scarcity of somatic and germ cell death is in contrast to 28-day-old ovaries (used as a positive control) in which granulosa cell apoptosis of atretic follicles is widespread (Fig. 2 H). As expected, all cells were TUNEL positive in sections treated with DNAse I before performing the TUNEL reaction (Fig. 2 I).
Figure 2.
Representative sections from 0-day-old ovaries (day of birth) showing that the decreased number of primordial follicles observed in trkA and trkB-null ovaries is not due to an earlier, postnatal increase in apoptotic oocyte death. Apoptosis was detected using a TUNEL assay (green color) and oocytes were identified with a monoclonal antibody against CD9, a membrane protein abundant in oocytes (red color). (A) trkA+/+ ovary. (B) trkA−/− ovary. (C) A trkB+/+ ovary. (D) A trkB−/− ovary. Arrows point to TUNEL-positive cells. (E) Higher magnification image showing a region of a WT ovary containing many naked oocytes. (F) Image showing that one of the oocytes shown in E is apoptotic (arrow). (G) Merged image. (H) Prepubertal 28-day-old ovary showing an abundance of apoptotic cell nuclei (green color, arrows) in granulosa cells of an atretic follicle, and the lack of apoptotic cells in healthy, adjacent follicles. (I) Section form a day 0 WT ovary treated with DNase I. Scale bars = 100 μm.
NGF acting via TrkA receptors restores formation of primordial follicles in Ngf −/− mice
To determine if the reduced number of primordial follicles observed in Ngf −/− ovaries is specifically related to the absence of NGF-initiated signaling, we cultured ovaries from 1-day-old Ngf −/− mice for four days in the presence of NGF and determined the number of primordial follicles at the end of this period. Exposure to the NT distinctly (p<0.01) increased the number of primordial follicles in comparison to untreated Ngf −/− ovaries (Fig. 3 A). In contrast, NGF was ineffective in trkA−/− ovaries (Fig. 3 B), indicating that the facilitatory effect of NGF on follicular assembly is mediated by TrkA receptors.
Figure 3.
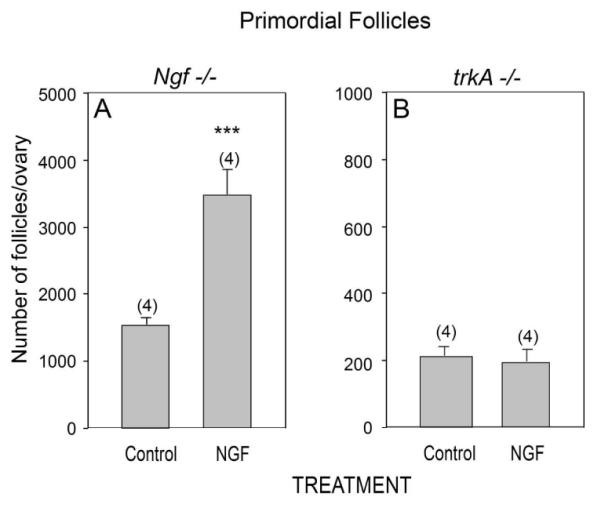
NGF acting via TrkA receptors rescues the number of primordial follicles that fail to form in Ngf −/− mice. (A) The number of primordial follicles is increased in Ngf −/− ovaries explanted on the day of birth (day 0) after a four day exposure to NGF (100 ng/ml). (B) The same treatment is ineffective in the absence of TrkA receptors. Vertical lines represent the SEM and numbers in parentheses are number of animals per group. *** = p<0.01 vs. untreated control group.
