Summary
CO2 present in exhaled air is the most important sensory cue for female blood-feeding mosquitoes, causing activation of long-distance host-seeking flight, navigation towards the vertebrate host1, and, in the case of Aedes aegypti, increased sensitivity to skin odours2. The CO2 detection machinery is therefore an ideal target to disrupt host seeking. We use electrophysiological assays to identify a volatile odourant that causes an unusual, ultra-prolonged activation of CO2-detecting neurons in three major disease-transmitting mosquitoes: Anopheles gambiae, Culex quinquefasciatus and A. aegypti. Importantly ultra-prolonged activation of this neuron severely compromises its ability to subsequently detect CO2 for several minutes. We also identify odours that strongly inhibit the CO2-sensitive neuron as candidates for use in disruption of host-seeking behaviour, as well as an odour that evokes CO2-like activity and thus has potential use as a lure in trapping devices. Analysis of responses to panels of structurally related odours across the three mosquitoes and Drosophila, which have related CO2-receptor proteins, reveals a pattern of inhibition that is often conserved. We use video tracking in wind-tunnel experiments to demonstrate that the novel ultra-prolonged activators can completely disrupt CO2-mediated activation as well as source-finding behaviour in Aedes mosquitoes, even after the odour is no longer present. Finally, semi-field studies demonstrate that use of ultra-prolonged activators disrupts CO2-mediated hut entry behaviour of Culex mosquitoes. The three classes of CO2 response modifying odours offer powerful instruments for developing new generations of insect repellents and lures, which even in small quantities can interfere with the ability of mosquitoes to seek humans.
Text
Each year A. gambiae, A. aegypti and C. quinquefasciatus mosquitoes transmit deadly diseases such as malaria and dengue to >half a billion people and cause millions of deaths. Female mosquitoes need to find and obtain a blood meal from an infected person and subsequently find an uninfected human-host to bite. Disruption of host-seeking in mosquitoes could therefore reduce incidence of both steps.
Female mosquitoes depend primarily on olfactory cues that are emitted from human breath, skin and sweat to find hosts3. Yet very few strategies that target the mosquito olfactory system, like the effective repellent DEET (N,N-Diethyl-m-toluamide)4,5, have emerged. However, the relatively high cost of DEET, and need for repeated application to the skin at high concentrations (10-70%) precludes its use in tropical countries. DEET is also known to melt plastics6, and to block cation channels4 and cholinesterase activity in mammals7..Moreover, DEET resistance has been observed in Drosophila melanogaster8 Anopheles albimanus9 and A. aegypti10, which reiterates a need to identify additional classes of repellents.
An ideal target for disrupting host-seeking is the carbon dioxide (CO2) detection machinery. CO2 exhaled by humans is a vital cue for mosquitoes. Female mosquitoes are exquisitely sensitive to minute changes in CO2 concentration11, which they potentially sense from long distances1,12. Upon encountering a plume of CO2, a female mosquito orients upwind using optomotor anemotaxis, while maintaining contact with the host-odour plume13. Remarkably, CO2 exposure also increases the sensitivity of A. aegypti to human skin odours2.
CO2 is detected by heteromeric receptor proteins that are highly conserved across dipterans including the three vector mosquitoes studied here and Drosophila14 (Supplemental Figure 1a,b), and are expressed in a dedicated labelled-line CO2-detection circuit15-18. This circuit provides means to disrupt key aspects of host-seeking behaviour in multiple species. Previously we identified odorants that inhibit electrophysiological and behavioural responses to CO2 in D. melanogaster, two of which also inhibited the CO2 response in C. quinquefasciatus mosquitoes19.
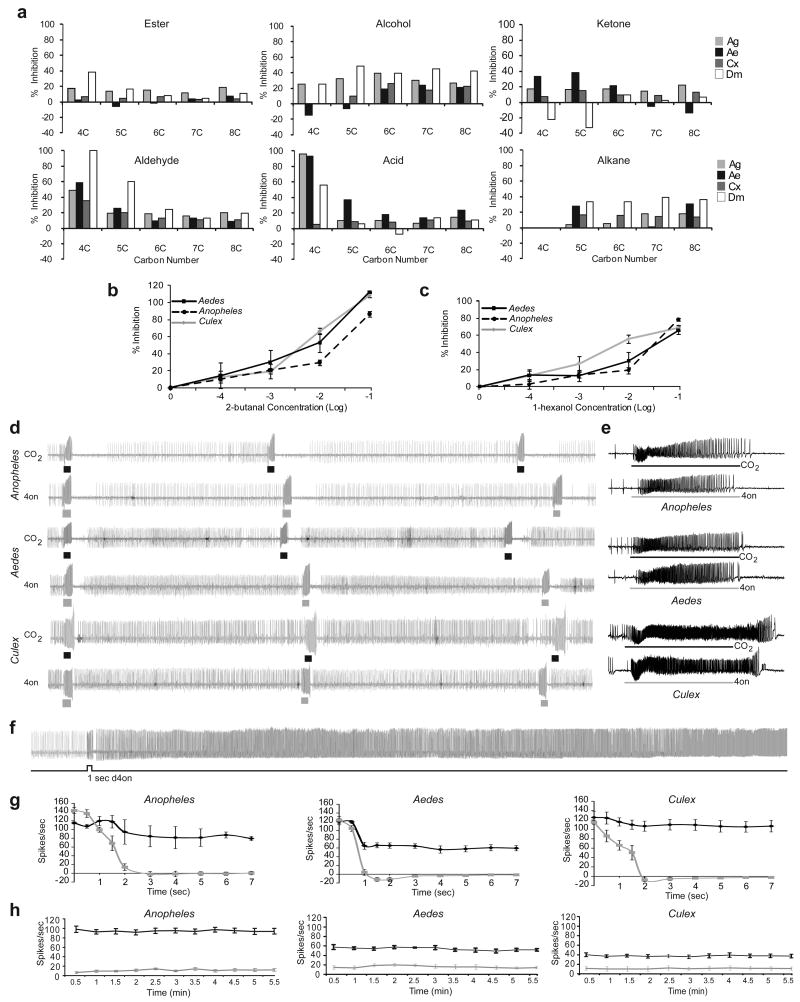
To identify more effective and across-species inhibitors of the conserved CO2 receptors14, we screened a panel of compounds structurally related to the known inhibitors using single-sensillum electrophysiology on A. gambiae, A. aegypti and C. quinquefasciatus females, and identified several efficient inhibitors for each (Figure 1a). The effects of some compounds were conserved across 250 MY of evolution20 while others were species-specific. Subtle changes in structure influenced the strength of inhibition (Figure 1a), but volatility or solubility did not appear to be as important (Supplemental Figure 2). Inhibition was dose-dependent (Figure 1b,c) and the temporal dynamics of inhibition were odour-dependent (Supplemental Figure 1b).
