Abstract
p38α MAPK is an important regulator of cellular responses induced by external cues, but the elucidation of physiological functions for p38α has been complicated by the possible functional redundancy in vivo with the related family member p38β. We found that mice with combined deletion of p38α and p38β display diverse developmental defects at midgestation, including major cardiovascular abnormalities, which are observed neither in single knockout nor in double heterozygous embryos. Expression analysis indicates specific functions of p38α and p38β in the regulation of cardiac gene expression during development. By using knock-in animals that express p38β under control of the endogenous p38α promoter, we also found that p38β cannot perform all of the functions of p38α during embryogenesis. Our results identify essential roles for p38α and p38β during development and suggest that some specific functions may be explained by differences in expression patterns.
Keywords: embryonic development, heart signaling
Proliferation and differentiation processes need to be tightly regulated during early development. The p38 mitogen-activated protein kinase (MAPK) pathway was originally identified as an important regulator of the stress responses, but it was later found to play an important role in coordinating cell cycle progression and differentiation of many cell types, including skeletal muscle, hepatocytes, and lung epithelial cells (reviewed by ref. 1). There are four p38 MAPK family members, which are encoded by different genes. p38α and p38β are ≈70% identical in their amino acid sequence and have similar substrate specificity, suggesting that they may have overlapping functions. Moreover, they are both sensitive to SB203580, a chemical inhibitor widely used to characterize p38 MAPK functions. However, whereas p38α is usually expressed at high levels in many cell types, the expression levels of p38β seem to be lower in most tissues. Mice knockout for p38α display embryonic lethality due to placental defects (2, 3), whereas conditional deletion of p38α in embryonic lineages results in animals that die soon after birth, probably due to defects in lung development (4). However, p38β knockout animals are fully viable and have no obvious phenotypes (5), except for the reduced bone mass recently reported (6). Previous reports using SB203580 have proposed the implication of p38α and p38β in various developmental processes, including limb morphogenesis (7), somitogenesis (8), and gastrulation (9). However, this inhibitor is known to target other signaling proteins in addition to p38α and p38β (10).
To investigate specific and overlapping functions of p38α and p38β during early development, we have generated double knockout animals that delete p38α in embryonic tissues, as well as knock-in animals expressing p38β under control of the endogenous p38α promoter. Our results indicate that p38α and p38β have synergistic roles during mouse development, cooperating for example in embryonic heart development. We also found that p38β cannot perform specific p38α functions during development.
Results and Discussion
Developmental Defects in p38α and p38β Double Knockout Mice.
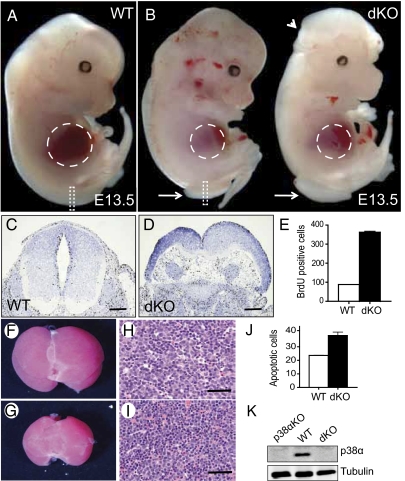
To identify new functions of p38α and p38β during development and to circumvent the early embryonic lethality of p38α null mice, we restricted p38α deletion to epiblast-derived tissues by crossing p38α(lox/lox) mice with the Sox2-Cre line and them with p38β(−/−) animals. No double knockout mice (p38α(Δ/Δ)p38β(−/−)Sox2-Cre) were observed after birth, suggesting that the elimination of both p38 MAPKs resulted in embryonic lethality. Moreover, we did not find any p38α and p38β double knockout embryos further than embryonic day (E) 16.5 in timed mating analysis (Table S1). Interestingly, all E13.5 embryos deficient in both p38α and p38β displayed spina bifida as well as reduced liver size compared with wild-type littermates. In addition, 30% of the double knockout embryos also showed exencephaly (Fig. 1 A and B and Table S1). Spina bifida correlated with neural hyperproliferation (Fig. 1 C–E). Haematoxylin and eosin staining of p38α and p38β-deficient livers showed abnormal cellularity, with dissociated hepatocytes and infiltration of hematopoietic cells (Fig. 1 F–I). Increased apoptosis in the double knockout livers was confirmed by TUNEL assays (Fig. 1J). We also confirmed that p38α was not detectable by immunoblotting in these embryos (Fig. 1K). The above developmental defects were observed neither in double heterozygous nor in p38α(Δ/Δ)Sox2-Cre embryos, in agreement with previous work (4), indicating that p38α and p38β have overlapping functions during mouse development (Table S1).