Early follicle development is impaired in the absence of TrkA and TrkB receptors
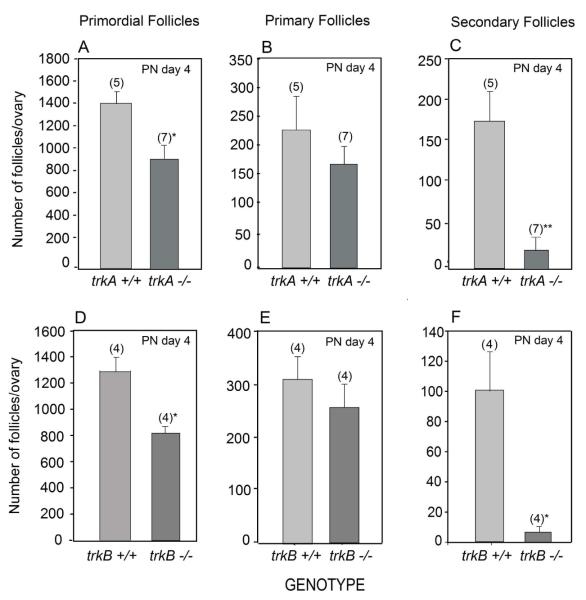
To determine if the lack of TrkA and TrkB receptors begins to impact follicle development as the follicles enter the proliferative pool, we examined the ovaries of 4-day-old mutant mice and compared the number of primordial, primary and secondary follicles with those of WT littermates. In both trkA and trkB-null ovaries the size of the primordial follicle population was still significantly reduced (p<0.02), but no significant differences in the number of primary follicles were detected (Fig. 4 A, B and D, E). In contrast, a marked decrease (p<0.01) in the number of secondary follicles was observed in both mutants (Fig. 4 C and F), suggesting that recruitment of primary follicles into the proliferative pool is compromised in the absence of either TrkA or TrkB signaling.
Figure 4.
A decreased number of primordial follicles persists 4 days after birth in both trkA−/− and trkB−/− ovaries, which in addition show a reduced number of secondary follicles. (A-C) trkA+/+ and trkA−/− ovaries. (D-F) trkB+/+ and trkB−/− ovaries. Vertical lines represent SEM and numbers in parentheses on top of bars are number of animals per group. * = p<0.05 and ** = p<0.02 vs. respective WT groups.
Both TrkA and TrkB receptors contribute to the acquisition of FSH receptors
We previously showed that FSHR mRNA is decreased in the ovaries of Ngf −/− and trkB−/− mice (Romero et al. 2002; Paredes et al. 2004), and that a short-term (8 h) exposure to NGF suffices to induce FSHR gene expression (Paredes et al. 2004). To determine if the supportive effect of NGF on FSHR gene expression is mediated by high-affinity TrkA receptors or by the common NT receptor p75NTR, we compared the abundance of FSHR mRNA in the ovaries of 7-day-old trkA−/− and p75NTR−/− mice to that of WT littermates. As shown in Figure 5, the absence of TrkA (panel A), but not that of p75NTR (panel B) resulted in significantly (p<0.05) lower FSHR mRNA levels than in WT ovaries, suggesting that NGF maintains FSHR expression (Romero et al. 2002) via activation of TrkA receptors.
Figure 5.
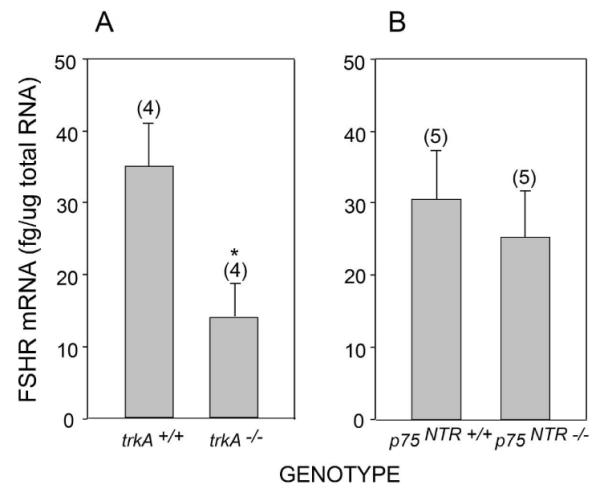
FSHR mRNA abundance is sustained by TrkA-mediated signaling, but does not require p75NTR. (A) FSHR mRNA levels in 7-day-old trkA−/− ovaries and their age-matched trkA+/+ controls. (B) FSHR mRNA in p75NTR−/− ovaries and their age-matched WT controls. Vertical lines represent SEM and numbers in parentheses on top of bars are number of animals per group. * = p<0.05 vs. trkA+/+ group.
