Abstract
Chaperones are proteins that assist the correct folding of other protein clients either when the clients are being synthesized or at their functional localities. Chaperones are responsible for certain diseases. The sigma-1 receptor is recently identified as a receptor chaperone whose activity can be activated/deactivated by specific ligands. Under physiological conditions, the sigma-1 receptor chaperones the functional IP3 receptor at the endoplasmic reticulum and mitochondrion interface to ensure proper Ca2+ signaling from endoplasmic reticulum into mitochondrion. However, under pathological conditions whereby cells encounter enormous stress that results in the endoplasmic reticulum losing its global Ca2+ homeostasis, the sigma-1 receptor translocates and counteracts the arising apoptosis. Thus, the sigma-1 receptor is a receptor chaperone essential for the metabotropic receptor signaling and for the survival against cellular stress. The sigma-1 receptor has been implicated in many diseases including addiction, pain, depression, stroke, and cancer. Whether the chaperone activity of the sigma-1 receptor attributes to those diseases awaits further investigation.
Keywords: Sigma receptor, chaperone, ER stress, mitochondrion, nucleus, calcium signaling, IP3 receptor, MAM
INTRODUCTION
The endoplasmic reticulum (ER) is the organelle where most of the proteins in the cell are synthesized. Nascent proteins enter the ER via translocons as linear peptide chains that need to be folded into correct three-dimensional configurations in the ER so that they can exit the ER for further modification and/or transport to reach their final destinations. The correct folding of nascent proteins is performed by chaperone proteins inside the ER. Very often, chaperones inside the ER work in concert with other chaperones to achieve the goal. This task is done either in the form of heteromeric chaperone complexes (co-chaperones) or multiple chaperones coordinated in series [1, 2]. Interestingly, the percentage of newly synthesized proteins that are correctly folded and thus are able to exit the ER is pretty low and is usually less than 10%. The low efficiency, puzzling as it is, makes one wonder if the low efficiency itself may serve for any specific unknown purposes in the living system.
Inasmuch as chaperones are important in maintaining the correct conformation of other protein clients and thus conferring their clients' biological activities, chaperones are present not only in the ER but also in the cytosol, mitochondria, and even the extracellular space [3]. Further, for the similar reason, chaperones are present in almost every species of the living system. Because the action of chaperones is fundamental to the cell, chaperones are implicated in many diseases including Huntington disease [4], Parkinsonism [5], stress disorders [6], bacterial infection [7], cystic fibrosis [8], and cancer [9]. The scope of the present review is however to focus on the chaperones inside the ER, in particular a chaperone called the sigma-1 receptor chaperone, and not in any attempt to provide an exhausted overview on chaperones in general. The readers can gain more in-depth information on chaperones from several other recent reviews on this subject [10–12]. This mini-review is also written to review sigma-1 receptors regarding their fundamental action as a molecular chaperone and thus is not meant to be an exhaustive review on sigma-1 receptors as a whole.
INSULTS TO THE ENDOPLASMIC RETICULUM
The ER, important as it is, is also the primary target of many biological insults. The insults include glucose deprivation, hyperhomocyateinemia, DNA damage, mutated secretory proteins, polyglutamine neuropathies, viral infection, inhibition of N-linked glycosylation, heat shock, free radicals, disruption of Ca2+ homeostasis, or drug intoxication [13]. The end-result of those insults is almost always the increase of misfolded proteins inside the ER which would ultimately end up in a pathological state if not properly rescued. Nevertheless, the nature has endowed the ER with at least three lines of defense against those insults.
LINES OF DEFENSE FROM THE ENDOPLASMIC RETICULUM
The first line of the ER defense is the chaperones inside the ER that attempt to chaperone and correct misfolded proteins. The second line of defense is the ER-nucleus signaling that involves three proteins PERK, IRE-1, and ATF6 at the ER membrane [13–16]. The three proteins in their inactive state bind to the same protein BiP (or GRP78). The misfolded proteins inside the ER, however, when accumulated in sufficient concentrations can displace BiP from each of the three proteins [13–16]. The displacement in turn causes the activation (i.e., dimerization/cleavage and phosphorylation) of the three proteins, each ensuing a downstream signaling from the ER to the nucleus that eventually calls for increases of gene transcriptions of survival proteins like redox enzymes, chaperones, endoplasmic reticulum-associated degradation enzymes, amino acid metabolism, and lipid synthesis [13–16]. The ER-nucleus signaling can also call for an increase of apoptotic protein CHOP [17]. In addition, the activation of PERK reduces the global protein biosynthesis by inactivating ribosomes in the cytosol [13, 14]. The above responses seen at the ER and nucleus as triggered by misfolded proteins are collectively called the unfolded protein response (UPR) [13–16]. The fine line defining survival or apoptosis of the cell as the end result of the UPR is however unknown at present.
The third line of the ER defense against insults is the ER-mitochondrion signaling [18–21]. As mitochondrion serves as the powerhouse of cells, one would imagine that the ER would communicate the status quo of its well-being to the mitochondrion. In fact that is exactly what happens in the living system, although the exact mode and mechanism of the communication need to be fully understood. This review focuses mainly on this third line of the ER defense that will thus be further expounded in the remaining portion of this review.