Fig. 1.
Developmental defects in p38α and p38β knockout embryos. (A and B) Wild-type (WT) and p38α(Δ/Δ)p38β(−/−) Sox2-Cre (dKO) E13.5 embryos are shown. Dashed lines outline the liver, arrows indicate spina bifida, and the arrowhead denotes exencephaly. (C and D) BrdU staining of the neural tube sections corresponding to the areas outlined by dashed rectangles in A and B. (E) Quantification of the BrdU staining in neural tubes (three sections of each genotype). (Scale bars: 500 μm.) (F and G) Liver gross morphology. (H and I) Haematoxylin and eosin staining in liver sections. (Scale bars: 50 μm.) (J) Quantification of TUNEL-positive cells in livers (three sections of each genotype). (K) Deletion of p38α by Sox2-Cre was analyzed by immunoblotting in whole lysates of E13.5 embryos. Error bars indicate SD.
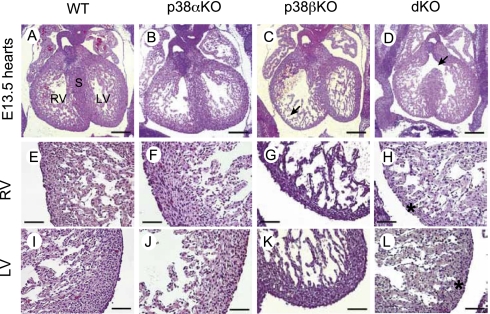
Histological analysis of embryos at E13.5 indicated that p38α and p38β null hearts displayed myocardial thinning in both ventricles, containing fewer myocardial cells compared with wild type hearts (compare Fig. 2 E and I with Fig. 2 H and L). Moreover, double knockout embryos displayed ventricular septal defects (Fig. 2D). The hearts of p38α(Δ/Δ)Sox2-Cre (p38α KO) embryos looked pretty much the same as in the wild-type embryos (Fig. 2B). Unexpectedly, p38β(−/−) (p38β KO) hearts displayed myocardial thinning in the right ventricle (Fig. 2C), contained fewer cell layers in the compact myocardium, and a possible excess of trabecules, although not as much as in the double knockout (Fig. 2G). This phenotype should be compatible with the normal survival reported for p38β KO mice (5).
Fig. 2.
Cardiac defects in p38α and p38β knockout embryos. (A–D) Hematoxylin and eosin staining of transverse sections of hearts from wild-type (WT), p38α(Δ/Δ) Sox2-Cre (p38αKO), p38β(−/−) (p38βKO), and p38α(Δ/Δ)p38β(−/−) Sox2-Cre (dKO) E13.5 littermate embryos. Note that ventricular septal defects are observed in double KO hearts (D, arrow). (E–H) Higher magnification of right ventricles showing thinner compact myocardium in p38β KO and dKO hearts (asterisk). (I–L) Higher magnification of left ventricles showing thinner myocardium in dKO hearts (asterisk). S, septum; RV, right ventricle; LV, right ventricle. (Scale bars: A–D, 500 μm; E–L, 100 μm.)
Analysis of Cardiac Gene Expression in p38α and p38β Null Mice.
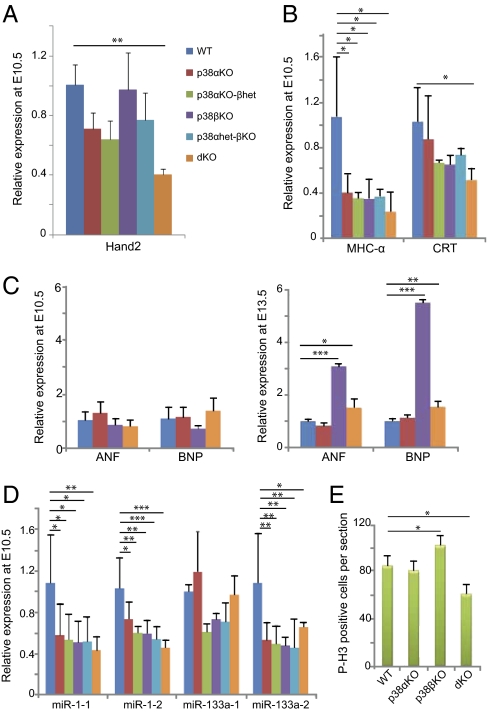
Previous reports have implicated p38α and p38β in different aspects of adult cardiogenesis (11), but the role of p38 MAPKs in embryonic heart development has been less studied. To investigate the basis for the cardiac defects observed in the p38α and p38β null embryos, we analyzed the expression levels of transcription factors that are important for normal heart development, such as Nkx2.5, Tbx5, and the GATA, Mef2, and Hand families (12–14). Transcription factors of the GATA and Mef2 families can be targeted by p38 MAPKs (15, 16). Quantitative RT-PCR analysis using RNA isolated from E10.5 hearts revealed no significant differences in most of these transcription factors, except for a reduction in Hand2 mRNA levels (Fig. 3A and Fig. S1A). Interestingly, Hand2 down-regulation has been associated with ventricular septal defects (17, 18), which agrees with the phenotype that we observed in double knockout hearts.