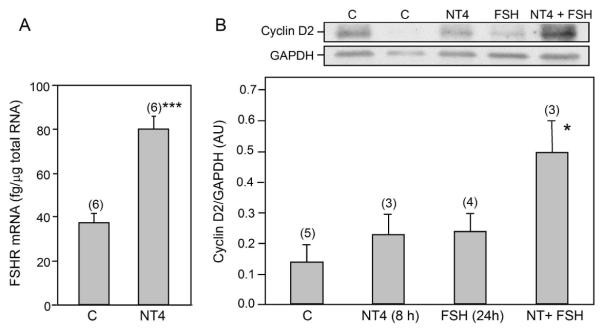
By the end of the first week of life, the ovaries from trkB−/− mice show a marked reduction in granulosa cell proliferation, and a sustained reduction in the number of secondary follicles (Paredes et al. 2004). It was, therefore, important to determine: a) if a transient exposure to the TrkB ligand NT4 can increase FSHR gene expression, as previously shown for NGF (Romero et al. 2002), and b) if this treatment also results in the formation of FSH receptors able to initiate a proliferative signal, as measured by the ability of FSH to increase the synthesis of cyclin D2, a cell-cycle protein known to mediate the stimulatory effect of FSH on granulosa cell proliferation (Sicinski et al. 1996). A short (8 h) in vitro exposure of 4-day-old WT ovaries to NT4 (100 ng/ml) significantly (p<0.01) increased FSHR mRNA levels (Fig. 6 A). Ovaries treated with NT4 for 8 h and then with FSH for 24 h, in the absence of the NT, responded to FSH with an increase (p<0.05) in cyclin D2 levels (Fig. 6 B). This increase was not seen in ovaries treated with NT4 for 8 h and then cultured in medium alone for 24 h or in ovaries cultured in medium alone for 8 h and then with FSH for 24 h (Fig. 6 B). These results suggest that the supportive effect of TrkB-mediated signaling on follicle development is mediated, at least in part, by facilitating the formation of biologically active FSH receptors capable of increasing the expression of a key cell cycle protein underlying the proliferative effect of FSH on the developing ovary.
Figure 6.
NT4 increases FSHR mRNA levels and induces the formation of biologically active FSHR (as measured by the ability of FSH to increase the synthesis of cyclin D2, an FSH-responsive gene) in explanted ovaries from 4-day-old mice. (A) FSHR mRNA abundance, determined by real-time PCR, increases after an 8 h of exposure to NT4 (100 ng/ml). (B) A short-term (8 h) exposure to NT4 (100 ng/ml) enhances the ability of the ovary to respond to FSH (500 ng/ml) with an increase in cyclin D2 formation, as determined by western blot analysis. A representative western blot is shown on top of the bar graph depicting the quantitative analysis of this experiment. Vertical lines are SEM and numbers in parentheses above bars are number of animals per group. * = p<0.05; *** = p<0.01 vs. untreated control groups (C).
Discussion
The present results demonstrate that mouse ovaries lacking either TrkA or TrkB receptors exhibit a reduced number of primordial follicles and a deficiency in early follicular development. The results also indicate that the defect in primordial follicle assembly is not due to premature oocyte death. Instead, it appears to results from a NT deficiency-dependent inability of somatic and germ cells to engage into the bidirectional communication process required for follicular organization. The subsequent delay of follicle development is likely related to the absence of a NT-dependent proliferative signal followed by reduced formation of FSHR.
The observation that folliculogenesis is impaired in mice lacking either NGF or its TrkA receptor complements and expands the results of a previous study examining follicular development in Ngf −/− mice (Dissen et al. 2001). In that study, we found that the number of primordial follicles of Ngf KO ovaries collected on postnatal day 7 was similar to that of WT littermates, implying that follicular assembly does not require NGF. However, an increased number of “naked” (i.e. non-encapsulated) oocytes was also noted, suggesting that, instead of preventing follicle assembly, the absence of NGF-mediated signaling may have delayed primordial follicle formation. The present results, obtained using ovaries collected at the time of initiation of folliculogenesis (PN day 2) and when follicle assembly is progressing in earnest (PN day 4), support this notion. Our findings also indicate that the supportive effect of NGF on follicular formation is mediated by TrkA receptors, because the absence of these receptors results in an ovarian phenotype indistinguishable from that of Ngf −/− mice.
Confirming the findings of Spears et. al. (Spears et al. 2003), we have now observed that the ovaries of 2-and 4-day-old trkB−/− mice contain fewer primordial follicles that WT controls. When the ovaries were examined at a later age (PN day 7), only a trend towards a lower number of primordial follicles was observed (Paredes et al. 2004). Together, these earlier findings and the present observations indicate that - as is the case of NGF and TrkA-deficient mice - the absence of TrkB-mediated signaling delays, but does not prevent follicle assembly.
