Abstract
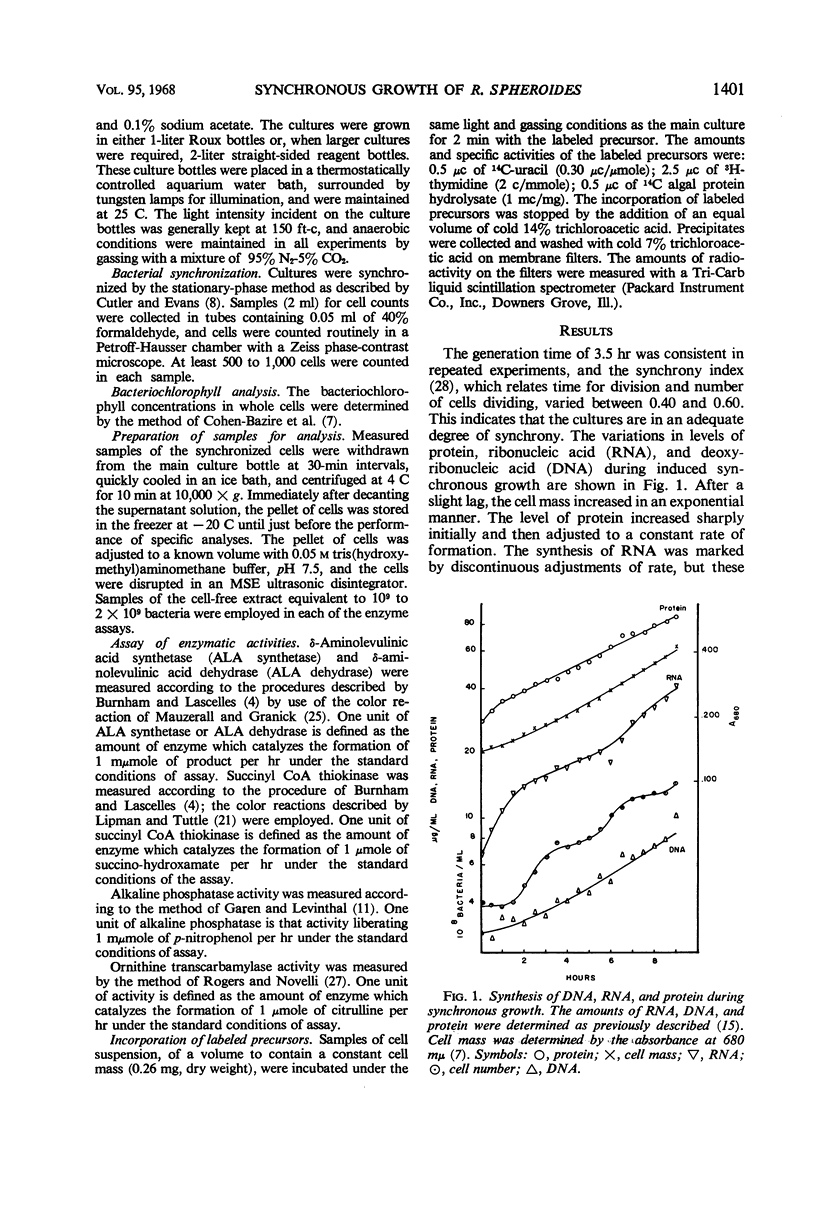
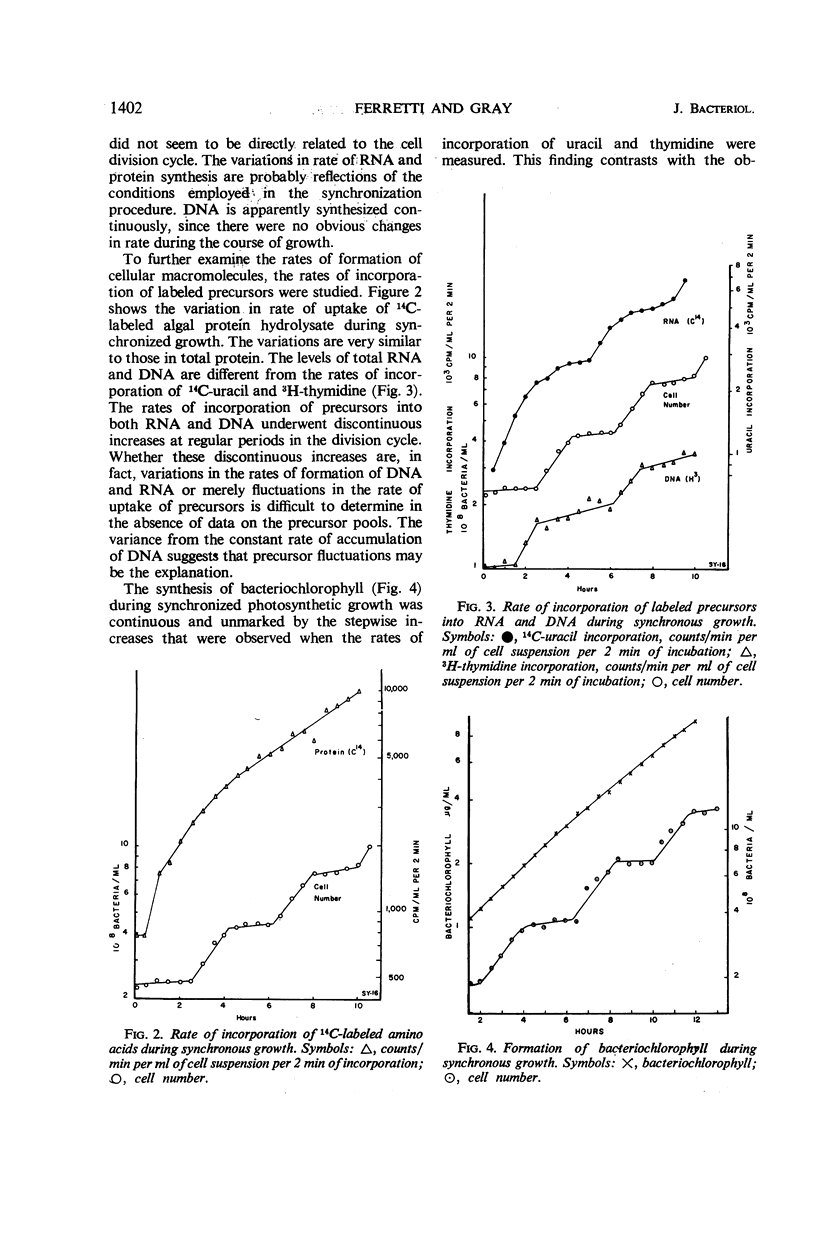
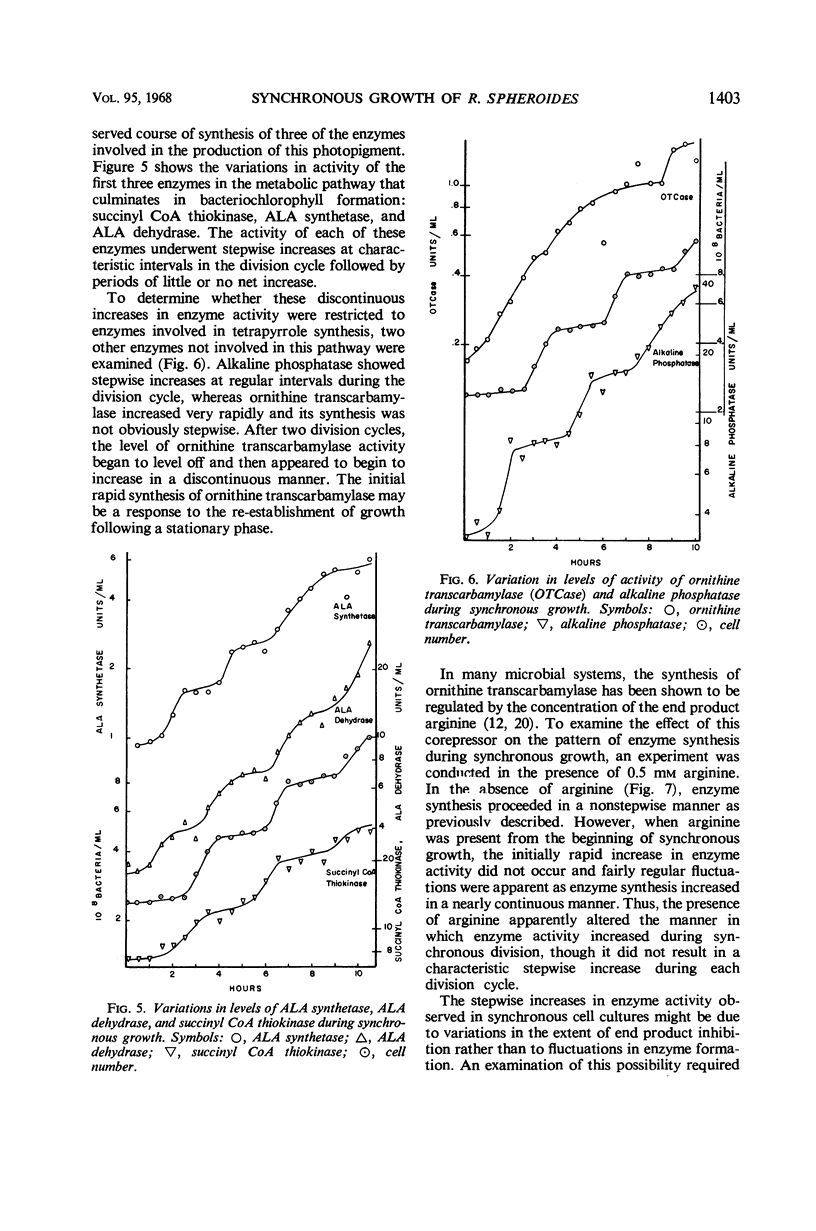
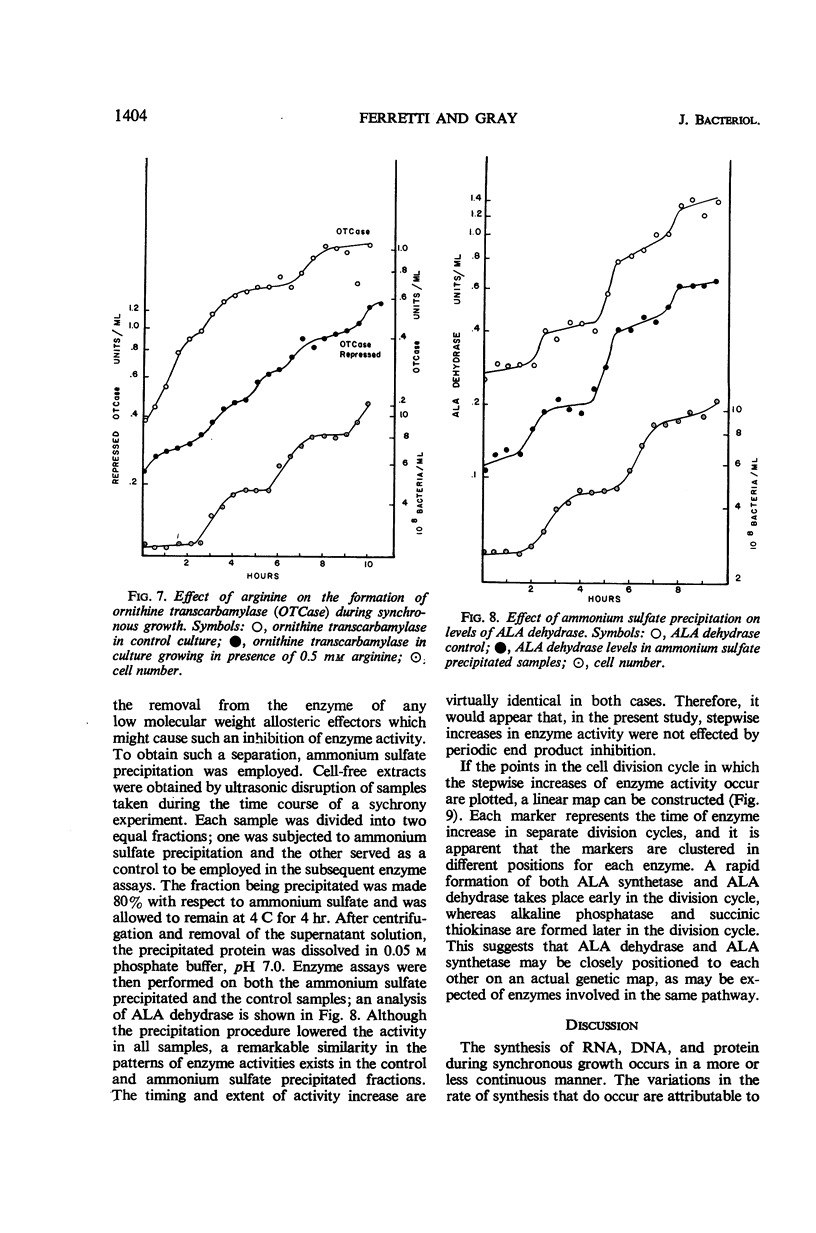
The synthesis of various cell components was examined during the anaerobic photosynthetic growth of synchronous populations of Rhodopseudomonas spheroides. Net deoxyribonucleic acid (DNA), ribonucleic acid (RNA), and protein increased continuously as did the rate of incorporation of radioactive precursors into protein. The rates of incorporation of radioactive precursors into RNA and DNA were marked by abrupt discontinuities. It is not clear whether these discontinuities represent changes in rates of synthesis or fluctuations in precursor pools. Although the synthesis of bacteriochlorophyll occurred in a continuous manner, those enzymes examined which are involved in the synthesis of tetrapyrroles, i.e., succinyl CoA thiokinase, δ-aminolevulinic acid synthetase, and δ-aminolevulinic acid dehydrase, increased discontinuously. Two other enzymes not involved in tetrapyrrole biosynthesis were examined. Alkaline phosphatase increased in a stepwise manner during the division cycle, whereas the synthesis of ornithine transcarbamylase increased rapidly before leveling off for a period of time until synthesis began again. In each instance of discontinuous enzyme synthesis, increases occurred at regular and characteristic times during the division cycle. Ammonium sulfate precipitation was employed to