Fig. 3.
Modulation of cardiac gene expression and proliferation. Expression of the indicated genes was analyzed by quantitative RT-PCR in biological triplicates of the following genotypes: wild-type (WT), p38α(Δ/Δ) Sox2-Cre (p38αKO), p38α(Δ/Δ) p38β(−/−) Sox2-Cre (p38αKO-βhet), p38β(−/−) (p38βKO), p38α(Δ/+) p38β(−/−) Sox2-Cre (p38αhet-βKO), and p38α(Δ/Δ)p38β(−/−) Sox2-Cre (dKO). RNA was isolated from three individual hearts in E10.5 embryos, and from three pools of three hearts each (nine hearts total) in E13.5 embryos. (A) Hand2 transcription factor is down-regulated in E10.5 dKO hearts. (B) Contractile protein MHC-α is down-regulated in all mutant hearts, whereas the differentiation marker CRT is down-regulated in dKO hearts. (C) Up-regulation of the hypertrophic markers ANF and BNP in p38βKO and dKO hearts at E13.5 but not at E10.5. (D) Down-regulation of miR-1-1, miR-1-2, and miR-133a-2 in all mutant hearts. (E) Phospho-histone H3 (P-H3) staining of E13.5 heart sections. MHC-α, myosin heavy chain-α; CRT; calreticulin; ANF, atrial natrium factor; BNP, B-type natriuretic protein. Error bars indicate SD. Statistical significance (n = 3) was determined by using one-way ANOVA-Tukey's test. Changes are referred to the expression levels in WT hearts (given the value of 1 for each gene) and indicated as *P < 0.05, **P < 0.01, and ***P < 0.001.
The expression of structural genes involved in normal heart function is controlled by cooperation of the above transcription factors at the promoter level (13, 14). We observed an important reduction in the expression level of the contractile protein myosin heavy chain α (MHC-α) in different p38α and p38β mutants, whereas the expression of calreticulin (CRT) was only significantly reduced in the absence of both p38α and p38β (Fig. 3B). However, expression of myosin heavy chain β (MHC-β) and myosin light chain 2V (Mlc2v) was not significantly altered (Fig. S1B). We also found that p38β KO hearts displayed a dramatic increase in expression levels of the differentiation markers atrial natriuretic factor (ANF) and B-type natriuretic peptide (BNP) at E13.5 compared with E10.5 (Fig. 3C), correlating with a possible excess in trabecular myocardium observed in p38β mutant hearts. This defect might be relevant for cardiac malfunction in adult p38β KO hearts as suggested by experiments using cultured cells and a mouse model of pressure-overload hypertrophy (19, 20).
Recently, microRNAs have been postulated as key players in the control of cardiac processes (21). In particular, the transcription factors Mef2c and Mef2d have been shown to control the miR-1 and miR-133a families (22), which, in turn, have been implicated in the regulation of heart development and function (23–25). Because Mef2 transcription factors can be phosphorylated by p38 MAPKs (16), we analyzed hearts of E10.5 embryos and observed that miR-1 and miR-133a expression levels were down-regulated in p38α and p38β mutant hearts (Fig. 3D). Surprisingly, p38β KO hearts of E13.5 embryos showed a dramatic reduction of miR-1-1, miR-133a-1, and miR-133a-2 transcripts (Fig. S1C). Reduced miR-133a expression levels have been associated with heart hypertrophy and ventricular septal defects (23, 25), and could therefore contribute to the phenotypes observed in p38α and p38β knockout mice.
Aberrant Proliferation and Deregulation of Cell Cycle Associated Genes in p38 MAPK Mutant Hearts.
