Abstract
A systematic study of the inverse electron demand Diels–Alder reactions of 1,2,3-triazines is disclosed, including an examination of the impact of a C5 substituent. Such substituents were found to exhibit a remarkable impact on the cycloaddition reactivity of the 1,2,3-triazine without altering, and perhaps even enhancing, the intrinsic cycloaddition regioselectivity. The study revealed that not only may the reactivity be predictably modulated by a C5 substituent (R = CO2Me > Ph > H), but that the impact is of a magnitude to convert 1,2,3-triazine (1) and its modest cycloaddition scope into a heterocyclic azadiene system with a reaction scope that portends extensive synthetic utility, expanding the range of participating dienophiles. Significantly, the studies define a now powerful additional heterocyclic azadiene, complementary to the isomeric 1,2,4-triazines and 1,3,5-triazines, capable of dependable participation in inverse electron demand Diels–Alder reactions, extending the number of complementary heterocyclic ring systems accessible with implementation of the methodology.
Introduction
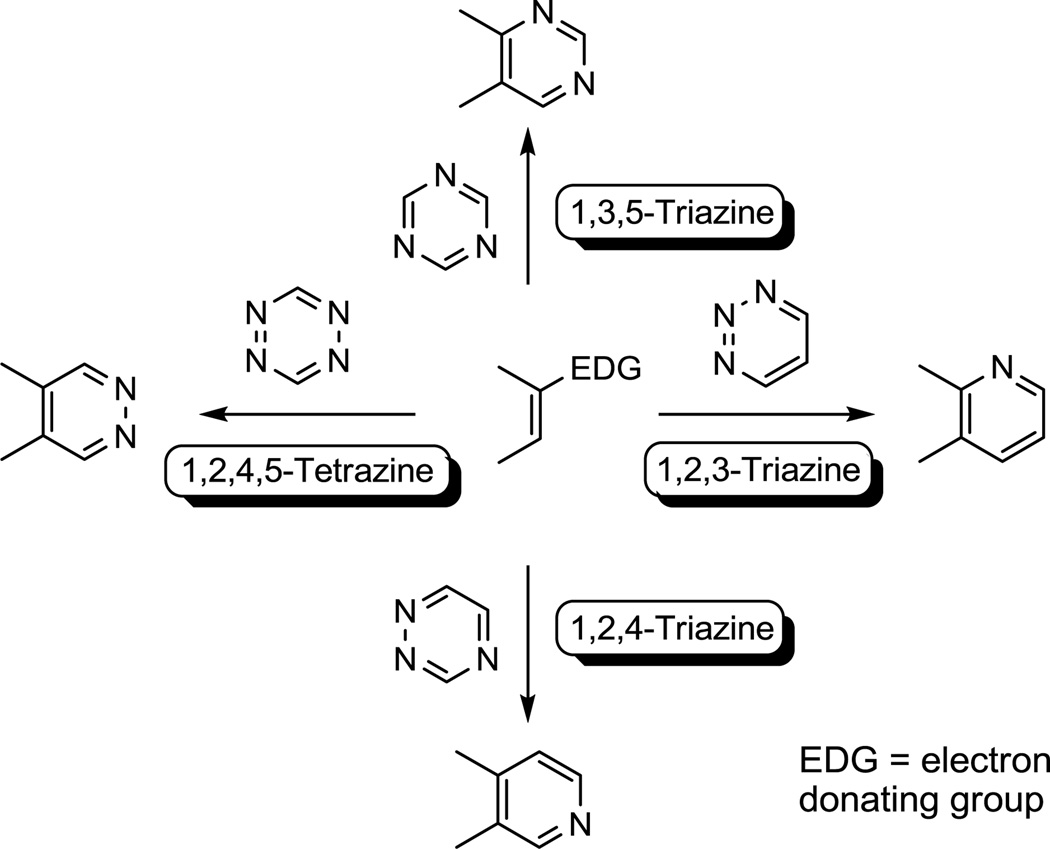
We have used the inverse electron demand Diels–Alder reactions of electron-deficient heterocyclic azadienes as powerful cycloaddition reactions central to a series of natural products total syntheses whose structures possess highly functionalized heterocyclic ring systems not easily accessed by conventional means.1 Such reactions have also found widespread use in the synthesis of highly substituted heterocycles not accessible by other means,2 in the divergent synthesis of screening libraries,3 and recently in bioconjugation reactions.4 To date, our own studies have focused largely on the fundamental cycloaddition reactions of 1,2,4,5-tetrazines,5 1,2,4-triazines,6 1,3,5-triazines,7 1,3,4-oxadiazoles,8 and occasionally 1,2-diazines,9 often followed by subsequent key transformations that now constitute general synthetic strategies for the preparation of five-membered10 as well as six-membered heterocyclic ring systems. In a continuation of our efforts to explore heterocyclic and acyclic11 azadiene Diels–Alder reactions and their applications, we recently examined the inverse electron demand Diels–Alder reactions of the parent 1,2,3-triazine (1), disclosing the first report of its unique capabilities for participating in previously unexplored [4 + 2] cycloaddition reactions with heterodienophiles.12 Several prior key studies have demonstrated the ability of a limited number of 1,2,3-triazines to participate in inverse electron demand Diels–Alder reactions, typically with enamine13 and ynamine14 dienophiles. These prior studies have been reported largely in the pioneering efforts of Okatani (Sugita)13 or Igeta and Ohsawa14 often times suggesting modest utility, and have not yet defined the role substituents may play in modulating the reactivity or regioselectivity of the cycloaddition reactions. Herein, we report a systematic study of the cycloaddition reactions of 1,2,3-triazines, focusing on the impact of 1,2,3-triazine C5 substituents. Such substituents were found to not only exhibit a remarkable impact on the now useful range of relative cycloaddition reactivities that may be predictably modulated by the substituents expanding the resulting range of participating dienophiles, but the studies also highlight the complementary nature of the heterocyclic ring systems generated by use of 1,2,3-triazines (Figure 1).
Figure 1.
Complementary cycloaddition reactions of key heterocyclic azadienes.
Results and Discussion
Synthesis of 1,2,3-triazines
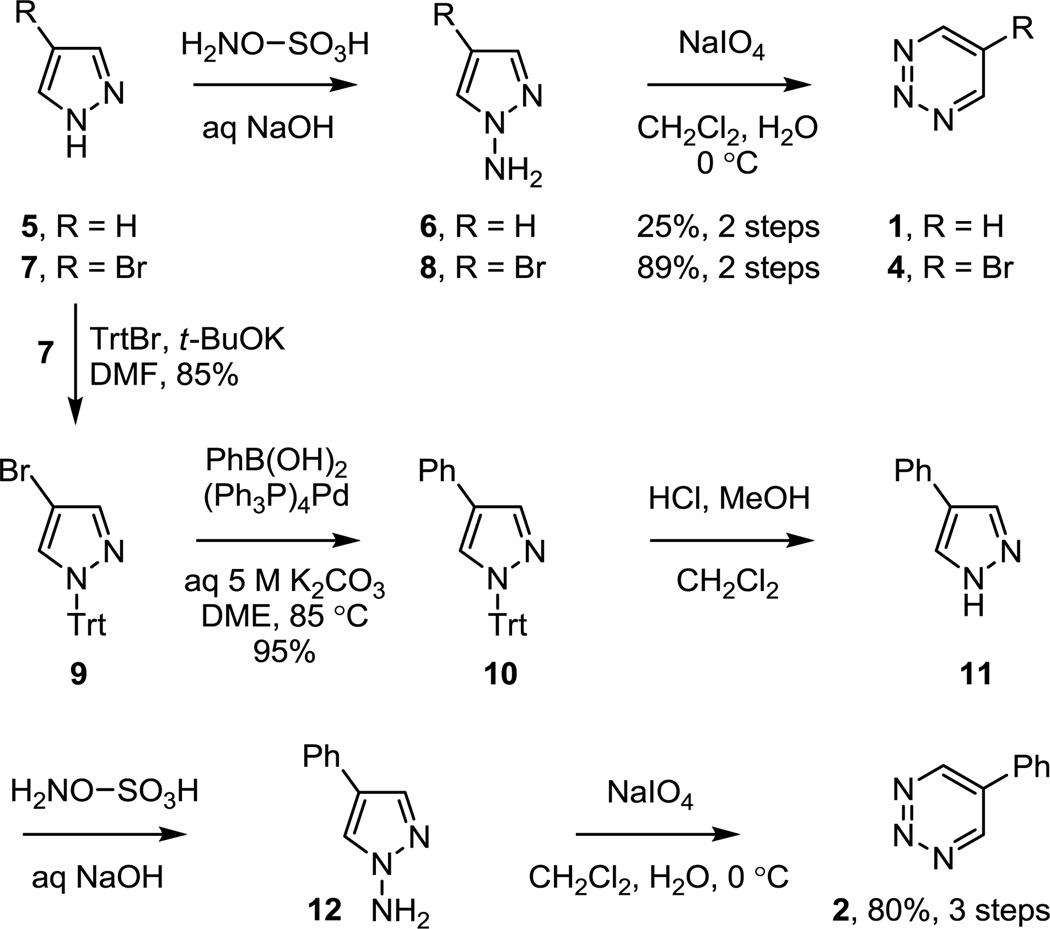
The key 1,2,3-triazines chosen to examine the inverse electron demand cycloaddition reactivity and regioselectivity impact of a C5 substituent were 5-phenyl-1,2,3-triazine (2) and 5-carbomethoxy-1,2,3-triazine (3), bearing a C5 phenyl and carbomethoxy group. This was complemented by the comparative examination of the parent 1,2,3-triazine 1 itself as well as 5-bromo-1,2,3-triazine (4) because of its availability as a result of the approaches examined for the preparation of 2 and 3. Since the unsubstituted 1,2,3-triazine 1 reacts with cycloaddition exclusively across N1/C4 in the studies disclosed to date, the C5 substitution was anticipated to further enhance this intrinsic regioselectivity while improving its modest reactivity. 1,2,3-Triazines are most directly accessed by N-amination of a symmetrical pyrazole followed by oxidative ring expansion and this approach could be used to prepare each of the four 1,2,3-triazines. The synthesis of 1,2,3-triazine (1) and 5-bromo-1,2,3-triazine (4) began with amination of the commercially available pyrazole (5) and 4-bromopyrazole (7), respectively, using hydroxylamine-O-sulfonic acid (3 equiv, aqueous 3.7 M NaOH, 30 min) and was followed by subsequent oxidative ring expansion of the N-aminopyrazoles 6 and 8 effected by treatment with NaIO4 (2 equiv, CH2Cl2–H2O, 12 h) at 0 °C to provide the desired 1,2,3-triazine 113–15 as detailed13e (25%, 2 steps) and 416 (89%, 2 steps), Scheme 1. Notably, the NaIO4 oxidative ring expansion introduced by Okatani13 for the synthesis of 1,2,3-triazines provides 1 in better conversions than alternative methods15 and proved much more effective for the preparation of 4 than earlier reported methods.16 Compounds 1 and 4 were isolated as tan solids and are stable for extended periods if kept free of moisture and stored at or below 0 °C.
