Abstract
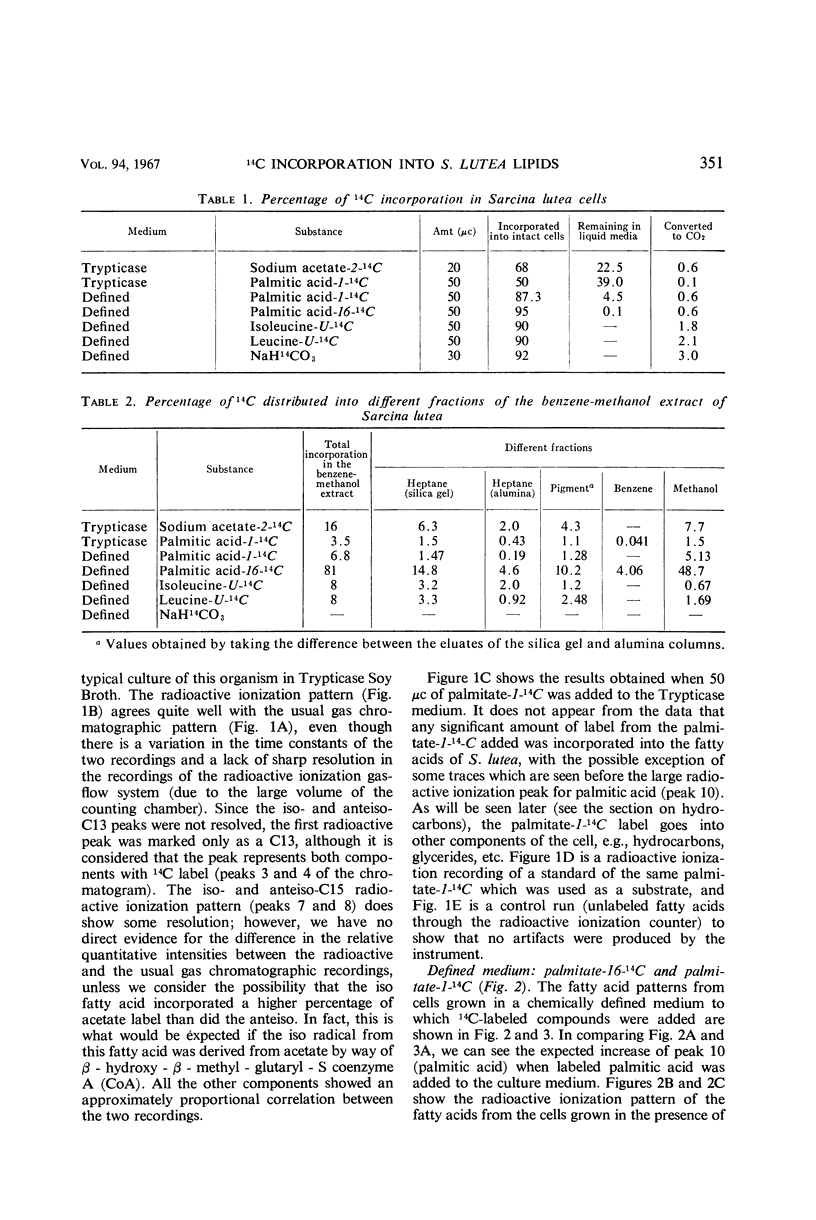
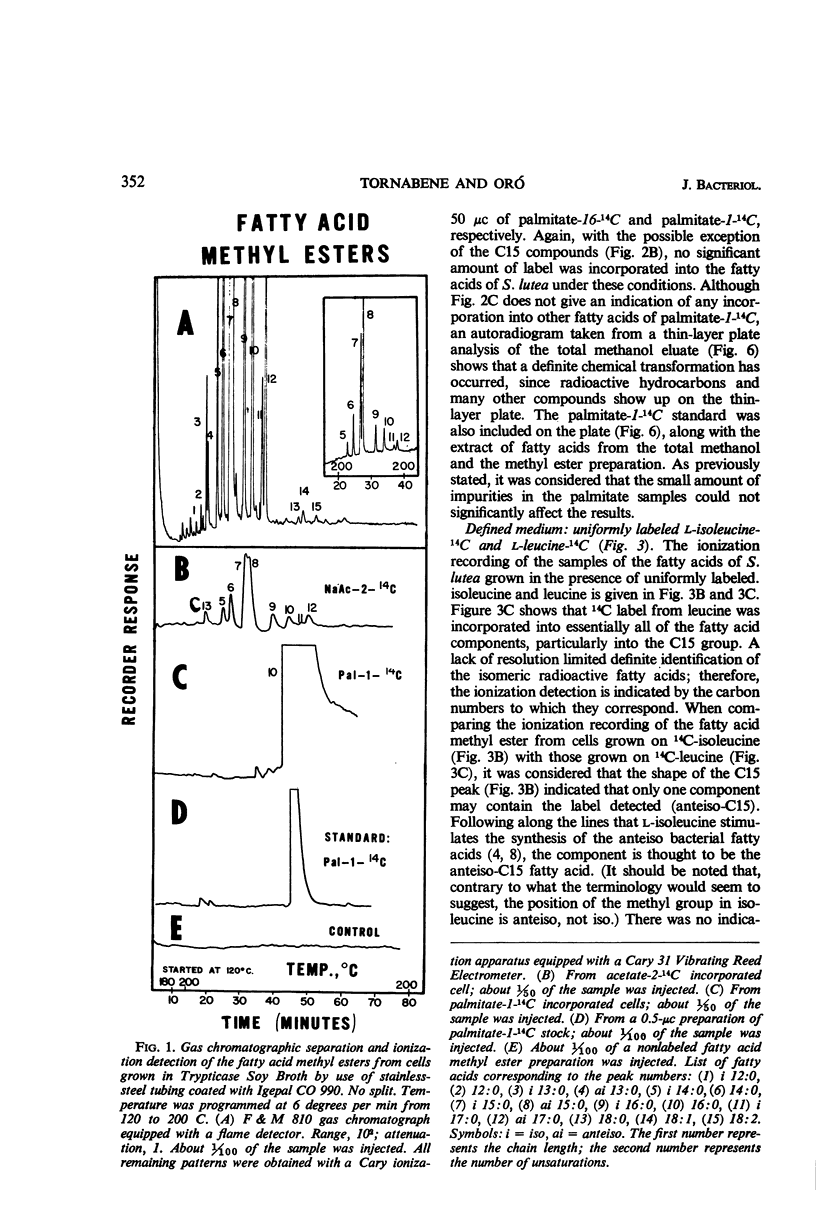
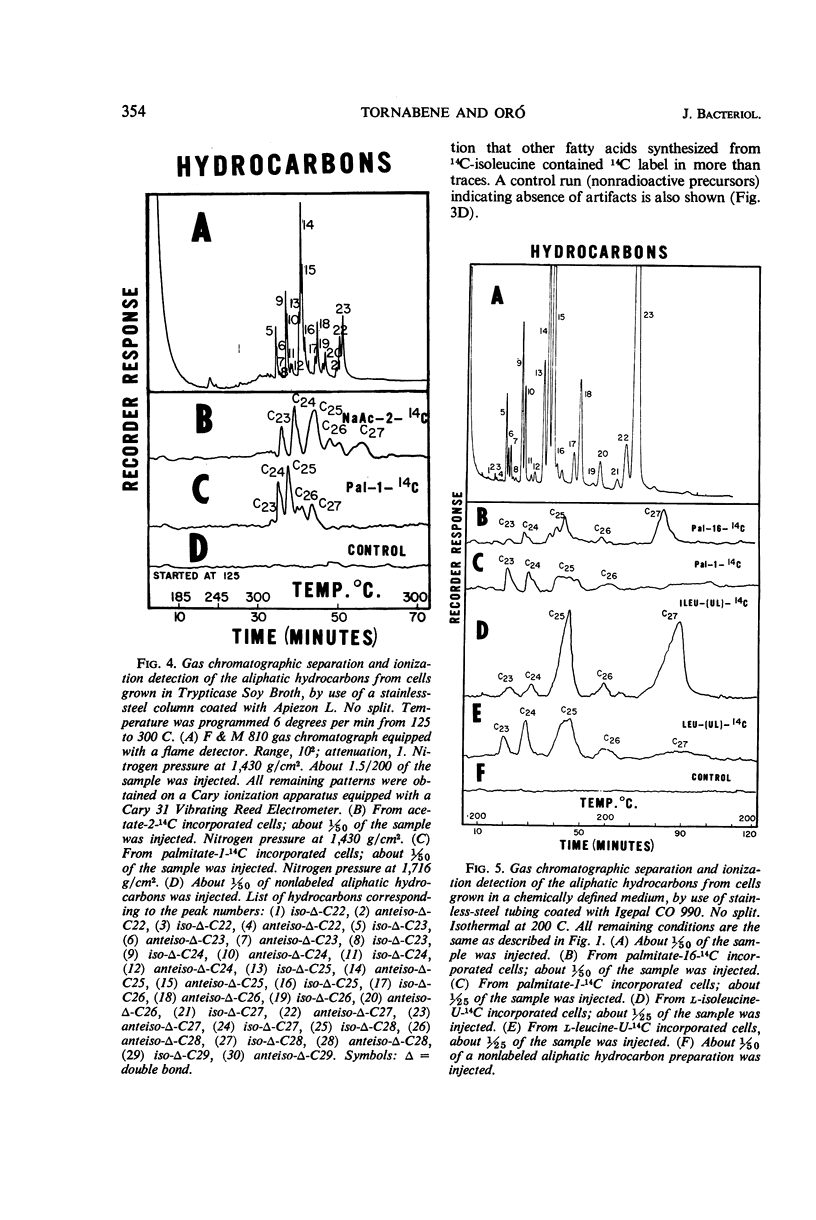
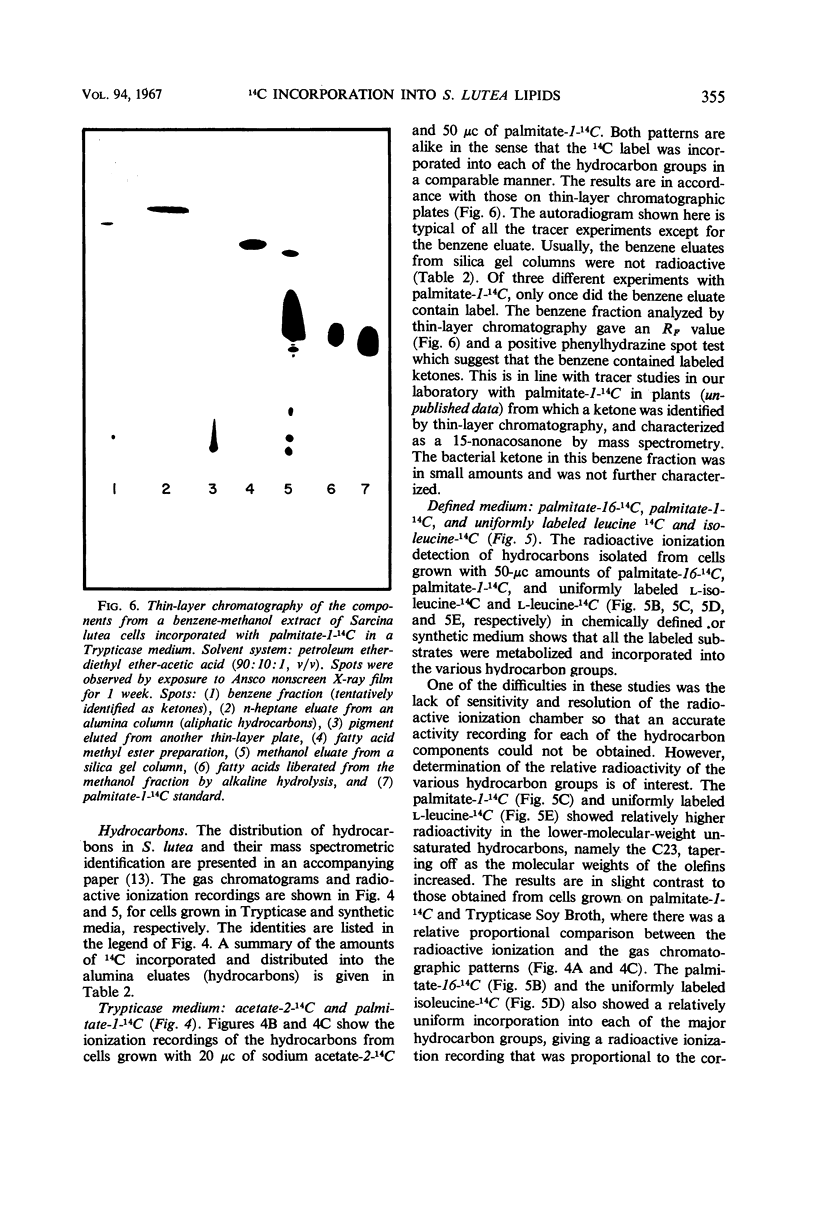
An initial investigation into the mechanism of hydrocarbon biosynthesis in Sarcina lutea was performed by measuring the amounts of 14C incorporated into the hydrocarbons and fatty acids by use of a combination gas chromatograph and high-temperature gas-flow ionization apparatus. Uniformly labeled l-isoleucine-14C was predominantly incorporated into the anteiso-branched chains. Palmitate-16-14C gave evidence that a direct correlation may exist between the nonpolar end of the palmitate and the biosynthesis of hydrocarbons and carotenoids. The label from palmitate-1-14C was incorporated into the various hydrocarbon groups as a compound, derived from