Figure 1. Odourants that dramatically change responses of the CO2-sensitive neuron in mosquitoes.
a, Comparison of percentage of CO2 response inhibition in the cpA neuron of A. gambiae (Ag), A. aegypti (Ae), C. quinquefasciatus (Cx), and D. melanogaster (Dm). Functional group is on the primary carbon atom except for ketones on C2, and the length of the carbon chain (carbon number) is indicated. A 1-sec stimulus of vapours from odourant diluted 10-2 applied on cotton-wool in cartridge, is overlaid on a 3-sec stimulus of 0.15% CO2. n=3. Data for Dm responses, using 0.33% CO2, is taken from19. b,c Dose-response of indicated inhibitors in the three species, in a similar overlay assay. n=5, error bars=s.e.m. d, Representative long-term recordings from the cpA neuron in response to repeated 1-sec pulses of CO2 (0.15%; black square) or 2-butanone (4on; 10-1; grey square).e, Expanded view of the traces for each during the stimulus period in the centre in d. f, Representative trace of A. aegypti peg sensillum to a 1-sec stimulus of 2,3-butanedione (10-1). g, Mean responses of the cpA neuron to 1-sec 2,3-butanedione (black line) or 0.15% CO2 (grey line) on Anopheles, Aedes, and Culex over 7 seconds. n=4, error bars =s.e.m. h, Mean baseline activity of the cpA neuron counted every 30-sec interval after pre-exposure to a 3-sec stimulus of 2,3-butanedione (10-1; black line) or paraffin oil (grey line). n=4, error bars =s.e.m. Baseline activity prior to initial stimulus subtracted in g and h.
Most trapping devices use CO2 to attract mosquitoes. Unfortunately, these are rarely used for surveillance or control in tropical countries because of high costs and difficulties in supplying CO2 via gas cylinders, dry ice, or propane combustion. Using a parallel electrophysiology screen we identified 2-butanone as a dose-dependent activator of the cpA neuron in all three mosquito species (Supplemental Figure 3a,b). Repeated stimuli of 2-butanone showed a temporal pattern of cpA neuron activation indistinguishable from that elicited by CO2 (Figure 1d,e). Such odours that mimic CO2 activity, are ideal candidates for formulating convenient, compact, and economical lures for use in trap-based mosquito control in developing countries.
In the electrophysiology screen we also identified an additional structurally related odourant, 2,3-butanedione, which activates the cpA neuron in the three mosquito species (Supplemental Figure 4a). 2,3-butanedione exhibits an unusual property: exposure to a brief 1-sec pulse results in ultra-prolonged activation of the cpA neuron (Figure 1f,g,h). The activity of the cpA neuron remained high for the entire 5 ½-minute recording period. In contrast, response to a comparable CO2 stimulus rapidly decays immediately after the end of stimulus (Figure 1g,h). Olfactory receptor neurons (ORNs) that express Or genes rarely show this property and rapidly adapt in the first second upon stimulus exposure, even with a continuous stimulus21. Not much has been reported quantitatively on temporal response termination kinetics for single odorants beyond two seconds post stimulus22. An individual neuron trace of a moth to a plant volatile suggests activation beyond the stimulus period23. In another example an ORN of a male moth showed activity for >10 minutes following a 1-sec pulse of a pheromone analog, but only with an increase of ∼3 spikes/sec24.
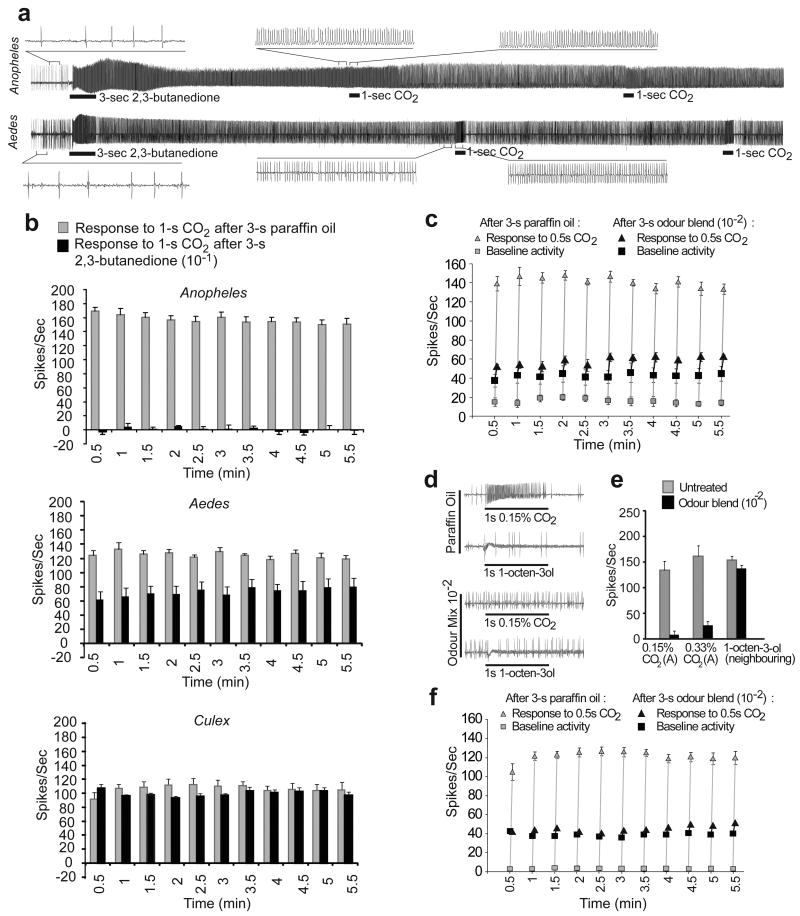
Most importantly, the brief 2,3-butanedione exposure evoked such a strong prolonged response in cpA neurons of A. gambiae and A. aegypti that responses to subsequent CO2 stimuli during the 5.5-min recordings were severely reduced, displaying little activation above the high baseline firing of the neuron (Figure 2a,b). This property is neither enhanced nor reversed efficiently by a subsequent intervening stimulus of another activator, or inhibitors (Supplemental Figure 4b,c) suggesting that an active state, perhaps of the receptor, G- protein signalling25, or OBP may be stabilized by 2,3-butanedione.
Figure 2. Ultra-prolonged activation disrupts ability to respond to CO2.
a, Representative traces of long-term recording from the cpA neuron in A. gambiae and A. aegypti response to a 3-sec stimulus of 2,3-butandione (10-1) followed by two 1-sec pulses of CO2 (0.15%). Expanded views of the indicated regions of the traces are shown. b, Mean increase in frequency of response of the cpA neuron to repeated stimuli of 1-sec CO2 (0.15%) applied approx. every 30-sec, following a 3-sec pre-exposure to 2,3-butanedione (10-1; black bars) or paraffin oil (grey bars) in A. gambiae, A. aegypti and C. quinquefasciatus. c, Mean baseline activity (square) and increase of A. aegypti cpA (triangle) in responses to 1-sec CO2 (0.15%) pulses applied approx. every 30-sec following a 3-sec pre-exposure to a 4-odour blend of 2,3-butanedione, 1-butanal, 1-pentanal, and 1-hexanol (10-2)(black) or paraffin oil (grey). d, Representative traces from peg sensillum and e, mean responses from the cpA neurons to CO2 (0.15%) and the cpB neuron to 1-octen-3-ol (10-3) with a pretreatment to 3-min of paraffin oil or 4-odour blend. f, Responses of cpA neuron in C. quinquefasciatus treated identically as in c. For b,c,e and f, n=5, error bars=s.e.m.