remove low molecular weight end product inhibitors from enzyme preparations. These studies suggested that the stepwise increases in enzyme activity observed in the present investigation were not affected by periodic end product inhibition. A temporal map of enzyme synthesis during the division cycle was constructed. Both δ-aminolevulinic acid synthetase and δ-aminolevulinic acid dehydrase appeared early in the division cycle, whereas alkaline phosphatase and succinyl CoA thiokinase appeared later on.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altenbern R. A. Apparent genomic mapping of Staphylococcus aureus by a new method. Biochem Biophys Res Commun. 1966 Nov 11;25(3):346–353. doi: 10.1016/0006-291x(66)90784-4. [DOI] [PubMed] [Google Scholar]
- BULL M. J., LASCELLES J. The association of protein synthesis with formation of pigments in some photosynthetic bacteria. Biochem J. 1963 Apr;87:15–28. doi: 10.1042/bj0870015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNHAM B. F., PIERCE W. S., WILLIAMS K. R., BOYER M. H., KIRBY C. K. delta-aminolaevulate dehydratase from Rhodopseudomonas spheroides. Biochem J. 1963 Jun;87:462–472. doi: 10.1042/bj0870462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock C. J., Donachie W. D., Masters M., Mitchison J. M. Synthesis of enzymes and DNA in synchronous cultures of Schizosaccharomyces pombe. Nature. 1966 May 21;210(5038):808–810. doi: 10.1038/210808a0. [DOI] [PubMed] [Google Scholar]