Scheme 1.
5-Phenyl-1,2,3-triazine (2) was also prepared from commercially available 4-bromopyrazole (7), Scheme 1. Trityl protection of 7 (85%), Suzuki coupling of 9 with phenylboronic acid (94%), trityl deprotection of 10 and N-amination of 11 with hydroxylamine-O-sulfonic acid (3 equiv, aq NaOH, 30 min) followed by subsequent oxidative ring expansion of 12 also effected by treatment with NaIO4 (2 equiv, CH2Cl2–H2O, 12 h) at 0 °C provided 5-phenyl-1,2,3-triazine17 (2, 80–85%, 3 steps) as small off-white crystals that proved stable to storage at room temperature.
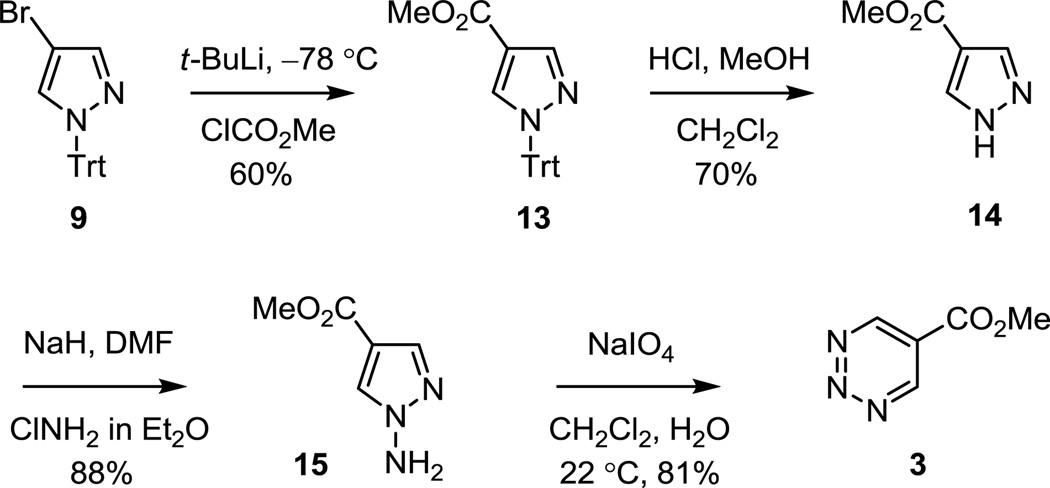
5-Carbomethoxy-1,2,3-triazine (3)15a was accessed by lithium–halogen exchange of N-trityl-4-bromopyrazole (9) and subsequent reaction of the 4-lithiopyrazole with methyl chloroformate to provide 13 (60%), Scheme 2. Trityl deprotection (HCl–MeOH, CH2Cl2, 70%) provided 14, which provided low and inconsistent conversions to the N-aminopyrazole 15 when treated with hydroxylamine-O-sulfonic acid under the required aqueous basic conditions (aq NaOH), even following reesterification (TMSCHN2) of the unavoidable in situ hydrolyzed methyl ester. In efforts to find a more reliable route to 3, the use of monochloroamine was examined, which has been reported for the N-amination of pyrroles and indoles.18 Deprotonation of 14 with NaH (DMF) followed by addition of monochloroamine (ClNH2 in ether, 30 min, 23 °C) provided the N-aminopyrazole 15 in superb conversions (88–93%) that was oxidized with NaIO4 (2 equiv, CH2Cl2–H2O, 2 h) at room temperature to provide 3 (81%). Although this synthesis was used to prepare most of 3 employed in our studies, we have more recently found that the N-amination of 14 to provide 15 may be accomplished even more conveniently with O-(4-nitrobenzoyl)hydroxylamine19 (1.1 equiv of KOBut, NMP, 20 min, 22 °C; 1.15 equiv of O-(4-nitrobenzoyl)hydroxylamine, NMP, 22 °C, 2 h, 75%). 5-Carbomethoxy-1,2,3-triazine proved to be especially reactive and was found to be sensitive to water. Consequently, 3 was handled typically in flame-dried glassware under an Ar atmosphere and flash chromatography was performed using oven-dried SiO2. Although not extensively investigated, alternative routes to 3, including Pd-catalyzed carbonylation in the presence of MeOH or lithium–halogen exchange of 4 followed by reaction with methyl chloroformate, proved unsuccessful likely due to the competitive reactions of 3 and/or 4 under the reaction conditions. In addition to their instability toward water, all four 1,2,3-triazines are unstable to protic solvents including MeOH with 3 and 4 reacting quickly even at −40 °C. 5-Bromo-1,2,3-triazine (4) was found to violently decompose at 112 °C while attempting to measure its melting point, whereas the 1,2,3-triazines 1–3 were all found to be stable at temperatures < 140 °C, the limit of our examination, as well as during our melting point determinations.
Scheme 2.