To find more effective long-term maskers we tested multiple combinations of CO2 response-modifying odours and identified a blend of four odourants, 2,3-butanedione, 1-hexanol, 1-butanal and 1-pentanal, which is effective at a 10-fold lower dilution (10-2). This blend caused prolonged activation and rendered the A. aegypti cpA neuron unresponsive to CO2 stimuli throughout the 5.5 minute duration of the assay (Figure 2c, Supplemental Figure 4d,e,5). The effect of the “ultra-prolonged” odour(s) is specific to the CO2-sensitive cpA neuron since the response of a neighbouring neuron, with a smaller spike amplitude to 1-octen-3-ol, was not affected (Figure 2 d,e). The same “ultra-prolonged” activating blend was also highly effective on the C. quinquefasciatus cpA neuron (Figure 2f).
Aedes females show rapid (<200msec) and robust responses to entering (turn upwind) and exiting (turn crosswind) a plume of CO2, both important for source finding2. In fact the cpA-neuron displays an exquisite temporal resolution of the presence or absence of above-baseline concentrations of CO218 (Figure 1d) as required for a system whose sensory input plays a key role in the control of flight behaviour. This form of rapid odour-evoked flight modulation (turning and surging) in insects26,27 involves both odour detection and discrimination dependent upon moment-to-moment changes in olfactory neuron activity27. Higher order projection neuron circuits are also particularly sensitive to the onset of odour stimuli28. Thus, an “ultra-prolonged activator” that compromises the ability of the sensory neuron to detect rapid changes in CO2 concentration could disrupt CO2-mediated attraction behaviour very effectively.
To test the effect of ultra-prolonged activators on behavioural attraction to CO2, mated A. aegypti females were pre-treated with odourant(s) vapours and tested in a wind tunnel (Figure 3a,b). Mock-treated A. aegypti females that were pre-exposed to solvent alone all left the holding cage, crossed the half-way point of the tunnel (Figure 3c) and navigated successfully along a turbulent CO2 plume and flew through the CO2-emitting ring (Figure 3d). In contrast, females pre-exposed to the “ultra-prolonged” odour blend were impaired in their ability to fly upwind (Figure 3c) as well as in finding the CO2 source, in a dose and time-dependent manner (Figure 3d,e,f, Supplemental Videos 1-3).
Figure 3. Exposure to ultra-prolonged activator causes long-term disruption of CO2-mediated attraction behaviour of female Aedes mosquitoes.
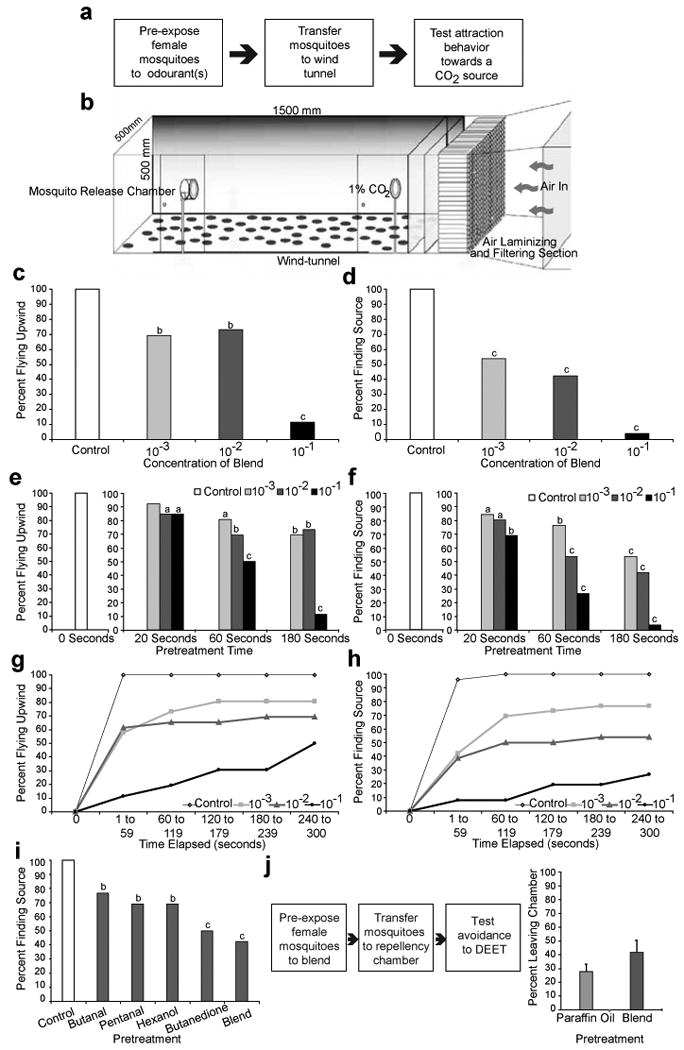
a, Schematic of the experimental strategy and b, the wind-tunnel apparatus. Percentage of female mosquitoes c, flying upwind to half-way point and d, flying through the CO2 emitting glass ring after pre-exposure for 3 minutes to paraffin oil (control) or ultra-prolonged activating blend at indicated concentrations. e, f, Similar experiment as above except three pre-exposure times were tested to the 4-odour ultra-prolonged blend (10-1). g, h, Time-course of percentages of female mosquitoes getting to half-way point and reaching the CO2 source after a 1 minute pre-exposure to paraffin oil or ultra-prolonged blend at the indicated concentrations. i, Percentage of female mosquitoes flying through the CO2-emitting glass ring after pre-exposure to 3 minutes of paraffin oil (control), indicated odourants (10-2) or ultra-prolonged activating blend (10-2). N=26 individuals for each condition. Pearsons χ2 test, compared to controls, a= P<0.05, b=P<0.01, c=P<0.001. j, Schematic of experimental strategy, and percentage of A. aegypti mosquitoes escaping repellency chamber within 5 mins in response to DEET (9.8%) after pre-exposure to paraffin oil (control) or ultra-prolonged activating blend (10-2) as above. N= 7 trails (20 mosquitoes/trial).
After pre-treatment with the odour blend for 1-min, not only did fewer mosquitoes reach the source (Figure 3g,h), they also took longer to get there (Supplemental Figure 6a,b,c). The proportion of pre-treated mosquitoes that reached the CO2 source increased as the assay progressed through 5 minutes (Figure 3g,h), suggesting that their ability to detect CO2 somewhat improves with time. Pre-exposure to 2,3-butanedione caused more pronounced behavioural defects in CO2 attraction than other components of the blend (Figure 3i).