- CAIRNS J. The bacterial chromosome and its manner of replication as seen by autoradiography. J Mol Biol. 1963 Mar;6:208–213. doi: 10.1016/s0022-2836(63)80070-4. [DOI] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- Cutler R. G., Evans J. E. Synchronization of bacteria by a stationary-phase method. J Bacteriol. 1966 Feb;91(2):469–476. doi: 10.1128/jb.91.2.469-476.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donachie W. D. Control of enzyme steps during the bacterial cell cycle. Nature. 1965 Mar 13;205(976):1084–1086. doi: 10.1038/2051084a0. [DOI] [PubMed] [Google Scholar]
- Ferretti J. J., Gray E. D. Control of enzyme synthesis during adaptation in synchronously dividing populations of Rhodopseudomonas spheroides. Biochem Biophys Res Commun. 1967 Nov 30;29(4):501–507. doi: 10.1016/0006-291x(67)90512-8. [DOI] [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- GORINI L., MAAS W. K. The potential for the formation of a biosynthetic enzyme in Escherichia coli. Biochim Biophys Acta. 1957 Jul;25(1):208–209. doi: 10.1016/0006-3002(57)90450-x. [DOI] [PubMed] [Google Scholar]
- Gorman J., Taruo P., LaBerge M., Halvorson H. Timing of enzyme synthesis during synchronous division in yeast. Biochem Biophys Res Commun. 1964 Feb 18;15(1):43–49. doi: 10.1016/0006-291x(64)90100-7. [DOI] [PubMed] [Google Scholar]
- Goto K., Higuchi M., Sakai H., Kikuchi G. Differential inhibition of induced syntheses of delta-aminolevulinate synthetase and bacteriochlorophyll in dark-aerobically grown Rhodopseudomonas spheroides. J Biochem. 1967 Feb;61(2):186–192. doi: 10.1093/oxfordjournals.jbchem.a128530. [DOI] [PubMed] [Google Scholar]