THE ENDOPLASMIC RETICULUM SIGNALING TO MITOCHONDRIA
The ER and mitochondrion have been recognized as two separate organelles of the cell, each performing its well-known functions. However in the past 20 years or so mainly through two seemingly unrelated areas of research, i.e., the phospholipid transport [22–24] and the Ca2+ signaling [18–21], the relationship between the ER and mitochondrion has taken a surprising turn. The ER and mitochondrion are in fact physically and functionally connected at some specialized areas contributed from each organelle [18–21]. The specialized area contributed from the ER is called the mitochondrion-associated ER membrane (MAM in short) [18–24]. It needs to be emphasized that the MAM belongs to the ER and is in no way implied to serve as a contiguous or fused membrane connecting the ER to mitochondrion. Less is known about the nature of the region in the mitochondrion that directly apposes the MAM of the ER. The distance between the MAM and mitochondrion outer membrane could be as short as 10–25 nm [20].
THE CA2+ SIGNALING BETWEEN THE ENDOPLASMIC RETICULUM AND MITOCHONDRIA
The MAM thus serves as a convenient region whereby the ER might communicate with the mitochondrion in a direct manner via exchanges of molecules. The most important aspect of the communication in this regard concerns the Ca2+. As readers are all aware of, Ca2+ plays important roles in the ER and also in the mitochondrion. In the ER, Ca2+ for example is important for the protein biosynthesis and for maintaining the chaperone activities inside the ER. In the mitochondrion, Ca2+ is, to say the least, important for the enzymatic activities for the tricarboxylic acid (TCA) cycle as well as for the electron transport chains for the respiratory production of ATP. Through pioneering works of several key scientists, it is now known that the ER directly transmits Ca2+ into mitochondrion via the MAM [18–21]. Mitochondria do have the capacity of directly transporting Ca2+ from the cytosol but the capacity is low. A more efficient way for mitochondria to receive Ca2+ is to receive it directly from the ER via the ER-mitochondrion contact MAM. The ER itself is a huge reservoir for Ca2+ that is able to uptake Ca2+ very efficiently. Therefore, together with the convenience of having the MAM directly apposing mitochondria, the ER serves to transmit Ca2+ supply directly into mitochondria. It is well-known that 1,4,5-trisphosphate inositol (IP3) receptors at the MAM on the side of the ER serve to release Ca2+ from the ER into mitochondrion [25]. By doing so, IP3 receptors open up, upon the stimulation by IP3, and generate thus high concentrations of Ca2+ as Ca2+ “puffs” at the MAM which can then be uptaken into mitochondrion [18, 19, 21].
PROBLEMS WITH THE IP3 RECEPTORS
However, three fundamental questions concerning IP3 receptors in this regard have never been well understood in the past: (1) IP3 receptors degrade rapidly after the binding of their ligand IP3 [26, 27]: How does nature deal with this problem in order to sustain the stability and the continuation of the function of the receptor? (2) The stability of IP3 receptors seems to be related to the Ca2+ level in the lumen of ER [27]: How exactly might the ER lumenal Ca2+ regulate the IP3 receptor's stability? (3) What happens to the IP3 receptor, or what happens to the Ca2+ signaling between the ER and the mitochondrion, when the ER is facing a pathological crisis such as a sustained global drop of ER lumenal Ca2+?
SIGMA-1 RECEPTORS COME TO RESCUE
The sigma-1 receptor at the ER provides answers to the above three questions.
We will skip the long history behind the sigma-1 receptor and focus instead primarily on what is known of its basic action on the regulation of IP3 receptors and the discovery of it, and speculate thereof how the chaperone activity of sigma-1 receptors at the ER may facilitate the cellular defense against diseases.
HOW SIGMA-1 RECEPTORS RELATE TO IP3 RECEPTORS?
The realization that sigma-1 receptors might have something to do with IP3 receptors started when Novakova et al [28] found that in cardiac myocytes sigma receptor ligands caused an increase of IP3 and the increase could be antagonized by the sigma receptor antagonist. Further, Vilner and Bowen [29] demonstrated a transient intracellular calcium rise in SK-N-SH neuroblastoma cells when cells were challenged with sigma receptor agonist and that the transient rise was blocked by the sigma-1 receptor antagonist N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-2(dimethylamino)ethylamine (BD1047).