Mice lacking the intracellular domain of the TrkB receptor show a reduced number of oocytes 4-6 days after birth (Spears et al. 2003), suggesting that the lower number of primordial follicles observed in these animals may be a consequence of prior oocyte death, which would reduce the pool of oocytes able to organize somatic cells into primordial follicles. We have now examined the ovaries of trkA−/− and trkB−/− mice lacking all isoforms of the TrkB receptor on the day of birth, i.e. 48 h before the initiation of folliculogenesis, and found no evidence for an increased incidence of oocyte death. In fact, we detected very few apoptotic oocytes in WT ovaries, and this low number remained unchanged in both trkA−/− and trkB−/− ovaries. Moreover, using Ngf −/− mice as a model to define the effects of NTs on primordial follicle formation, we observed that ovaries treated with NGF from the day of birth, respond to the NT with increased formation of primordial follicles, but only when TrkA receptors are present. These findings indicate that the loss of primordial follicles resulting from the absence of NT signaling can be prevented by restoring NTs to the deficient ovary, as long as the corresponding high-affinity Trk receptors are also present. This conclusion does not exclude the possibility of a prenatal increase in oocyte death caused by the absence of Trk receptors. Such a loss, if it occurs, may exacerbate the natural loss of oocytes that occurs before birth (McClellan et al. 2003). Because in the present study we did not count the total number of “non-encapsulated” follicles present on the day of birth, we cannot formally rule out this possibility. Nevertheless, our findings are consistent with the notion that the loss of primordial follicles seen in NT/Trk receptor-deficient ovaries is not an irretrievable event, because it can be reversed by exposing the ovary to a NT (NGF), and it is no longer evident by the end of the first postnatal week of life (Dissen et al. 2001; Paredes et al. 2004). The cellular mechanisms underlying the supportive effect of NTs on primordial follicle formation remain to be identified.
Both trkA- and trkB- null ovaries, in addition to the ovaries from Ngf −/− mice, exhibit a reduced number of secondary follicles. Although 7-day-old Ngf −/− ovaries also have a reduced number of primary follicles (Dissen et al. 2001), this was not the case of the younger ovaries examined in the present study. The most parsimonious explanation for this difference is that the size of the primary follicle population reflects a equilibrium between the number of primordial follicles that differentiate into follicles containing one-cell layer of cuboidal granulosa cells and those that enter the proliferative pool. If a defect in primordial-to-primary follicle differentiation is more pronounced than a defect in primary follicle recruitment to the proliferative pool (primary to secondary follicle transition), as it happens in 7-day-old NGF-deficient mice (Dissen et al. 2001), the number of primary follicles is reduced. However, if the recruitment defect is more prevalent, then the number of primary follicles may not change.
The cellular mechanisms underlying the supportive effect of NTs on follicle development have not been elucidated. However, in both NGF- and TrkB-deficient ovaries, somatic cells proliferation is reduced (Romero et al. 2002; Paredes et al. 2004), suggesting that follicle development is stunted because follicular cells fail to proliferate. This functional relationship appears more obvious in the case of the TrkB receptor, because the proliferative defect observed in TrkB-deficient ovaries takes place in the granulosa cell compartment (Paredes et al. 2004). The present results show that a transient (8 h) exposure of 4-day-old ovaries to NT4 enhances the ability of the ovary to respond to FSH with formation of cyclin D2, a cell cycle protein known to mediate the stimulatory effect of FSH on granulosa cell proliferation (Sicinski et al. 1996). Such an effect of NT4, coupled to the proliferative deficit observed in trkB-null ovaries, strongly suggest that one mechanism by which activation of TrkB receptors supports follicular development is by endowing the developing follicles with the capacity to respond to FSH with proliferation. It is also clear, however, that the defect in follicle development becomes evident before the follicles reach the secondary stage and, consequently, acquire responsiveness to FSH (Dierich et al. 1998). This early deficiency strongly suggests that NTs exert proliferative actions on their own, in the absence of FSH. The present report does not directly address this likely possibility, which needs to be examined in detail by future studies.
In contrast to trkB−/− ovaries, the ovaries of Ngf −/− mice show a reduced proliferation of mesenchymal cells (Romero et al. 2002), implying the existence of a directional communication pathway that, initiated in these cells and acting on granulosa cell/oocytes, support follicle development. Such a pathway was previously postulated based on morphological evidence (Rajah et al. 1992), but the molecules involved remain to be identified.