Cycloaddition reactions with ynamines
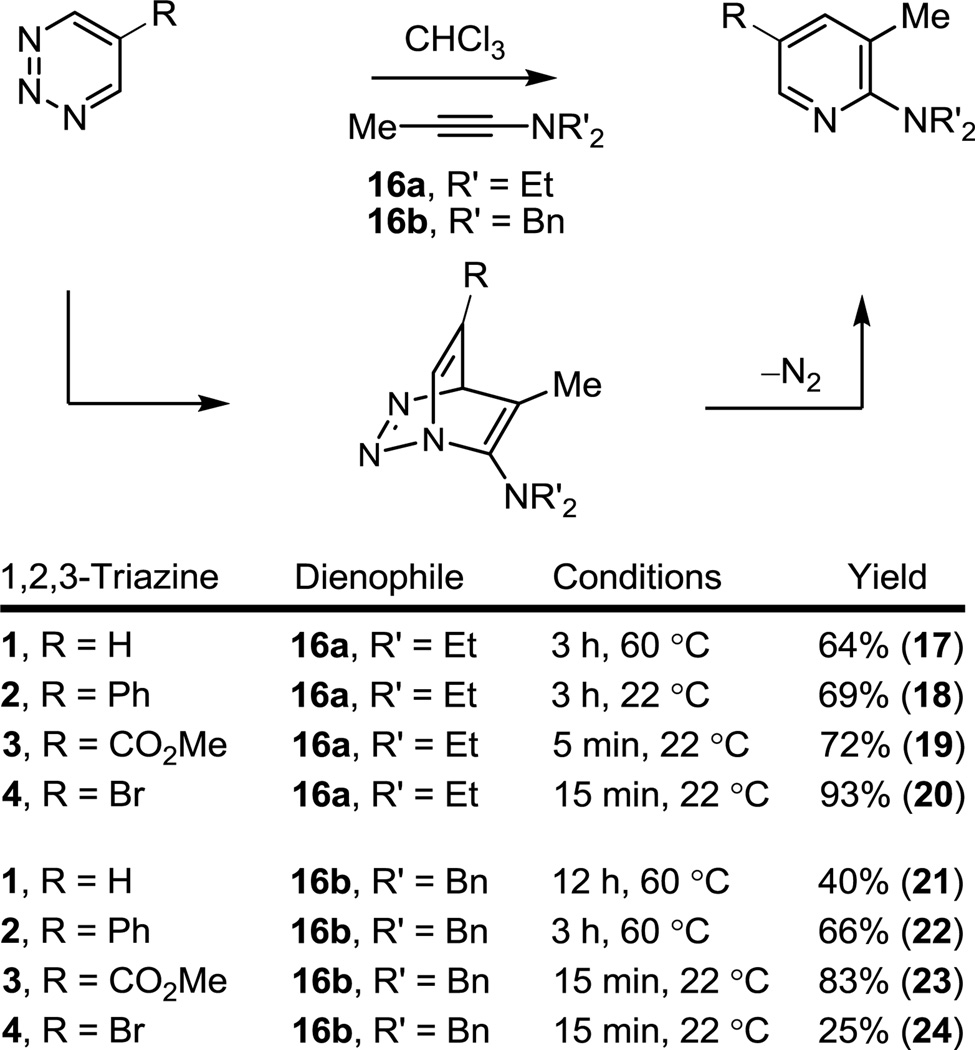
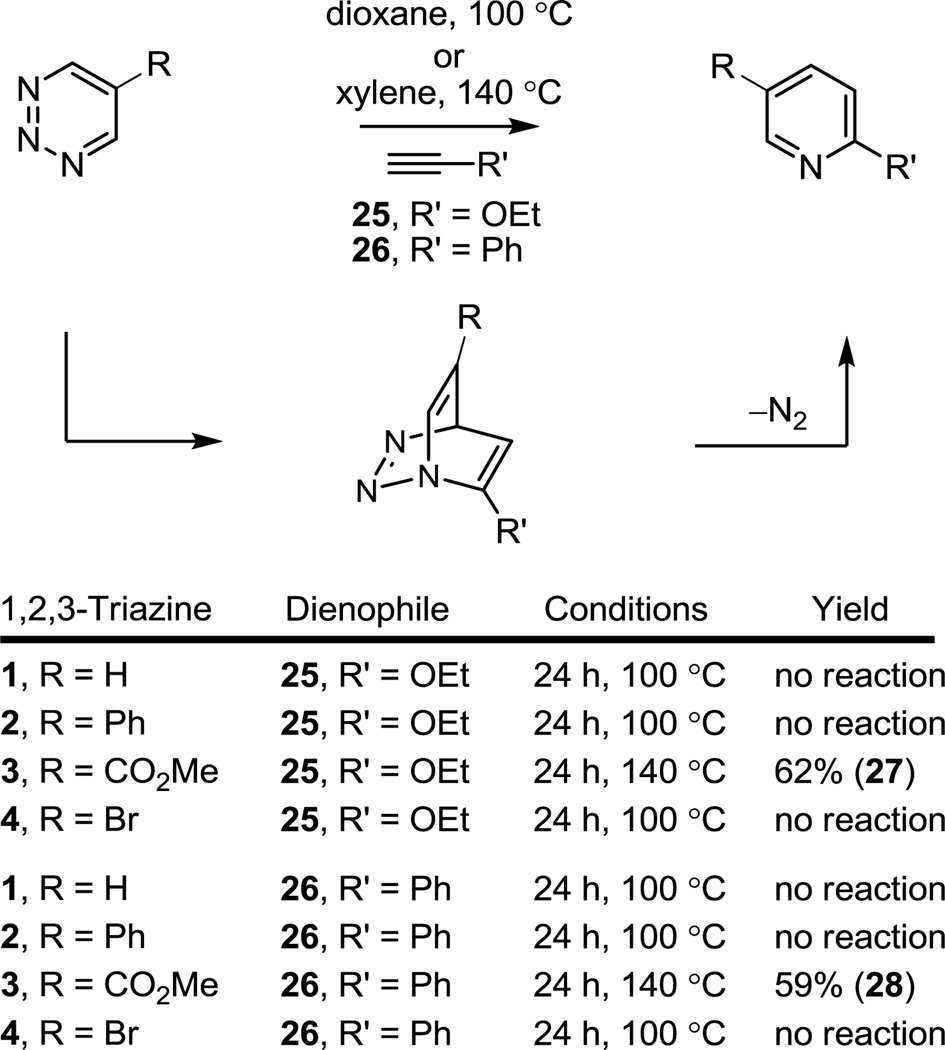
Consistent with prior reports, the Diels–Alder reaction of the unsubstituted 1,2,3-triazine (1) with ynamines14 proceeded well, but required warming the reaction mixtures at 60 °C (CHCl3, 3–12 h) for complete reaction indicative of a modest reactivity. The reactions proceeded with clean regioselectivity with cycloaddition across N1/C4 of the 1,2,3-triazine (Figure 2). The C5 substituted 1,2,3-triazines 2 and 3, bearing a conjugated phenyl substituent and a more strongly electron-withdrawing methoxycarbonyl substituent, respectively, exhibited a progressively enhanced reactivity (R = CO2Me > Ph > H) and the same characteristic exclusive N1/C4 cycloaddition regioselectivity, benefiting from the complementary azadiene substitution. Remarkably, the modest reactivity of the parent 1,2,3-triazine (1, 60 °C, 3–12 h) is transformed into a reaction that proceeds in minutes at room temperature, providing improved conversions to the corresponding pyridines, with the introduction of the C5 methoxycarbonyl group (3, 5–15 min, 72–83%). Although the comparisons based on the reaction with ynamines are limited, the results also suggest that 4-bromo-1,2,3-triazine (4) exhibits a reactivity intermediate that of 2 and 3 (R = CO2Me > Br > Ph > H).
Figure 2.
Diels–Alder reactions of 1,2,3-triazines 1–4 with ynamines.
Cycloaddition reactions with additional acetylenic dienophiles
Representative of potential cycloaddition reactions with less electron-rich or even conjugated acetylenic dienophiles, the reactions of the 1,2,3-triazines with ethoxyacetylene (25) and phenylacetylene (26) were examined. Whereas both 1,2,3-triazine (1) and 5-phenyl-1,2,3-triazine (2) failed to exhibit a detectable reactivity toward either ethoxyacetylene or phenylacetylene in dioxane at 100 °C, the more electron-deficient 5-methoxycarbonyl-1,2,3-triazine (3) displayed a measurable reactivity, Figure 3. This was optimized by conducting the reactions at higher temperatures (xylenes, 140 °C), requiring a shorter reaction time for completion (< 24 h), and provided the Diels–Alder products 27 (62%) and 28 (59%), respectively, in good conversions and as single regioisomers. 1,2,3-Triazines 1 and 2 were examined at even higher reaction temperatures (200 °C) under concentrated conditions and failed to provide evidence of cycloaddition. Clearly and especially because of the regiospecific reaction with phenylacetylene that one would not ordinarily expect with an electron-deficient heterocyclic azadiene, the scope of cycloadditions of 3 with acetylenic dienophiles is broad and portends extensive usage.
Figure 3.
Reaction with additional acetylenes.
Cycloaddition reactions with enamines
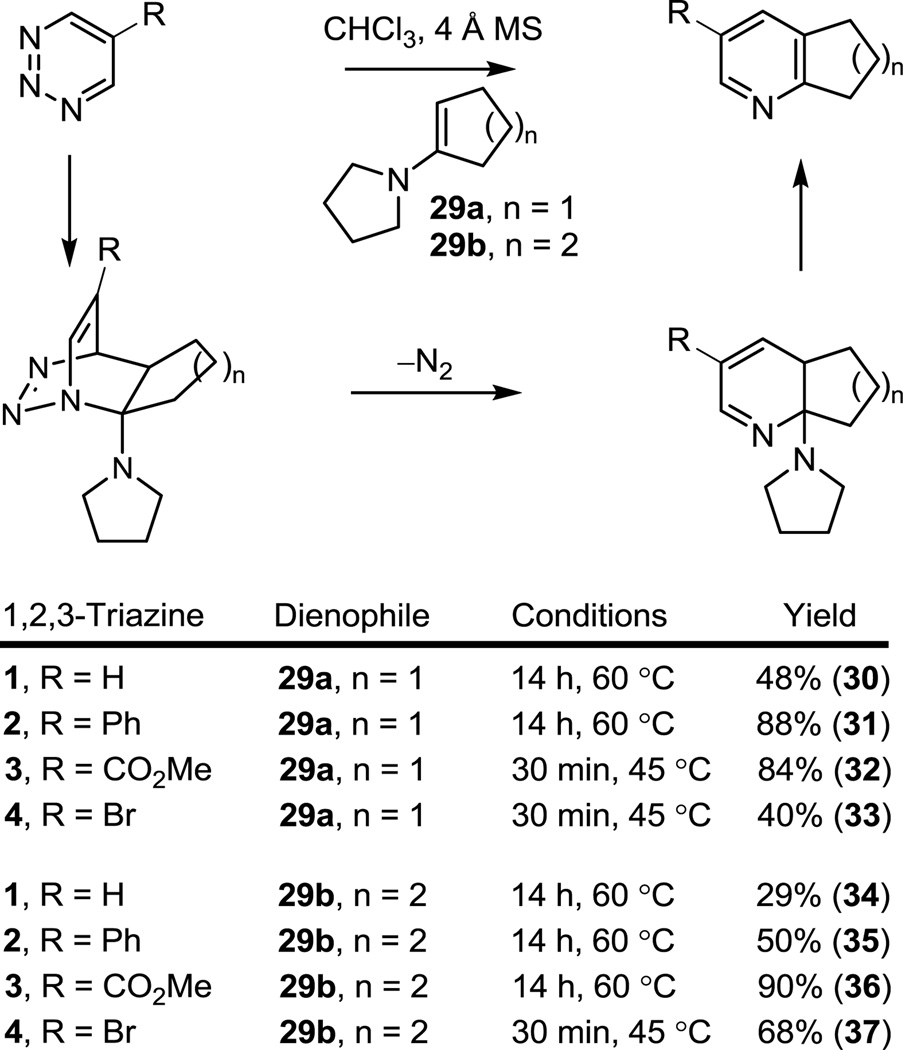
Consistent with Okatani’s prior reports13 and as detailed in our recent study,12 we found that the reaction of the parent 1,2,3-triazine 1 with enamines (CHCl3, 60 °C) proved more limited than the analogous reactions of 1,2,4- or 1,3,5-triazines,6,7 providing modest yields of the expected pyridine products and a disappointing scope, Figure 4. In contrast, the C5 substituted 1,2,3-triazines 2 and 3, bearing a conjugated phenyl substituent and a more strongly electron-withdrawing methoxycarbonyl substituent, respectively, exhibited a progressively enhanced reactivity (R = CO2Me > Ph > H), providing good yields of the corresponding pyridine products and a generalized scope accommodating enamines typically problematic (29b). Products derived from liberated pyrrolidine addition to the starting 1,2,3-triazine were detected with 1 (in the reaction with both 29a and 29b) and occasionally with 2 (in the reaction with 29b) that reflect a slow [4 + 2] cycloaddition reaction relative to aromatization and that may account in part for their more modest conversions. By contrast, no evidence of liberated pyrrolidine addition was observed with 3 or 4, presumably reflecting their more rapid [4 + 2] cycloaddition that leads to complete consumption of the 1,2,3-triazine prior to liberation of any pyrrolidine. Consistent with this behavior, the reaction of 1,2,3-triazines 3 and 4 with enamines