In our own research, the realization that the sigma-1 receptor might have any bearing at all with the IP3 receptor came from an experiment in which MK-801-induced special memory impairment in mice was blocked by a selective sigma-1 receptor ligand 2-(4-morpholinethyl)1-phenylcyclohexanecarboxylate hydrochloride (PRE-084) [30]. MK-801 is a channel blocker and thus an antagonist for the NMDA receptor. It had been known that the sigma-1 receptor is an ER protein. The question arose then: how can an ER protein affect the behavior caused by the blockade of a plasma membrane receptor? As MK-801 causes a reduction of intracellular Ca2+ by blocking the NMDA receptor, an intuitive guess would be that the sigma-1 receptor at the ER might counteract this effect of MK-801 by increasing the Ca2+ efflux from the ER to compensate for the drop of Ca2+ caused by MK-801. As the IP3 receptor is one of the major Ca2+ release channels at the ER, the sigma-1 receptor might in a way increase the ER Ca2+ efflux by regulating IP3 receptors. One possibility would be that the sigma-1 receptor is in proximity to the IP3 receptor thereby regulating the latter via a direct protein-protein interaction in a mechanism that needs to be fully understood. To provide a preliminary proof of concept that the two receptors are close-by to each other, heparin, a stretched molecule known to inhibit the IP3 receptor, was used. The idea was that heparin, by binding IP3 receptors, may also extend itself to the nearby sigma-1 receptor with the possibility, if any, that it might also block the access of the sigma-1 receptor to its ligand. Indeed, heparin blocked the binding of [H3] (+) SKF-10047 to sigma-1 receptors in brain homogenates [31]. Whether the sigma-1 receptor has a relation with another Ca2+ releasing channel at the ER, the ryanodine receptor is unknown at present.
SIGMA-1 RECEPTORS COLOCALIZE WITH IP3 RECEPTORS
With the successful cloning of the sigma-1 receptor [32] and the availability of antibodies against the receptor, immunocytochemistry further confirmed that the sigma-1 receptor indeed colocalizes with the IP3 receptor [21]. More specifically, the sigma-1 receptor resides primarily at the ER-mitochondrion contact MAM where the two receptors colocalize [21]. The two receptors also coimmunoprecipitate [21]. These results suggest a direct interaction of the two receptors. The exact mechanism of action of the sigma-1 receptor on the IP3 receptor was unraveled when it was found that the sigma-1 receptor at the MAM also colocalizes and coimmunoprecipitates with another ER chaperone called GRP78 or BiP [21]. Because chaperones coordinate with each other to perform the chaperone activity, the sigma-1 receptor forming a complex with BiP suggested that the sigma-1 receptor itself is a chaperone. Indeed, the sigma-1 receptor itself is a chaperone as clearly demonstrated in that report [21].
SIGMA-1 RECEPTORS CHAPERONE IP3 RECEPTORS
How does the chaperone activity of the sigma-1 receptor provide answers to the three questions mentioned above then? Firstly, concerning the stability of the IP3 receptor during the metabolic receptor signaling, it was found that the IP3 receptor is the client of the sigma-1 receptor chaperone [21]. The sigma-1 receptor chaperone, in a free form as to be explained below, stabilizes the conformation of the IP3 receptor thereby preventing the latter from the proteasomal degradation. Secondly, concerning the relation between the ER Ca2+ level and the stability of the IP3 receptor, the sigma-1 receptor-BiP complex turns out to be a Ca2+ sensor [21]. By sensing the drop of local ER lumenal Ca2+ after the opening of IP3 receptor, the sigma-1 receptor dissociates itself from BiP and translocates to the IP3 receptor thereafter to chaperone the IP3 receptor to ensure proper Ca2+ signaling from the ER to mitochondrion through the MAM [21]. Thirdly, concerning the prolonged pathological drop of ER Ca2+, it was demonstrated that the sigma-1 receptor departs from the MAM and translocates throughout the whole ER reticular network upon when the ER lumenal Ca2+ is nearly depleted [21]. The translocation of the sigma-1 receptor correlates with an enhanced survival of cells against apoptosis induced by the prolonged ER Ca2+ depletion [21].