We determined the specificity of the effect of the odour blend by examining its effect on a different odour-mediated behaviour, and found that avoidance to DEET in a non-contact repellency chamber29 was not reduced by pre-exposure to this blend (Figure 3j, Supplemental Figure 7). This result also suggests that, if desired, a long-range CO2-masking strategy might be used effectively in combination with short-range repellency to DEET.
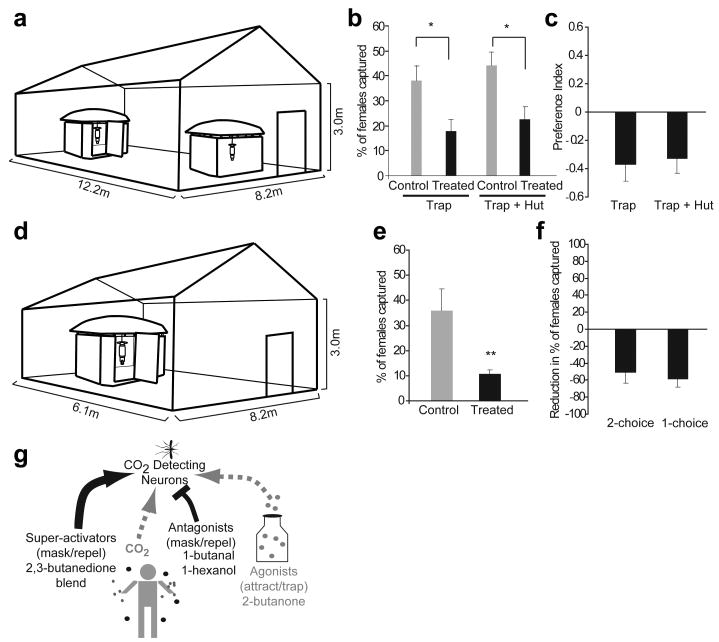
We then tested for disruption of mosquito hut-entry behaviour in a semi-field environment in Kenya30. Host-seeking C. quinquefasciatus females were released overnight in a large enclosed greenhouse that contained two faux huts with CO2-emitting counter-flow geometry traps placed in each of them; females were attracted by the traps and entered the huts through the eaves, and a large majority were then trapped (Figure 4a, Supplemental Figure 8). Inclusion of a source of the “ultra-prolonged” activating blend at 1% (dissolved in paraffin oil) in the form of small fan-driven odour dispensers (Figure 4b, Supplemental Figure 8) resulted in a significant reduction in the number of females that entered the CO2-trap in the treated hut. Moreover, the total number of mosquitoes entering the treated hut but not trapped was also significantly lower than in the control hut, both indicating that “ultra prolonged activators” masked CO2–mediated attraction to the hut (Figure 4b,c). Additionally, odour treatment in one hut did not lead to an increase in the number of mosquitoes entering the control hut (44.25 mosquitoes/night), compared to numbers entering a similarly CO2-baited hut (56 mosquitoes/night) when odour-treatment was not applied to either hut.
Figure 4. Ultra-prolonged activators disrupt attraction behaviour of female Culex mosquitoes in semi-field conditions.
a, Schematic of 2-choice MalariaSphere experiment with counter-flow CO2-traps placed to attract female Culex mosquitoes released from the centre inside each experimental hut. One hut contains ultra-prolonged blend dispensers (1%, treated) while the other dispenses paraffin oil (control). b, Mean percentage of released mosquitoes and c, mean preference index of female mosquitoes captured in trap and captured in trap plus hut in treated vs. control huts. N= 4 trials,100 females /trial, error bars=s.e.m., T-test, *=p<0.05. d, Schematic of 1-choice MalariaSphere experiment. Trials were conducted with (treated) or without (control) ultra-prolonged blend (3%) dispensers e, Mean percentage of mosquitoes in MalariaSphere that are trapped in control and treated huts. N=4 trial nights each for control and treatment, 100 females added every evening, error bars=s.e.m., T-test, **=p<0.005. f, Mean reduction in percentage of Culex females captured in traps from ultra-prolonged blend treated huts in b and d. j, Proposed model of odourant application for mosquito control. Inhibitory odourants or antagonists (red dots) can be used to mask mosquito attraction, agonist (green dots) can be used as a lure or attractant in a trap application, and ultra-prolonged activators (blue dots) can be used to block CO2 detection through persistent strong activation of the neuron. Gray dots represent other human odours.
In a separate greenhouse experiment, mosquitoes were given unrestricted access to a CO2-baited trap in a hut through open door, window and eaves (Figure 4d). There was a significant reduction in capture of female C. quinquefasciatus mosquitoes in the trap when the “ultra-prolonged” activating blend (3% in paraffin oil) was also continuously dispensed inside the hut (Figure 4e) as compared to control experiments in the untreated hut. Thus, the odorant blend reduced the number of female mosquitoes inside a hut in both 2-choice and 1-choice semi-field behaviour assays (Figure 4f).
Taken together, we show that several aspects of CO2-mediated behaviour, including activation of upwind flight and navigation towards the CO2 source, are severely reduced when detection of CO2 by the cpA neuron is disrupted by exposure to an “ultra-prolonged” activating blend at concentrations as low as 0.1%. Importantly, the “ultra-prolonged” blend is absent from the behaviour arena in the wind-tunnel assays. This implies that the reduced attraction towards the CO2 source cannot be attributed to detection of blend-components via other ORNs but is due to the inability of the cpA neurons in pre-treated mosquitoes to detect the CO2 source.
To test whether an “ultra-prolonged” activator like 2,3-butanedione can become attractive at lower concentrations (10-3 and 10-4), we tested it overnight as an evaporating lure in a counterflow-geometry trap for Culex females in a large enclosed arena in California as well as on female Aedes mosquitoes for attraction towards a turbulent plume of 2,3-butanedione at 10-3 in a wind tunnel. Lower concentrations of the ultra-prolonged activator did not act as an attractive cue for mosquitoes in either assay (Supplemental Table 1).
In summary we describe a novel coding mechanism where “ultra-prolonged” activation of a sensory neuron modifies its ability to detect and transmit information about changes in concentrations of its ligands in the environment. Along with this class of ligands we have also identified inhibitors and activators of the mosquito CO2 neurons (Figure 4g). Several characteristics of these novel classes of volatile chemicals offer powerful advantages as potential tools for reducing mosquito-human contact. First, volatile odorants that mask or repel mosquitoes at low concentrations, and that can be easily dispersed in the air, may protect several individuals within a large area. Second, area masking agents and repellents may be effective without application on skin, and thus the convenience may lead to widespread adoption in developing countries. Third, the use of multiple-odour blends to modify behaviour might delay the emergence of resistant strains, a common concern with other methods of mosquito control. Compounds reported in this initial proof-of-principle investigation such as 2,3-butanedione have undesirable safety profiles at high concentrations and are not ideal for human use without further testing. However the structures of the agonists and antagonists we have described in this study have enabled us to identify important structural features and pharmacophores (Supplemental Figure 9) that provide a rational foundation to find additional odorants with improved efficacy and safety profiles. Identification of such molecules is of extremely high priority, which may be effective at lower concentrations, is environmentally friendly, economical and useful in preventing mosquito borne diseases.
