Abstract
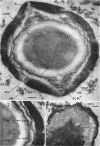
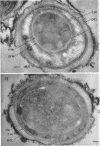
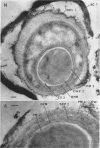
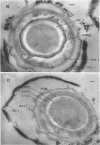
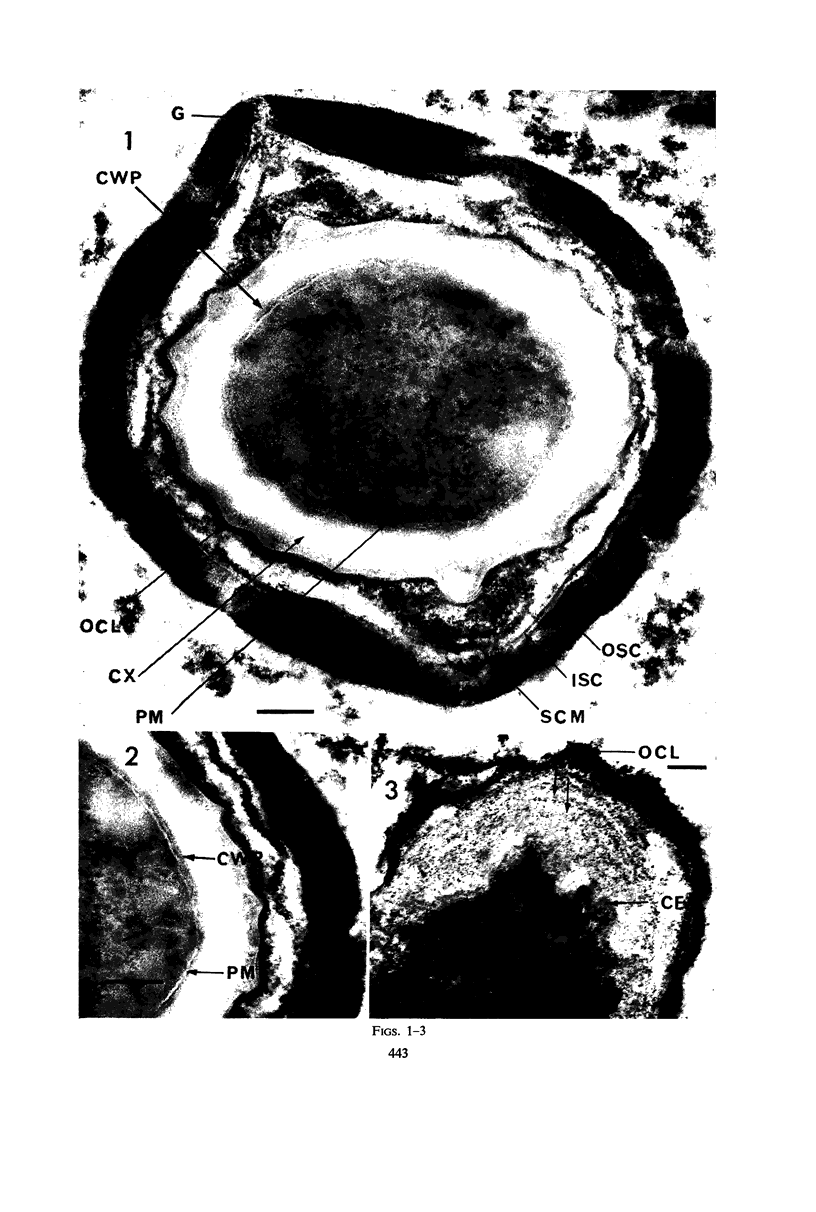
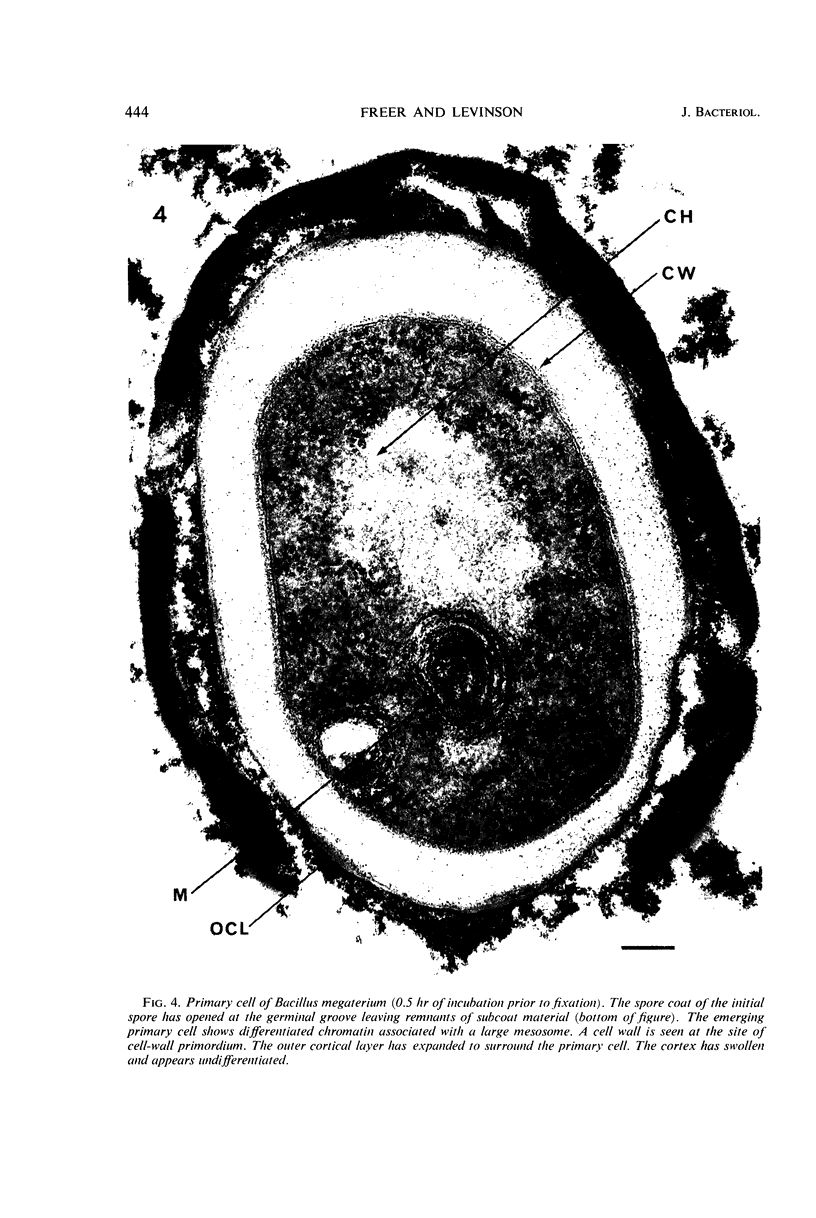
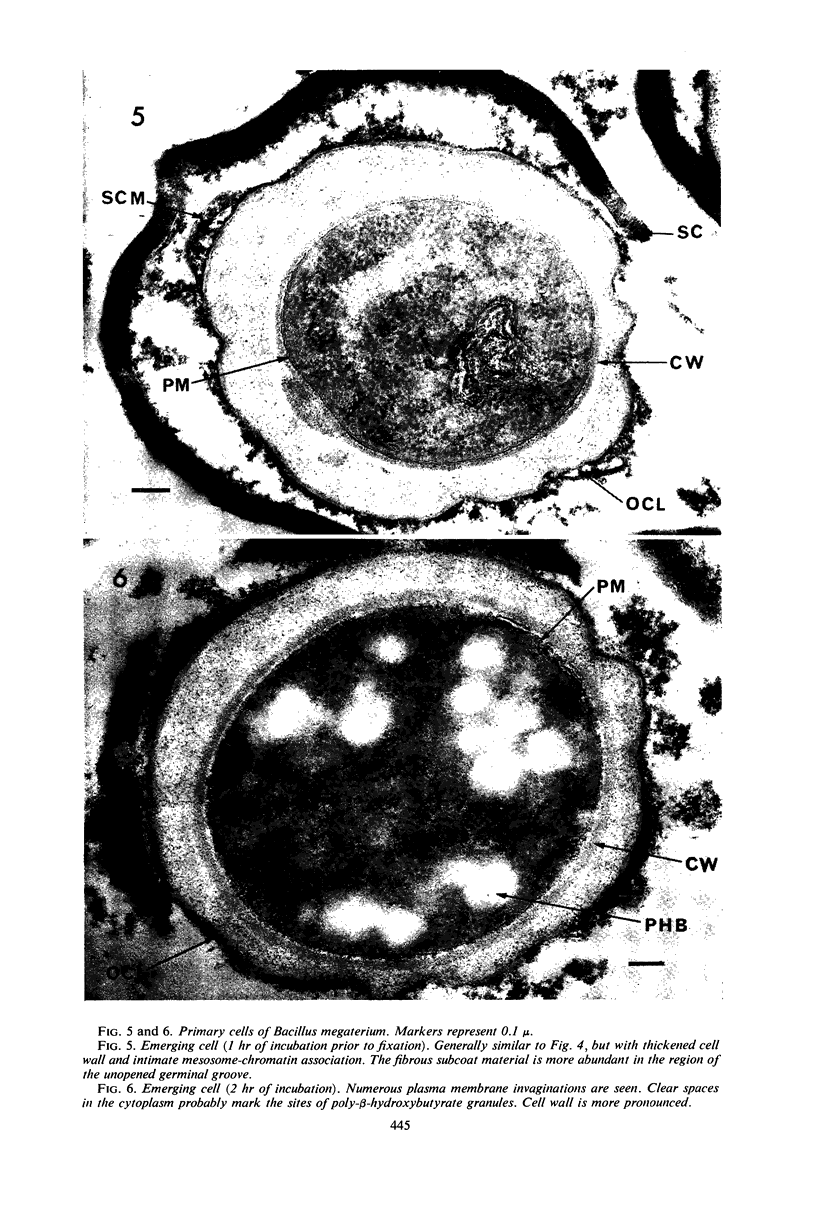
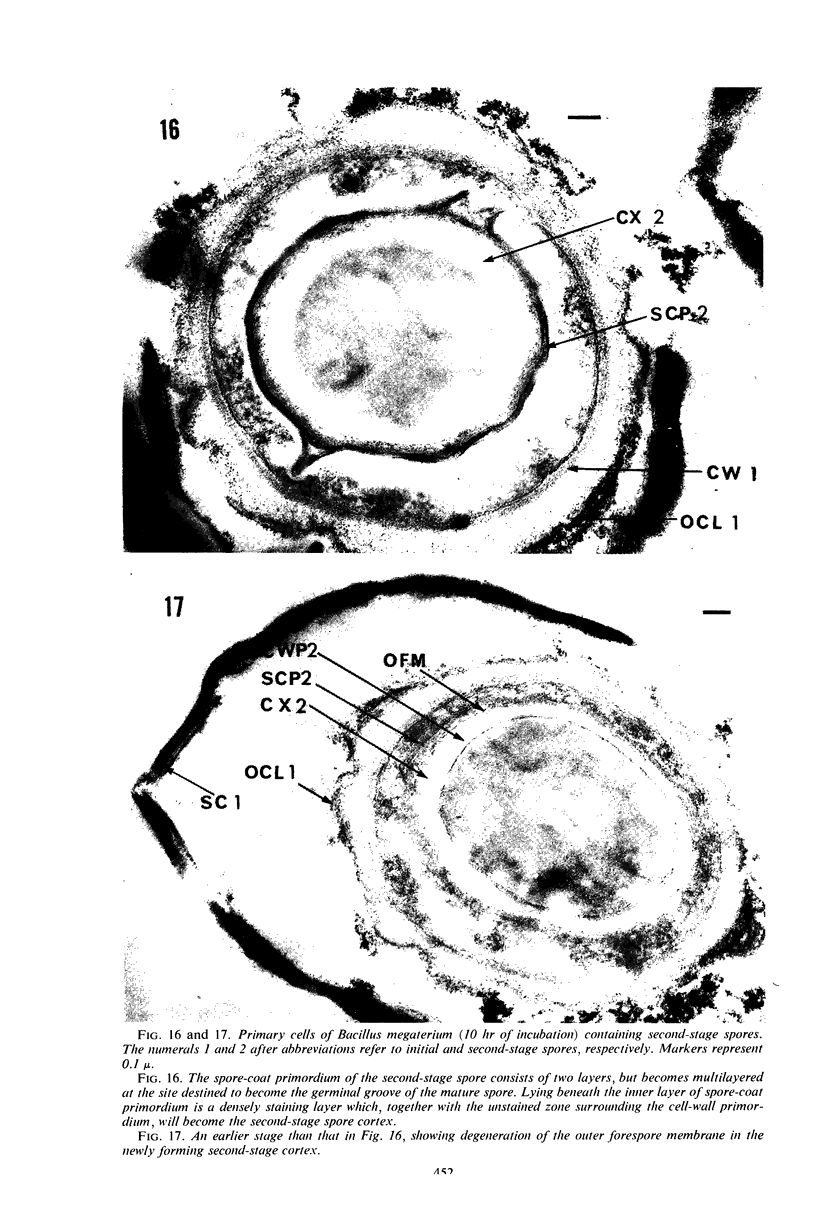
Ultrathin sections were prepared from cultures of Bacillus megaterium QM B1551 undergoing microcycle sporogenesis (initial spore to primary cell to second-stage spore without intervening cell division) on a chemically defined medium. The cytoplasmic core of the dormant spore was surrounded by plasma membrane, cell-wall primordium, cortex, outer cortical layer, and spore coats. Early in the cycle, the coat opened at the germinal groove, the cortex swelled, ribosomes and a chromatinic area associated with large mesosomes (which may later be incorporated into the expanding plasma membrane) appeared in the core, and the cell wall became defined at the site of the cell wall primordium. Poly-β-hydroxybutyrate granules began to appear in the primary cell at about 3 hr. By 7 hr, the forespore of the second-stage spore was delineated by typical double membranes. Between 7 and 12 hr, second-stage cell-wall primordium and cortex developed between the separating forespore membranes. The inner