the polar end of the palmitate, consisting of more than two carbon atoms. Palmitate-16-14C and -1-14C gave no detectable evidence that transformed products were incorporated into other fatty acids. Sodium acetate-2-14C and uniformly labeled l-leucine-14C gave evidence of a nonspecific incorporation into both the aliphatic hydrocarbons and fatty acids of Sarcina lutea.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBRO P. W., HUSTON C. K. LIPIDS OF SARCINA LUTEA. II. HYDROCARBON CONTENT OF THE LIPID EXTRACTS. J Bacteriol. 1964 Oct;88:981–986. doi: 10.21236/ad0437968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUSTON C. K., ALBRO P. W. LIPIDS OF SARCINA LUTEA. I. FATTY ACID COMPOSITION OF THE EXTRACTABLE LIPIDS. J Bacteriol. 1964 Aug;88:425–432. doi: 10.1128/jb.88.2.425-432.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen S. L. Biosynthesis and function of carotenoid pigments in microorganisms. Annu Rev Microbiol. 1965;19:163–182. doi: 10.1146/annurev.mi.19.100165.001115. [DOI] [PubMed] [Google Scholar]
- Kaneda T. Biosynthesis of branched-chain fatty acids. IV. Factors affecting relative abundance of fatty acids produced by Bacillus subtilis. Can J Microbiol. 1966 Jun;12(3):501–514. doi: 10.1139/m66-073. [DOI] [PubMed] [Google Scholar]
- Kaneda T. Biosynthesis of branched-chain fatty acids. V. Microbial stereospecific syntheses of D-12-methyltetradecanoic and D-14-methylhexadecanoic acids. Biochim Biophys Acta. 1966 Aug 3;125(1):43–54. doi: 10.1016/0005-2760(66)90142-1. [DOI] [PubMed] [Google Scholar]
- Kolattukudy P. E. Biosynthesis of wax in Brassica oleracea. Relation of fatty acids to wax. Biochemistry. 1966 Jul;5(7):2265–2275. doi: 10.1021/bi00871a015. [DOI] [PubMed] [Google Scholar]
- LENNARZ W. J. The role of isoleucine in the biosynthesis of branched-chain fatty acids by Micrococcus lysodeikticus. Biochem Biophys Res Commun. 1961 Nov 1;6:112–116. doi: 10.1016/0006-291x(61)90395-3. [DOI] [PubMed] [Google Scholar]
- Oró J., Tornabene T. G., Nooner D. W., Gelpi E. Aliphatic hydrocarbons and fatty acids of some marine and freshwater microorganisms. J Bacteriol. 1967 Jun;93(6):1811–1818. doi: 10.1128/jb.93.6.1811-1818.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornabene T. G., Bennett E. O., Oró J. Fatty acid and aliphatic hydrocarbon composition of Sarcina lutea grown in three different media. J Bacteriol. 1967 Aug;94(2):344–348. doi: 10.1002/path.1700940212. [DOI] [PMC free article] [PubMed] [Google Scholar]