Because cardiac phenotypes are usually associated with altered cell proliferation that results in abnormal heart formation, we performed phospho-histone H3 staining in hearts of E13.5 embryos. We found no differences in proliferation between p38α KO and wild-type hearts, whereas p38β KO hearts showed increased cardiomyocyte proliferation (Fig. 3E). However, double knockout hearts showed reduced proliferation but normal apoptosis levels compared with wild-type hearts (Fig. 3E and Fig. S1D). These differences in cardiomyocyte proliferation correlated with the differential expression of cell cycle regulators (26). Thus, the increased proliferation in p38β KO hearts correlated with the up-regulation of Cyclin A2, Cyclin D2, and Cdk4 (Fig. S1E), which have been reported to promote cell cycle progression in cardiomyocytes (27, 28). Reduced expression of miR-133a transcripts (25) may contribute to the elevated expression of cyclin D2 in p38β KO hearts. However, cyclins A and D were generally down-regulated in double knockout hearts (Fig. S1E), in agreement with the reduced proliferation observed. Interestingly, ventricular septal defects have been also reported in Cyclin D1, D2, and D3 triple knockout hearts (29).
Generation of p38βKIα Mice.
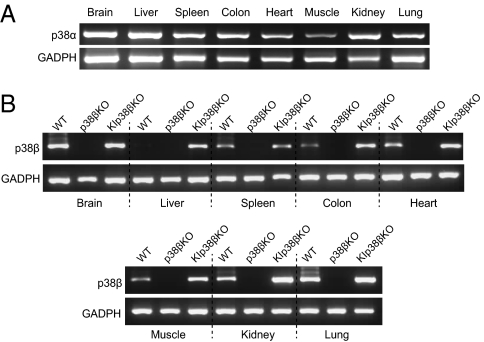
To explore whether p38α and p38β are functionally interchangeable, we generated a knock-in model with p38β integrated in the p38α chromosomal locus (p38βKIα), so that p38β expression was driven by the endogenous p38α promoter (Fig. S2). Previous studies have reported that p38β protein levels are high in adult brain but rather low in other adult tissues (5). To confirm that p38β was expressed under the control of the endogenous p38α promoter, we analyzed expression in several tissues obtained from p38α(βKI/+) p38β(−/−) mice. In agreement with the ubiquitous expression of the p38α mRNA (Fig. 4A), we found that the p38βKIα allele sufficed to recover p38β expression, in a p38β null background, in all tissues analyzed (Fig. 4B). Quantitative RT-PCR analysis confirmed increased p38β mRNA levels in several tissues of the p38α(βKI/+) p38β(−/−) mice compared with wild-type animals (Fig. S3A). We also detected by immunoblotting expression of the p38β protein in adult brain and spleen of p38α(βKI/+) p38β(−/−) mice (Fig. S3B). The enhanced p38β expression levels detected in most of the p38βKIα knock-in tissues analyzed agrees with the fact that p38α is the p38 MAPK family member expressed at higher levels in the majority of adult tissues (5). Taken together, these results demonstrate that the p38βKIα allele can express p38β under control of the endogenous p38α promoter.
Fig. 4.
Generation of mice expressing p38β under control of the endogenous p38α promoter (p38βKIα allele). (A) RT-PCR analysis of p38α mRNA extracted from the indicated WT adult tissues. (B) RT-PCR analysis of p38β mRNA extracted from the indicated adult tissues of mice wild-type (WT), p38β(−/−) (p38βKO), and p38α(βKI/+)p38β(−/−) (KIp38βKO).
Identification of p38α and p38β Specific Functions.
To investigate whether the p38βKIα allele was able to rescue the embryonic lethality of p38α knockouts, we generated p38α(βKI/βKI) mice. We found that none of these animals were alive at birth, and timed mating analysis showed that E11.5 p38α(βKI/βKI) embryos suffered an important developmental retardation compared with p38α-expressing littermates (Fig. S4A). Histological analysis of the placenta revealed reduced size of the labyrinthine layer, whereas the labyrinthine trophoblast was thicker (Fig. S4 B–E), as it has been reported for the p38α KO mice (2, 3). These results indicate that the function of p38α in placenta was not compensated by enhanced expression of p38β (Fig. S4F), although p38α(βKI/βKI) placentas displayed similar phospho-p38 MAPK levels as the p38α-expressing placentas (Fig. S4 G–I). It therefore appears that p38β cannot perform p38α functions during placental development, most likely due to differences in downstream target activation.