- Gray E. D. Studies on the adaptive formation of photosynthetic structures in Rhodopseudomonas spheroides. I. Synthesis of macromolecules. Biochim Biophys Acta. 1967 May 30;138(3):550–563. doi: 10.1016/0005-2787(67)90551-5. [DOI] [PubMed] [Google Scholar]
- HALVORSON H., GORMAN J., TAURO P., EPSTEIN R., LABERGE M. CONTROL OF ENZYME SYNTHESIS IN SYNCHRONOUS CULTURES OF YEAST. Fed Proc. 1964 Sep-Oct;23:1002–1008. [PubMed] [Google Scholar]
- LASCELLES J. Adaptation to form bacteriochlorophyll in Rhodopseudomonas spheroides: changes in activity of enzymes concerned in pyrrole synthesis. Biochem J. 1959 Jul;72:508–518. doi: 10.1042/bj0720508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LASCELLES J. The synthesis of enzymes concerned in bacteriochlorophyll formation in growing cultures of Rhodopseudomonas spheroides. J Gen Microbiol. 1960 Dec;23:487–498. doi: 10.1099/00221287-23-3-487. [DOI] [PubMed] [Google Scholar]
- LEHRER H. I., JONES M. E. Repression of ornithine transcarbamoyltransferase of Bacillus subtilis. Biochim Biophys Acta. 1962 Dec 4;65:360–362. doi: 10.1016/0006-3002(62)91059-4. [DOI] [PubMed] [Google Scholar]
- MAUZERALL D., GRANICK S. The occurrence and determination of delta-amino-levulinic acid and porphobilinogen in urine. J Biol Chem. 1956 Mar;219(1):435–446. [PubMed] [Google Scholar]