membrane became the plasma membrane of the second-stage spore, and the outer membrane eventually disintegrated within the second-stage spore cortex. A densely staining double layer (spore-coat primordium) developed external to the outer forespore membrane. The inner spore coat and the outer cortical layer of the second-stage spore developed from this primordium. The outer part of the spore coat, probably of sporangial origin, was laid down on the external surface of the inner spore coat. By 12 hr, second-stage spores were almost mature. By 20 hr, the mature endospores, with a thickened outer coat, were often still enclosed by degenerate primary cell wall and by the outer cortical layer and spore coat of the initial spore.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson A. Membrane-bound messenger RNA and polysomes in sporulating bacteria. J Mol Biol. 1965 Aug;13(1):92–104. doi: 10.1016/s0022-2836(65)80082-1. [DOI] [PubMed] [Google Scholar]
- CALLAHAN W. P., HORNER J. A. THE USE OF VANADIUM AS A STAIN FOR ELECTRON MICROSCOPY. J Cell Biol. 1964 Feb;20:350–356. doi: 10.1083/jcb.20.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellar D. J., Lundgren D. G. Fine structure of sporulation in Bacillus cereus grown in a chemically defined medium. J Bacteriol. 1966 Dec;92(6):1748–1764. doi: 10.1128/jb.92.6.1748-1764.