In mice lacking FSHR, follicular development proceeds normally until follicles reach the secondary stage (Dierich et al. 1998), indicating that they become gonadotropin-dependent only at this time. Our results suggest that NTs acting via TrK receptors are intraovarian factors that promote this biochemical differentiation process because FSHR expression is low in the absence of NGF (Romero et al. 2002) or its TrkA receptor (present results), and also in ovaries lacking TrkB receptors (Paredes et al. 2004). Conversely, FSHR mRNA levels increase within 8 h of exposing ovaries to either NGF, the trkA ligand (Romero et al. 2002) or NT4 (present results), one of the TrkB ligands. The common NT receptor p75NTR does not appear to play a role in this process, because p75NTR null mice have normal FSHR mRNA levels. This result was not entirely unexpected because in the rodent ovary p75NTR is expressed only in mesenchymal cells long before the initiation of follicle assembly (Dissen et al. 1995), and remains highly expressed in thecal cells throughout the natural history of follicle development (Dissen et al. 1991). Because the loss of p75NTR expression is only partial in existing p75NTR KOs (von Schack et al. 2001; Paul et al. 2004), it may be necessary to use conditional KO mice in which the cell-specific loss of the receptor is complete to fully understand the contribution that this receptor may have to ovarian development.
The cell-to-cell signaling pathways underlying the action of NTs on follicle organization and subsequent development are not known. Because in the perinatal mouse ovary, Trk receptors are located in both somatic and germ cells (Dissen et al. 2001; Paredes et al. 2004; Anderson et al. 2002; Abir et al. 2005) it appears plausible that part of the mechanism used by NTs to facilitate folliculogenesis and the progression of follicular development to the secondary stage involves the activation of reciprocal mesenchyma-granulosa cell and granulosa cell-oocyte communication pathways. The nature of the cell-cell signaling molecules involved remains to be established, but in recent studies examining the changes in gene expression that accompany the absence of TrkA and TrkB receptors, we have observed that a cell-cell communication pathway affected in both mutant ovaries involves the Jagged1 ligand and its Notch receptor (Kerr et. al., unpublished results).
Altogether, the present results provided substantial support to the concept that NT are intraovarian factors that promote ovarian development before the ovary becomes subjected to gonadotropin control.
Acknowledgements
We thank Mr. Ricardo A. Ojeda for morphometric analysis of ovarian follicles.
Funding This work was supported by NIH grants HD24870 (SRO), the Eunice Kennedy Shriver NICHD/NIH through cooperative agreement HD18185 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research (SRO), and RR00163 for the operation of the Oregon National Primate Research Center (GAD, SRO). CG-R was a visiting scientist and BK was a postdoctoral fellow, both supported by the NICHD TW/HD00668 Fogarty International Training & Research in Population & Health grant.
Footnotes
Publisher's Disclaimer: Disclaimer. This is not the definitive version of record of this article. This manuscript has been accepted for publication in Reproduction, but the version presented here has not yet been copy edited, formatted or proofed. Consequently, the journal accepts no responsibility for any errors or omissions it may contain. The definitive version is available at http://dx.doi.org/10.1530/REP-08-0474.