SIGMA-1 RECEPTORS ARE LIGAND-REGULATED CHAPERONES
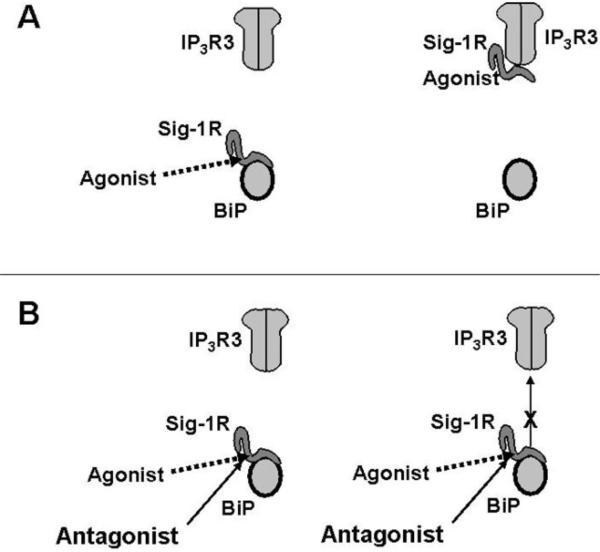
Thus, the fundamental action of the sigma-1 receptor is, at least for one, functioning as a chaperone. The sigma-1 receptor chaperone not only maintains the proper Ca2+ signaling due the downstream signaling of the metabotropic receptor activation but also serves as a survival protein against apoptosis induced by the prolonged ER stress. More interestingly, the traditionally recognized sigma-1 receptor agonists dissociate the sigma-1 receptor from BiP and thus facilitate the association of the free form of sigma-1 receptors with IP3 receptors [21]. The sigma-1 receptor antagonists block this action of the agonists [21]. These actions of the sigma-1 receptor agonists and antagonists take place in normal physiological conditions in which the Ca2+ concentration is at its physiological level [21]. These results suggest that the sigma-1 receptor agonist or antagonist may compete with Ca2+ for the same binding domains on either the sigma-1 receptor or BiP. However, the possibility is not excluded that the ligands may regulate the sigma-1 receptor-BiP association/dissociation via an allosteric effect by binding to a different site from that for the Ca2+. The results suggest that, under normal physiological conditions, the sigma-1 receptor agonist, either endogenously existing or exogenously added, may increase the proportion of the sigma-1 receptor in its free form that would be available to bind and chaperone IP3 receptors when in times of need. In other words, the sigma-1 receptor agonists may predispose more readily available protective power against the degradation of IP3 receptors when needed. These results also suggest that the sigma-1 receptor ligands may have therapeutic usages in regulating the stability of IP3 receptors as well as the associated interorganelle Ca2+ signaling from the ER to mitochondrion under normal or otherwise pathological conditions. More studies are needed to ascertain the potential therapeutic usages of the sigma-1 receptor based on the mechanisms mentioned as such (Figure 1).
Fig. (1).
Modes of action of the sigma-1 receptor agonist and antagonist
(A) Agonist causes the sigma-1 receptor to dissociate from BiP and begin to bind the IP3 receptor. (B) Antagonist blocks the action of agonist, thus preventing the sigma-1 receptor to dissociate from BiP.
FUTURE CONSIDERATIONS
Although the basic molecular action of the sigma-1 receptor has thus been identified as the chaperone, many questions remain that need to be addressed. The questions are as follows.
The sigma-1 receptor is implicated in many diseases including addiction [33–35], depression [36–39], amnesia [40–42], analgesia [43–45], stroke [46–48], and cancer [49–51]. One question naturally arises: is the chaperone activity of the sigma-1 receptor attributing to those diseases? Or, may there be other as yet to-be-discovered activities of the sigma-1 receptor that may relate the receptor to those diseases? Similarly, there are other related questions to be addressed. For example, inasmuch as the sigma-1 receptor is a chaperone, is the IP3 receptor the only client for the sigma-1 receptor? Are there other client proteins for the sigma-1 receptor chaperone? What are they? Are they thus involved in the diseases implicated upon the activation of the sigma-1 receptor? Further, as the IP3 receptors have been shown to be modulated by other proteins such as cytochrome c [52, 53], is there a relationship between the sigma-1 receptor chaperoning the IP3 receptor and the “feed forward” action of cytochrome c at the IP3 receptor [52, 53]?
Equally important are the questions concerning the translocation of sigma-1 receptors. What causes the sigma-1 receptor to translocate from the MAM? Is there a molecular messenger that senses the prolonged ER stress and thus calls for the sigma-1 receptor to translocate from the MAM to the whole ER network to fight against apoptosis? If yes, what is the messenger? Further, what are the molecular and the biochemical bases for the sigma-1 receptor to be able to move and translocate throughout the ER? The sigma-1 receptor has been reported to be present at the plasma membrane and regulate the ion channels therein via direct protein-protein interactions [54, 55]. How would a supposedly ER protein be present at the plasma membrane? What is the exact relation between the plasma membrane sigma-1 receptor and the ER sigma-1 receptor?
What is the downstream effect of the sigma-1 receptor at the mitochondria beyond that fact that the receptor chaperone is able to supply Ca2+ for the enzymes in the TCA cycle and the electron transfer chains? Or, does the sigma-1 receptor act and thus involve with the functioning of mitochondria more than what we have described and understood so far? Are there other aspects of the action of sigma-1 receptors that we do not know yet that may serve to regulate the function of mitochondria by the ER?
The sigma-1 receptor exists not only in the CNS but also in the peripheral organs [21] including the liver, adrenal gland, pancreas, and spleen wherein the receptor has been implicated in the hepatocyte survival [56], norepinephrine uptake [57], insulin secretion [58], and immune response [59] respectively. One cannot help but asking: Does the sigma-1 receptor act the same as a ligand-operated receptor chaperone in all those organs just as shown in the model system using CHO cells [21]? Would the chaperone action of the sigma-1 receptor, if so observed, relate to the described effects seen in those organs?