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZ-JAMES P. C. Participation of the cytoplasmic membrane in the growth and spore fromation of bacilli. J Biophys Biochem Cytol. 1960 Oct;8:507–528. doi: 10.1083/jcb.8.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZ-JAMES P. C. SPORULATION IN PROTOPLASTS AND ITS DEPENDENCE ON PRIOR FORESPORE DEVELOPMENT. J Bacteriol. 1964 Mar;87:667–675. doi: 10.1128/jb.87.3.667-675.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZ-JAMES P. FATE OF THE MESOSOMES OF BACILLUS MEGATERIUM DURING PROTOPLASTING. J Bacteriol. 1964 Jun;87:1483–1491. doi: 10.1128/jb.87.6.1483-1491.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz-James P. C. MORPHOLOGY OF SPORE DEVELOPMENT IN CLOSTRIDIUM PECTINOVORUM. J Bacteriol. 1962 Jul;84(1):104–114. doi: 10.1128/jb.84.1.104-114.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLAUERT A. M., GLAUERT R. H. Araldite as an embedding medium for electron microscopy. J Biophys Biochem Cytol. 1958 Mar 25;4(2):191–194. doi: 10.1083/jcb.4.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASHIMOTO T., BLACK S. H., GERHARDT P. Development of fine structure, thermostability, and dipicolinate during sporogenesis in a bacillus. Can J Microbiol. 1960 Apr;6:203–212. doi: 10.1139/m60-022. [DOI] [PubMed] [Google Scholar]