Methods Summary
Electrophysiology
Extracellular single-unit recordings were performed as described previously 19 with few modifications noted in Full Methods section. Odour delivery system was specifically designed to ensure steady levels of CO2 concentration as described previously19 and minimize changes in airflow over insect preparation during single-odour stimulus by switching stimulus airflow from a blank delivery cartridge to an odour-laden delivery cartridge.
Behaviour
Wind-tunnel behaviour experiments were performed as described previously2 with some modifications. Avoidance assays with 9.8% DEET were performed in the dark using a repellency chamber which was designed as in29 with some modifications. Briefly, 20 female A. aegypti mosquitoes between 1-2 weeks of age were released inside the repellency chamber and allowed 5 minutes to “escape” from a spout in response to a 9.8% DEET impregnated filter paper which was placed out of range for physical contact. Greenhouse behaviour trials were conducted in two MalariaSphere greenhouses30 at the ICIPE field station in Mbita Point, western Kenya. Trapping experiments were also conducted in a netted enclosure inside a greenhouse in the Agricultural Research Station, University of California Riverside. Details of all behaviour experiments are noted in the Full Methods section.
Full Methods
Electrophysiology
Extracellular single-unit recordings were performed as described previously19 with few modifications. Chemicals were of the highest purity available, typically >99% (Sigma-Aldrich). Odourants were diluted in paraffin oil at indicated concentration. Unless indicated 50 μl of diluted odorant is applied/ cartridge, and each cartridge used for no more than 3 stimuli. A controlled volume of air 5 ml/sec was puffed through the odour cartridge containing vapours, and was delivered into a constant humidified airstream of 10ml/sec that flowed over the fly antenna. The odourant vapour present in the cartridge was thus diluted ∼3-fold before being passed over the fly. CO2 stimulus was pulsed through a separate delivery system that delivered controlled pulses using a PSM 8000 microinjector (variable 2.5 ml/sec – 6.5 ml/ sec) into the same humidified airstream, from either a 1% or 5% tank of CO2 (Airgas). The baseline constant humidified airstream (10 ml/sec) was generated from a purified air tank (Airgas) and mixed with a constant controlled volume (5 ml/sec) of filtered room air (∼0.035% CO2). For delivery of binary mixtures of CO2 with another odorant, we ensured a steady concentration of CO2 to the fly preparation as described in detail in19. Unless mentioned, responses were quantiӿed by subtraction of baseline activity immediately preceding stimulus application from activity during the stimulus. Odour stimuli without CO2 was also delivered using the system described above to minimize changes in airflow over insect preparation by switching filtered room air (5 ml/sec) from a blank delivery cartridge to an odour-laden delivery cartridge for indicated duration using the CS-55 stimulus delivery system (Syntech). For each odorant or blend that had a long-term effect on CO2 response, each recording was obtained from a naive insect.
Behaviour in wind tunnel
Wind-tunnel behaviour experiments were performed as described previously 2 with some modifications. Briefly, female mated non-blood fed A. aegypti mosquitoes between 7-14 days of age raised in a 14:10 hour light-dark cycle were individually collected in release cages and held for 21 hours at 25°C and 70% relative humidity. Mosquitoes in the cage were then transferred into an upended 1 L glass beaker with 100 μL of odour diluted in paraffin oil at the indicated concentration. The mosquito in the release cage was removed from the odour and beaker and transferred in room air across the room to the wind tunnel (within ∼15 sec) where it was carefully manipulated into the release cage. Air from outside the building was presented in the wind tunnel in a laminar flow at a controlled rate of 30 cm3/sec, at ∼70% relative humidity, temperature ∼23°C. A turbulent plume of 1% CO2 was generated by mixing purified CO2 and air from cylinders (Airgas) and delivered through a glass ring with 8 outlets pointing inwards. The cage was opened remotely to release an individual female mosquito. Two video cameras were used to record the flight path. Analyses of videos were performed on computers offline.
Behaviour in repellency chamber
A stainless steel repellency chamber used a standard design29(Supplemental Figure 7). A stainless steel plate was added underneath the smaller screened inner cage to hold the stimulus-loaded paper thus preventing contact with mosquitoes. A blotting paper (12″×7″ inches) was impregnated with 5 ml of 9.8% DEET (Jungle Juice 98% DEET, REI diluted in acetone) and the solvent allowed to evaporate inside a fume hood for 30 mins before placement on the steel plate inside the repellency chamber. Twenty female A. aegypti between 1-2 weeks old were transferred to a release cage 30-mins prior to assay start, and pre-exposed to odourant blend exactly as described previously right before start of assay. Mosquitoes were released inside the inner chamber from the release cage and the lights turned off, and the number of mosquitoes escaping from the exit spout at the end of 5 mins counted. Equipment was cleaned with acetone or ethanol and dried to avoid contamination.
Two-Choice Behaviour in MalariaSphere
Experiments were conducted at the Thomas Odhiambo Field Station of the International Centre of Insect Physiology and Ecology in Mbita Point, western Kenya in late April to June. Culex quinquefasciatus were obtained from the existing rabbit-blood fed colonies at the station insectary. At 18:00 hours 100 female and 20 male Culex were released remotely for every experiment from the centre of a large glass-covered MalariaSphere and allowed to enter either of two huts on two sides until 07:00 (Figure 4a), each running one CO2-baited (250ml/min) counterflow geometry trap (Supplemental Figure 8). Each hut also ran two TimeMist® Fan dispensers starting 30-mins prior to mosquito release and run for duration of the assay, the test with a container containing 15 ml of odour (1% in paraffin oil), and the control with container of 15 ml paraffin oil. The huts were fitted with one-way entry ports in the eaves as the only mosquito entry points in order to enable us to count all entering mosquitoes, with windows covered by nets and doors closed. Additionally, huts were lined with clean black cotton sheets that could be removed and washed to reduce odour contamination between trials. Treatment huts and trap positions were randomized. At end of trial mosquitoes were counted in each trap and inside each hut. Additional non-participating mosquitoes inside the MalariaSphere were removed.
One-Choice Behaviour in MalariaSphere
A smaller single-hut net-covered MalariaSphere (Figure 4d) was used. At 18:00 hours, 100 female and 20 male Culex mosquitoes were released inside the MalariaSphere and allowed to enter the hut freely through open eaves, door and window. A CO2-baited (250ml/min) counterflow geometry trap was started at 18:00 inside the hut and trapped mosquitoes were counted at 07:00. One TimeMist® Fan dispenser ran continuously during the 4-day duration of the 4 overnight trials with a container containing 15 ml of odour (3%) /paraffin oil which was replaced every 24 hours. Because we observed possible long-term contamination effects in thatched roofs and netting in pilot experiments, the no-odour controls were run before odour-treatment to avoid contamination. Untrapped mosquitoes were not removed from the MalariaSphere and their approximate numbers taken into account while calculating percentage caught for each night.