One last note concerns the physiological significance of the discovery from CHO cells that sigma-1 receptors are molecular chaperons. A particular question would be: what phenotype sigma-1 receptor knockout mice might display? Recent publications shed light on this question. Although the sigma-1 receptor-knockout mice do not show apparent phenotypes in the standard screening procedures, the knockout mice showed decreased locomotor activities when challenged with the sigma-1 receptor agonist (+)SKF-10047 [60]. Further, the knockout mice showed an enhanced sensitivity to morphine-induced antinociception [61] and showed also a depressive-like behavior in forced swimming test [62]. We do not know at present how the chaperone activity might relate to those behavioral alterations in the sigma-1 receptor-knockout mice. But the results of those behavioral studies teach us a lesson: In knockout studies, in addition to standard primary phenotyping, secondary phenotypings such as those by using pharmacological challenges should be employed to come up with a complete phenotyping in knockout animals.
CONCLUSIONS
In summary, 26 years after its discovery, the sigma-1 receptor is now identified as a receptor chaperone in a cellular system using CHO cells. It has to be noted that the sigma-1 receptor derived from Martin's [63] original concept of the existence of an opioid/sigma receptor but was later identified as a separate receptor entity that is not opioid in nature. In spite of the hallmark discovery, many questions arise that demand answers. The foremost of those questions concerns how an ER receptor chaperone relates to so many diseases. It is our hope that through more investigations and research the full nature of action of the sigma-1 receptor can be unraveled and that in the end the full understanding of which may benefit the well-being of humans by providing new opportunities and insights for combating a wide spectrum of human diseases.
ACKNOWLEDGEMENT
This work supported by the Intramural Research Program of the National Institute on Drug Abuse, NIH, DHHS.
ABBREVIATIONS
- BD1047
N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-2(dimethylamino)ethylamine
- ER
Endoplasmic reticulum
- IP3
1,4,5-Trisphosphotidyl inositol
- MAM
Mitochondria-associated ER membrane
- MK-801
Dizocilpine
- NMDA
N-methyl-D-aspartate
- SKF-10047
N-allylnormetazocine
- PERK
Pancreatic ER kinase
- PRE-084
2-(4-Morpholinethyl)1-phenylcyclohexanecarboxylate hydrochloride
- TCA
Tricarboxylic acid
- UPR
Unfolded protein response
REFERENCES
- [1].Ellis RJ. Molecular chaperones: assisting assembly in addition to folding. Trends Biochem. Sci. 2006;31:395–401. doi: 10.1016/j.tibs.2006.05.001. [DOI] [PubMed] [Google Scholar]
- [2].Fink AL. Chaperone-mediated protein folding. Physiol. Rev. 1999;79:425–449. doi: 10.1152/physrev.1999.79.2.425. [DOI] [PubMed] [Google Scholar]
- [3].Ranford JC, Coates ARM, Henderson B. Chaperones are cell-signalling proteins: the unfolding biology of molecular chaperones. Expert Rev. Mol. Med. 2000;2:1–17. doi: 10.1017/S1462399400002015. [DOI] [PubMed] [Google Scholar]
- [4].Carmichael J, Chatellier J, Woolfson A, Milstein C, Fersht AR, Rubinsztein DC. Bacterial and yeast chaperones reduce both aggregate formation and cell death in mammalian cell models of Huntington's disease. Proc. Natl. Acad. Sci. USA. 2000;97:9701–9705. doi: 10.1073/pnas.170280697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kabuta T, Wada K. Insights into links between familial and sporadic Parkinson's disease: physical relationship between UCH-L1 variants and chaperone-mediated autophagy. Autophagy. 2008;4:827–829. doi: 10.4161/auto.6560. [DOI] [PubMed] [Google Scholar]
- [6].Caamano CA, Morano MI, Akil H. Corticosteroid receptors: a dynamic interplay between protein folding and homeostatic control. Possible implications in psychiatric disorders. Psychopharmacol. Bull. 2001;35:6–23. [PubMed] [Google Scholar]
- [7].Cabanes D, Sousa S, Cebria A, Lecuit M, Garcia-del Portillo F, Cossart P. Gp96 is a receptor for a novel Listeria monocyto-genes virulence factor, Vip, a surface protein. EMBO J. 2005;24:2827–2838. doi: 10.1038/sj.emboj.7600750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang X, Venable J, LaPointe P, Hutt DM, Koulov AV, Coppinger J, Gurkan C, Kellner W, Matteson J, Plutner H, Riordan JR, Kelly JW, Yates JR, Balch WE. Hsp90 cochaperone Aha1 downregulation rescues misfolding of CFTR in cystic fibrosis. Cell. 2006;127:803–815. doi: 10.1016/j.cell.2006.09.043. [DOI] [PubMed] [Google Scholar]