- Masters M., Donachie W. D. Repression and the control of cyclic enzyme synthesis in Bacillus subtilis. Nature. 1966 Jan 29;209(5022):476–479. doi: 10.1038/209476a0. [DOI] [PubMed] [Google Scholar]
- Masters M., Kuempel P. L., Pardee A. B. Enzyme synthesis in synchronous cultures of bacteria. Biochem Biophys Res Commun. 1964 Feb 18;15(1):38–42. doi: 10.1016/0006-291x(64)90099-3. [DOI] [PubMed] [Google Scholar]
- Masters M., Pardee A. B. Sequence of enzyme synthesis and gene replication during the cell cycle of Bacillus subtilis. Proc Natl Acad Sci U S A. 1965 Jul;54(1):64–70. doi: 10.1073/pnas.54.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGATA T. The molecular synchrony and sequential replication of DNA in Escherichia coli. Proc Natl Acad Sci U S A. 1963 Apr;49:551–559. doi: 10.1073/pnas.49.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGERS P., NOVELLI G. D. Formation of ornithine transcarbamylase in cells and protoplasts of Escherichia coli. Biochim Biophys Acta. 1959 Jun;33(2):423–436. doi: 10.1016/0006-3002(59)90132-5. [DOI] [PubMed] [Google Scholar]
- SCHERBAUM O. H. COMPARISON OF SYNCHRONOUS AND SYNCHRONIZED CELL DIVISION. Exp Cell Res. 1964 Jan;33:89–98. doi: 10.1016/s0014-4827(64)81016-8. [DOI] [PubMed] [Google Scholar]
- Tauro P., Halvorson H. O. Effect of gene position on the timing of enzyme synthesis in synchronous cultures of yeast. J Bacteriol. 1966 Sep;92(3):652–661. doi: 10.1128/jb.92.3.652-661.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]