Trapping Responses in Greenhouse
A greenhouse at the Agricultural research station of UC Riverside (California) was modified to enclose a 3 × 6m area in netting where 50 laboratory reared female Culex quinquifasciatus were released each evening and counterflow geometry traps were run from 17:00 to 09:00. CO2 was dispensed as described before. Odourants were dissolved in paraffin oil and dispensed from a 50 ml uncapped tube containing 6 perforations and two 15 cm nylon wicks, which were attached to the air flow outlet tube of the trap. The odorant had largely evaporated from the container at the end of each trial as observed from a sniff test and from change in colour of solution from light yellow to transparent.
Supplementary Material
Supplemental figure 1: CO2 receptors are highly conserved in insects.
a, Alignment of the amino acid sequences of CO2 receptor orthologs using ClustalW from D. melanogaster (Dmel), A. gambiae (Agam), A.aegypti (Aaeg) and C. quinquefasciatus (Cpip). Recreated using sequences from Robertson et. al. 2009. b Mean spikes per second of cpA neuron for 3-sec stimulus of 0.15% CO2 overlayed with 0.5-sec odour, counted in 100-msec bins, n=5, error bars=s.e.m.
Supplemental figure 2: Volatility and solubility of CO2 response inhibitors
Percentage inhibition of the CO2 response as a function of a, vapor pressure, or b, solubility. Information obtained using Scifinder Scholar. Differing shapes indicate same compounds across different insects; A. gambiae (□), A. aegypti (▲), C. quinquefascitus (—), D. melanogaster (◊). Differing colors indicate chemical class; alcohol (red), aldehyde (orange), ester (pink), alkane (blue), ketone (green), acid (purple). n=3.
Supplemental figure 3: Butanone activates cpA neuron in mosquito and mimics CO2.
a, Representative trace from A. gambiae peg sensillum to a 1-sec stimulus of solvent (PO) or 2-butanone (10-1 dilution). b, Mean responses of the cpA neuron to 1-sec 2-butanone at indicated dilutions on A. gambiae (Ag), A. aegypti (Ae), and C. quinquefasciatus (Cx). n=5, error bars=s.e.m.
Supplemental Figure 4: Effect of individual odor mixture components on ultra-prolonged activation.
a, Mean responses of the cpA neuron to 1-sec 2,3-butanedione at indicated dilutions on A. gambiae (Ag), A. aegypti (Ae), and C. quinquefasciatus (Cx). n=5. error bars=s.e.m. b, Schematic of odour exposure sequence. c, Mean response of A. aegypti cpA neuron to a 1-sec CO2 stimulus after pre-exposure as indicated on X-axis with odorants at t=0 and t=15 sec. d, Representative traces (left) from peg sensillum and mean responses (right) from the cpA neurons to 0.15% and 0.33% CO2 after a pretreatment to 3-min of paraffin oil, or individual odor mixture components indicated. n=5, error bars=s.e.m.
Supplemental figure 5: Long-term Inhibition of CO 2 response in A. aegypti after pre-exposure to combinations of odorants.
Mean increase in frequency of response of the cpA neuron to stimulus of 1-sec 0.15% CO2 applied approx. every 30-sec, following a 3-sec pre-exposure to odor mixtures (10-2 dilution) or paraffin oil (PO). d4on=2,3-butanedione, 4al=butanal, 5al=pentanal, 6ol=hexanol. n=5, error bars=s.e.m. Spontaneous activity subtracted.
Supplemental figure 6: Behavior disruption in wind-tunnel
a, Scatterplot of time elapsed from start of assay required to find CO2 source for individual female Aedes mosquitoes according to the various ultra-prolonged blend treatments as indicated. Mosquitoes that did not find source are not included. Mean times and s.e.m. are indicated with line and error bars. b, Mean flight-time (after takeoff from holding cage) to the half-way point, and c, mean flight-time (after takeoff from holding cage) to the CO2 source. Error bars =s.e.m. Note the data for the 10-1 pre-exposure for 60-sec and 180-sec are severely skewed since very few individuals performed the behaviour, only 1 in the last condition. N=26 individuals for each condition were tested.
Supplemental Figure 7:
a,b Schematic diagram of the components of the stainless steel repellency chamber and use for the DEET avoidance assay.
Supplemental Figure 8:
Schematic and pictures of the a, experimental hut inside MalariaSphere with close-up pictures of the one-way entry traps installed in the eaves and TimeMist odour; b, remote mosquito release system; c, counter-flow geometry trap.
Supplemental Figure 9:
Pharmacophores of a, antagonists (1-hexanol, 1-pentanol, 1-butanal, 1-butanoic acid), and b, agonists (2-butanone and 2,3-butanedione). Pharmacophores are shown in two orientations at right-angles. Grey=hydrophobic, green=acceptors. Pharmacophores generated using PharmaGist program as described in Inbar, Y., et. al.. Deterministic Pharmacophore Detection via Multiple Flexible Alignment of Drug-Like Molecules. In Proc. of RECOMB (2007), vol. 3692 of Lecture Notes in Computer Science, pp. 423-434. Springer Verlag.
Supplemental Table 1:
a, Percentage of female C. quinquifasciatus mosquitoes trapped overnight in a counter-flow geometry trap using the indicated lures. N=5 trials per lure treatment, 50 mosquitoes/trial. b, Percentage of female C. quinquifasciatus mosquitoes flying through the odor emitting ring upwind in wind-tunnel assays. N= 20 per trial.
Supplemental Video 1:
Representative video of a mock-treated female A. aegypti mosquito navigating upwind and through a CO2 turbulent-plume releasing ring in real time.
Supplemental Video 2:
Representative video of a pretreated (ultra-prolonged odour blend, 10-2) female A. aegypti mosquito, unable to find upwind CO2 source in real time. For the remaining duration of the 300 sec assay the mosquito does not move after coming to rest on the glass wall.
Supplemental Video 3:
Representative video of a pretreated (ultra-prolonged odour blend, 10-1) female A. aegypti mosquito, unable to activate upwind flight from the cage and find upwind CO2 source during the entire duration of the 300 sec assay (video speeded up).
Acknowledgments
We thank E. Lacey and S. McInally for help setting up behaviour experiments; all the Malaria Group team members in ICIPE Mbita Point Research Station and G. Yan for logistical support and mosquitoes; S. M. Boyle for help with Pharmacophores; K. Klingler for help with statistics; J. Perecko for building traps and excitorepellency chambers; A. Dahanukar and G. Tauxe for comments on the manuscript; W. Walton, P. Atkinson, P. Wirth and A. Khalon for mosquitoes. Part of this work was funded by a grant to A. Ray from the Bill & Melinda Gates Foundation through the Grand Challenges Exploration Initiative, and part supported by a grant to A. Ray, Award Number R01AI087785, from the NIAID (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID or NIH.