- [9].Powers MV, Clarke PA, Workman P. Dual targeting of HSC70 and HSP72 inhibits HSP90 function and induces tumor-specific apoptosis. Cancer Cell. 2008;14:250–262. doi: 10.1016/j.ccr.2008.08.002. [DOI] [PubMed] [Google Scholar]
- [10].Vos MJ, Hageman J, Carra S, Kampinga HH. Structural and functional diversities between members of the human HSPB, HSPH, HSPA, and DNAJ chaperone families. Biochemistry. 2008;47:7001–7011. doi: 10.1021/bi800639z. [DOI] [PubMed] [Google Scholar]
- [11].Kim J, Löwe T, Hoppe T. Protein quality control gets muscle into shape. Trends Cell Biol. 2008;18:264–272. doi: 10.1016/j.tcb.2008.03.007. [DOI] [PubMed] [Google Scholar]
- [12].Palotai R, Szalay MS, Csermely P. Chaperones as integrators of cellular networks: changes of cellular integrity in stress and diseases. IUBMB Life. 2008;60:10–18. doi: 10.1002/iub.8. [DOI] [PubMed] [Google Scholar]
- [13].Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol. 2004;14:20–28. doi: 10.1016/j.tcb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- [14].Lai E, Teodoro T, Volchuk A. Endoplasmic reticulum stress: signaling the unfolded protein response. Physiology. 2007;22:193–201. doi: 10.1152/physiol.00050.2006. [DOI] [PubMed] [Google Scholar]
- [15].Ron D, Hubbard SR. How IRE1 reacts to ER stress. Cell. 2008;132:24–26. doi: 10.1016/j.cell.2007.12.017. [DOI] [PubMed] [Google Scholar]
- [16].Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein respons. Nat. Rev. Mol. Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- [17].Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J. Clin. Invest. 2008;118:3378–3389. doi: 10.1172/JCI34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- [19].Walter L, Hajnoczky G. Mitochondria and endoplasmic reticulum: the lethal interorganelle cross-talk. J. Bioenerg. Biomembr. 2005;37:191–206. doi: 10.1007/s10863-005-6600-x. [DOI] [PubMed] [Google Scholar]
- [20].Csordas G, Renken C, Varnai P, Walter L, Buttle KF, Balla T, Mannella CA, Hajnoczky G. Structural and functional features and significance of the physical linkage between ER and mitochondria. J. Cell Biol. 2006;174:915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hayashi T, Su T-P. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca (2+) signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- [22].Vance JE. Phospholipid synthesis in a membrane fraction associated with mitochondria. J. Biol. Chem. 1990;265:7248–7256. [PubMed] [Google Scholar]
- [23].Voelker DR. Reconstitution of phosphatidylserine import into rat liver mitochondria. J. Biol. Chem. 1989;264:8019–8025. [PubMed] [Google Scholar]
- [24].Kuge O, Nishijima M. Biosynthetic regulation and intracellular transport of phosphatidylserine in mammalian cells. J. Biochem. 2003;133:397–403. doi: 10.1093/jb/mvg052. [DOI] [PubMed] [Google Scholar]
- [25].Szabadkai G, Duchen MR. Mitochondria: the hub of cellular Ca2+ signaling. Physiology (Bethesda) 2008;23:84–94. doi: 10.1152/physiol.00046.2007. [DOI] [PubMed] [Google Scholar]
- [26].Alzayady KJ, Wojcikiewicz RJH. The role of Ca2+ in triggering inositol 1,4,5-trisphosphate receptor ubiquitination. Biochem. J. 2005;392:601–606. doi: 10.1042/BJ20050949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bhanumathy CD, Nakao SK, Joseph SK. Mechanism of proteasomal degradation of inositol trisphosphate receptors in CHOK1 cells. J. Biol. Chem. 2006;281:3722–3730. doi: 10.1074/jbc.M509966200. [DOI] [PubMed] [Google Scholar]
- [28].Novakova M, Ela C, Bowen WD, Hasin Y, Eilam Y. Highly selective sigma receptor ligands elevate inositol 1, 4, 5-trisphosphate production in rat cardiac myocytes. Eur. J. Pharmacol. 1998;353:315–327. doi: 10.1016/s0014-2999(98)00398-7. [DOI] [PubMed] [Google Scholar]
- [29].Vilner BJ, Bowen WD. Modulation of cellular calcium by sigma-2 receptors: release from intracellular stores in human SKN-SH neuroblastoma cells. J. Pharmacol. Exp. Ther. 2000;292:900–911. [PubMed] [Google Scholar]
- [30].Maurice T, Su TP, Parish DW, Nabeshima T, Privat A. PRE-084, a sigma selective PCP derivative, attenuates MK-801-induced impairment of learning in mice. Pharmacol. Biochem. Behav. 1994;49:859–869. doi: 10.1016/0091-3057(94)90235-6. [DOI] [PubMed] [Google Scholar]