We next investigated the ability of the p38βKIα allele to compensate for the development defects observed in the absence of p38α and p38β. First, we confirmed by immunoblotting the expression of the p38β protein in p38α(βKI/+) p38β(−/−) embryos (Fig. 5A). Interestingly, the endogenous p38β protein appeared to be significantly expressed in several embryo tissues. We then generated the p38α(βKI/Δ) p38β(−/−) Sox2-Cre line, where one p38α allele was normally expressed in placenta to preserve its function and the other was replaced by the p38βKIα allele, in a p38β null background. We found that expression of p38β under control of the endogenous p38α promoter rescued the spina bifida phenotype, and we also observed the expected number of embryos alive at E18.5, when all p38α and p38β double knockout embryos were dead (Table S2). However, we could find no p38α(βKI/Δ) p38β(−/−) Sox2-Cre animals at weaning. We confirmed that p38β was significantly expressed in the heart, liver, and lung of p38α(βKI/Δ) p38β(−/−) Sox2-Cre embryos (Fig. 5 A and B), but the heart defects were still present in these embryos (Fig. 5C). These results indicate that p38β expression driven by the endogenous p38α promoter is not sufficient to support heart development in the absence of endogenous p38α and p38β expression. One possibility is that the p38βKIα allele cannot reconstitute appropriate levels of p38 MAPK activity in heart cells required for ventricular septal formation. An alternative, more interesting speculation would be that p38α and p38β are active in different cell types required to form a proper heart (12, 30). The later possibility is supported by the observation that p38β KO hearts have a phenotype, which is absent in p38α KO hearts (Fig. 2C).
Fig. 5.
Expression of p38β under control of the endogenous p38α promoter does not rescue cardiac defects in embryos. (A) Immunoblotting of p38β protein in the indicated whole embryos and embryo tissues wild-type (WT), p38α(Δ/Δ)Sox2-Cre (p38αKO), p38β(−/−) (p38βKO), and p38α(βKI/+)p38β(−/−) (KIp38βKO, one copy p38α, and the other p38βKIα). (B) Quantitative RT-PCR analysis of p38β mRNA expression in heart, liver, and lung of E13.5 embryos p38αKO, p38βKO, and p38αKI/Δ p38βKO, which express p38β only under control of the p38α promoter (p38α(βKI/Δ)p38β(−/−)Sox2-Cre). Expression levels of p38β were referred to those in WT tissues, which were given the value of 1. (C) Histological analysis showing that p38αKI/Δ p38βKO embryos have similar cardiac defects as the p38α and p38β double knockout embryos.
We also analyzed the compensatory effect of the p38βKIα allele in mice where the p38α function was preserved in placenta but not in the embryo, and the endogenous p38β expression was also maintained. For this purpose, we generated the p38α(βKI/Δ) p38β(+/+) Sox2-Cre line. Down-regulation of embryonic p38α has been reported to result in perinatal death associated with severe lung defects (4). Surprisingly, ≈30% of the expected number of p38α(βKI/Δ) p38β(+/+) Sox2-Cre mice were able to survive to adulthood, suggesting that enhanced p38β expression might compensate, to certain extent, the essential function of p38α in lung development.
Our genetic analysis shows that p38α and p38β play key roles in embryonic heart development with double knockout embryos displaying serious heart abnormalities, such as myocardial thinning, trabecular disorganization, and ventricular septal defects. Heart development is extremely sensitive to gene dosage effects, with ventricular septal defects being the most frequent cardiac malformation in humans (31). Expression analysis of p38α and p38β knockout embryos revealed deregulation of important controllers of normal heart development. For example, down-regulation of the bHLH transcription factor Hand2 has been implicated in ventricular development and heart septation (18), and subtle differences in the expression levels of miRNAs have been reported to perturb heart development (21). The GATA and Mef2 families of transcription factors are also known to play important roles in vertebrate heart development (13, 32). Accordingly, Mef2c is required for early heart development, but dispensable in late development (33, 34), and Mef2a null mice die perinatally from a spectrum of heart effects (35). Both p38α and p38β have been reported to phosphorylate and activate the transcription factors Mef2a and Mef2c (16). Subtle differences in expression levels of the transcription factors GATA6 and GATA4, which can be both targeted by p38 MAPKs (15), have been also reported to perturb ventricular septal formation (36). Our analysis did not reveal deregulated expression of these transcription factors, but we did detect altered expression of some of their targets, such as Hand2, CRT, and members of the miR-1 and miR-133a families (13, 22, 33, 37), suggesting a reduction in GATA and Mef2 transcriptional activities, probably due to impaired posttranslational modifications. In double knockout embryonic hearts, Hand2 down-regulation could account for the ventricular septal defect and reduced compact myocardium. However, these embryonic hearts do not present the hypoplastic right ventricule associated with Hand2 depletion (18). We speculate that the residual Hand2 expression observed in the double knockout hearts might compensate for the right ventricule defect but is not sufficient to support ventricular septum formation. In agreement with this idea, Hand2 has been reported to play a role in cardiac neural crest cells and the formation of the ventricular septum without affecting right ventricule size (17). Identification of the heart cell type where Hand2 expression is regulated by p38α and p38β will help to clarify this issue.