Footnotes
Supplemental Information is provided which contains detailed experimental methods, 9 additional figures, 1 addition table and 3 videos.
Author contributions: S.L.T planned the electrophysiology experiments, performed the experiments, collected and analyzed the data and helped write the paper. N.L performed the wind tunnel, repellency, MalariaSphere, and Greenhouse behaviour experiments and analyzed the data. T.G performed one-choice behaviour experiments. J. G helped plan MalariaSphere experiments. R.T.C helped plan the behaviour experiments, and helped write the paper. A.R helped plan the experiments, analyzed the data, managed the project and wrote the paper.
References
- 1.Gillies MT. The role of carbon dioxide in host-finding by mosquitoes: a review. Bull Entomol Res. 1980;70:525–532. [Google Scholar]
- 2.Dekker T, Geier M, Carde RT. Carbon dioxide instantly sensitizes female yellow fever mosquitoes to human skin odours. J Exp Biol. 2005;208:2963–2972. doi: 10.1242/jeb.01736. [DOI] [PubMed] [Google Scholar]
- 3.Zwiebel LJ, Takken W. Olfactory regulation of mosquito-host interactions. Insect Biochem Mol Biol. 2004;34:645–652. doi: 10.1016/j.ibmb.2004.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ditzen M, Pellegrino M, Vosshall LB. Insect odorant receptors are molecular targets of the insect repellent DEET. Science. 2008;319:1838–1842. doi: 10.1126/science.1153121. [DOI] [PubMed] [Google Scholar]
- 5.Syed Z, Leal WS. Mosquitoes smell and avoid the insect repellent DEET. Proc Natl Acad Sci U S A. 2008;105:13598–13603. doi: 10.1073/pnas.0805312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krajick K. Medical entomology - Keeping the bugs at bay. Science. 2006;313:36–38. doi: 10.1126/science.313.5783.36. [DOI] [PubMed] [Google Scholar]
- 7.Corbel V, et al. Evidence for inhibition of cholinesterases in insect and mammalian nervous systems by the insect repellent DEET. BMC Biol. 2009;7:47. doi: 10.1186/1741-7007-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reeder NL, Ganz PJ, Carlson JR, Saunders CW. Isolation of a DEET-insensitive mutant of Drosophila melanogaster (Diptera: Drosophilidae) J Econ Entomol. 2001;94:1584–1588. doi: 10.1603/0022-0493-94.6.1584. [DOI] [PubMed] [Google Scholar]
- 9.Klun JA, et al. Comparative resistance of Anopheles albimanus and Aedes aegypti to N,N-diethyl-3-methylbenzamide (Deet) and 2-methylpiperidinyl-3-cyclohexen-1-carboxamide (AI3-37220) in laboratory human volunteer repellent assays. Journal of Medical Entomology. 2004;41:418–422. doi: 10.1603/0022-2585-41.3.418. [DOI] [PubMed] [Google Scholar]
- 10.Stanczyk NM, Brookfield JF, Ignell R, Logan JG, Field LM. Behavioral insensitivity to DEET in Aedes aegypti is a genetically determined trait residing in changes in sensillum function. Proc Natl Acad Sci U S A. 2010;107:8575–8580. doi: 10.1073/pnas.1001313107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grant AJ, OConnell RJ. Electrophysiological responses from receptor neurons in mosquito maxillary palp sensilla. Ciba F Symp. 1996;200:233–253. doi: 10.1002/9780470514948.ch17. [DOI] [PubMed] [Google Scholar]
- 12.Zollner GE, Torr SJ, Ammann C, Meixner FX. Dispersion of carbon dioxide plumes in African woodland: implications for host-finding by tsetse flies. Physiological Entomology. 2004;29:381–394. [Google Scholar]
- 13.Carde RT, Gibson G. In: Olfaction in Vector-Host Interactions. Takken W, Knols BGF, editors. Vol. 2. Wageningen Academic Publishers; 2010. pp. 115–141. [Google Scholar]
- 14.Robertson HM, Kent LB. Evolution of the gene lineage encoding the carbon dioxide receptor in insects. J Insect Sci. 2009;9:19. doi: 10.1673/031.009.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu T, et al. Odor coding in the maxillary palp of the malaria vector mosquito Anopheles gambiae. Curr Biol. 2007;17:1533–1544. doi: 10.1016/j.cub.2007.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones WD, Cayirlioglu P, Kadow IG, Vosshall LB. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature. 2007;445:86–90. doi: 10.1038/nature05466. [DOI] [PubMed] [Google Scholar]
- 17.Kwon JY, Dahanukar A, Weiss LA, Carlson JR. The molecular basis of CO2 reception in Drosophila. Proc Natl Acad Sci U S A. 2007;104:3574–3578. doi: 10.1073/pnas.0700079104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Syed Z, Leal WS. Maxillary palps are broad spectrum odorant detectors in Culex quinquefasciatus. Chem Senses. 2007;32:727–738. doi: 10.1093/chemse/bjm040. [DOI] [PubMed] [Google Scholar]
- 19.Turner SL, Ray A. Modification of CO2 avoidance behaviour in Drosophila by inhibitory odorants. Nature. 2009;461:277–281. doi: 10.1038/nature08295. [DOI] [PubMed] [Google Scholar]
- 20.Zdobnov EM, et al. Comparative genome and proteome analysis of Anopheles gambiae and Drosophila melanogaster. Science. 2002;298:149–159. doi: 10.1126/science.1077061. [DOI] [PubMed] [Google Scholar]
- 21.de Bruyne M, Foster K, Carlson J. Odor coding in the Drosophila antenna. Neuron. 2001;30:537–552. doi: 10.1016/s0896-6273(01)00289-6. [DOI] [PubMed] [Google Scholar]
- 22.Hallem EA, Carlson JR. Coding of odors by a receptor repertoire. Cell. 2006;125:143–160. doi: 10.1016/j.cell.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 23.Rostelien T, Borg-Karlson AK, Faldt J, Jacobsson U, Mustaparta H. The plant sesquiterpene germacrene D specifically activates a major type of antennal receptor neuron of the tobacco budworm moth Heliothis virescens. Chem Senses. 2000;25:141–148. doi: 10.1093/chemse/25.2.141. [DOI] [PubMed] [Google Scholar]
- 24.Kaissling KE, Meng LZ, Bestmann HJ. Responses of bombykol receptor cells to (Z,E)-4,6-hexadecadiene and linalool. J Comp Physiol A. 1989;165:147–154. [Google Scholar]
- 25.Yao CA, Carlson JR. Role of G-proteins in odor-sensing and CO2-sensing neurons in Drosophila. J Neurosci. 2010;30:4562–4572. doi: 10.1523/JNEUROSCI.6357-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mafra-Neto A, Carde RT. Fine-scale structure of pheromone plumes modulates upwind orientation of flying moths. Nature. 1994;369:142–144. [Google Scholar]
- 27.Bhandawat V, Maimon G, Dickinson MH, Wilson RI. Olfactory modulation of flight in Drosophila is sensitive, selective and rapid. J Exp Biol. 2010;213:3625–3635. doi: 10.1242/jeb.040402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhandawat V, Olsen SR, Gouwens NW, Schlief ML, Wilson RI. Sensory processing in the Drosophila antennal lobe increases reliability and separability of ensemble odor representations. Nat Neurosci. 2007;10:1474–1482. doi: 10.1038/nn1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noosidum A, Prabaripai A, Chareonviriyaphap T, Chandrapatya A. Excito-repellency properties of essential oils from Melaleuca leucadendron L., Litsea cubeba (Lour.) Persoon, and Litsea salicifolia (Nees) on Aedes aegypti (L.) mosquitoes. J Vector Ecol. 2008;33:305–312. doi: 10.3376/1081-1710-33.2.305. [DOI] [PubMed] [Google Scholar]
- 30.Njiru BN, Mukabana WR, Takken W, Knols BG. Trapping of the malaria vector Anopheles gambiae with odour-baited MM-X traps in semi-field conditions in western Kenya. Malar J. 2006;5:39. doi: 10.1186/1475-2875-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figure 1: CO2 receptors are highly conserved in insects.