leads to an instantaneous color change and evolution of N2 even at −20 °C, but provides poor conversions to product at these low temperatures (3 + 29b: 22 °C, 2 h, 0%; 22 °C for 1 min and 45 °C, 30 min, 54%; 22 °C for 1 min and 60 °C, 12 h, 90%). Because of this, the inclusion of 4 Å molecular sieves (4 Å MS) in the reaction mixture first utilized with 1,2,4-triazines6 was found to aid the aromatization step and improve the overall conversions, especially with the dienophile 29b. Using this modification, the reactions summarized in Figure 4 were initiated at 22 °C (for 3) and 0 °C (for 4) and only after the exothermic evolution of N2 was complete were the reaction mixtures warmed to promote aromatization and completion of the reaction. In each case, the 1,2,3-triazines exhibited the now characteristic exclusive N1/C4 cycloaddition regioselectivity with the nucleophilic carbon of the electron-rich dienophile attached to C4, benefiting from the additional complementary azadiene substitution in the case of 2–4. Importantly, the reactivity of 2–4 imparted by the C5 substituents provide substantially improved cycloaddition reactions with enamine dienophiles realizing a synthetic scope and efficiency first envisioned by Okatani.13
Figure 4.
Cycloaddition reactions of 1,2,3-triazines 1–4 with enamines.
Diels–Alder reactions with additional electron-rich olefinic dienophiles
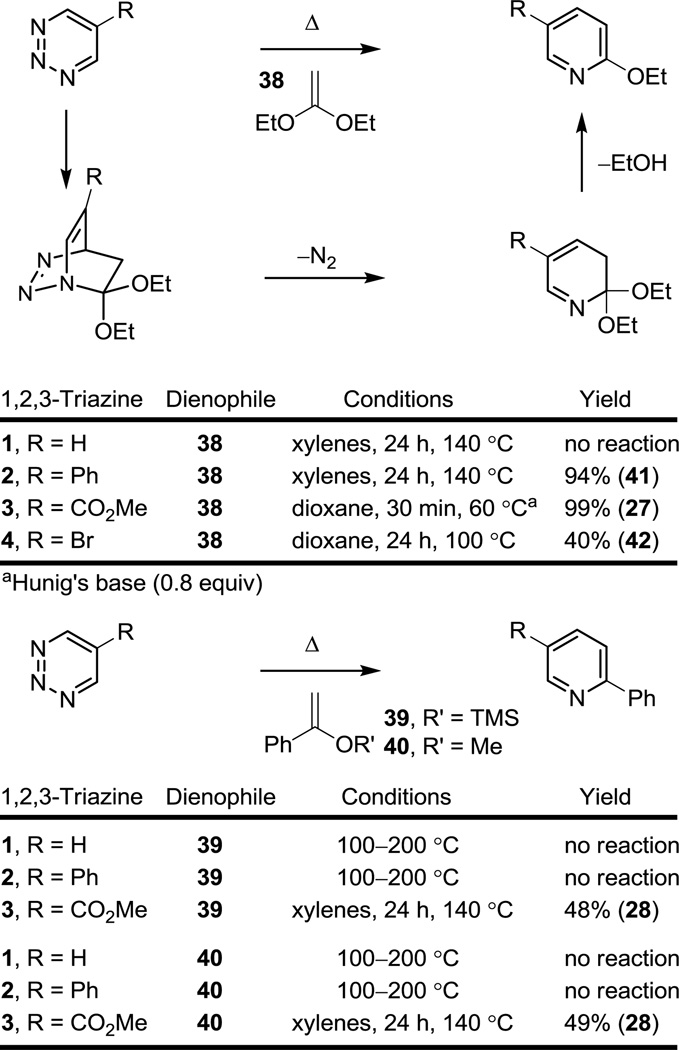
In our recent survey of the cycloaddition reactions of the parent 1,2,3-triazine (1),12 a key series of additional potential electron-rich dienophiles were examined, including ketene acetals ((EtO)2C=CH2), and enol ethers (Ph(OMe)C=CH2 and Ph(OTMS)C=CH2), that failed to react with 1 under the conditions examined. As a result, the reactivity of 2–4 with such dienophiles was of special interest potentially expanding the synthetic scope of the 1,2,3-triazine cycloaddition reactions, Figure 5.
Figure 5.
Diels–Alder reactions of 1,2,3-triazines 1–4 with ketene acetals and enol ethers.
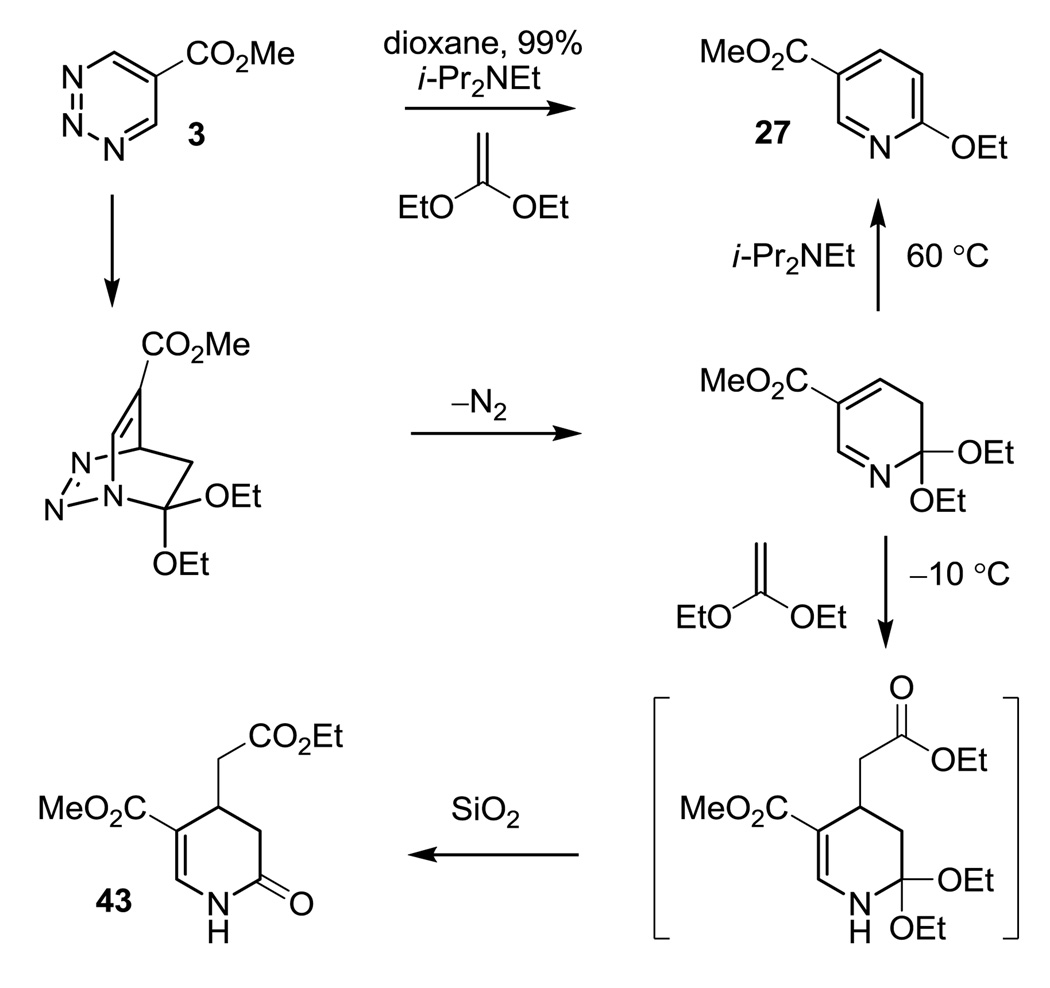
Consistent with the relative reactivity of the dienophiles and the anticipated impact of the 1,2,3-triazine C5 substituents examined (R = CO2Me > Ph > H), 5-carbomethoxy-1.2.3-triazine (3) was found to react with both the ketene acetal 38 and the enol ether dienophiles 39 and 40 under conditions that reflect the relative reactivities of the electron-rich olefins (ketene acetals > enol ethers), whereas 5-phenyl-1,2,3-triazine (2) was found to only react with the ketene acetal 38 and not with the less reactive enol ethers 39 and 40 under the conditions examined. Thus, the reaction of 2 with the ketene acetal 38 proceeds slowly in dioxane at 100 °C (24 h, 88%; 98% based on recovered starting material) and proceeds to completion when run in xylenes at 140 °C (24 h, 94%), whereas the reaction of 3 with 38 is complete in dioxane at 60 °C within 30 min. In fact, the initial Diels–Alder reaction of 3 with 38 (5 equiv) occurs at temperatures as low as −10 °C where N2 evolution and a distinct color change is observed, but initially provided only low conversions to the expected pyridine product 27 (18%). Instead, the lactam 43,20 the product of a subsequent nucleophilic addition of the ketene acetal 38 to the intermediate cycloaddition product prior to aromatization, was isolated as the major product (80%), Scheme 3. Beautifully, reducing the amount of dienophile (1.0 equiv) and the addition of Hunig’s base (i-Pr2NEt, 0.8 equiv) to the reaction mixture combined with its heating (60 °C, dioxane) led to rapid aromatization to provide 27 in superb yield (99%) with complete suppression of the formation of lactam 43. Significantly, this series of dienophiles clearly differentiates the reactivity of the three 1,2,3-triazines 1–3, indicating that the incorporation of an appropriate C5 substituent can convert substrates incapable of reaction with 1 even at temperatures of 200 °C, to those that exhibit remarkably effective cycloaddition rates without altering the intrinsic cycloaddition regioselectivity. This is especially evident with 1,2,3-triazine 3 that initially reacts with the ketene acetal 38 (1,1-diethoxyethylene) at temperatures as low as −10 °C.