Declaration of interest The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Abir R, Fisch B, Jin G, Barnnet M, Ben-Haroush A, Felz C, Kessler-Icekson G, Feldberg D, Nitke S, Ao A. Presence of NGF and its receptors in ovaries from human fetuses and adults. Mol.Hum.Reprod. 2005;11:229–236. doi: 10.1093/molehr/gah164. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Robnson LLL, Brooks J, Spears N. Neurotropins and their receptors are expressed in the human fetal ovary. Journal of Clinical Endocrinology and Metabolism. 2002;87:890–897. doi: 10.1210/jcem.87.2.8221. [DOI] [PubMed] [Google Scholar]
- Anesetti G, Lombide P, D’Albora H, Ojeda SR. Intrinsic neurons in the human ovary. Cell and Tissue Research. 2001;306:231–237. doi: 10.1007/s004410100451. [DOI] [PubMed] [Google Scholar]
- Berkemeier LR, Winslow JW, Kaplan DR, Nikolics K, Goeddel DV, Rosenthal A. Neurotrophin-5: A novel neurotrophic factor that activates trk and trkB. Neuron. 1991;7:857–866. doi: 10.1016/0896-6273(91)90287-a. [DOI] [PubMed] [Google Scholar]
- Crowley C, Spencer SD, Nishimura MC, Chen KS, Pitts-Meek S, Armanini MP, Ling LH, McMahon SB, Shelton DL, Levinson AD, Phillips HS. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell. 1994;76:1001–1011. doi: 10.1016/0092-8674(94)90378-6. [DOI] [PubMed] [Google Scholar]
- Dierich A, Sairam MR, Monaco L, Fimia GM, Gansmuller A, LeMeur M, Sassone-Corsi P. Impairing follicle-stimulating hormone (FSH) signaling in vivo: Targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proceedings of the National Academy of Science USA. 1998;95:13612–13617. doi: 10.1073/pnas.95.23.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissen GA, Hill DF, Costa ME, Dees WL, Lara HE, Ojeda SR. A role for trkA nerve growth factor receptors in mammalian ovulation. Endocrinology. 1996;137:198–209. doi: 10.1210/endo.137.1.8536613. [DOI] [PubMed] [Google Scholar]
- Dissen GA, Hill DF, Costa ME, Ma YJ, Ojeda SR. Nerve growth factor receptors in the peripubertal rat ovary. Molecular Endocrinology. 1991;5:1642–1650. doi: 10.1210/mend-5-11-1642. [DOI] [PubMed] [Google Scholar]
- Dissen GA, Hirshfield A Newman, Malamed S, Ojeda SR. Expression of neurotrophins and their receptors in the mammalian ovary is developmentally regulated: Changes at the time of folliculogenesis. Endocrinology. 1995;136:4681–4692. doi: 10.1210/endo.136.10.7664689. [DOI] [PubMed] [Google Scholar]
- Dissen GA, Paredes A, Romero C, Dees WL, Ojeda SR. Neural and neurotrophic control of ovarian development. In: Leung P, Adashi E, editors. The Ovary. 2nd Edition Academic Press; San Diego, CA: 2004. pp. 3–23. [Google Scholar]
- Dissen GA, Romero C, Hirshfield A Newman, Ojeda SR. Nerve growth factor is required for early follicular development in the mammalian ovary. Endocrinology. 2001;142:2078–2086. doi: 10.1210/endo.142.5.8126. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Wetmore C, Olson L, Persson H. Identification of cells in rat brain and peripheral tissues expressing mRNA for members of the nerve growth factor family. Neuron. 1990;5:511–526. doi: 10.1016/0896-6273(90)90090-3. [DOI] [PubMed] [Google Scholar]
- Hallböök F, Ibañez CF, Persson H. Evolutionary studies of the nerve growth factor family reveal a novel member abundantly expressed in Xenopus ovary. Neuron. 1991;6:845–858. doi: 10.1016/0896-6273(91)90180-8. [DOI] [PubMed] [Google Scholar]
- Hirshfield AN. Development of follicles in the mammalian ovary. International Review of Cytology. 1991;124:43–101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- Hirshfield AN, DeSanti AM. Patterns of ovarian cell proliferation in rats during the embryonic period and the first three weeks postpartum. Biology of Reproduction. 1995;53:1208–1221. doi: 10.1095/biolreprod53.5.1208. [DOI] [PubMed] [Google Scholar]
- Klein R, Parada LF, Coulier F, Barbacid M. trkB a novel tyrosine protein kinase receptor expressed during mouse neural development. EMBO Journal. 1989;8:3701–3709. doi: 10.1002/j.1460-2075.1989.tb08545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamballe F, Klein R, Barbacid M. trkC a new member of the trk family of tyrosine protein kinases, is a receptor for neurotrophin-3. Cell. 1991;66:967–979. doi: 10.1016/0092-8674(91)90442-2. [DOI] [PubMed] [Google Scholar]