- [31].Tsao LI, Su TP. IP3 receptor antagonist heparin uncompetitively inhibits [3H] (+)-SKF-10047 binding to sigma receptors. Eur. J. Pharmacol. 1996;311:R1–2. doi: 10.1016/0014-2999(96)00533-x. [DOI] [PubMed] [Google Scholar]
- [32].Hanner M, Moebius FF, Flandorfer A, Knaus HG, Striessnig J, Kempner E, Glossmann H. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc. Natl. Acad. Sci. USA. 1996;93:8072–8077. doi: 10.1073/pnas.93.15.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Maurice T, Martin-Fardon R, Romieu P, Matsumoto RR. Sigma(1) (sigma(1)) receptor antagonists represent a new strategy against cocaine addiction and toxicity. Neurosci. Biobehav. Rev. 2002;26:499–527. doi: 10.1016/s0149-7634(02)00017-9. [DOI] [PubMed] [Google Scholar]
- [34].Martin-Fardon R, Maurice T, Aujla H, Bowen WD, Weiss F. Differential effects of sigma1 receptor blockade on self-administration and conditioned reinstatement motivated by cocaine vs natural reward. Neuropsychopharmacology. 2007;32:1967–1973. doi: 10.1038/sj.npp.1301323. [DOI] [PubMed] [Google Scholar]
- [35].Liu Y, Matsumoto RR. Alterations in fos-related antigen 2 and sigma1 receptor gene and protein expression are associated with the development of cocaine-induced behavioral sensitization: time course and regional distribution studies. J. Pharmacol. Exp. Ther. 2008;327:187–195. doi: 10.1124/jpet.108.141051. [DOI] [PubMed] [Google Scholar]
- [36].Bergeron R, Debonnel G, De Montigny C. Modification of the N-methyl-D-aspartate response by antidepressant sigma receptor ligands. Eur. J. Pharmacol. 1993;240:319–323. doi: 10.1016/0014-2999(93)90918-8. [DOI] [PubMed] [Google Scholar]
- [37].Stahl SM, Grady MM, Moret C, Briley M. SNRIs: their pharmacology, clinical efficacy, and tolerability in comparison with other classes of antidepressants. CNS Spectr. 2005;10:319–323. doi: 10.1017/s1092852900019726. [DOI] [PubMed] [Google Scholar]
- [38].Skuza G, Rogoz Z. Antidepressant-like effect of combined treatment with selective sigma receptor agonists and a 5-HT1A receptor agonist in the forced swimming test in rats. Pharmacol. Rep. 2007;59:773–777. [PubMed] [Google Scholar]
- [39].Kulkarni SK, Dhir A. On the mechanism of antidepressant-like action of berberine chloride. Eur. J. Phramacol. 2008;589:163–172. doi: 10.1016/j.ejphar.2008.05.043. [DOI] [PubMed] [Google Scholar]
- [40].Maurice T, Phan VL, Noda Y, Yamada K, Privat A, Nabeshima T. The attenuation of learning impairments induced after exposure to CO or trimethyltin in mice by sigma (sigma) receptor ligands involves both sigma1 and sigma2 sites. Br. J. Pharmacol. 1999;127:335–342. doi: 10.1038/sj.bjp.0702553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kitaichi K, Chabot JG, Moebius FF, Flandorfer A, Glossmann H, Quirion R. Expression of the purported sigma(1) (sigma(1)) receptor in the mammalian brain and its possible relevance in deficits induced by antagonism of the NMDA receptor complex as revealed using an antisense strategy. J. Chem. Neuroanat. 2000;20:375–387. doi: 10.1016/s0891-0618(00)00106-x. [DOI] [PubMed] [Google Scholar]
- [42].Skuza G, Wedzony K. Behavioral pharmacology of sigma-ligands. Pharmacopsychiatry. 2004;37(Suppl 3):S183–188. doi: 10.1055/s-2004-832676. [DOI] [PubMed] [Google Scholar]
- [43].King MA, Bradshaw S, Chang AH, Pintar JE, Pasternak GW. Potentiation of opioid analgesia in dopamine2 receptor knock-out mice: evidence for a tonically active anti-opioid system. J. Neurosci. 2001;21:7788–7792. doi: 10.1523/JNEUROSCI.21-19-07788.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gear RW, Lee JS, Miaskowski C, Gordon NC, Paul SM, Levine JD. Neuroleptics antagonize nalbuphine antianalgesia. J. Pain. 2006;7:187–191. doi: 10.1016/j.jpain.2005.10.005. [DOI] [PubMed] [Google Scholar]
- [45].Prezzavento O, Parenti C, Marrazzo A, Ronsisvalle S, Vittorio F, Arico G, Scoto GM, Ronsisvalle G. A new sigma ligand, (+/−)-PPCC, antagonizes kappa opioid receptor-mediated antinociceptive effect. Life Sci. 2008;82:549–553. doi: 10.1016/j.lfs.2007.11.032. [DOI] [PubMed] [Google Scholar]
- [46].Bhardwaj A, Sawada M, London ED, Koehler RC, Traystman RJ, Kirsch JR. Potent sigma1-receptor ligand 4-phenyl-1-(4-phenylbutyl) piperidine modulates basal and N-methyl-D-aspartate-evoked nitric oxide production in vivo. Stroke. 1998;29:2404–2410. [PubMed] [Google Scholar]