Scheme 3.
Cycloaddition reactions with heterodienophiles
Especially interesting was the reactivity of the 1,2,3-triazines toward heterodienophiles. Amidines, imidates and related reagents have been found to react with many heteroaromatic dienes by a reaction course that is dependent on the nature of the reactants and reaction conditions. Such reagents have been shown to react as either C=N or isomeric N,N- or N,O-ketene acetal dienophiles depending on the heterocyclic azadiene and reaction conditions examined (equation 1), and typically are characterized as cleanly proceeding through a single pathway.
 |
(1) |
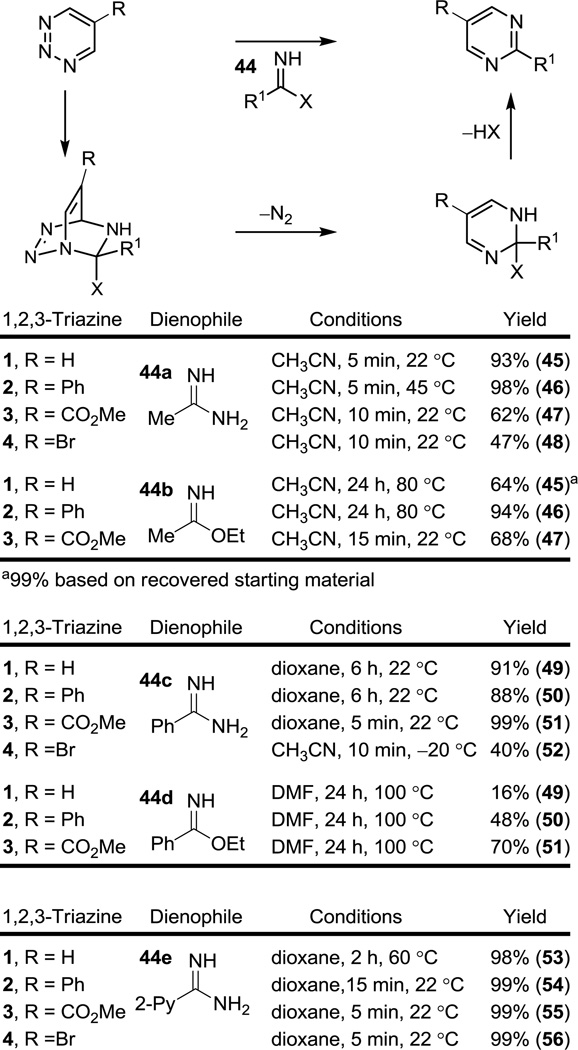
Given the modest reactivity of 1 and like the behavior of 1,3,5-triazines,7d one might have anticipated that 1–4 would be a superb candidates for reaction with such reagents through their more reactive, in situ generated N,N- or N,O-ketene acetals. Remarkably and like 1,2,4,5-tetrazines,5a the aliphatic acetamidine (44a) and ethoxy acetimidate (44b) both underwent clean, rapid [4 + 2] cycloaddition with 1–4 as C=N dienophiles to provide the corresponding pyrimidines 45–48 in superb conversions with no evidence of reaction through their in situ generated and more reactive 1,1-diaminoethylene or 1-amino-1-ethoxyethylene tautomers, Figure 6. The reaction of even 1, the least reactive of the 1,2,3-triazines, with the aliphatic amidine 44a (CH3CN, 25 °C, 5 min, 93%) was extraordinarily fast, proceeding in minutes at room temperature, whereas the reaction with the less reactive aliphatic imidate 44b required higher temperatures and longer times for reaction with 1 (CH3CN, 60 °C, 24 h, 64%, 99% based on recovered starting material). Even here, only the product derived from a regiospecific cycloaddition of the C=N dienophile across C4/N1 was detected independent of the reaction conditions examined (temperature, solvent polarity, free base vs HCl salt). Although complete conversion to the pyrimidine product required higher reaction temperatures, most remarkable of the initial observations was that even 1 reacts with the aliphatic amidine 44a at −30 °C in minutes with an instantaneous evolution of N2 and distinct color change indicative of a remarkably facile [4 + 2] cycloaddition. Similarly, the aryl amidines 44c and 44e provided the corresponding pyrimidines 49–56 in good to superb conversions in reactions where the disappearance of 1–4 precedes completion of the reaction, indicating that aromatization with loss of ammonia versus [4 + 2] cycloaddition is the slow step in the overall reaction sequence.
Figure 6.
Reactions of 1,2,3-triazines 1–4 with amidines and imidates.
The reactions were completely regioselective with regard to both the amidine/imidate, providing only pyrimidine product with no trace of the corresponding pyridazine (1,2-diazine), as well as the 1,2,3-triazine, providing no trace of 1,2,4-triazine product consistent with cycloaddition only across C4/N1 versus C5/N2. In all cases and in contrast to our observations made with 1,3,5-triazines,7 it was important to use the amidine or imidate free base rather than their HCl salts, which provided less reproducible and substantially lower yields. Perhaps the clearest depiction of the relative rates of reaction among the 1,2,3-triazines toward the C=N dienophiles emerged from the examination of the aliphatic and aryl imidates 44b and 44d (R = CO2Me > Ph > H), the more electron-rich amidines were expectedly and considerably much more reactive than the corresponding imidates (44a vs 44b, and 44c vs 44d), and the aliphatic amidine or imidate were more reactive than the corresponding conjugated aryl amidine or imidate (44a vs 44c and 44b vs 44d). The reactions of the amidines with the more electron-deficient 1,2,3-triazines 3 and 4 are highly exothermic and are accompanied by the rapid N2 evolution upon mixing. Often times, these reactions benefited from their mixing at low temperature to contain the exotherm followed by exposure to higher reaction temperatures to effect the slower aromatization (e,g., 4 + 44c). Additionally, the most effective substrate examined was the 2-pyridylamidine 44e (98–99% for 1–4) in which the 2-pyridyl substituent presumably participates in the final, typically slow, aromatization step via an internal deprotonation to facilitate the elimination of ammonia.