- Lara HE, Hill DF, Katz KH, Ojeda SR. The gene encoding nerve growth factor is expressed in the immature rat ovary: Effect of denervation and hormonal treatment. Endocrinology. 1990;126:357–363. doi: 10.1210/endo-126-1-357. [DOI] [PubMed] [Google Scholar]
- Lee K-F, Li E, Huber LJ, Landis SC, Sharpe AH, Chao MV, Jaenisch R. Targeted mutation of the gene encoding the low affinity NGF receptor p75 leads to deficits in the peripheral sensory nervous system. Cell. 1992;69:737–749. doi: 10.1016/0092-8674(92)90286-l. [DOI] [PubMed] [Google Scholar]
- Liebl DJ, Klesse LJ, Tessarollo L, Wohlman T, Parada LF. Loss of brain-derived neurotrophic factor-dependent neural crest-derived sensory neurons in neurotrophin-4 mutant mice. Proceedings of the National Academy of Science USA. 2000;97:2297–2302. doi: 10.1073/pnas.040562597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan KA, Gosden R, Taketo T. Continuous loss of oocytes throughout meiotic prophase in the normal mouse ovary. Developmental Biology. 2003;258:334–348. doi: 10.1016/s0012-1606(03)00132-5. [DOI] [PubMed] [Google Scholar]
- Morse SM, Shaw G, Larner SF. Concurrent mRNA and protein extraction from the same experimental sample using a commercially available column-based RNA preparation kit. BioTechniques. 2006;40:54, 56, 58. doi: 10.2144/000112100. [DOI] [PubMed] [Google Scholar]
- Paredes A, Romero C, Dissen GA, DeChiara TM, Reichardt L, Cornea A, Ojeda SR, Xu B. TrkB receptors are required for follicular growth and oocyte survival in the mammalian ovary. Developmental Biology. 2004;267:430–449. doi: 10.1016/j.ydbio.2003.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul CE, Vereker E, Dickson KM, Barker PA. A pro-apoptotic fragment of the p75 neurotrophin receptor is expressed in p75NTRExonIV null mice. Journal of Neuroscience. 2004 Feb 25;24:1917–1923. doi: 10.1523/JNEUROSCI.5397-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters H. The development of the mouse ovary from birth to maturity. Acta Endocrinologica. 1969;62:98–116. doi: 10.1530/acta.0.0620098. [DOI] [PubMed] [Google Scholar]
- Rajah R, Glaser EM, Hirshfield AN. The changing architecture of the neonatal rat ovary during histogenesis. Dev.Dyn. 1992;194:177–192. doi: 10.1002/aja.1001940303. [DOI] [PubMed] [Google Scholar]
- Romero C, Paredes A, Dissen GA, Ojeda SR. Nerve growth factor induces the expression of functional FSH receptors in newly formed follicles of the rat ovary. Endocrinology. 2002;143:1485–1494. doi: 10.1210/endo.143.4.8711. [DOI] [PubMed] [Google Scholar]
- Sicinski P, Donaher JL, Geng Y, Parker SB, Gardner H, Park MY, Robker RL, Richards JS, McGinnis LK, Biggers JD, Eppig JJ, Bronson RT, Elledge SJ, Weinberg RA. Cyclin D2 is an FSH-responsive gene involved in gonadal cell proliferation and oncogenesis. Nature. 1996 May 12;384:470–474. doi: 10.1038/384470a0. [DOI] [PubMed] [Google Scholar]
- Spears N, Molinek MD, Robinson LL, Fulton N, Cameron H, Shimoda K, Telfer EE, Anderson RA, Price DJ. The role of neurotrophin receptors in female germ-cell survival in mouse and human. Development. 2003;130:5481–5491. doi: 10.1242/dev.00707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolosa JM, Schjenken JE, Civiti TD, Clifton VL, Smith R. Column-based method to simultaneously extract DNA, RNA, and proteins from the same sample. BioTechniques. 2007;43:799–804. doi: 10.2144/000112594. [DOI] [PubMed] [Google Scholar]
- von Schack D, Casademunt E, Schweigreiter R, Meyer M, Bibel M, Dechant G. Complete ablation of the neurotrophin receptor p75NTR causes defects both in the nervous and the vascular system. Nat.Neurosci. 2001;4:977–978. doi: 10.1038/nn730. [DOI] [PubMed] [Google Scholar]
- Zhu G-Z, Miller BJ, Boucheix C, Rubinstein E, Liu CC, Hynes RO, Myles DG, Primakoff P. Residues SFQ (173-175) in the large extracellular loop of CD9 are required for gamete fusion. Development. 2002;129:1995–2002. doi: 10.1242/dev.129.8.1995. [DOI] [PubMed] [Google Scholar]