- Holmes P. K., Levinson H. S. Metabolic requirements for microcycle sporogenesis of Bacillus megaterium. J Bacteriol. 1967 Aug;94(2):434–440. doi: 10.1128/jb.94.2.434-440.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F., BRENNER S. [On the regulation of DNA synthesis in bacteria: the hypothesis of the replicon]. C R Hebd Seances Acad Sci. 1963 Jan 2;256:298–300. [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINSON H. S., HYATT M. T. EFFECT OF SPORULATION MEDIUM ON HEAT RESISTANCE, CHEMICAL COMPOSITION, AND GERMINATION OF BACILLUS MEGATERIUM SPORES. J Bacteriol. 1964 Apr;87:876–886. doi: 10.1128/jb.87.4.876-886.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson H. S., Wrigley A. S. Spore Germination and Emergence of Bacillus megaterium. Science. 1960 May 6;131(3410):1382–1382. doi: 10.1126/science.131.3410.1382. [DOI] [PubMed] [Google Scholar]
- Moberly B. J., Shafa F., Gerhardt P. Structural details of anthrax spores during stages of transformation into vegetative cells. J Bacteriol. 1966 Jul;92(1):220–228. doi: 10.1128/jb.92.1.220-228.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OHYE D. F., MURRELL W. G. Formation and structure of the spore of Bacillus coagulans. J Cell Biol. 1962 Jul;14:111–123. doi: 10.1083/jcb.14.