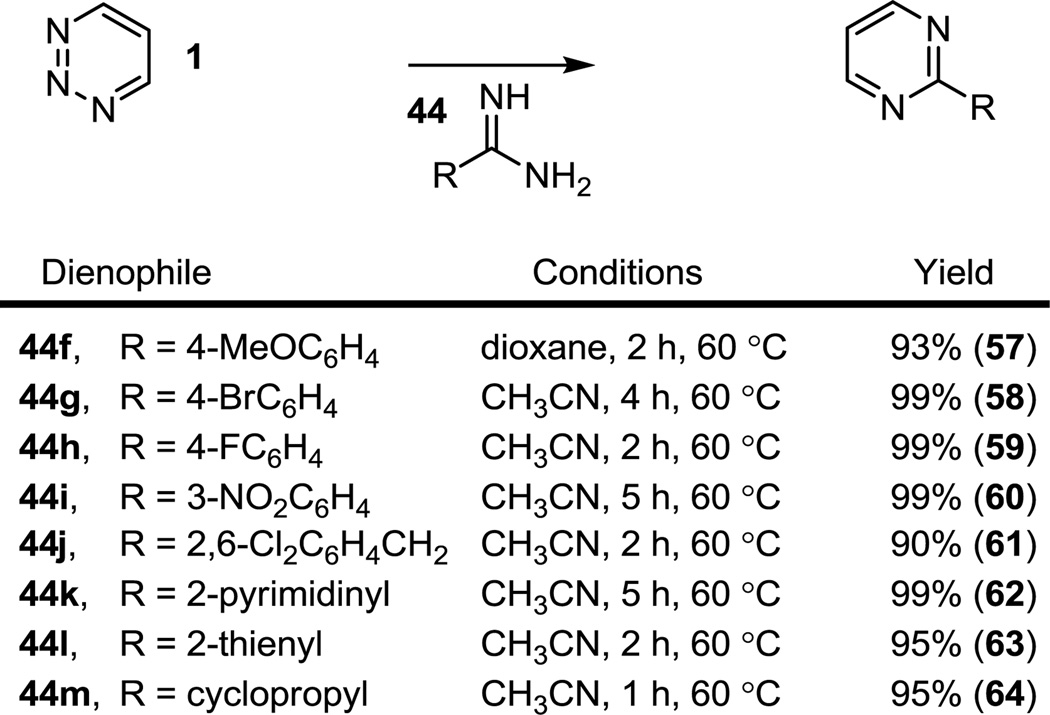
Since the cycloaddition reaction of 1,2,3-triazine (1) itself with amidines had not been examined or reported prior to our studies,12 its generality was further established with the amidines 44f–44m, providing the corresponding pyrimidines 57–64 in superb conversions (90–99%), Figure 7. Given that 1 is the least reactive of the four 1,2,3-triazines examined, its broad scope portends extensive usage across the entire series.
Figure 7.
Additional reactions of 1,2,3-triazine (1) with amidines.
Computational studies
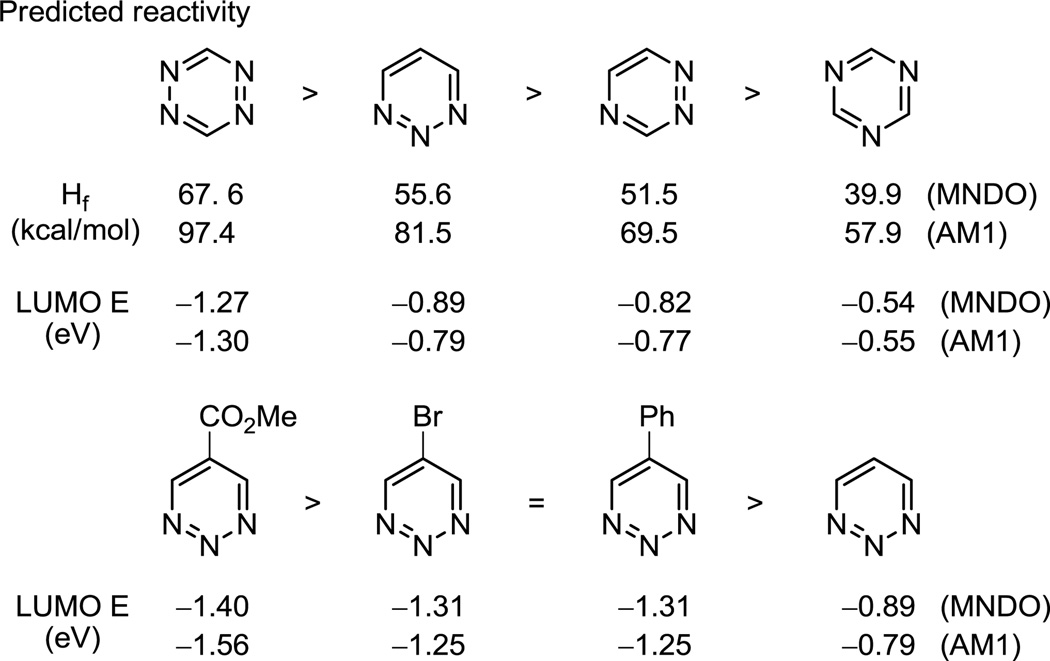
Semiempirical computational studies (MNDO, AM1) of the parent ring systems as well as the substituted 1,2,3-triazines were carried out in efforts to shed insight into the relative behavior of the heterocyclic azadienes, Figure 8. Consistent with intuitive expectations, a comparison of the computed heats of formation (Hf) indicate that 1,2,3-triazine with three vicinal nitrogen atoms and their adjacent repulsive lone pairs is predicted to be more unstable (more reactive) than 1,2,4-triazine with two vicinal nitrogens that in turn is more unstable than 1,3,5-triazine. Potentially predictive of the relative reactivity in LUMO-controlled cycloaddition reactions, the computed LUMO energies followed this same trend. It was not possible to find a single class of dienophiles that have been reported to participate in Diels–Alder reactions with each of the isomeric parent triazines, rendering a direct experimental comparison difficult. The closest comparison that could be made was in their reactions with enamines, where both 1,2,4-triazine6 and 1,3,5-triazine7 perform much better and more dependably than 1,2,3-triazine. However, here it is not clear whether this is due to their intrinsic reactivity or whether the ensuing slow aromatization step complicates the comparison. What is clear from the computational studies is that 1,2,3-triazine would be expected to participate in effective inverse electron demand Diels–Alder reactions. This was observed most effectively with the C5 substituted 1,2,3-triazines 2 and 3 bearing the conjugating phenyl group and electron-withdrawing methoxycarbonyl group, respectively. Here, the computational studies accurately predict the relative reactivity of the 1,2,3-triazines examined (R = CO2Me > Ph = Br > H), and define substantial differences in the LUMO energies that are of a magnitude that reflect the experimental observations.
Figure 8.
Computational comparisons.
Interestingly and whereas the LUMO coefficients accurately predict the cycloaddition regioselectivity of both 1,2,4-triazine and 1,3,5-triazine, they predict cycloaddition across C5/N2 (vs C4/N1) for 1,2,3-triazine. Additionally, all computational measures of electron density (net atomic charge, Mulliken charge, electrostatic potential charge) indicate C4 is more electron deficient than C5, suggesting it would be the preferential site of nucleophilic attack consistent with qualitative predictions based on preferential subsequent charge delocalization onto nitrogen(s) following addition. As a result, the simple computational studies would suggest that the cycloaddition regioslectivity observed with 1,2,3-triazines is more consistent with stepwise addition-cyclization reactions than concerted cycloaddition reactions. To date, however, we have not isolated reaction byproducts to support this possibility.
Conclusions
Herein, a systematic study of the inverse electron demand Diels–Alder reactions of 1,2,3-triazines is disclosed, including an examination of the impact of 1,2,3-triazine C5 substituents. Such substituents were found to exhibit a remarkable impact on the relative cycloaddition reactivity of the 1,2,3-triazine without altering, and perhaps even enhancing, the intrinsic cycloaddition regioselectivity. The study revealed that not only may the reactivity be predictably modulated by the C5 substituent (R = CO2Me > Ph > H), but that the impact is sufficient to convert 1,2,3-triazine (1) itself and its modest cycloaddition scope into a heterocyclic azadiene system with a reaction scope that portends extensive synthetic utility, substantially expanding the range of participating dienophiles. Significantly, the studies provide a now powerful additional heterocyclic azadiene, complementary to the isomeric 1,2,4- and 1,3,5-triazines, capable of predicable and useful participation in inverse electron demand Diels–Alder reactions, extending the range of complementary heterocyclic ring systems accessible with implementation of the methodology. Such applications in the total synthesis of natural products, complementing our past efforts,21–24 are in progress and will be reported in due course.
Supplementary Material
Acknowledgements
We gratefully acknowledge the financial support of the National Institutes of Health (CA042056) and the Skaggs Institute for Chemical Biology. E.D.A. is a Skaggs Fellow and recipient of a Novartis Graduate fellowship (2010–2011).
Abbreviations
- DME
1,2-dimethoxyethane
- DMF
N,N-dimethylformamide
- Py
pyridine
- TMS
trimethylsilyl
- Trt
trityl or triphenylmethyl
Footnotes
Supporting Information Available. Full experimental details and compound characterizations are provided. This material is available free of charge via the internet at http://pubs.acs.org.
References and Notes
- 1.(a) Boger DL. Tetrahedron. 1983;39:2869. [Google Scholar]; (b) Boger DL. Chem. Rev. 1986;86:781. [Google Scholar]; (c) Boger DL, Weinreb SM. Hetero Diels–Alder Methodology in Organic Synthesis. San Diego: Academic; 1987. [Google Scholar]; (d) Boger DL. Chemtracts: Org. Chem. 1996;9:149. [Google Scholar]
- 2. Carboni RA, Lindsey RV. J. Am. Chem. Soc. 1959;81:4342. For recent examples, see: Raw SA, Taylor RJK. J. Am. Chem. Soc. 2004;126:12260. doi: 10.1021/ja045780g. Benson SC, Lee L, Yang L, Snyder JK. Tetrahedron. 2000;56:1165. Girardot M, Nomak R, Snyder JK. J. Org. Chem. 1998;63:10063. Klindert T, Stroetmann I, Seitz G, Hofner G, Wanner KT, Frenzen G, Eckhoff B. Arch. Pharm. 1997;330:163. doi: 10.1002/ardp.19973300602.