In summary, we have identified unique overlapping functions for p38α and p38β that are essential during embryonic development. We also show that placental development requires specific p38α functions that cannot be performed by p38β, even when expressed under control of the endogenous p38α promoter. Intriguingly, embryos that express p38β only under control of the p38α promoter display a similar heart phenotype as the double knockout embryos, implying that heart development requires endogenous p38β expression. Taken together, our results indicate that the pattern of expression makes an important contribution to the specific roles performed by p38α and p38β, although intrinsic differences between both proteins also impinge on their functions. Of note, we found that p38α and p38β double knockout embryos display no limb defects, undergoing normal somitogenesis and gastrulation processes compatible with embryonic development until midgestation, in contrast with previous reports based on the use of the chemical inhibitor SB203580 (7–9).
Materials and Methods
Gene Targeting and Mouse Breeding.
p38α(lox/lox) (38, 39), p38β(−/−) (5), and Sox2-Cre (40) lines were bred to give rise to p38α(Δ/+)p38β(+/−)Sox2-Cre males and p38α(lox/lox)p38β(+/−) females, which were interbred to obtain p38α(Δ/Δ)p38β(−/−)Sox2-Cre embryos. Generation of the p38βKIα targeting vector is described in Fig. S2. Genotyping of p38α(βKI/+) mice was performed by PCR on genomic tail DNA. Primers and conditions are available upon request. p38α(βKI/+) mutant mice were maintained in a C57BL/6 background. p38α(βKI/βKI) and p38α(βKI/+)p38β(−/−) animals were obtained crossing p38α(βKI/+) p38β(+/−) animals. p38α(βKI/Δ)Sox2-Cre and p38α(βKI/Δ)p38β(−/−) Sox2-Cre animals were obtained crossing p38α(βKI/+) p38β(+/−) Sox2-Cre males with p38α(lox/lox)p38β(+/−) females. Adults, newborns, and embryos were genotyped for p38α, p38β, and Sox2-Cre by gene-specific PCR (5, 39, 40).
mRNA Expression.
Total RNA was purified from tissues by using TRIzol reagent according to the manufacturer's instructions. RNA was treated with RNase-free DNase (Roche) before the reverse transcription step. Quantitative RT-PCRs were performed in duplicates by using SuperScript kit (Invitrogen) and SybrGreen probes. To analyze cardiac gene expression in E13.5 embryos, three pools of three embryonic hearts each were used for every genotype. In the case of E10.5 embryos, RNA from three single hearts of each genotype was extracted and amplified by using the TransPlex Complete Whole Transcriptome Amplification Kit (Sigma-Aldrich). Primers are indicated in Table S3.
Histology and Immunohistochemistry.
Hematoxylin and eosin staining was carried out by using E13.5 embryos and E11.5 placentas, which were fixed overnight in 4% paraformaldehyde, dehydrated, embedded in paraffin, and sectioned at 6 μm (41).
For BrdU labeling, an i.p. injection (2 mg of BrdU) was administrated 2 h before killing. BrdU staining was performed by using a mouse monoclonal antibody (GE Healthcare; clone BU-1; dilution 1:50) and the GaM HRP secondary antibody (Dako). Phospho-histone H3 staining was performed by using rabbit antibody against Ser10 (Millipore; dilution 1:200) together with anti-rabbit from ABC method (Vector). Phospho-p38 MAPK staining was performed by using a rabbit antibody (Cell Signaling no. 4631; dilution 1:50) and the BrightVision Poly-HRP-Anti Rb secondary antibody (Immunologic).
TUNEL staining was performed on 12-mm frozen sections by using the Apop Tag reagents (Chemicon) and rhodamine-conjugated anti-digoxigenin antibodies. Apoptotic cells in liver sections were counted at 200× magnification on 3–5 randomly chosen fields of 600 mm2. Statistical comparisons were made by using the paired Student t test.
Immunoblotting.
Lysates were prepared from whole E13.5 and E10.5 embryos or from different organs and were analyzed as described (39) by using antibodies against p38α (1:1,000; Cell Signaling no. 9218), p38β (1:30; affinity purified home-made rabbit antiserum raised against bacterially expressed GST-p38β), tubulin (1:1,000; Santa Cruz Biotechnologies) or GADPH (1:10,000; Sigma).
Supplementary Material
Acknowledgments
We thank Jalaj Gupta for providing embryo lysates; Antonio Nuñez for purification of p38β antibodies; Sagrario Ortega for ES cell electroporation and the injection of blastocystes; Irene Chicote, Lorena Ramirez, and Sylvie Richelme for excellent technical help; and Diego Franco (Universidad de Jaen) for fruitful discussions. This work was funded by Spanish Ministerio de Ciencia e Innovacion Grants BFU2007-60575 and BFU2010-17850; European Commission FP7 program Inflammation and Cancer Research in Europe (INFLA-CARE) Grant 223151; and the Fundación Científica de la AECC.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1015013108/-/DCSupplemental.