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYTER A., LANDMAN O. E. ELECTRON MICROSCOPE STUDY OF THE RELATIONSHIP BETWEEN MESOSOME LOSS AND THE STABLE L STATE (OR PROTOPLAST STATE) IN BACILLUS SUBTILIS. J Bacteriol. 1964 Aug;88:457–467. doi: 10.1128/jb.88.2.457-467.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rode L. J., Williams M. G. Utility of sodium hypochlorite for ultrastructure study of bacterial spore integuments. J Bacteriol. 1966 Dec;92(6):1772–1778. doi: 10.1128/jb.92.6.1772-1778.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau M., Fléchon J., Hermier J. Etude au microscope électronique de la germination de la spore chex Bacillus subtilis. Ann Inst Pasteur (Paris) 1966 Aug;111(2):149–160. [PubMed] [Google Scholar]
- Vinter V., Slepecky R. A. Direct Transition of Outgrowing Bacterial Spores to New Sporangia Without Intermediate Cell Division. J Bacteriol. 1965 Sep;90(3):803–807. doi: 10.1128/jb.90.3.803-807.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARTH A. D., OHYE D. F., MURRELL W. G. Location and composition of spore mucopeptide in Bacillus species. J Cell Biol. 1963 Mar;16:593–609. doi: 10.1083/jcb.16.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARTH A. D., OHYE D. F., MURRELL W. G. The composition and structure of bacterial spores. J Cell Biol. 1963 Mar;16:579–592. doi: 10.1083/jcb.16.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]