- [47].Anderson TR, Jarvis CR, Biedermann AJ, Molnar C, Andrew RD. Blocking the anoxic depolarization protects without functional compromise following simulated stroke in cortical brain slices. J. Neurophysiol. 2005;93:963–979. doi: 10.1152/jn.00654.2004. [DOI] [PubMed] [Google Scholar]
- [48].Ajmo CT, Vernon DO, Collier L, Pennypacker KR, Cuevas J. Sigma receptor activation reduces infarct size at 24 hours after permanent middle cerebral artery occlusion in rats. Curr. Neurovasc. Res. 2006;3:89–98. doi: 10.2174/156720206776875849. [DOI] [PubMed] [Google Scholar]
- [49].Bowen WD. Sigma receptors: recent advances and new clinical potentials. Pharm. Acta Helv. 2000;74:211–218. doi: 10.1016/s0031-6865(99)00034-5. [DOI] [PubMed] [Google Scholar]
- [50].Palmer CP, Mahen R, Schnell E, Djamgoz MB, Aydar E. Sigma-1 receptors bind cholesterol and remodel lipid rafts in breast cancer cell lines. Cancer Res. 2007;67:11166–11175. doi: 10.1158/0008-5472.CAN-07-1771. [DOI] [PubMed] [Google Scholar]
- [51].Achison M, Boylan MT, Hupp TR, Spruce BA. HIF-1alpha contributes to tumour-selective killing by the sigma receptor antagonist rimcazole. Oncogene. 2007;26:1137–1146. doi: 10.1038/sj.onc.1209890. [DOI] [PubMed] [Google Scholar]
- [52].Boehning D, Patterson RL, Sedaghat L, Glebova NO, Kurosaki T, Snyder SH. Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat. Cell Biol. 2003;5:1051–1061. doi: 10.1038/ncb1063. [DOI] [PubMed] [Google Scholar]
- [53].Sedlak TW, Snyder SH. Messenger molecules and cell death: therapeutic implications. JAMA. 2006;295:81–89. doi: 10.1001/jama.295.1.81. [DOI] [PubMed] [Google Scholar]
- [54].Aydar E, Palmer CP, Klyachko VA, Jackson MB. The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron. 2002;34:399–410. doi: 10.1016/s0896-6273(02)00677-3. [DOI] [PubMed] [Google Scholar]
- [55].Herrera Y, Katnik C, Rodriguez JR, Hall AA, Willing A, Pannypacker KR, Cuevas J. Sigma-1 receptor modulation of acid-sensing ion channel a (ASIC1a) and ASIC1a-induced Ca2+ influx in rat cortical neurons. J. Pharmacol. Exp. Ther. 2008;327:491–502. doi: 10.1124/jpet.108.143974. [DOI] [PubMed] [Google Scholar]
- [56].Klouz A, Said DB, Ferchichi H, Kourda N, Ouanes L, Lakhal M, Tillement JP, Morin D. Protection of cellular and mitochondrial functions against liver ischemia by N-benzyl-N'-(2-hydroxy-3,4-dimethoxybenzyl)-piperazine (BHDP), a sigma1 ligand. Eur. J. Pharmacol. 2008;578:292–299. doi: 10.1016/j.ejphar.2007.09.038. [DOI] [PubMed] [Google Scholar]
- [57].Rogers C, Lemaire S. Role of the sigma receptor in the inhibition of [3H]-noradrenaline uptake in brain synaptosomes and adrenal chromaffin cells. Br. J. Pharmacol. 1991;103:1917–1922. doi: 10.1111/j.1476-5381.1991.tb12352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Chan SL, Morgan NG. Sigma receptor ligands and imidazoline secretagogues mediate their insulin secretory effects by activating distinct receptor systems in isolated islets. Eur. J. Pharmacol. 1998;350:267–272. doi: 10.1016/s0014-2999(98)00263-5. [DOI] [PubMed] [Google Scholar]
- [59].Zhu LX, Sharma S, Gardner B, Escuadro B, Atianzar K, Tashkin DP, Dubinett SM. IL-10 mediates sigma 1 receptor-dependent suppression of antitumor immunity. J. Immunol. 2003;170:3585–3591. doi: 10.4049/jimmunol.170.7.3585. [DOI] [PubMed] [Google Scholar]
- [60].Langa F, Codony X, Tovar V, Lavado A, Giménez E, Cozar P, Cantero M, Dordal A, Hernández E, Pérez R, Monroy X, Zamanillo D, Guitart X, Montoliu L. Generation and phenotypic analysis of sigma receptor type I (sigma 1) knockout mice. Eur. J. Nuerosci. 2003;18:2188–2196. doi: 10.1046/j.1460-9568.2003.02950.x. [DOI] [PubMed] [Google Scholar]
- [61].Cendan CM, Pujalte JM, Portillo-Salido E, Montoliu L, Baeyens JM. Formalin-induced pain is reduced in sigma (1) receptor knockout mice. Eur. J. Pharmacol. 2005;511:73–74. doi: 10.1016/j.ejphar.2005.01.036. [DOI] [PubMed] [Google Scholar]
- [62].Sabino V, Cottone P, Parylak SL, Steardo L, Zorrilla EP. Sigma-1 receptor knockout mice display a depressive-like phenotype. Behav. Brain Res. 2008;198:472–476. doi: 10.1016/j.bbr.2008.11.036. Epub 2008 Nov 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J. Pharmacol. Exp. Ther. 1976;197:517–532. [PubMed] [Google Scholar]