References
- 1.Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochem J. 2010;429:403–417. doi: 10.1042/BJ20100323. [DOI] [PubMed] [Google Scholar]
- 2.Adams RH, et al. Essential role of p38alpha MAP kinase in placental but not embryonic cardiovascular development. Mol Cell. 2000;6:109–116. [PubMed] [Google Scholar]
- 3.Mudgett JS, et al. Essential role for p38alpha mitogen-activated protein kinase in placental angiogenesis. Proc Natl Acad Sci USA. 2000;97:10454–10459. doi: 10.1073/pnas.180316397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hui L, et al. p38alpha suppresses normal and cancer cell proliferation by antagonizing the JNK-c-Jun pathway. Nat Genet. 2007;39:741–749. doi: 10.1038/ng2033. [DOI] [PubMed] [Google Scholar]
- 5.Beardmore VA, et al. Generation and characterization of p38beta (MAPK11) gene-targeted mice. Mol Cell Biol. 2005;25:10454–10464. doi: 10.1128/MCB.25.23.10454-10464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenblatt MB, et al. The p38 MAPK pathway is essential for skeletogenesis and bone homeostasis in mice. J Clin Invest. 2010;120:2457–2473. doi: 10.1172/JCI42285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zuzarte-Luís V, et al. A new role for BMP5 during limb development acting through the synergic activation of Smad and MAPK pathways. Dev Biol. 2004;272:39–52. doi: 10.1016/j.ydbio.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 8.de Angelis L, et al. Regulation of vertebrate myotome development by the p38 MAP kinase-MEF2 signaling pathway. Dev Biol. 2005;283:171–179. doi: 10.1016/j.ydbio.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Zohn IE, et al. p38 and a p38-interacting protein are critical for downregulation of E-cadherin during mouse gastrulation. Cell. 2006;125:957–969. doi: 10.1016/j.cell.2006.03.048. [DOI] [PubMed] [Google Scholar]
- 10.Godl K, et al. An efficient proteomics method to identify the cellular targets of protein kinase inhibitors. Proc Natl Acad Sci USA. 2003;100:15434–15439. doi: 10.1073/pnas.2535024100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engel FB, et al. p38 MAP kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes Dev. 2005;19:1175–1187. doi: 10.1101/gad.1306705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buckingham M, Meilhac S, Zaffran S. Building the mammalian heart from two sources of myocardial cells. Nat Rev Genet. 2005;6:826–835. doi: 10.1038/nrg1710. [DOI] [PubMed] [Google Scholar]
- 13.Peterkin T, Gibson A, Loose M, Patient R. The roles of GATA-4, -5 and -6 in vertebrate heart development. Semin Cell Dev Biol. 2005;16:83–94. doi: 10.1016/j.semcdb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Dunwoodie SL. Combinatorial signaling in the heart orchestrates cardiac induction, lineage specification and chamber formation. Semin Cell Dev Biol. 2007;18:54–66. doi: 10.1016/j.semcdb.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Charron F, et al. Tissue-specific GATA factors are transcriptional effectors of the small GTPase RhoA. Genes Dev. 2001;15:2702–2719. doi: 10.1101/gad.915701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han J, Molkentin JD. Regulation of MEF2 by p38 MAPK and its implication in cardiomyocyte biology. Trends Cardiovasc Med. 2000;10:19–22. doi: 10.1016/s1050-1738(00)00039-6. [DOI] [PubMed] [Google Scholar]
- 17.Holler KL, et al. Targeted deletion of Hand2 in cardiac neural crest-derived cells influences cardiac gene expression and outflow tract development. Dev Biol. 2010;341:291–304. doi: 10.1016/j.ydbio.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsuchihashi T, et al. Hand2 function in second heart field progenitors is essential for cardiogenesis. Dev Biol. 2011;351:62–69. doi: 10.1016/j.ydbio.2010.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, et al. Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J Biol Chem. 1998;273:2161–2168. doi: 10.1074/jbc.273.4.2161. [DOI] [PubMed] [Google Scholar]