- 3.(a) Panek JS, Zhu B. Tetrahedron Lett. 1996;37:8151. [Google Scholar]; (b) Moisan L, Odermatt S, Gombosuren N, Carella A, Rebek J., Jr Eur. J. Org. Chem. 2008:1673. [Google Scholar]; (c) Volonterio A, Moisan L, Rebek J., Jr Org. Lett. 2007;9:3733. doi: 10.1021/ol701487g. [DOI] [PubMed] [Google Scholar]; (d) Biros SM, Moisan L, Mann E, Carella A, Zhai D, Reed J, Rebek J., Jr Bioorg. Med. Chem. Lett. 2007;17:4641. doi: 10.1016/j.bmcl.2007.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Lahue BR, Lo S-M, Wan Z-K, Woo GHC, Snyder JK. J. Org. Chem. 2004;69:7171. doi: 10.1021/jo040193z. [DOI] [PubMed] [Google Scholar]
- 4.(a) Blackman ML, Royzen M, Fox JM. J. Am. Chem. Soc. 2008;130:13518. doi: 10.1021/ja8053805. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Han H, Devaraj NK, Lee J, Hilderbrand SA, Weissleder R, Bawendi MG. J. Am. Chem. Soc. 2010;132:7838. doi: 10.1021/ja101677r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zibo L, Cai H, Hassink M, Blackman ML, Brown RCD, Conti PS, Fox JM. Chem. Commun. 2010;46:8043. doi: 10.1039/c0cc03078c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Devaraj NK, Upadhyay R, Haun JB, Hilderbrand SA, Weissleder R. Angew. Chem., Int. Ed. 2009;48:7013. doi: 10.1002/anie.200903233. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Devaraj NK, Weissleder, Hilderbrand SA. Bioconjugate Chem. 2008;19:2297. doi: 10.1021/bc8004446. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Schoch J, Weissler M, Jäschke A. J. Am. Chem. Soc. 2010;132:8846. doi: 10.1021/ja102871p. [DOI] [PubMed] [Google Scholar]; (g) Pipkorn R, Waldemar W, Didinger B, Koch M, Mueller G, Wiessler M, Braun K. J. Pept. Sci. 2009;15:235. doi: 10.1002/psc.1108. [DOI] [PubMed] [Google Scholar]; (h) Rossin R, Verkerk PR, van den Bosch SM, Vulders RCM, Verel I, Lub J, Robillard MS. Angew. Chem., Int. Ed. 2010;49:3375. doi: 10.1002/anie.200906294. [DOI] [PubMed] [Google Scholar]; (i) Haun JB, Devaraj NK, Hilderbrand SA, Lee H, Weissleder R. Nat. Nanotech. 2010;5:660. doi: 10.1038/nnano.2010.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Boger DL, Panek JS. Tetrahedron Lett. 1983;24:4511. [Google Scholar]; (b) Boger DL, Panek JS, Coleman RS, Sauer J, Huber FX. J. Org. Chem. 1985;50:5377. [Google Scholar]; (c) Boger DL, Sakya SM. J. Org. Chem. 1988;53:1415. [Google Scholar]; (d) Boger DL, Panek JS, Patel M. Org. Synth. 1992;70:79. [Google Scholar]; (e) Sakya SM, Groskopf KK, Boger DL. Tetrahedron Lett. 1997;38:3805. [Google Scholar]; (f) Boger DL, Schaum RP, Garbaccio RM. J. Org. Chem. 1998;63:6329. doi: 10.1021/jo980795g. [DOI] [PubMed] [Google Scholar]; (g) Soenen DR, Zimpleman JM, Boger DL. J. Org. Chem. 2003;68:3593. doi: 10.1021/jo020713v. [DOI] [PubMed] [Google Scholar]; (h) Hamasaki A, Ducray R, Boger DL. J. Org. Chem. 2006;71:185. doi: 10.1021/jo051832o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Boger DL, Panek JS. J. Org. Chem. 1981;46:2179. [Google Scholar]; (b) Boger DL, Panek JS, Meier MM. J. Org. Chem. 1982;47:895. [Google Scholar]; (c) Boger DL, Panek JS. J. Org. Chem. 1982;47:3763. [Google Scholar]; (d) Boger DL, Panek JS. Tetrahedron Lett. 1984;25:3175. [Google Scholar]; (e) Boger DL, Panek JS, Yasuda M. Org. Synth. 1987;66:142. [Google Scholar]
- 7.(a) Boger DL, Schumacher J, Panek JS, Mullican MD, Patel M. J. Org. Chem. 1982;47:2673. [Google Scholar]; (b) Boger DL, Dang Q. Tetrahedron. 1988;44:3379. [Google Scholar]; (c) Boger DL, Dang Q. J. Org. Chem. 1992;57:1631. [Google Scholar]; (d) Boger DL, Kochanny MJ. J. Org. Chem. 1994;59:4950. [Google Scholar]
- 8.(a) Wilkie GD, Elliott GI, Blagg BSJ, Wolkenberg SE, Soenen DR, Miller MM, Pollack S, Boger DL. J. Am. Chem. Soc. 2002;124:11292. doi: 10.1021/ja027533n. [DOI] [PubMed] [Google Scholar]; (b) Elliott GI, Fuchs JR, Blagg BSJ, Ishikawa H, Tao H, Yuan Z-Q, Boger DL. J. Am. Chem. Soc. 2006;128:10589. doi: 10.1021/ja0612549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Kessler SN, Wegner HA. Org. Lett. 2010;12:4062. doi: 10.1021/ol101701z. [DOI] [PubMed] [Google Scholar]; (b) Boger DL, Coleman RS. J. Org. Chem. 1984;49:2240. [Google Scholar]
- 10.Review: Joshi U, Pipelier M, Naud S, Dubreuil D. Curr. Org. Chem. 2005;9:261. Applications: Bach NJ, Kornfeld EC, Jones ND, Chaney MO, Dorman DE, Paschal JW, Clemens JA, Smalstig EB. J. Med. Chem. 1980;23:481. doi: 10.1021/jm00179a003. Boger DL, Coleman RS, Panek JS, Yohannes D. J. Org. Chem. 1984;49:4405. Boger DL, Baldino CM. J. Org. Chem. 1991;56:6942. Yeung BKS, Boger DL. J. Org. Chem. 2003;68:5249. doi: 10.1021/jo034326c. Daly K, Nomak R, Snyder JK. Tetrahedron Lett. 1997;38:8611. Joshi U, Josse S, Pipeleir M, Chevallier F, Pradere JP, Hazard R, Legoupy S, Huet F, Dubreuil D. Tetrahedron Lett. 2004;45:1031. Muller J, Troschutz R. Synthesis. 2006:1513. Naud S, Pipelier M, Chaumette C, Viault G, Adjou A, Huet F, Legoupy S, Aubertin AM, Evain M, Dubreuil D. Eur. J. Org. Chem. 2007:3296. Moisan L, Odermatt S, Gombosuren N, Carella A, Rebek J., Jr Eur. J. Org. Chem. 2008:1673. Bakkali H, Marie C, Ly A, Thobie-Gautier C, Graton J, Pipelier M, Sengmany S, Leonel E, Medelec JV, Evain M, Dubreuil D. Eur. J. Org. Chem. 2008:2156.
- 11.(a) Boger DL, Kasper AM. J. Am. Chem. Soc. 1989;111:1517. [Google Scholar]; (b) Boger DL, Corbett WL, Wiggins JM. J. Org. Chem. 1990;55:2999. [Google Scholar]; (c) Boger DL, Curran TT. J. Org. Chem. 1990;55:5439. [Google Scholar]; (d) Boger DL, Corbett WL, Curran TT, Kasper AM. J. Am. Chem. Soc. 1991;113:1713. [Google Scholar]; (e) Boger DL, Nakahara S. J. Org. Chem. 1991;56:880. [Google Scholar]; (f) Boger DL, Zhang M. J. Org. Chem. 1992;57:3974. [Google Scholar]; (g) Boger DL, Corbett WL. J. Org. Chem. 1992;57:4777. [Google Scholar]; (h) Boger DL, Corbett WL. J. Org. Chem. 1993;58:2068. [Google Scholar]; (i) Clark RC, Pfeiffer SS, Boger DL. J. Am. Chem. Soc. 2006;128:2587. doi: 10.1021/ja0571646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson ED, Boger DL. Org. Lett. 2011;13:2492. doi: 10.1021/ol2007428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sugita T, Koyama J, Tagahara K, Suzuta Y. Heterocycles. 1985;23:2789. Sugita T, Koyama J, Tagahara K, Suzuta Y. Heterocycles. 1986;24:29. Okatani T, Koyama J, Tagahara K, Suzuta Y. Heterocycles. 1987;26:595. Okatani T, Koyama J, Suzuta Y, Tagahara K. Heterocycles. 1988;27:2213. Okatani T, Koyama J, Tagahara K. Heterocycles. 1989;29:1809. Lewis acid catalysis: Koyama J, Ogura T, Tagahara K. Heterocycles. 1994;38:1595. Microwave heating: Diaz-Oritz A, de la Hoz A, Prieto P, Carrillo JR, Moreno A, Neunhoeffer H. Synlett. 2001;2:236.