a, Alignment of the amino acid sequences of CO2 receptor orthologs using ClustalW from D. melanogaster (Dmel), A. gambiae (Agam), A.aegypti (Aaeg) and C. quinquefasciatus (Cpip). Recreated using sequences from Robertson et. al. 2009. b Mean spikes per second of cpA neuron for 3-sec stimulus of 0.15% CO2 overlayed with 0.5-sec odour, counted in 100-msec bins, n=5, error bars=s.e.m.
Supplemental figure 2: Volatility and solubility of CO2 response inhibitors
Percentage inhibition of the CO2 response as a function of a, vapor pressure, or b, solubility. Information obtained using Scifinder Scholar. Differing shapes indicate same compounds across different insects; A. gambiae (□), A. aegypti (▲), C. quinquefascitus (—), D. melanogaster (◊). Differing colors indicate chemical class; alcohol (red), aldehyde (orange), ester (pink), alkane (blue), ketone (green), acid (purple). n=3.
Supplemental figure 3: Butanone activates cpA neuron in mosquito and mimics CO2.
a, Representative trace from A. gambiae peg sensillum to a 1-sec stimulus of solvent (PO) or 2-butanone (10-1 dilution). b, Mean responses of the cpA neuron to 1-sec 2-butanone at indicated dilutions on A. gambiae (Ag), A. aegypti (Ae), and C. quinquefasciatus (Cx). n=5, error bars=s.e.m.
Supplemental Figure 4: Effect of individual odor mixture components on ultra-prolonged activation.
a, Mean responses of the cpA neuron to 1-sec 2,3-butanedione at indicated dilutions on A. gambiae (Ag), A. aegypti (Ae), and C. quinquefasciatus (Cx). n=5. error bars=s.e.m. b, Schematic of odour exposure sequence. c, Mean response of A. aegypti cpA neuron to a 1-sec CO2 stimulus after pre-exposure as indicated on X-axis with odorants at t=0 and t=15 sec. d, Representative traces (left) from peg sensillum and mean responses (right) from the cpA neurons to 0.15% and 0.33% CO2 after a pretreatment to 3-min of paraffin oil, or individual odor mixture components indicated. n=5, error bars=s.e.m.
Supplemental figure 5: Long-term Inhibition of CO 2 response in A. aegypti after pre-exposure to combinations of odorants.
Mean increase in frequency of response of the cpA neuron to stimulus of 1-sec 0.15% CO2 applied approx. every 30-sec, following a 3-sec pre-exposure to odor mixtures (10-2 dilution) or paraffin oil (PO). d4on=2,3-butanedione, 4al=butanal, 5al=pentanal, 6ol=hexanol. n=5, error bars=s.e.m. Spontaneous activity subtracted.
Supplemental figure 6: Behavior disruption in wind-tunnel
a, Scatterplot of time elapsed from start of assay required to find CO2 source for individual female Aedes mosquitoes according to the various ultra-prolonged blend treatments as indicated. Mosquitoes that did not find source are not included. Mean times and s.e.m. are indicated with line and error bars. b, Mean flight-time (after takeoff from holding cage) to the half-way point, and c, mean flight-time (after takeoff from holding cage) to the CO2 source. Error bars =s.e.m. Note the data for the 10-1 pre-exposure for 60-sec and 180-sec are severely skewed since very few individuals performed the behaviour, only 1 in the last condition. N=26 individuals for each condition were tested.
Supplemental Figure 7:
a,b Schematic diagram of the components of the stainless steel repellency chamber and use for the DEET avoidance assay.
Supplemental Figure 8:
Schematic and pictures of the a, experimental hut inside MalariaSphere with close-up pictures of the one-way entry traps installed in the eaves and TimeMist odour; b, remote mosquito release system; c, counter-flow geometry trap.
Supplemental Figure 9:
Pharmacophores of a, antagonists (1-hexanol, 1-pentanol, 1-butanal, 1-butanoic acid), and b, agonists (2-butanone and 2,3-butanedione). Pharmacophores are shown in two orientations at right-angles. Grey=hydrophobic, green=acceptors. Pharmacophores generated using PharmaGist program as described in Inbar, Y., et. al.. Deterministic Pharmacophore Detection via Multiple Flexible Alignment of Drug-Like Molecules. In Proc. of RECOMB (2007), vol. 3692 of Lecture Notes in Computer Science, pp. 423-434. Springer Verlag.
Supplemental Table 1:
a, Percentage of female C. quinquifasciatus mosquitoes trapped overnight in a counter-flow geometry trap using the indicated lures. N=5 trials per lure treatment, 50 mosquitoes/trial. b, Percentage of female C. quinquifasciatus mosquitoes flying through the odor emitting ring upwind in wind-tunnel assays. N= 20 per trial.
Supplemental Video 1:
Representative video of a mock-treated female A. aegypti mosquito navigating upwind and through a CO2 turbulent-plume releasing ring in real time.
Supplemental Video 2:
Representative video of a pretreated (ultra-prolonged odour blend, 10-2) female A. aegypti mosquito, unable to find upwind CO2 source in real time. For the remaining duration of the 300 sec assay the mosquito does not move after coming to rest on the glass wall.
Supplemental Video 3:
Representative video of a pretreated (ultra-prolonged odour blend, 10-1) female A. aegypti mosquito, unable to activate upwind flight from the cage and find upwind CO2 source during the entire duration of the 300 sec assay (video speeded up).