- 20.Zhang S, et al. The role of the Grb2-p38 MAPK signaling pathway in cardiac hypertrophy and fibrosis. J Clin Invest. 2003;111:833–841. doi: 10.1172/JCI16290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu N, Olson EN. MicroRNA regulatory networks in cardiovascular development. Dev Cell. 2010;18:510–525. doi: 10.1016/j.devcel.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu N, et al. An intragenic MEF2-dependent enhancer directs muscle-specific expression of microRNAs 1 and 133. Proc Natl Acad Sci USA. 2007;104:20844–20849. doi: 10.1073/pnas.0710558105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carè A, et al. MicroRNA-133 controls cardiac hypertrophy. Nat Med. 2007;13:613–618. doi: 10.1038/nm1582. [DOI] [PubMed] [Google Scholar]
- 24.Zhao Y, et al. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2. Cell. 2007;129:303–317. doi: 10.1016/j.cell.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 25.Liu N, et al. microRNA-133a regulates cardiomyocyte proliferation and suppresses smooth muscle gene expression in the heart. Genes Dev. 2008;22:3242–3254. doi: 10.1101/gad.1738708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pasumarthi KB, Field LJ. Cardiomyocyte cell cycle regulation. Circ Res. 2002;90:1044–1054. doi: 10.1161/01.res.0000020201.44772.67. [DOI] [PubMed] [Google Scholar]
- 27.Chaudhry HW, et al. Cyclin A2 mediates cardiomyocyte mitosis in the postmitotic myocardium. J Biol Chem. 2004;279:35858–35866. doi: 10.1074/jbc.M404975200. [DOI] [PubMed] [Google Scholar]
- 28.Cheng RK, et al. Cyclin A2 induces cardiac regeneration after myocardial infarction and prevents heart failure. Circ Res. 2007;100:1741–1748. doi: 10.1161/CIRCRESAHA.107.153544. [DOI] [PubMed] [Google Scholar]
- 29.Kozar K, et al. Mouse development and cell proliferation in the absence of D-cyclins. Cell. 2004;118:477–491. doi: 10.1016/j.cell.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 30.Martin-Puig S, Wang Z, Chien KR. Lives of a heart cell: Tracing the origins of cardiac progenitors. Cell Stem Cell. 2008;2:320–331. doi: 10.1016/j.stem.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Hoffman JI, Kaplan S, Liberthson RR. Prevalence of congenital heart disease. Am Heart J. 2004;147:425–439. doi: 10.1016/j.ahj.2003.05.003. [DOI] [PubMed] [Google Scholar]
- 32.Potthoff MJ, Olson EN. MEF2: A central regulator of diverse developmental programs. Development. 2007;134:4131–4140. doi: 10.1242/dev.008367. [DOI] [PubMed] [Google Scholar]
- 33.Lin Q, Schwarz J, Bucana C, Olson EN. Control of mouse cardiac morphogenesis and myogenesis by transcription factor MEF2C. Science. 1997;276:1404–1407. doi: 10.1126/science.276.5317.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vong LH, Ragusa MJ, Schwarz JJ. Generation of conditional Mef2cloxP/loxP mice for temporal- and tissue-specific analyses. Genesis. 2005;43:43–48. doi: 10.1002/gene.20152. [DOI] [PubMed] [Google Scholar]
- 35.Naya FJ, et al. Mitochondrial deficiency and cardiac sudden death in mice lacking the MEF2A transcription factor. Nat Med. 2002;8:1303–1309. doi: 10.1038/nm789. [DOI] [PubMed] [Google Scholar]
- 36.Xin M, et al. A threshold of GATA4 and GATA6 expression is required for cardiovascular development. Proc Natl Acad Sci USA. 2006;103:11189–11194. doi: 10.1073/pnas.0604604103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lynch J, et al. Calreticulin signals upstream of calcineurin and MEF2C in a critical Ca(2+)-dependent signaling cascade. J Cell Biol. 2005;170:37–47. doi: 10.1083/jcb.200412156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heinrichsdorff J, Luedde T, Perdiguero E, Nebreda AR, Pasparakis M. p38 alpha MAPK inhibits JNK activation and collaborates with IkappaB kinase 2 to prevent endotoxin-induced liver failure. EMBO Rep. 2008;9:1048–1054. doi: 10.1038/embor.2008.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ventura JJ, et al. p38alpha MAP kinase is essential in lung stem and progenitor cell proliferation and differentiation. Nat Genet. 2007;39:750–758. doi: 10.1038/ng2037. [DOI] [PubMed] [Google Scholar]
- 40.Hayashi S, Lewis P, Pevny L, McMahon AP. Efficient gene modulation in mouse epiblast using a Sox2Cre transgenic mouse strain. Mech Dev. 2002;119(Suppl 1):S97–S101. doi: 10.1016/s0925-4773(03)00099-6. [DOI] [PubMed] [Google Scholar]
- 41.Hogan B, Beddington R, Costantini F, Lacy E, editors. Manipulating the Mouse Embryo. A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1994. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.