- 14.(a) Itoh T, Ohsawa A, Okada M, Kaihoh T, Igeta H. Chem. Pharm. Bull. 1985;33:3050. [Google Scholar]; (b) Itoh T, Okada M, Nagata K, Yamaguchi K, Ohsawa A. Chem. Pharm. Bull. 1990;38:2108. [Google Scholar]
- 15.(a) Neunhoeffer H, Bopp R, Diehl W. Liebigs Ann. Chem. 1993:367. [Google Scholar]; (b) Neunhoeffer H, Clausen M, Voetter H, Ohl H, Krueger C, Angermund K. Liebigs Ann. Chem. 1985:1732. [Google Scholar]; (c) Ohsawa A, Heihachiro A, Itoh T, Kaihoh T, Okada M, Igeta H. J. Org. Chem. 1985;50:5520. [Google Scholar]; (d) Ohsawa A, Heihachiro A, Ohnishi H, Igeta H. J. Chem. Soc., Chem. Commun. 1981:1174. [Google Scholar]; (e) Ohsawa A, Heihachiro A, Ohnishi H, Igeta H. J. Chem. Soc., Chem. Commun. 1980:1182. [Google Scholar]
- 16.Ohsawa A, Kaihoh T, Itoh T, Okada M, Kawabata C, Yamaguchi Y, Igeta H. Chem. Pharm. Bull. 1988;36:3838. [Google Scholar]
- 17.(a) Itoh T, Nagata K, Kaihoh T, Okada M, Kawabata C, Arai H, Ohnishi H, Yamaguchi K, Igeta H, Ohsawa A, Iitaka Y. Heterocycles. 1992;33:631. [Google Scholar]; (b) Mättner M, Neunhoeffer H. Synthesis. 2003:413. [Google Scholar]
- 18.Hynes J, Doubleday WW, Dyckman AJ, Godfrey JD, Grosso JA, Kiau S, Leftheris K. J. Org. Chem. 2004;69:1368. doi: 10.1021/jo035587p. [DOI] [PubMed] [Google Scholar]
- 19.Parlanti L, Discordia RP, Hynes J, Jr, Miller MM, O’Grady HR, Shi Z. Org. Lett. 2007;9:3821. doi: 10.1021/ol701730r. [DOI] [PubMed] [Google Scholar]
- 20.For 43: 1H NMR (CDCl3, 500 MHz) δ 8.19 (s, 1H), 7.34 (s, 1H), 4.20 (q, J = 7.0 Hz, 2H), 3.73 (s, 3H), 3.36 (m, 1H), 2.71 (m, 1H), 2.63 (m, 1H), 2.50 (m, 1H), 2.40 (m, 1H); 13C NMR (CDCl3, 100 MHz) δ 171.6, 171.0, 166.6, 136.1, 110.6, 61.1, 52.1, 37.5, 35.5, 28.5, 14.6; IR vmax 3276, 2952, 1693, 1645, 1440, 1367, 1303, 1254, 1195, 1099, 1032, 914, 765, 639 cm−1; ESI-TOF HRMS m/z 242.1027 ([M + H]+, C11H15NO5 + H+ requires 242.1023). Its immediate intermediate N,O,O-orthoester that hydrolyzes upon SiO2 chromatography was also characterized: 1H NMR (CDCl3, 400 MHz) δ 7.67 (s, 1H), 4.33 (q, J = 7.2 Hz, 2H), 4.14 (q, J = 6.8 Hz, 2H), 3.77 (s, 3H), 3.25 (m, 1H), 2.58–2.20 (m, 4H), 1.35 (t, J = 7.2 Hz, 3H), 1.27 (t, J = 7.2 Hz, 3H); 13C NMR (CDCl3, 100 MHz) δ 171.8, 170.3, 167.5, 146.3, 116.2, 63.4, 61.0, 51.9, 36.2, 30.5, 27.5, 14.6, 14.5.
- 20.Streptonigrin: Boger DL, Panek JS. J. Am. Chem. Soc. 1985;107:5745. Boger DL, Panek JS. J. Org. Chem. 1983;48:621. Lavendamycin: Boger DL, Panek JS, Duff SR, Yasuda M. J. Org. Chem. 1985;50:5790. Boger DL, Duff SR, Panek JS, Yasuda M. J. Org. Chem. 1985;50:5782. PDE I and II: Boger DL, Coleman RS. J. Am. Chem. Soc. 1987;109:2717. Boger DL, Coleman RS. J. Org. Chem. 1986;51:3250. CC-1065: Boger DL, Coleman RS. J. Am. Chem. Soc. 1987;110:1321. Boger DL, Coleman RS. J. Am. Chem. Soc. 1987;110:4796. Trikentrin A: Boger DL, Zhang M. J. Am. Chem. Soc. 1991;113:4230. Pyrimidoblamic acid: Boger DL, Menezes RF, Honda T. Angew. Chem., Int. Ed. Engl. 1993;32:273. P-3A: Boger DL, Honda T, Menezes RF, Colletti SL, Dang Q, Yang W. J. Am. Chem. Soc. 1994;116:82. Bleomycin A2: Boger DL, Honda T. J. Am. Chem. Soc. 1994;116:5647. Boger DL, Colletti SL, Honda T, Menezes RF. J. Am. Chem. Soc. 1994;116:5607. Boger DL, Honda T, Dang Q. J. Am. Chem. Soc. 1994;116:5619. Boger DL, Honda T, Menezes RF, Colletti SL. J. Am. Chem. Soc. 1994;116:5631. Phomazarin: Boger DL, Hong J, Hikota M, Ishida M. J. Am. Chem. Soc. 1999;121:2471. Anhydrolycorinone: Boger DL, Wolkenberg SE. J. Org. Chem. 2000;65:9120. doi: 10.1021/jo0012546. Boger DL, Wolkenberg SE. J. Org. Chem. 2002;67:7361. doi: 10.1021/jo020437k. Minovine: Yuan Q, Ishikawa H, Boger DL. Org. Lett. 2005;7:741. doi: 10.1021/ol050017s. Vindorosine: Elliott GI, Velcicky J, Ishikawa H, Li Y, Boger DL. Angew. Chem., Int. Ed. 2006;45:620. doi: 10.1002/anie.200503024. Vindoline: Choi Y, Ishikawa H, Velcicky J, Elliott GI, Miller MM, Boger DL. Org. Lett. 2005;7:4539. doi: 10.1021/ol051975x. Ishikawa H, Elliott GI, Velcicky J, Choi Y, Boger DL. J. Am. Chem. Soc. 2006;128:10596. doi: 10.1021/ja061256t. Vinblastine and vincristine: Ishikawa H, Colby DA, Seto S, Va P, Tam A, Kakei H, Rayl TJ, Hwang I, Boger DL. J. Am. Chem. Soc. 2009;131:4904. doi: 10.1021/ja809842b. Ishikawa H, Colby DA, Boger DL. J. Am. Chem. Soc. 2008;130:420. doi: 10.1021/ja078192m. Fendleridine and N-acetylaspidoalbidine: Campbell EL, Zuhl AM, Liu CM, Boger DL. J. Am. Chem. Soc. 2010;132:3009. doi: 10.1021/ja908819q. Vindoline and vindorosine: Kato D, Sasaki Y, Boger DL. J. Am. Chem. Soc. 2010;132:3685. doi: 10.1021/ja910695e. Sasaki Y, Kato D, Boger DL. J. Am. Chem. Soc. 2010;132:13533. doi: 10.1021/ja106284s.
- 21.Prodigiosin: Boger DL, Patel M. Tetrahedron Lett. 1987;28:2499. Boger DL, Patel M. J. Org. Chem. 1988;53:1405. Isochrysohermidin: Boger DL, Baldino CM. J. Am. Chem. Soc. 1993;115:11418. Ningalin A, lamellarin O, lukianol A, and storniamide A: Boger DL, Boyce CW, Labroli MA, Sehon CA, Jin Q. J. Am. Chem. Soc. 1999;121:54. Ningalin B: Boger DL, Soenen DR, Boyce CW, Hedrick MP, Jin Q. J. Org. Chem. 2000;65:2479. doi: 10.1021/jo9916535. Roseophilin: Boger DL, Hong J. J. Am. Chem. Soc. 2001;123:8515. doi: 10.1021/ja011271s. Ningalin D: Hamasaki A, Zimpleman JM, Hwang I, Boger DL. J. Am. Chem. Soc. 2005;127:10767. doi: 10.1021/ja0526416. Lycogarubin C and lycogalic acid: Oakdale JS, Boger DL. Org. Lett. 2010;12:1132. doi: 10.1021/ol100146b.
- 22.Streptonigrone: Boger DL, Cassidy KC, Nakahara S. J. Am. Chem. Soc. 1993;115:10733. Fredericamycin: Boger DL, Hüter O, Mbiya K, Zhang M. J. Am. Chem. Soc. 1995;117:11839. Nothapodytine B and mappicine: Boger DL, Hong J. J. Am. Chem. Soc. 1998;120:1218. Rubrolone: Boger DL, Ichikawa S, Jiang H. J. Am. Chem. Soc. 2000;122 Camptothecin: Blagg BSJ, Boger DL. Tetrahedron. 2002;58:6343. Piericidins: Schnermann MJ, Boger DL. J. Am. Chem. Soc. 2005;127:15704. doi: 10.1021/ja055041f. Schnermann MJ, Romero FA, Hwang I, Nakamaru-Ogiso E, Yagi T, Boger DL. J. Am. Chem. Soc. 2006;128:11799. doi: 10.1021/ja0632862.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.